Abstract
Objective
Brain death induces dramatic changes in hemodynamics. Ischemic injury and inflammation resulting from inadequate resuscitation might influence organ yield for transplantation. Using functional hemodynamic monitoring in brain-dead organ donors, we test the hypothesis that donor preload (fluid) responsiveness is associated with increased inflammatory response and lower organ yield for transplantation.
Design
Prospective, observational, pilot study.
Setting
A large intensive care unit of a university hospital in the United States.
Patients
Twenty-one brain-dead organ donors between July 2006 and April 2007.
Interventions
None.
Measurements and Main Results
Following declaration of brain death, we collected data on donor demographics, mechanism of brain death, and number of organs procured and transplanted. Functional hemodynamics were monitored using pulse contour analysis technique. Plasma tumor necrosis factor, interleukin-6, and interleukin-10 concentrations were measured at study enrollment, after 4 hrs, and immediately before organ procurement for transplantation. Preload responsiveness (pulse pressure variation >13%) was observed in 48% of donors (mean ± sd pulse pressure variation, 19.2% ± 4.8%). Plasma interleukin-6 and tumor necrosis factor concentrations at study enrollment were greater in preload responsive donors: mean concentrations of interleukin-6 in preload responsive vs. unresponsive donors were 5420 ± 9102 vs. 378 ± 631 pg/mL (p = .009), and mean concentrations of tumor necrosis factor were 60.5 ± 103.6 vs. 15.7 ± 10.1 pg/mL (p = .048). Preload responsive compared with unresponsive donors had significantly increased interleukin-6 (p = .013) and tumor necrosis factor (p = .044) concentrations over time. Fewer organs were transplanted from preload responsive donors: mean organs transplanted from preload responsive vs. unresponsive donors were 1.8 ± 0.9 vs. 3.7 ± 2.5 (p = .034). In multivariable regression, older donor age (p = .028) and increased plasma interleukin-6 concentration (p = .035) were significantly associated with lower number of organs transplanted.
Conclusions
Preload responsiveness is common in brain-dead organ donors and is associated with higher inflammatory response and lower organ yield. A controlled trial of preload optimization is warranted in brain-dead donors. (Crit Care Med 2009; 37:2387–2393)
Keywords: brain-dead organ donors, inflammation, organ transplantation, interleukin-6, cytokine, pulse pressure variation
Solid organ transplantation is currently the only definitive treatment option available for patients with end-stage organ failure. In the United States, approximately 100,000 candidates were on the transplant waiting list for various organs in 2008 (1). However, there were only 5,000 brain-dead organ donors, who donated approximately >14,000 organs for transplantation, an average of only 2.8 organs per donor (1). This large disparity between the transplant waiting-list candidates and the limited number of available organs poses a significant public health crisis. Although there are many reasons why not all organs are transplanted, such as donor age, comorbidities, and inflammation (2), hemodynamic instability in brain-dead organ donors is an important cause (3, 4).
Hemodynamic instability in potential organ donors is caused by several factors, such as autonomic dysfunction, hypovolemia, cardiac dysfunction, release of vasoactive inflammatory molecules, and secondary adrenal insufficiency (5), and may result in ischemia and reperfusion injury leading to organ dysfunction and loss (6). Hemodynamic instability can lead to increased inflammation and cardiac ischemia, which in turn can result in further hemodynamic instability, producing a vicious cycle (7–9). Therefore, optimal donor resuscitation may salvage many borderline organs and is critical to improve the number and quality of organs for transplantation (10). However, little is known about organ donor resuscitation practices because many donors are not invasively monitored.
Although resuscitation with fluids, vasopressors, and inotropes is the only practical and effective management strategy available to optimize donor hemodynamics, traditional tools for assessing preload and preload (fluid) responsiveness (i.e., increase in cardiac output following fluid infusion) using central venous pressure and pulmonary artery occlusion pressure are notoriously inaccurate (11, 12). Recently, less invasive, functional hemodynamic variables, such as pulse pressure variation (PPV), derived from the arterial waveform analysis, have been found to be more sensitive and specific in assessing preload responsiveness (13).
In this observational pilot study in brain-dead organ donors, we use PPV to assess donor preload responsiveness and test two hypotheses: 1) donor preload responsiveness is associated with increased inflammatory response; and 2) donor preload responsiveness is associated with lower number of organs procured and transplanted. Although preload responsiveness is a normal state, it also exists in hypovolemia, and we hypothesized that in resuscitation of organ donors, the presence of preload responsiveness would be a marker of underresuscitation. We have previously shown that inflammation in the donor, as measured by circulating interleukin (IL)-6 concentration, is associated with decreased organ yield and reduced hospital-free survival in recipients of organs from donors with increased IL-6 (2).
