Abstract
Differentiation-inducing factors (DIFs) are well known to modulate formation of distinct communal cell types from identical Dictyostelium discoideum amoebas, but DIF biosynthesis remains obscure. We report complimentary in vivo and in vitro experiments identifying one of two ~3,000-residue D. discoideum proteins, termed ‘steely’, as responsible for biosynthesis of the DIF acylphloroglucinol scaffold. Steely proteins possess six catalytic domains homologous to metazoan type I fatty acid synthases (FASs) but feature an iterative type III polyketide synthase (PKS) in place of the expected FAS C-terminal thioesterase used to off load fatty acid products. This new domain arrangement likely facilitates covalent transfer of steely N-terminal acyl products directly to the C-terminal type III PKS active sites, which catalyze both iterative polyketide extension and cyclization. The crystal structure of a steely C-terminal domain confirms conservation of the homodimeric type III PKS fold. These findings suggest new bioengineering strategies for expanding the scope of fatty acid and polyketide biosynthesis.
During the life cycle of the social amoeba D. discoideum, starvation triggers a cyclic AMP–mediated process in which as many as 105 undifferentiated unicellular amoebas aggregate to form a multicellular ‘slug’ that can migrate en masse toward light and heat1. Via differentiation of these identical slime-mold cells into two main classes (prestalk and prespore), this mobile slug form of D. discoideum then transforms into a stationary fruiting body comprised of spore cells perched atop a vertical pedestal of vacuolated stalk cells. DIFs are polyketide-derived signaling molecules in D. discoideum that are critical for orchestration of this cellular differentiation2,3. In addition to their physiological role in the D. discoideum life cycle, DIFs and DIF analogs have attracted medical interest by virtue of their ability to inhibit cell proliferation and induce differentiation of mammalian cells4.
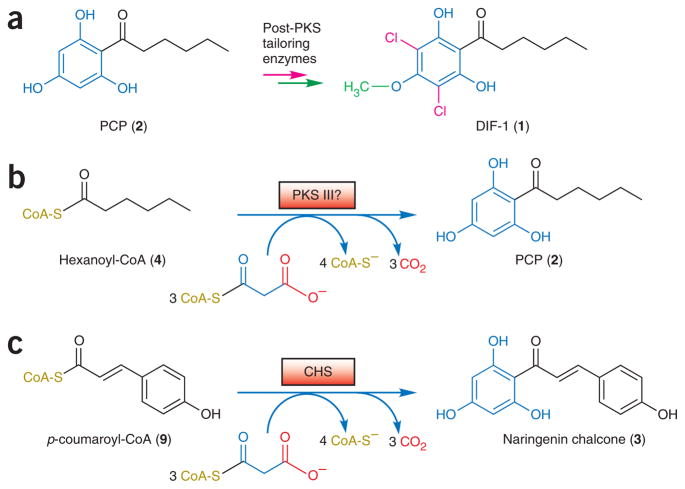
Biosynthesis of the DIF-1 (1) chemical signal requires chlorination and O-methylation of a polyketide-derived phlorocaprophenone (PCP, 2) intermediate5 (Fig. 1a), but the only enzyme of the DIF biosynthetic pathway identified so far is the O-methyltransferase that catalyzes the final step in DIF functionalization3. Notably, PCP resembles the substituted phloroglucinol rings of naringenin chalcone (3) and similar polyketides biosynthesized by chalcone synthase (CHS)6 and related plant type III (CHS-like) PKS enzymes7,8. We postulated that an unknown type III PKS in D. discoideum might synthesize the phloroglucinol core of PCP via a CHS-like intramolecular cyclization6 after three iterative acetyl extensions of a hexanoyl coenzyme A (4) starter molecule (Fig. 1b,c) that is likely formed either by a specialized FAS or as a byproduct of fatty acid β-oxidation.
Figure 1.
Biosynthesis of D. discoideum DIF-1. (a) DIF-1 results from chlorination (red) and O-methylation (green) of PCP. The presence of saturated (black) and hydroxylated (blue) portions of PCP suggests two stages of biosynthesis. (b) Hypothetical type III PKS biosynthesis of PCP from hexanoyl-CoA, suggested by similarity to the phloroglucinol core of chalcone. (c) In plants, CHS transfers p-coumaroyl from CoA to a catalytic cysteine, then catalyzes sequential decarboxylative condensations with three malonyl-CoA molecules. Chalcone is formed and off loaded by an intramolecular Claisen cyclization of the resulting linear tetraketide.
Subsequent bioinformatic analysis of the publicly available raw sequencing data from the ongoing D. discoideum genome project9 indeed revealed two candidate type III PKS sequences. Unexpectedly, as all other known type III PKSs occur as independent homodimers8 (also see PF02797 at http://www.sanger.ac.uk/Software/Pfam), each D. discoideum CHS-like domain occurs as a C-terminal fusion to a multidomain polypeptide homologous to metazoan type I FASs10–12 and related type I PKSs13,14. Notably, these N-terminal FAS-like domains are good candidates for producing the hexanoyl precursor necessary for CHS-like biosynthesis of PCP. In reference to their hybrid nature and to their discovery in D. discoideum, we termed these type I FAS–type III PKS fusion enzymes “steely.”
