Reactive oxygen species (ROS) are a consequence of living in an oxygen-rich atmosphere and are byproducts of oxygen metabolism.1 ROS can be generated from both endogenous and exogenous sources such as mitochondria2 and ionizing radiation,3 respectively. ROS include superoxide (O2•−) and hydrogen peroxide (H2O2) and are important for the execution of many normal cellular processes, including cell growth and differentiation, adhesion, immune responses and apoptosis. Low levels of ROS are essential for carrying out these cellular functions. However, over-production of ROS and generation of additional highly reactive ROS, such as hydroxyl (•OH) radicals, numerous diseases, including arthritis, various neurological disorders and cancer.4
ROS resulting from ionizing radiation are formed from either direct interactions with cellular targets or radiolysis of water, leading to DNA damage and eventually cell death.3 The effects of ionizing radiation on cells make it a powerful tool for treating cancer. Nevertheless, the exposure of normal tissues to ionizing radiation can have deleterious consequences days, months and even years after exposure due to chronic oxidative stress,4 increasing the risk of cancer development.3
The cell is equipped with a variety of enzyme systems to detoxify ROS.4 Superoxide dismutases (SODs), primary ROS detoxification enzymes in the cell, catalyze the dismutation of superoxide radicals to molecular oxygen and hydrogen peroxide.5,6 The SOD family of enzymes is made up of three structurally unrelated proteins encoded by different genes.4 Copper- and zinc-containing SOD (CuZnSOD, SOD1) is a homodimeric enzyme found primarily in the cytoplasm (with small amounts within the mitochondria).4 Extracellular SOD (ECSOD, SOD3) shares 40–60% homology with CuZnSOD but resides in the extracellular compartment of the cell.7 Manganese-containing SOD (MnSOD, SOD2) is a homotetramer found exclusively in the mitochondrial matrix.8
MnSOD is the only SOD indispensable to aerobic life and is not compensated by the presence of CuZnSOD. The importance of MnSOD is due to its strategic location in the mitochondrial matrix, as both ROS generation via respiration and ROS removal by respiration occur into the mitochondria. Increased expression of MnSOD protects E. coli B cells from hyperbaric concentrations of oxygen (20 atm) and confers resistance to streptonigrin (a superoxide-generating antibiotic).9 Similar results have been observed for the yeast strain Saccharomyces cerevisiae var. ellipsoideus.10 Deletion of the gene responsible for MnSOD production or expression of inactive mutants causes early death in both mouse11 and Drosophila12 models due to reduced mitochondrial activity, resulting in both neurological and cardiac abnormalities that lead to a diminished lifespan.13,14 Heterozygous MnSOD knockout mice have roughly a 50% reduction in MnSOD enzyme activity. This decrease in MnSOD activity does not affect the lifespan of these animals but results instead in an age-dependent increase in oxidative damage to both mitochondrial and nuclear DNA.15
The role of MnSOD in preventing cancer development has been documented. A seminal review by Oberley and Buettner16 identified reduced MnSOD expression in many types of cancer. Heterozygous MnSOD knock-out mice have a 100% increased risk of developing cancer compared to wild-type controls.15 Overexpression of MnSOD in cancer cells results in a more differentiated morphology, a reduced growth rate, and decreased tumorigenicity in a variety of in vitro and in vivo models. MnSOD affects cancer cell growth by altering the levels of ROS17 in these models and modulating the activity of transcription factors involved in both proliferation18,14 and apoptosis.19 Increased expression of MnSOD protects normal tissues and immortalized cells against neoplastic transformation by chemical carcinogens that generate ROS20 and ionizing radiation.21
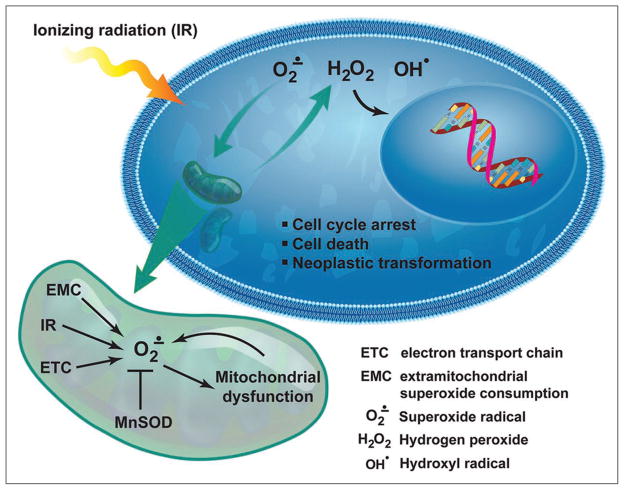