Materials and Methods
Study Design and Population
Prior to executing a large randomized clinical trial of protocolized resuscitation of organ donors, we conducted this prospective, observational, pilot study in 21 brain-dead organ donors admitted to the intensive care unit (ICU). The pilot study was approved by the Committee for Oversight of Research Involving the Dead and was conducted in coordination with the local organ procurement organization (OPO) between July 25, 2006, and April 24, 2007 at the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania. We initially intended to enroll 30 subjects over a period of 9 months based on our prior study in organ donors (2). However, because enrollment was slower than expected, we concluded the study as planned at 9 months after enrolling 21 subjects. Because this was a pilot study, we did not attempt to power the study for the outcome.
Patients were pronounced brain-dead by the clinical team providing care as per the local hospital criteria for brain death. Once consent for organ donation was provided by next of kin, consent for participation in the study was obtained from the same individuals. We established 11 exclusion criteria in regard to donors: 1) age <16 yrs; 2) inability to obtain informed consent; 3) deemed unsuitable for organ donation by OPO; 3) HIV infection; 4) leucopenia; 5) received cancer chemotherapy 7 days preceding brain death; 6) receiving antileukocyte and cytokine-modulating drug therapy; 7) receiving lithium therapy; 8) non-heart-beating organ donor; 9) patients on venoarterial extracorporeal membrane oxygenator; 10) pregnancy; and 11) conditions in which pulse contour waveform analysis has been shown to be inaccurate, such as atrial arrhythmias, aortic regurgitation, patients with intra-aortic balloon pump, and intracardiac shunt.
Study Procedures
We recorded donor demographics, comorbidities, etiology of brain death, and severity of illness at study enrollment. All donors were managed by the clinical team caring for them in cooperation with the local OPO coordinator. Following enrollment, all donors were connected to a hemodynamic monitor (LiDCOplus, Cambridge, UK), which provides continuous beat-to-beat assessment of functional hemodynamic profile from an indwelling arterial catheter (14), in addition to regular hemodynamic monitoring in the ICU. The LiDCO hemodynamic monitor uses a continuous arterial pulse contour analysis system that estimates functional hemodynamic variables, such as PPV, by analyzing and processing the arterial pressure signal obtained from the primary blood pressure monitor. PPV was measured using validated method by Michard et al (15). Donors were deemed to be preload responsive if they had PPV >13% and unresponsive if they had PPV ≤13% for >20 secs. Cardiac output was estimated using lithium indicator dilution technique after a single-point calibration once every 24 hrs.
The organ procurement coordinators, the clinical team providing care, and the transplant surgeons were blinded to all functional hemodynamic data via use of monitor shields as well as changing the display screen. No interventions were performed using functional hemodynamic data, and monitoring was continued until transfer to the operating room for organ explantation. The surgical team along with the OPO made decisions regarding organ procurement as well as transplantation. The study investigators performed no interventions and followed all donors until transfer to the operating room for organ procurement.
Cytokine Measurement
Specimens for measurement of plasma cytokines (IL-6, IL-10, and tumor necrosis factor [TNF]) were obtained from all donors immediately following enrollment into the study (baseline), after the first 4 hrs following baseline, and again just before organ procurement. Samples were drawn from preexisting arterial cannula. We drew each blood sample into pyrogen-free vials containing heparin, separated the plasma by centrifugation, and divided the plasma into four separate 1.5-mL tubes, which we stored frozen at −80°C until they were assayed in batches. We measured cytokines using an automated immunoassay analyzer (IMMULITE System, Siemens Medical Solutions Diagnostics, Deerfield, IL). All laboratory personnel were blinded to clinical information and donor hemodynamics.
Statistical Methods
We compared baseline characteristics, hemodynamics, and organs transplanted between preload responsive and unresponsive donors. We used Student's t test to compare normally distributed continuous variables and chi-square test for categorical variables with continuity correction or non-parametric counterpart. For modeling purposes, cytokine data were log-transformed (natural logarithm) to reduce skewness. Longitudinal cytokine concentrations for each donor and their association with other patient characteristics were modeled using generalized estimating equations. The generalized estimating equation analysis takes into account the correlation between repeated observations from the same donor. Cytokine levels over time were modeled as a linear function of age, gender, and preload responsiveness. To assess the relationship between the number of organs transplanted and donor characteristics such as age, gender, and plasma cytokine concentration, we used a log-linear regression (Poisson regression) of number of organs transplanted (as the dependant variable) on explanatory variables. In the Poisson regression model, the natural log of the mean number of organs transplanted is assumed to be a linear function of the donor characteristics. For all analyses, the level of statistical significance was assumed to be 5%, and all analyses were performed using statistical software packages SAS 9.1 (SAS, Cary, NC).
Results
Donor Characteristics and Hemodynamics
Table 1 shows donor demographics, mechanism of brain death, severity of illness, duration of ICU stay, and organs transplanted, stratified by donor preload response characteristics. Preload responsive donors (PPV >13%) were older, predominantly male gender, and had significant history of hypertension when compared with preload unresponsive donors (PPV ≤13%). Preload responsive donors received less fluid resuscitation when compared with preload unresponsive donors, although this was not statistically significant. Similarly, there was no difference in total fluids infused among donors who had history of hypertension and those who did not (p = 0.93). Seven donors (35%) had developed diabetes insipidus after brain death, and the distribution of diabetes insipidus between preload responsive and unresponsive donors was not different.