Working independently, both the Noel and the Kay laboratories identified the same two genes as possibly encoding the PKS responsible for DIF polyketide biosynthesis. Here we report the combined results of the complimentary experimental approaches taken by these laboratories, each confirming the biosynthetic role of one of these steely hybrid enzymes in the biosynthesis of the PCP acylphloroglucinol skeleton of DIF-1. These experiments include the cloning, heterologous expression, in vitro enzyme assay and structural determination of steely C-terminal type III PKS domains, as well as the generation, in vivo analysis and chemical complementation of steely null mutants in D. discoideum.
RESULTS
Bioinformatic discovery of D. discoideum hybrid FAS-PKSs
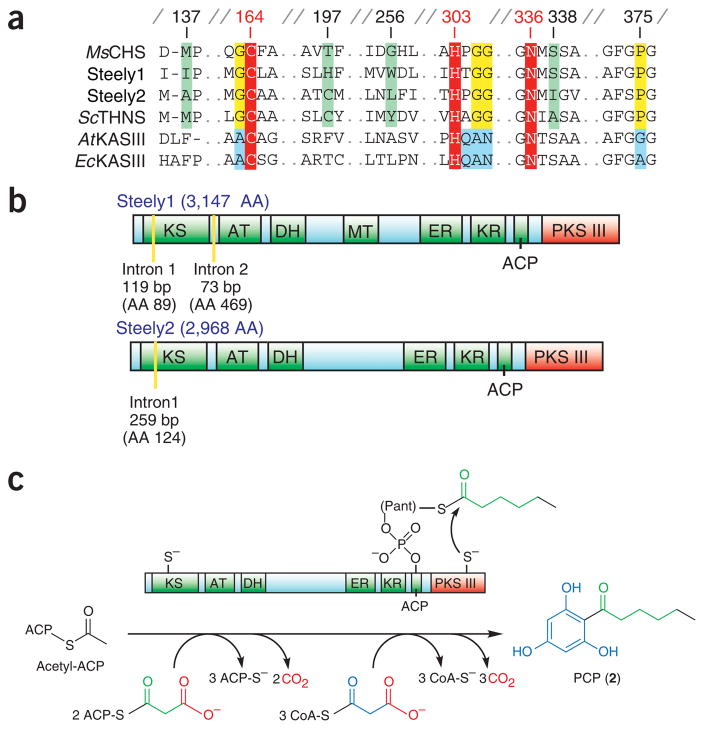
We independently identified candidate CHS-like genes in D. discoideum either through Basic Local Alignment Search Tool (BLAST) searches against translated raw shotgun reads available on the Internet or through a whole-genome shotgun assembly with attendant gene predictions and annotations, including the results of matches to hidden Markov models. Surprisingly, as no CHS-like enzymes were then known outside of plants and bacteria, these searches uncovered two open reading frames (ORFs) encoding enzymes that are substantially similar to the type III PKS query. Both D. discoideum protein sequences possess the conserved Cys-His-Asn catalytic triad common to both type III PKSs and the noniterative and structurally related β-ketoacyl–acyl carrier protein synthase III (KAS III) enzymes of type II FASs (found in plants and bacteria), but they also contain type III PKS–specific signature sequences8 (Fig. 2a). However, there is extensive sequence divergence between these two D. discoideum type III PKS domains (~30% amino acid identity); notably, this is the same evolutionary distance between either of these sequences and their closest plant or bacterial relatives. Moreover, this sequence divergence includes unique combinations of residues at positions lining the predicted active site cavity that are known to mediate substrate and product specificities of type III PKSs (Fig. 2a)8,15,16.
Figure 2.
D. discoideum type III PKSs occur as C-terminal domains of steely FAS-PKS hybrids. (a) Active site and signature sequence comparison of steely type III PKSs with plant and bacterial type III PKSs (Medicago sativa (alfalfa) CHS and Streptomyces coelicolor 1,3,6,8-tetrahydroxynaphthalene synthase, THNS) and related type II (plant and bacterial) FAS enzymes (Arabidopsis thaliana and E. coli KAS III enzymes). Signature sequences are highlighted in yellow or blue, red marks the conserved catalytic triad, and green indicates type III PKS specificity-determining positions. Numbering is for alfalfa CHS2. (b) Predicted enzymatic domains of D. discoideum steely proteins, including N-terminal type I FAS-PKS and C-terminal type III PKS domains. Type I FAS-PKS intermediates are tethered by a thioester linkage to either a KS cysteine or the prosthetic Ppant arm of the ACP-like domain. AA (amino acid) labels indicate the total number of residues in each steely protein or the amino acid position of intron boundaries. (c) Proposed PCP biosynthesis by a steely FAS I–PKS III hybrid. Direct transfer of a hexanoyl intermediate to the type III PKS domain based on analogous off loading of conventional type I FAS-PKS products via activity of thioesterase (TE) domains (Supplementary Fig. 1).