The current study by Du et al. in this issue of Cancer Biology & Therapy further confirms and expands on the importance of MnSOD as a tumor-suppressing enzyme in response to ionizing radiation. Mouse embryonic fibroblasts (MEFs) expressing wild-type MnSOD (SOD2+/+), or heterozygous (SOD2+/−) or homozygous (SOD2−/−) MEF knockouts, were irradiated with equitoxic doses (10% survival) of ionizing radiation. SOD2−/− MEFS produced significantly higher ROS levels 72 hr post-irradiation, had more micronuclei (a marker of DNA damage), exited from the G2 phase of the cell cycle more rapidly, and demonstrated greater transformation efficiency compared to wild-type controls. These results suggest that mitochondria-generated ROS induced after irradiation are important for the late response of cells to ionizing radiation and the neoplastic transformation of these cells. This study, while lacking experimental evidence revealing the mechanisms of MnSOD-mediated protection from the late effects of ionizing radiation, demonstrates the importance of MnSOD in attenuating the effects of ionizing radiation on the transformation of normal cells long after the exposure to radiation (Fig. 01). Du et al.’s findings imply that mitochondrial ROS are a key component of the general signaling pathways that detect cellular damage and translate them into biological responses. It also provides an important framework for future studies assessing the mechanisms of MnSOD-mediated protection against the late effects of ionizing radiation, which may eventually lead to improved methodologies for cancer radio-therapy that diminish the harmful effects of ionizing radiation on normal tissues long after the radiation exposure.
Figure 1.
A model depicting how MnSOD acts to prevent neoplastic transformation.
References
- 1.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–80. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 2.Kakkar P, Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem. 2007;305:235–53. doi: 10.1007/s11010-007-9520-8. [DOI] [PubMed] [Google Scholar]
- 3.Mettler FA, Upton AC. Medical Effects of Ionizing Radiation. Philadelphia, PA: Saunders Elsvier; 2008. [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 5.Klug D, Rabini J. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972;247:4839–42. [PubMed] [Google Scholar]
- 6.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–4. [PubMed] [Google Scholar]
- 7.Hjalmarsson K, Marklund SL, Engstrom A, Edlund T. Isolation and sequence of complimentary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci USA. 1987;84:6340–4. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–6. [PubMed] [Google Scholar]
- 9.Gregory EM, Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973;114:1193–7. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory EM, Goscin SA, Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974;117:456–60. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Huang T-T, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995:11. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 12.Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in drosophila. Genetics. 2003;165:2295–9. doi: 10.1093/genetics/165.4.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul A, Belton A, Nag S, Martin I, Grotewiel MS, Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128:706–16. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, et al. Neurodegeneration, mycardial injury and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–7. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiolog Genom. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 16.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–9. [PubMed] [Google Scholar]
- 17.Oberley LW. Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomed Pharmacotherap. 2005;59:143–8. doi: 10.1016/j.biopha.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Kiningham KK, St Clair DK. Overexpression of manganese superoxide dismutase selectively modulates the activity of Jun-associated transcription factors in fibrosarcoma cells. Cancer Res. 1997;57:5265–71. [PubMed] [Google Scholar]
- 19.Zhao Y, Oberley TD, Chaiswing L, Lin S-m, Epstein CJ, Huang T-T, et al. Manganese superoxide dismutase deficiency enhances cell turnover via tumor promoter-induced alterations in AP-1 and p53-mediated pathways in a skin cancer model. Oncogene. 2002;21:3836–46. doi: 10.1038/sj.onc.1205477. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Xue Y, Oberley TD, Kiningham KK, Lin S-M, Yen H-C, et al. Overexpression of manganese superoxide dismutase suppresses tumor formation by modulation of activator protein-1 signaling in a multistage skin carcinogenesis model. Cancer Res. 2001;61:6082–8. [PubMed] [Google Scholar]
- 21.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcinogen. 1992;6:238–42. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- 22.Du C, Gao Z, Venkatesha VA, Kalen AL, Chaudhuri L, Spitz DR, et al. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol Therap. 2009 doi: 10.4161/cbt.8.20.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]