Table 1.
Demographic and clinical characteristics of brain-dead organ donors
| Characteristic | Preload Unresponsive Donors (PPV ≤13%) (n = 11) | Preload Responsive Donors (PPV >13%) (n = 10) | All Donors (n = 21) | p Value |
|---|---|---|---|---|
| Age, yrs, mean (sd) | 48.5 (20) | 58.2 (13.5) | 53 (18) | NS |
| Male gender, n (%) | 5 (45.5) | 7 (70) | 12 (58) | NS |
| Race, n (%) | ||||
| Caucasian | 9 (82) | 7 (70) | 16 (76) | NS |
| African American | 2 (18) | 3 (30) | 5 (24) | |
| Mechanism of brain death, n (%) | ||||
| Intracerebral hemorrhage | 4 (36.4) | 4 (40) | 8 (38.2) | NS |
| Stroke | — | 1 (10) | 1 (5) | |
| Subarachnoid hemorrhage | 2 (18.2) | 3 (30) | 5 (24.1) | |
| Head injury | 1 (9.1) | 1 (10) | 2 (9.6) | |
| Gunshot injury | 2 (18.2) | 1 (10) | 3 (14.1) | |
| Other | 2 (18.2) | — | 2 (9.1) | |
| Acute Physiology and Chronic Health Evaluation II score, mean (sd) | 22.4 (5.4) | 19.2 (9.4) | 21 (7.4) | NS |
| Comorbidities, n (%) | ||||
| Hypertension | 3 (27.3) | 7 (70) | 10 (48.6) | .05 |
| Diabetes mellitus | 3 (27.27) | 2 (20) | 5 (23.6) | NS |
| Peripheral vascular disease | 1 (9.1) | 1 (10) | 2 (9.6) | NS |
| Smoking history | 7 (64) | 4 (40) | 11 (52) | NS |
| Coronary artery disease | 2 (18.2) | — | 2 (9.1) | NS |
| Chronic kidney disease | 1 (9.1) | — | 1 (4.5) | NS |
| Cancer history | 3 (27.3) | 1 (10) | 4 (18.3) | NS |
| Medication use, n (%) | ||||
| Corticosteroid | 9 (82) | 5 (50) | 14 (66) | NS |
| Thyroxine | 11 (100) | 9 (90) | 20 (95) | NS |
| Fluid balance, L, mean (sd) | ||||
| Total input | 6.3 (3.4) | 5.0 (3.3) | 5.8 (3.3) | NS |
| Urine output | 3.5 (2.1) | 3.9 (2.9) | 3.7 (2.40) | NS |
| Net balance | 2.7 (2.31) | 1.4 (1.6) | 2.1 (2.1) | NS |
| Time from brain death to first cytokine assay, hrs, mean (sd) | 8.4 (8.6) | 5.1 (2.9) | 6.8 (6.4) | NS |
| Time from brain death to organ procurement, hrs, mean (sd) | 20.3 (8.0) | 19.3 (9.5) | 20 (8.4) | NS |
| Organs | ||||
| Procured, n (mean) | 52 (4.7) | 32 (3.2) | 84 (4.0) | NS |
| Transplanted, n (mean) | 41 (3.7) | 18 (1.8) | 59 (2.8) | .034 |
PPV, pulse pressure variation; NS, not significant.
The average PPV among all the donors for the entire duration of the study was 13.5% ± 7%, and 48% of all donors were preload responsive characterized by mean PPV >13%. Table 2 shows the distribution of donor hemodynamics by preload response characteristics. The average PPV among preload responsive and unresponsive donors were 19.2% ± 5% and 8.1% ± 3.4%, respectively. Traditional hemodynamic variables, such as heart rate, blood pressure, central venous pressure, cardiac index, lactate, and superior vena caval oxygen saturation, were not different between the two groups. Of the entire cohort, 81% required vasopressors to maintain mean arterial pressure of 65 mm Hg. However, preload responsive donors required significantly higher vasopressor doses to maintain mean arterial pressure when compared with preload unresponsive donors (p = .04).
Table 2.