Unexpectedly, each CHS-like sequence is fused to the C terminus of a predicted multidomain FAS-PKS protein, so that they constitute the final ~400 residues of novel 3,147- and 2,968-residue predicted proteins, respectively (Fig. 2b). We termed these D. discoideum hybrid ORFs ‘Steely1’ (gene name stlA, located on chromosome 1) and ‘Steely2’ (stlB, on chromosome 5). The encoded N-terminal regions of each steely ORF are predicted to contain several covalently linked catalytic domains (Fig. 2b) whose order and spacing resembles the enzyme architecture of the first six of seven domains comprising the type I FAS of metazoans17. Sequentially from the N termini, these FAS-like steely domains constitute a β-ketoacyl synthase (KS or KAS I), an acyltransferase (AT), a dehydratase (DH), an enoyl reductase (ER), a ketoreductase (KR) and a phosphopantetheine (Ppant) attachment site, the latter of which serves in type I FASs10–12 and related PKS enzymes13,14 as a covalently tethered acyl carrier protein (ACP) to shuttle biosynthetic intermediates between the active sites of the various catalytic domains (Fig. 2b,c). Each of these steely FAS-like enzymatic domains is predicted to possess intact and likely functional catalytic active sites. However, the seventh and final domain of metazoan-like type I FAS proteins is a thioesterase (TE) that terminates further FAS chain extension by catalyzing hydrolytic cleavage of the thioester bond tethering the full-length acyl product to the Ppant arm of the ACP-like sixth domain (Supplementary Fig. 1 online). The entire FAS C-terminal TE domain is replaced in Steely1 and Steely2 by a structurally unrelated type III PKS domain (Fig. 2b,c). Like many of the ~40 other type I PKS ORFs in the sequenced D. discoideum genome9, the encoded Steely1 also contains a potential methyltransferase (MT) domain (between the DH and ER domains) that is homologous to those used in microcystin biosynthesis18 and might catalyze methylation of acyl coenzyme A (CoA) extender units.
The natural covalent fusion of type III PKS enzymes with multi-domain FAS-like enzymes strongly suggests, based on bioinformatic comparisons with analogous type I FAS–PKS C-terminal domain interactions10–14,19 (Supplementary Fig. 1), that a streamlined and efficient PCP biosynthetic pathway exists in which FAS-like acylthioester end products derived from the N-terminal catalytic domains of Steely1 and Steely2 are directly transferred from the pantetheine arm of the ACP-like domain to the catalytic cysteine of the immediately adjacent type III PKS domain (Fig. 2c). Furthermore, the implied direct transfer of fatty acyl products to the C-terminal type III PKS active site eliminates the need for a TE domain to off load acyl thioester products as free acids, while simultaneously bypassing the subsequent requirement for a CoA ligase to activate the free carboxylate moiety for type III PKS catalysis. Thus, the novel steely domain architecture might indeed be sufficient for the entire assembly-line production of PCP from primary metabolic precursors.
In vitro activities of C-terminal type III PKS domains
Given the size of each full-length steely ORF, as well as the occurrence of 5′ introns, we focused our cloning and in vitro biochemical analyses on each steely C-terminal type III PKS domain, either alone or as didomain constructs incorporating the adjacent ACP-like domains. All D. discoideum heterologous constructs expressed poorly in Escherichia coli, even in strains optimized for rare codon expression. Moreover, the attempted purification of didomain constructs yielded no soluble protein (data not shown). Constructs comprising only the CHS-like ~400-residue C-terminal domain of either steely protein along with a thrombin-cleavable N-terminal polyhistidine affinity tag yielded small quantities of relatively pure soluble protein, which we used for subsequent in vitro biochemical characterization.
All substrates used in this study were provided as CoA-activated thioesters (see Discussion). We confirmed by standard in vitro enzyme assays using radiolabeled malonyl-CoA (5) and a representative set of type III PKS substrates that both heterologously expressed steely C-terminal domains catalyze iterative polyketide extension when primed with hexanoyl-CoA or similar medium-length aliphatic starters (Supplementary Fig. 2 online). However, only Steely1 accepted a longer octanoyl-CoA (6) starter, whereas only Steely2 turned over isovaleryl-CoA (7), a shorter branched aliphatic starter. These differences in in vitro starter specificity are consistent with the substantial divergence of these steely active sites predicted by homology modeling. Neither enzyme showed substantial polyketide chain extension activity with malonyl-CoA alone or when primed with either acetyl-CoA (8) or the larger CHS starter p-coumaroyl-CoA (9) (Supplementary Fig. 2).
