Abstract
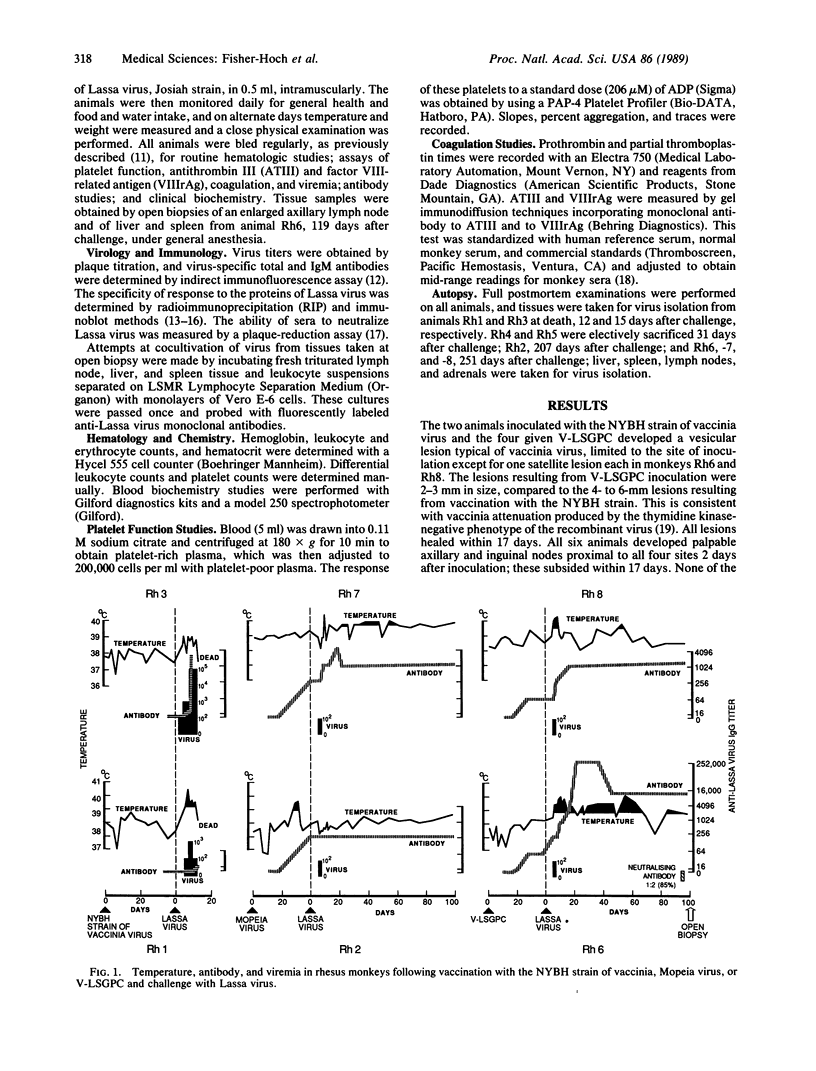
Lassa fever is an acute febrile disease of West Africa, where there are as many as 300,000 infections a year and an estimated 3000 deaths. As control of the rodent host is impracticable at present, the best immediate prospect is vaccination. We tested as potential vaccines in rhesus monkeys a closely related virus, Mopeia virus (two monkeys), and a recombinant vaccinia virus containing the Lassa virus glycoprotein gene, V-LSGPC (four monkeys). Two monkeys vaccinated with the New York Board of Health strain of vaccinia virus as controls died after challenge with Lassa virus. The two monkeys vaccinated with Mopeia virus developed antibodies measurable by radioimmunoprecipitation prior to challenge, and they survived challenge by Lassa virus with minimal physical or physiologic disturbances. However, both showed a transient, low-titer Lassa viremia. Two of the four animals vaccinated with V-LSGPC had antibodies to both Lassa glycoproteins, as determined by radioimmunoprecipitation. All four animals survived a challenge of Lassa virus but experienced a transient febrile illness and moderate physiologic changes following challenge. Virus was recoverable from each of these animals, but at low titer and only during a brief period, as observed for the Mopeia-protected animals. We conclude that V-LSGPC can protect rhesus monkeys against death from Lassa fever.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auperin D. D., Esposito J. J., Lange J. V., Bauer S. P., Knight J., Sasso D. R., McCormick J. B. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988 Feb;9(2-3):233–248. doi: 10.1016/0168-1702(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Auperin D. D., Sasso D. R., McCormick J. B. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology. 1986 Oct 15;154(1):155–167. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch S. P., Mitchell S. W., Sasso D. R., Lange J. V., Ramsey R., McCormick J. B. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J Infect Dis. 1987 Mar;155(3):465–474. doi: 10.1093/infdis/155.3.465. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Peters C. J., Stephen E. L. Enhanced treatment of Lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis. 1984 Mar;149(3):420–427. doi: 10.1093/infdis/149.3.420. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kiley M. P., Lange J. V., Johnson K. M. Protection of rhesus monkeys from Lassa virus by immunisation with closely related Arenavirus. Lancet. 1979 Oct 6;2(8145):738–738. doi: 10.1016/s0140-6736(79)90659-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCormick J. B. Epidemiology and control of Lassa fever. Curr Top Microbiol Immunol. 1987;134:69–78. doi: 10.1007/978-3-642-71726-0_3. [DOI] [PubMed] [Google Scholar]
- McCormick J. B., King I. J., Webb P. A., Johnson K. M., O'Sullivan R., Smith E. S., Trippel S., Tong T. C. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987 Mar;155(3):445–455. doi: 10.1093/infdis/155.3.445. [DOI] [PubMed] [Google Scholar]
- McCormick J. B., King I. J., Webb P. A., Scribner C. L., Craven R. B., Johnson K. M., Elliott L. H., Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986 Jan 2;314(1):20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- McCormick J. B., Webb P. A., Krebs J. W., Johnson K. M., Smith E. S. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987 Mar;155(3):437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- Monath T. P., Newhouse V. F., Kemp G. E., Setzer H. W., Cacciapuoti A. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science. 1974 Jul 19;185(4147):263–265. doi: 10.1126/science.185.4147.263. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Elango N., Prince G. A., Murphy B. R., Johnson P. R., Moss B., Chanock R. M., Collins P. L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. E., Fisher-Hoch S. P., Craven R. B., McCormick J. B. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ. 1988 Sep 3;297(6648):584–587. doi: 10.1136/bmj.297.6648.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Wulff H., Lange J. V., Murphy F. A. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull World Health Organ. 1975;52(4-6):523–534. [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H., McIntosh B. M., Hamner D. B., Johnson K. M. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull World Health Organ. 1977;55(4):441–444. [PMC free article] [PubMed] [Google Scholar]