Donor hemodynamics by preload response characteristics
| Hemodynamic Variablea | Preload Unresponsive Donors (PPV ≤13%) (n = 11) | Preload Responsive Donors (PPV >13%) (n = 10) | All Donors (n = 21) | p Value |
|---|---|---|---|---|
| Heart rate, beats/min | 91 (17) | 107 (17) | 95.3 (17.3) | NS |
| Systolic blood pressure, mm Hg | 134 (25.5) | 131.4 (19.4) | 133 (22.4) | NS |
| Diastolic blood pressure, mm Hg | 64.3 (18) | 66 (10.5) | 65 (15) | NS |
| Mean arterial pressure, mm Hg | 88 (19.5) | 84 (13) | 86 (16) | NS |
| Cardiac index, L/min/m2 | 4.3 (3) | 3.3 (1.2) | 3.8 (2.2) | NS |
| Stroke volume index, mL/m2/beat | 49 (36.2) | 31.4 (9.6) | 41 (28.4) | NS |
| Systemic vascular resistance index, dyne·sec/cm5/m2 | 2008.5 (1071) | 2267 (917.66) | 2125 (987) | NS |
| Oxygen delivery index, mL/min/m2 | 585.2 (365) | 468.6 (214) | 526.9 (290) | NS |
| PPV, % | 8.1 (3.4) | 19.2 (5.0) | 13.5 (7.0) | — |
| Superior vena caval oxygen saturation, % | 87.4 (8.6) | 86 (6.3) | 87 (7.6) | NS |
| Lactate, mmol/L | 2.6 (0.8) | 2.7 (2.5) | 2.7 (1.8) | NS |
| Vasopressor, norepinephrine unitsb | 0.1 (0.3) | 0.4 (0.4) | 0.2 (0.4) | .04 |
PPV, pulse pressure variation; NS, not significant.
All hemodynamic variables are reported as mean (sd);
vasopressor doses among various donors were standardized using the following criteria: We assumed 5 μg/kg/min of dopamine = 1 μg/kg of phenylephrine = 0.2 norepinephrine units, and 0.04 μg/min of vasopressin = 0.3 norepinephrine units.
Fourteen donors had central venous pressure >12 mm Hg and were thought by OPO coordinators to have been adequately resuscitated; among these donors the mean PPV was 14% ± 5.7%. There were 14 donors in the entire cohort who received corticosteroids, and no difference in mean PPV was found among donors who received corticosteroids (12.5% ± 7.4%) and donors who did not (15.2% ± 6.2%) (p = 0.42). Twenty donors received thyroxine supplementation, and the mean PPV among the donors who received thyroxine supplementation was 13% ± 6.6%.
Cytokine Response Between Preload Responsive Versus Unresponsive Donors
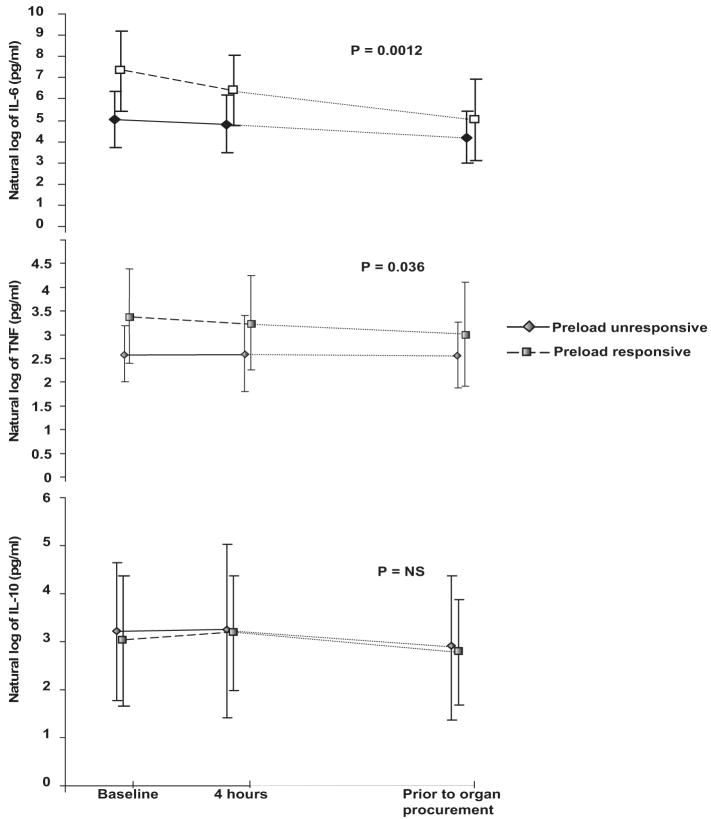
All donors exhibited increased plasma concentrations of IL-6, IL-10, and TNF following brain death, and these patterns persisted until organ procurement (Fig. 1). At study enrollment, preload responsive donors had significantly elevated concentrations of plasma IL-6 when compared with preload unresponsive donors (5420 ± 9102 vs. 378 ± 631 pg/mL, p = .009). Increased plasma concentrations of IL-6 were significantly correlated with elevated PPV (r = .4, p = .038). On average, from the time of study enrollment to organ procurement, preload responsive donors had a larger increase in log IL-6 concentrations when compared with preload unresponsive donors (mean difference 1.7 log, p = .013) (Table 3; Figs. 1 and 2. Although log IL-6 concentrations decreased over time for all donors (rate of decline 1/10 of a log per hour for entire cohort, p < .0001), the rate of decline did not differ between preload responsive and unresponsive donors (data not shown). Female donors had a trend toward increased log IL-6 concentrations over time when compared with male donors (p = .067). Although corticosteroid administration lowered IL-6 concentration, this was not statistically significant (Table 3).
Figure 1.