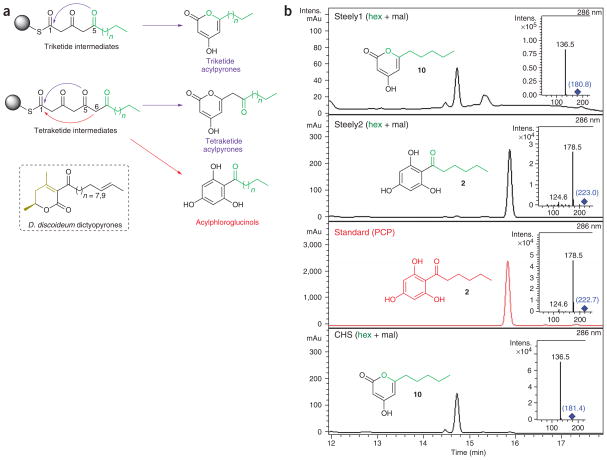
HPLC-MS-MS analyses of in vitro assays using unlabeled malonyl-CoA in conjunction with an authentic PCP standard unambiguously confirmed that the hexanoyl-primed Steely2 type III PKS domain catalyzes three rounds of polyketide chain extension and the final CHS-like intramolecular C6 to C1 Claisen condensation that is necessary to synthesize and off load the DIF-1 skeleton (Fig. 3). Despite a similar preference for medium-length acyl starters (Supplementary Fig. 1), hexanoyl-primed assays of the Steely1 type III PKS domain produced only triketide (10) and tetraketide (11) lactonization-derived pyrones (Fig. 3 and Supplementary Fig. 3 online). The related D. discoideum DIF-2 acylphloroglucinol scaffold seems to be derived from a pentanoyl intermediate. Therefore, we also primed in vitro assays of each steely C-terminal domain with butanoyl-CoA (12), as pentanoyl-CoA is not commercially available. Although changing the starter moiety in this manner often alters type III PKS product cyclization8, our use of a four-carbon (rather than six-carbon) acyl starter had no effect on the cyclization fate of in vitro–generated products (13, 14 and 15) of either enzyme (Supplementary Fig. 4 online). Variation of pH and of enzyme and substrate concentrations also had no effect on the in vitro cyclization specificities reported here, although Steely1 showed reduced catalytic activity in HEPES-buffered assays (data not shown). Though extracted ion chromatogram (EIC) analyses revealed trace amounts of malonyl-primed triacetic acid lactone (TAL) in CHS assays, Steely1 and Steely2 assays lacking an acyl starter (that is, either hexanoyl- or butanoyl-CoA) showed no evidence of TAL production. These assay results suggest that Steely2 may be responsible for the in vivo biosynthesis of both known acylphloroglucinol DIF scaffolds.
Figure 3.
Hexanoyl-primed in vitro product specificity of steely C-terminal type III PKS domains. (a) Polyketide cyclization routes leading to acylpyrones (blue arrows) and acylphloroglucinols (red arrows). Carbons 1, 5 and 6 are involved in cyclization. Sphere represents CoA or active site cysteine. Starter-derived moieties are green; n = 3 and n = 2 for hexanoyl and pentanoyl moieties (respectively) of known D. discoideum acylphloroglucinols, and n = 3 and n = 1 for hexanoyl- and butanoyl-CoA substrates (respectively) tested here (see b and Supplementary Figs. 3 and 4). Conversely, dictyopyrone biosynthesis may involve condensation of a diketide (black) with another small molecule (gold). (b) Acylphloroglucinol (PCP) biosynthesis by Steely2, but not Steely1. Main enzymatic products of hexanoyl-CoA–primed in vitro type III PKS assays with malonyl-CoA as determined by negative-mode LC-MS-MS (insets). Parent (MS) masses for each MS-MS spectrum are given in blue. We also analyzed minor hexanoyl-primed enzymatic products and all butanoyl-primed enzymatic products (see Supplementary Figs. 3 and 4).
In vivo genetic disruption of steely genes
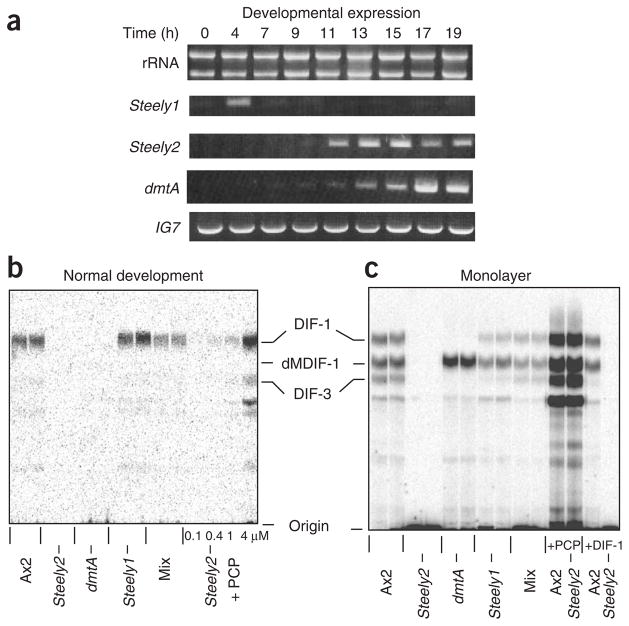
We used D. discoideum genetics to directly test the involvement of the steely proteins in DIF-1 biosynthesis. Both steely genes (stlA and stlB) are expressed during development of the standard laboratory strain, Ax2, suggesting that they encode functional gene products. The Steely1 gene (stlA) is expressed maximally in early development before cellular aggregation. The Steely2 gene (stlB) is coordinately expressed with the dmtA gene, which encodes the O-methyltransferase that carries out the terminal step in DIF-1 biosynthesis (Fig. 4a); this expression occurs later in development, at precisely the time when DIF-1 itself is detected20. We disrupted both steely genes in strain Ax2 by inserting plasmids into the coding region adjacent to the ACP domain (Supplementary Fig. 5 online), and we tested the resulting mutants for their ability to make DIF-1 using in vivo labeling with 36Cl−. We obtained a single disruptant of the Steely1 gene (stlA−) and several disruptants of Steely2 (stlB−), the latter of which had similar phenotypes. Both steely mutants developed to the slug stage, in which DIF-1 accumulation is maximal, though the Steely2− mutant slugs were thin and tended to break up (see below). Slugs of the Steely1− mutant strain still made DIF-1 (Fig. 4b). However, two independent Steely2− mutant strains both failed to accumulate any detectable DIF-1 at this or any other stage of development, indicating that the Steely2 protein probably makes the DIF acylphloroglucinol scaffold in vivo.