Cytokine response in brain-dead organ donors. Plasma cytokine concentrations in natural logarithm scale between preload responsive (dashed lines with squares) and preload unresponsive (solid line with diamonds) donors. Baseline represents the cytokine level at enrollment in the study, and 4 hrs is the time following baseline. The cytokine level before organ procurement represents the level just before the explantation of organs. Plasma concentrations of interleukin (IL)-6 and tumor necrosis factor (TNF) were significantly (p = .0012 and p = .036, respectively) increased in preload responsive donors compared with preload unresponsive donors over time. Log indicates the natural logarithm of individual cytokine levels.
Table 3.
Longitudinal analysis of cytokine concentrations over time
| Characteristics | Interleukin-6 Slope Estimatea (95% Confidence Interval) | p Value | Tumor Necrosis Factor Slope Estimatea (95% Confidence Interval) | p Value |
|---|---|---|---|---|
| Donor age, per 5 yrs | 0.06 (−0.08, 0.20) | .44 | −0.02 (−0.10, 0.06) | .64 |
| Female gender | 0.92 (−0.06, 1.91) | .067 | 0.56 (−0.25, 1.30) | .19 |
| Time after brain death, hrs | −0.10 (−0.14, −0.06) | <.0001 | −0.008 (−0.019, 0.003) | .14 |
| Corticosteroid use | −0.25 (−1.57, 1.08) | .71 | −0.37 (−1.05, 0.32) | .29 |
| Preload responsiveness | 1.69 (0.36, 3.03) | .013 | 0.78 (0.02, 1.53) | .044 |
Cytokine concentrations are expressed as natural logarithms in pg/mL and modeled as a linear function of donor age, gender, corticosteroid use, duration after brain death, and preload responsiveness over time. Log IL-6 levels over time are not significantly associated with donor age or corticosteroid use. However, female donors have a trend of increased log IL-6 concentrations compared with male donors over time (p = .067). On average, preload responsive donors have significantly higher log IL-6 concentrations compared with preload unresponsive donors (mean difference 1.7 log, p = .013). Whereas log IL-6 concentrations decreased over time for all donors (overall rate of decline 1/10 of a log per hour, p < .0001), the rate of decline was not different between preload responsive and unresponsive donors (data not shown). Similarly, log TNF concentrations were significantly higher for preload responsive donors compared with preload unresponsive donors (p = .044); however, there were no differences in TNF concentrations by age, gender, corticosteroid use, or duration after brain death.
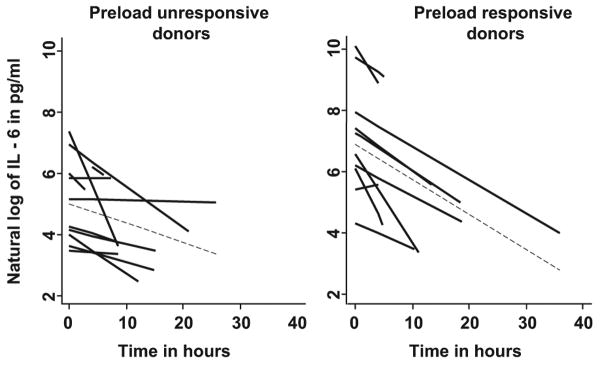
Figure 2.
Spaghetti plots illustrating interleukin (IL)-6 concentrations over time in individual donors. Preload responsive donors had higher log IL-6 concentrations at baseline compared with preload unresponsive donors. Time in hours represents the cytokine levels drawn after study enrollment. Log indicates the natural logarithm of individual IL-6 levels. Dashed lines indicate the mean slope of decline in IL-6 concentrations among all donors over time in the two groups.
Plasma TNF concentrations were higher in preload responsive donors compared with preload unresponsive donors at baseline (60.5 ± 103.6 vs. 15.7 ± 10.1 pg/mL, p = .048; Fig. 1), and these differences persisted over time between the two groups (p = .044; Table 3). TNF concentrations did not differ, however, by donor age, gender, corticosteroid use, or duration after brain death. Log IL-10 concentrations significantly differed over time (p < .0001) among the entire cohort of donors; however, the changes were similar across donor age, gender, corticosteroid use, and preload response characteristics (data not shown).
Organs Transplanted Between Preload Responsive Versus Unresponsive Donors
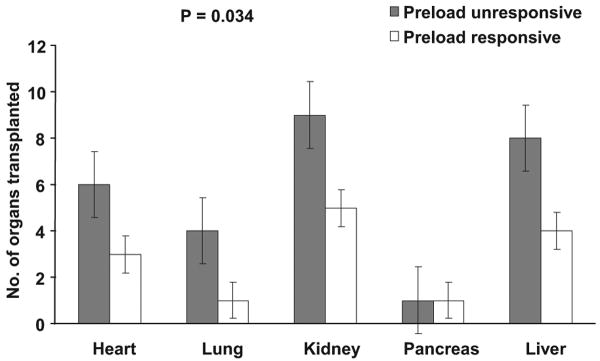
Number of organs transplanted from donors ranged from zero to eight, with an average of three organs per donor. On average, only 4 ± 2.1 organs were procured from each donor and only 2.8 ± 2.1 were transplanted. The youngest donor was 17 yrs old, from whom seven organs were transplanted, whereas the oldest donor was 75 yrs old and did not contribute any organ for transplantation. Preload responsive donors donated fewer organs for transplantation when compared with preload unresponsive donors: average number of organs transplanted from preload responsive vs. unresponsive donors was 1.8 ± 0.9 vs. 3.7 ± 2.5 (p = .034; Fig. 3). In unadjusted log-linear regression analysis, older age (p = .001), history of hypertension (p = .02), increased mean plasma log IL-6 (p = .003), mean plasma log TNF concentrations (p = .042), and preload responsiveness (p = .028) were significantly associated with lower number of organs transplanted (Table 4). In contrast, IL-10 concentrations did not predict the number of organs transplanted (data not shown).