Figure 4.
Steely gene expression and DIF-1 synthesis by steely− null mutants. (a) Expression of the steely genes during development of wild-type Ax2 cells determined by reverse transcription PCR. Hours of development are given; rRNA and the IG7 gene are shown as loading and PCR controls. (b,c) DIF-1 synthesis detected by labeling with 36Cl− during normal development on an agar surface or development as a submerged monolayer of cells in tissue culture dishes. Cells: Ax2 is parental control; HM1154 is Steely2− (stlB−); HM1030 is dmtA−, blocked in the last step of DIF-1 biosynthesis; HM1157 is Steely1− (stlA−); mix is a 1:1 mix of HM1154 and HM1030 cells, which develop intermingled with each other and can therefore exchange membrane-permeable metabolites. Additions: (b) PCP added to the medium at the concentrations indicated; (c) 4 μM PCP and 100 nM DIF-1 added to the medium as indicated. Markers: dMDIF-1 is the immediate precursor of DIF-1; DIF-3 is the first breakdown product of DIF-1; the other labeled compounds are likely to be more derived metabolites. Incorporation of radioactivity (36Cl−) into DIF-1 by Ax2 cells: (b) 6 c.p.m. by 108 cells per lane; (c) 40 c.p.m. by 4 × 107 cells per lane.
A conceivable alternative interpretation for these results is that the Steely2− mutant produces a polyketide that is not PCP but is essential for normal development and hence indirectly for DIF-1 production. To rule out this possibility, we performed rescue and feeding experiments. Allowing Steely2− mutant cells to develop on agar containing DIF-1 restored normal development, but not the production of DIF-1, as expected if the Steely2 protein is required directly for DIF-1 biosynthesis (Figs. 4c and 5). By contrast, Steely2− cells were able to produce DIF-1 and its breakdown products21 when supplied with the polyketide PCP in the medium (Fig. 4). Likewise, Steely2− cells produced DIF-1 when mixed with cells of the dmtA− mutant. In this mixture, the dmtA− cells, lacking the terminal O-methyltransferase of the biosynthetic pathway, cannot produce DIF-1 but can still supply PCP to the Steely2− cells, which then convert it into DIF-1 (Fig. 4). To confirm these results at greater sensitivity, we developed cells as submerged monolayers, in which they release much more DIF-1 into the medium than is present in aggregates during normal development on agar. Both cell culture methods produced similar biological results (Fig. 4c).
Figure 5.
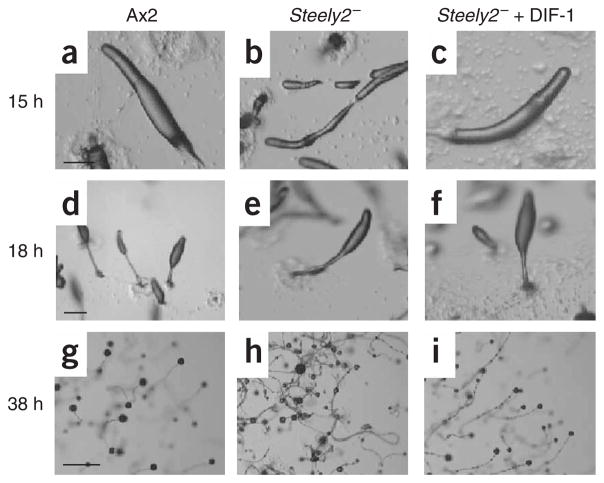
Development of Steely2− mutant and rescue by DIF-1.