Figure 3.
Solid organ transplantation by donor preload responsiveness. Preload responsive donors donated significantly fewer organs in every organ category when compared with preload unresponsive donors (p = .034). The p values refer to comparison of organs transplanted between preload responsive and unresponsive donors in the entire cohort. Error bars represent standard error.
Table 4.
Univariable and multivariable log-linear regression analysis on organ transplantation
| Covariate | Unadjusteda Slope Estimate (95% Confidence Interval) | p Value | Adjustedb Slope Estimate (95% Confidence Interval) | p Value |
|---|---|---|---|---|
| Donor age (per 5 yrs) | −0.12 (−0.19, −0.05) | .001 | v0.10 (−0.18, −0.01) | .028 |
| Female gender | −0.10 (−0.41, 0.61) | .70 | ||
| History of hypertension | −0.59 (−1.13, −0.06) | .02 | ||
| Acute Physiology and Chronic Health Evaluation II | −0.02 (−0.06, 0.01) | .17 | ||
| Vasopressor (norepinephrine units) | 0.20 (−0.43, 0.83) | .54 | ||
| Interleukin-6c | −0.28 (−0.47, −0.10) | .003 | −0.20 (−0.39, −0.01) | .035 |
| Tumor necrosis factorc | −0.37 (−0.74, −0.01) | .042 | ||
| Interleukin-10c | −0.05 (−0.26, 0.16) | .62 | ||
| Preload responsiveness | −0.62 (−1.18, −0.07) | .028 |
In unadjusted analysis, younger donor age was significantly associated with higher number of organs transplanted, such that for each 5-yr increase in donor age from mean age of 53 yrs there was an approximately 11% decrease in the number of organs transplanted. Higher mean IL-6 was also significantly associated with lower number of organs transplanted, such that mean number of organs transplanted decreased by about 25% for each 1-log pg/mL increase in mean IL-6 concentration from 5 log pg/mL to 6 log pg/mL. Similarly, increased mean TNF concentration was significantly associated with lower number of organs transplanted. On average, preload responsive donors donated fewer organs compared with unresponsive donors;
in multivariable analysis, younger donor age was significantly associated with higher number of organs transplanted such that for each 5-yr increase in donor age from the mean age of 53 yrs, there was a 10% decrease in the number of organs transplanted. Mean IL-6 concentration was also significantly associated with the number of organs transplanted. In particular, mean organ transplanted decreased by about 18% for each 1-log pg/mL increase in mean IL-6 concentration. Although mean TNF concentrations and preload responsiveness were significantly associated with lower number of organs transplanted, when adjusted for age, mean log IL-6 concentrations, history of hypertension, and vasopressors, the associations were not statistically significant. Variables that were not statistically significant (p > .05) predictors are not shown;
all cytokine concentrations are expressed in means of natural logarithm/pg/mL.
For every 5-yr increase in donor age, there was an approximately 11% decrease in the average number of organs transplanted. Higher mean log IL-6 concentrations were also significantly associated with lower numbers of organs transplanted, such that the number of mean organ transplanted decreased by about 25% for a 1-log pg/mL increase in mean IL-6 concentration from 5 log pg/mL to 6 log pg/mL. Similarly, increased mean TNF concentration was also significantly associated with lower number of organs transplanted. The number of organs transplanted from preload responsive donors was significantly lower than that of preload unresponsive donors (mean difference 0.62, p = .028).
In multivariable log linear regression analysis, younger donor age was significantly associated with higher number of organs transplanted such that for each 5-yr increase in donor age from the mean age of 53 yrs, there was a 10% decrease in the number of organs transplanted (p = .028). Mean IL-6 concentration was also significantly associated with the number of organs transplanted, such that the mean organ transplanted decreased by 18% for a 1-log pg/mL increase in mean IL-6 concentration from 5 log pg/mL to 6 log pg/mL. Although mean TNF concentration and preload responsiveness were significantly associated with lower number of organs transplanted in the univariable analysis, when adjusted for age, mean log IL-6 concentration, history of hypertension, and vasopressor use, mean TNF concentrations and preload responsiveness did not predict the number of organs transplanted.