(a–c) Migratory slugs; those of the mutant are long and thin (and they break apart) but are restored by 100 nM DIF-1 in the agar. (d–f) Early culminates. (g–i) Mature fruiting bodies; those of the wild type each have a spore mass supported by a stalk, but the mutant stalks lay down on the surface and the spores slip down to their base, giving an untidy appearance, which is again restored by DIF-1. Cells used are Ax2 (parental control) and HM1154 (Steely2−). Each horizontal set of panels is at the same magnification; scale bars used are (a,d) 0.2 mm, (g) 1 mm.
The phenotype of the Steely2− mutant resembles the classic ‘DIF-less’ phenotype of dmtA− null cells3; aggregation and tipped mound formation are similar to wild type, but the resulting slugs break up. Stalks often lie directly on the agar substratum. When stalks rise above the agar, the spore mass often slips down the stalk, giving a generally untidy appearance (Fig. 5). Supplying DIF-1 in the agar restores normal development, confirming that the phenotype is due to a lack of DIF.
These in vivo results clearly demonstrate that the Steely2 hybrid FAS-PKS does indeed make the polyketide skeleton of DIF-1.
Structure of the Steely1 C-terminal type III PKS domain
To compare the three-dimensional architectures of the steely C-terminal domains to those of other type III PKSs, we solved the crystal structure of the heterologously expressed and purified Steely1 type III PKS domain and refined the atomic coordinates, with all data extending to 2.9 Å resolution (Supplementary Table 1 online). The Steely1 crystal structure reveals conservation of the overall fold and homodimeric domain assembly (Fig. 6a) observed in all type III PKSs that have been structurally characterized so far6,15,22–24, including conservation of the internal active site cavity containing the Cys-His-Asn catalytic triad8,22 (Fig. 6b). Notably, this first crystal structure of a slime mold type III PKS also reveals subtle conformational divergence of the Steely1 protein backbone relative to alfalfa CHS and to our initial CHS-derived Steely1 homology model.
Figure 6.
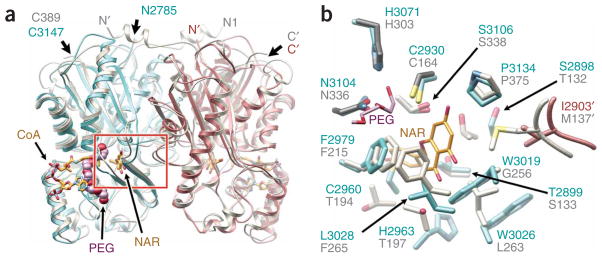
Crystal structure of Steely1 C-terminal domain confirms conservation of the PKS III fold, homodimeric assembly and internal active site cavity. (a) Structure of D. discoideum Steely1 homodimeric C-terminal domain (cyan and rose ribbons) overlaid with alfalfa CHS2 (gray ribbons, PDB ID 1CJK). A PEG molecule (space-filling lavender and red) is bound in the Steely1 pantetheine-binding tunnel, illustrated here by CHS-bound CoA (gold stick, superimposed from PDB ID 1BQ6), which presents thioester substrates to the internal active site cavity, delineated here by CHS-bound naringenin (NAR). The red box indicates area detailed in b. Apostrophes designate the dyad-related protein chain. (b) Active site comparison confirms homology-predicted assignments of important active site residues (Fig. 2a and text), but with subtle conformational changes. One end of the Steely1-bound PEG molecule (shown here as a stick model) is positioned in the ‘oxyanion hole’ of the conserved catalytic triad.
These observed backbone conformational differences, though not unexpected in proteins sharing ~30% sequence identity, also affect the position and orientation of side chains lining the active site cavity (Fig. 6b). This result suggests that the Steely2 C-terminal domain, which also shares ~30% sequence identity with either CHS or the Steely1 type III PKS, likely possesses similarly unpredictable conformational nuances that will require experimental elucidation. Indeed, attempts to ascertain the determinants of acylphloroglucinol product specificity by automated docking of PCP into the homology-modeled Steely2 active site have produced conflicting results (not shown) depending on whether CHS or Steely1 was chosen as structural template for model building.
However, the Steely1 crystal structure confirms our prediction of the unique set of residues that define the active site surface of the Steely1 C-terminal domain (Figs. 2a and 6b). Notably, substitution at key positions lining the active site cavity (CHS residues 132, 137, 197, 256 and 338) often modulates mechanistic divergence of type III PKS substrate and product specificities8,15,16. Our experimental elucidation of the Steely1 type III PKS active site likewise lends support for our complementary homology-based prediction of an entirely different set of residues lining the Steely2 active site cavity (Fig. 2a). Consistent with observed in vitro (Fig. 3 and Supplementary Figs. 2–4) and in vivo (Figs. 4 and 5) functional differences, these predicted and observed active site differences provide a starting point for mutagenic exploration of the distinct substrate and product specificities of D. discoideum type III PKS domains, including their interactions with the adjacent ACP-like domains of each multidomain enzyme.
DISCUSSION
The two D. discoideum type III PKS domains experimentally characterized here are the first type III PKSs to be identified in a slime mold (fungal type III PKSs were also only recently discovered25) as well as the only known covalent inclusions of CHS-like enzymes within multidomain proteins. Although both steely type III PKSs similarly occur as C-terminal fusions with multidomain type I FASs or PKSs, the extensive evolutionary sequence divergence between Steely1 and Steely2 suggests that several related but unsequenced dictyostelid species may also contain steely homologs.
This work illuminates the previously obscure initial steps of DIF-1 biosynthesis. In vitro assays of the isolated C-terminal domain of Steely2 demonstrate that this type III PKS efficiently synthesizes PCP (Fig. 3 and Supplementary Fig. 3), the acylphloroglucinol skeleton of both DIF-1 and its in vivo degradation product DIF-3, via three polyketide extensions of a hexanoyl (C6) acyl precursor and a final CHS-like intramolecular C6-to-C1 Claisen condensation (Fig. 1). Abrogation of both in vivo DIF-1 biosynthesis (Fig. 4b,c) and normal development (Fig. 5) in the D. discoideum Steely2− genetic knockout, as well as rescue of this null mutant by supply of exogenous PCP (Fig. 4b,c), confirms Steely2-mediated catalysis of PCP biosynthesis in vivo.
Although analogous type I PKS–FAS domain interactions10–14 strongly suggest that the Steely2 N-terminal type I domains indeed provide the hexanoyl intermediate necessary for PCP biosynthesis catalyzed by the C-terminal type III PKS domain, further experiments are necessary to determine whether these N-terminal domains act iteratively on an acetyl precursor or instead catalyze only the second modular extension of a four-carbon intermediate. Likewise, although our in vitro type III PKS assays with CoA-activated substrates succeeded in demonstrating iterative PKS activity, starter preference and cyclization specificity, it remains to be seen whether CoA- or ACP-thioesters are used in vivo to deliver malonyl extender units to these C-terminal domains. Moreover, the similar in vitro product specificity of the Steely2 C-terminal domain when alternatively primed with a C4 acyl substrate (Supplementary Fig. 4) suggests this same enzyme may also catalyze the in vivo biosynthesis of the DIF-2 acylphloroglucinol skeleton (from a C5 acyl intermediate26). It is feasible that the Steely2 N-terminal FAS domains could catalyze either two chain extensions of a C2 starter or one chain extension of an alternative C3 starter to produce both hexanoyl and pentanoyl fatty acyl intermediates, respectively. Given the results of our in vitro enzyme assays, we propose naming the Steely2 type III PKS domain differentiation acylphloroglucinol synthase (DAPS), to most accurately reflect its possible role in biosynthesizing both DIF polyketide precursors.
In contrast, although the Steely1 C-terminal domain also catalyzes in vitro iterative polyketide extension and prefers similar acyl starters (Supplementary Fig. 2), as might be expected from both enzymes’ covalent fusion to N-terminal FAS-like domains, under no conditions did this type III PKS synthesize DIF-like cyclized tetraketide products (that is, acylphloroglucinols) from any starter moiety tested. Instead, Steely1 formed only acylpyrones in vitro, via lactonization of predominantly triketide intermediates (Fig. 3 and Supplementary Figs. 3 and 4). This difference in the in vitro product specificity of Steely1 and Steely2 type III PKSs is consistent with the unperturbed in vivo biosynthesis of DIF-1 (Fig. 4) by the Steely1− null mutant. These experimental results are further reinforced by the extensive divergence of residues lining the respective C-terminal domain active site cavities of the encoded Steely1 and Steely2 proteins (Figs. 2a and 6b). Specifically, important steric differences in residues corresponding to the key CHS specificity-determining positions 137, 197, 256 and 338 (Fig. 2a) make it extremely unlikely that Steely1 and Steely2 catalyze identical physiological reactions.
If Steely2 (DAPS) biosynthesizes both acylphloroglucinol DIF scaffolds, what might be the physiological role of Steely1, whose C-terminal domain produced only acylpyrones in vitro (Fig. 3 and Supplementary Figs. 3 and 4)? Although type III PKS–lactonized products can result from derailment of an in vitro reaction owing to improper assay conditions or nonphysiological substrates8, pyrone formation is also the bona fide physiological function of some type III PKS enzymes15, as might be suggested here by the resilience of the observed Steely1 cyclization specificity to in vitro assay conditions and starter molecule variation. Interestingly, in addition to the hexanoyl- and pentanoyl-derived acylphloroglucinols discussed above, other as-yet-unidentified differentiation-controlling polyketides in D. discoideum have recently been discovered27. The Steely1 in vitro acylpyrone cyclization specificity shown here might prove to be relevant to the in vivo biosynthesis of these unknown DIF molecules. Notably, previously characterized ‘dictyopyrone’ in vivo products of D. discoideum species are structurally distinct from these Steely1 in vitro acylpyrones28 (Fig. 3a). Specifically, dictyopyrone rings lack the typical β-hydroxylation pattern that results from cyclization of linear polyketide intermediates, though this chemical difference might be a result of extensive in vivo post-PKS processing of Steely1-derived acylpyrones. Although the current study provides insight into the physiological role of Steely1, additional characterization is necessary.