Discussion
Our major finding is that nearly half of the brain-dead organ donors exhibited preload responsiveness. Given that the vast majority of donors are in shock, preload responsiveness is likely to equate with inadequate fluid resuscitation. Further support for this contention comes from the fact that preload responsiveness was significantly associated with increased IL-6 concentrations and lower organ yield for transplantation. To our knowledge, this is the first time a sensitive measure of functional hemodynamic monitoring has been shown to predict inflammatory response and organ outcome in brain-dead humans. However, our findings are consistent with other studies using functional hemodynamic monitoring in that nearly half of the hypotensive, critically ill patients in the ICU are preload responsive and therefore may have been inadequately fluid resuscitated (13, 15, 16).
Our results also confirm the findings of our prior study showing that brain death is associated with a massive inflammatory response, which is associated with lower organ yield (2). Our current study extends these prior results by establishing a relationship between volume status and inflammation. The inflammatory response associated with hypotension and ischemia may have directly contributed to poor viability, dysfunction, and organ loss. Indeed, many of the organs that were discarded after procurement were unusable because of organ dysfunction at the time of procurement, as assessed by biopsy; other physiologic variables; and quality of the organs, as assessed visually by the transplant surgeons.
Preload responsiveness has been demonstrated in critically ill patients with sepsis (15), after major high-risk surgery (17), and after cardiac surgery (18, 19). Indeed, preload responsive state in our brain-dead donors may have contributed to an increased cytokine response leading to more capillary leak and thus more hypovolemia. Supporting this hypothesis is the finding that despite fluid resuscitation, many of our donors continued to demonstrate preload responsiveness, remaining hypotensive on high-dose vasopressor support with persistent elevations of plasma IL-6 and TNF concentrations until organ procurement (Fig. 1). Preload responsiveness was associated with lower organ yield despite apparently “normal” standard hemodynamic variables (e.g., central venous pressure). Surprisingly, we found that although IL-10 concentrations were elevated in all donors, there was no difference in IL-10 concentration between preload responsive and unresponsive donors. We speculate that the stimulus for IL-10 release in donors may not have been affected by fluid resuscitation in the same way that the stimulus for IL-6 and TNF release is affected. This is perhaps not surprising, because IL-10 has a very different regulation pathway compared with IL-6 and TNF, which are closely linked to nuclear factor-κB DNA binding (20, 21).
There are important limitations to our study. First, because this was an exploratory, observational, pilot study, we were unable to infer causality between donor preload responsiveness, inflammatory response, and their association with organ transplantation. It is unclear whether the preload responsiveness causes increased IL-6 levels or vice versa or whether IL-6 or preload responsiveness is part of the causal pathway for lower number of organs transplanted. Second, it is unknown whether manipulating preload responsiveness (by giving fluid) will modulate IL-6 concentrations and increase organ yield. Further study is needed to examine the influence of fluid loading on inflammation and organ yield in preload responsive donors. Third, residual confounding cannot be excluded despite detailed evaluation of donor characteristics. Residual confounding may occur because we did not obtain more detailed organ specific indices of dysfunction in donors. Fourth, our results may be difficult to generalize to other populations because the decisions to procure and transplant organs were primarily based on local OPO criteria as well as the judgment of transplant surgeons because there are no unified objective criteria for hemodynamic management and organ transplantation in brain-dead donors. Nonetheless, in our study all OPO staff, clinical teams, and transplant surgeons were blinded to cytokine and hemodynamic data, and so these data could not have influenced care decisions. Fifth, given the small sample size, we had inadequate power to assess the effect of preload responsiveness on the function of individual organs. It is possible that the effect of ischemia and inflammation in the donors disadvantaged some organs more than others, and further study is needed to examine this aspect.
Our study also has several strengths. First, to our knowledge this investigation is the first to explore the relationships among donor hemodynamics, inflammatory response, and organ transplantation using highly sensitive functional hemodynamic monitoring variables in organ donors. We were able to analyze the effects of donor demographics, mechanisms of brain death, hemodynamics, preload responsiveness, and its effects on inflammation and organ transplantation. Second, the cohort study design allowed longitudinal assessment of all donors from the time of brain death until organ procurement. Third, we were able to perform cytokine profiling on these donors to characterize inflammatory response after brain death and to analyze its association with preload responsiveness and organ transplantation. To our knowledge, this study is the first to correlate preload response characteristics, cytokine response, and organ outcome in brain-dead organ donors.
Conclusions
Preload responsiveness is common and is associated with increased plasma concentrations of IL-6 and decreased organ yield from brain-dead donors. Our data provide the rationale that a protocol-guided donor resuscitation strategy using functional hemodynamic monitoring following brain death may have the potential to reduce ischemic and inflammatory organ injury and improve organ viability and yield for transplantation. Although the results of our study are not sufficient to recommend changes in clinical practice at this time, controlled trials of donor resuscitation using functional hemodynamic monitoring seem an appropriate next step.
Acknowledgments
We are indebted to the Center for Organ Recovery and Education (CORE), Pittsburgh, Pennsylvania; the nurses, respiratory therapists, phlebotomists, physicians, and other healthcare professionals who participated; and the subjects and their families who made this study possible.
Supported, in part, by grant R380T01300 from the Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services, Rockville, Maryland; and by LiDCO, Cambridge, United Kingdom, which generously provided LiDCO monitors. Neither HRSA nor LiDCO had any role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation or approval of the manuscript. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
See also p. 2480.
Presented, in part, at the Society of Critical Care Medicine's 37th Critical Care Congress, Honolulu, Hawaii, February 2–6, 2008, and published, in part, as an abstract in Crit Care Med 2007; 35(12 suppl):A5.
The authors have not disclosed any potential conflicts of interest.
Contributor Information
Raghavan Murugan, The CRISMA Laboratory (Clinical Research, Investigation, and Systems Modeling of Acute Illness), Department of Critical Care Medicine), University of Pittsburgh, Pittsburgh, Pennsylvania.
Ramesh Venkataraman, The CRISMA Laboratory (Clinical Research, Investigation, and Systems Modeling of Acute Illness), Department of Critical Care Medicine), University of Pittsburgh, Pittsburgh, Pennsylvania.
Abdus S. Wahed, The Department of Biostatistics, University of Pittsburgh, Pittsburgh, Pennsylvania.
Michele Elder, The CRISMA Laboratory (Clinical Research, Investigation, and Systems Modeling of Acute Illness), Department of Critical Care Medicine), University of Pittsburgh, Pittsburgh, Pennsylvania.
Melinda Carter, The CRISMA Laboratory (Clinical Research, Investigation, and Systems Modeling of Acute Illness), Department of Critical Care Medicine), University of Pittsburgh, Pittsburgh, Pennsylvania.
Nicholas J. Madden, The CRISMA Laboratory (Clinical Research, Investigation, and Systems Modeling of Acute Illness), Department of Critical Care Medicine), University of Pittsburgh, Pittsburgh, Pennsylvania.
John A. Kellum, The CRISMA Laboratory (Clinical Research, Investigation, and Systems Modeling of Acute Illness), Department of Critical Care Medicine), University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.United Network for Organ Sharing: United States facts about transplantation. Richmond, VA: [December 7, 2008]. http://www.optn.org/latestData/step2.asp.? [Google Scholar]
- 2.Murugan R, Venkataraman R, Wahed AS, et al. Increased plasma interleukin-6 in donors is associated with lower recipient hospital-free survival after cadaveric organ transplantation. Crit Care Med. 2008;36:1810–1816. doi: 10.1097/CCM.0b013e318174d89f. [DOI] [PubMed] [Google Scholar]
- 3.Pratschke J, Wilhelm MJ, Kusaka M, et al. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67:343–348. doi: 10.1097/00007890-199902150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Herijgers P, Leunens V, Tjandra-Maga TB, et al. Changes in organ perfusion after brain death in the rat and its relation to circulating catecholamines. Transplantation. 1996;62:330–335. doi: 10.1097/00007890-199608150-00005. [DOI] [PubMed] [Google Scholar]
- 5.Richards PS, Nelson KA, Frazier OH, et al. Why referred potential heart donors aren't used. Tex Heart Inst J. 1993;20:218–222. [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss S, Kotsch K, Francuski M, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm MJ, Pratschke J, Beato F, et al. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation. 2000;102:2426–2433. doi: 10.1161/01.cir.102.19.2426. [DOI] [PubMed] [Google Scholar]
- 8.Birks EJ, Burton PB, Owen V, et al. Elevated tumor necrosis factor-alpha and interleukin-6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102:III352–III358. doi: 10.1161/01.cir.102.suppl_3.iii-352. [DOI] [PubMed] [Google Scholar]
- 9.Torre-Amione G, Kapadia S, Benedict C, et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 10.Salim A, Velmahos GC, Brown C, et al. Aggressive organ donor management significantly increases the number of organs available for transplantation. J Trauma. 2005;58:991–994. doi: 10.1097/01.ta.0000168708.78049.32. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Anel R, Bunnell E, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–699. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 12.Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 13.Michard F, Teboul JL. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care. 2000;4:282–289. doi: 10.1186/cc710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LiDCOplus; Cambridge, UK: [December 7, 2008]. www.lidco.com. [Google Scholar]
- 15.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 16.Tavernier B, Makhotine O, Lebuffe G, et al. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–1321. doi: 10.1097/00000542-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Lopes MR, Oliveira MA, Pereira VO, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander M, Spies CD, Berger K, et al. Prediction of volume response under open-chest conditions during coronary artery bypass surgery. Crit Care. 2007;11:R121. doi: 10.1186/cc6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofer CK, Muller SM, Furrer L, et al. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005;128:848–854. doi: 10.1378/chest.128.2.848. [DOI] [PubMed] [Google Scholar]
- 20.Clarke CJ, Hales A, Hunt A, et al. IL-10-mediated suppression of TNF-alpha production is independent of its ability to inhibit NF kappa B activity. Eur J Immunol. 1998;28:1719–1726. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Wu P, Siegel MI, et al. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes: IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]