The crystal structure of the Steely1 C-terminal domain confirms conservation of the type III PKS αβαβα fold and homodimeric assembly (Fig. 6a), despite unprecedented covalent fusion to a type I biosynthetic system. A conserved interdigitation of loops from opposing monomers at the interface of the type III PKS dimer contributes to active site cavity size and shape, and therefore also to specificity. Steely1 conservation of this intimate interface, together with the consistency of our in vitro (C-terminal domain) and in vivo (full-length protein) functional results, indicates that the observed Steely1 homodimeric assembly is not merely a crystallographic artifact and strongly suggests homodimeric physiological architectures for both Steely1 and Steely2 C-terminal domains. These results support our homology-based predictions of important Steely2 (DAPS) active site residues (Fig. 2a), facilitating future mutagenic analyses of their relative contributions to this enzyme’s substrate and product specificities. Unfortunately, subtle main chain conformational variations between the CHS and Steely1 structures, extensive Steely2 sequence divergence, and our inconclusive docking results with Steely2 homology models based on either structural template (not shown) all suggest that the precise topology of the Steely2 (DAPS) active site will require direct experimental elucidation.
Although the hexanoyl intermediate produced by the Steely2 N-terminal domains is a canonical (unbranched and saturated) fatty acid, the homodimeric association of Steely1 and Steely2 C-terminal type III PKS domains (Fig. 6a) suggests that the functional interaction of type I and type III domains at the novel hybridization interface more closely resembles the intramodular C-terminal architecture of type I PKSs than that of type I FASs. A recently published 4.5- Å-resolution crystallographic elucidation of the domain architecture of an animal type I FAS shows a parallel (head-to-head) dimeric assembly of the FAS protein chain involving homodimerization of the KS, DH and ER domains along a central axis12. This recent discovery disproves a controversial11,17,29 and older antiparallel (head-to-tail) model for animal FAS assembly10 that was worryingly inconsistent with head-to-head dimeric-assembly models of related type I PKSs13,14. However, this definitive new FAS model validates the predicted monomeric state of FAS TE domains12, highlighting an apparently genuine architectural difference between type I FAS and PKS C-terminal TE domains. Despite sharing a common function and the αβ-hydrolase fold with these non-interacting FAS TE domains, type I PKS TE domains are homodimeric19, like the evolutionarily unrelated Steely1 C-terminal domain described here and all other structurally characterized type III PKSs6,15,22–24.
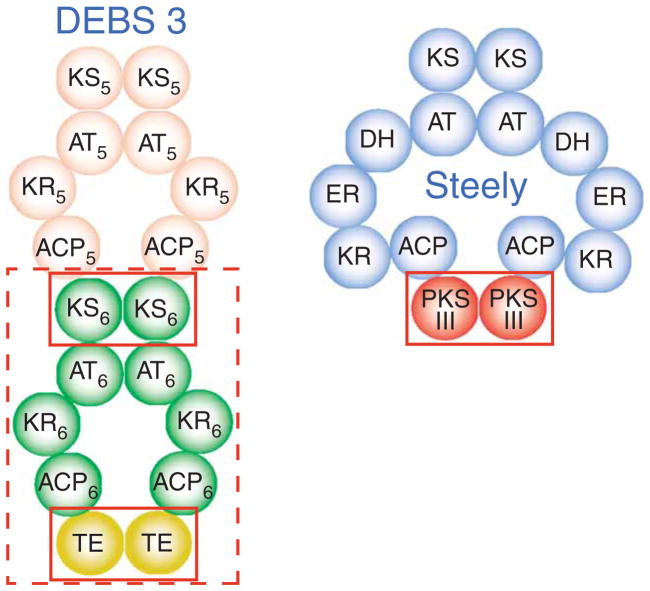
This type I PKS intramodular interaction of ACP domains with homodimeric C-terminal TE domains is likewise similar to the inter-modular interface of ACPs with downstream KS domains in modular type I PKS systems. Such type I PKS intermodular associations (which are sometimes covalently enforced) allow transfers of thioester-linked acyl intermediates from the C-terminal ACP of one module to the N-terminal homodimeric KS domain of the next module13,14. Notably, all KS domains (including CHS-like domains) share the αβαβα fold8, which is evolutionarily unrelated to the αβ-hydrolase fold of TE domains19. Given their KS-conserved αβαβα fold and catalytic activities, including both iterative polyketide extension and product off loading, the ~400-residue steely C-terminal type III PKS domains might more accurately be envisioned as functional replacements for one or more complete multidomain type I PKS modules (Fig. 7).
Figure 7.
Steely1 type III PKS homodimer suggests functional similarity of steely hybridization interface to modular type I PKS interfaces. Domain interaction schematic (adapted from ref. 14) showing a current model for the parallel dimeric assembly of modular type I PKS proteins. Shown here is the the final polypeptide chain of DEBS, chain 3, which is composed of modules 5 and 6 (numbered subscripts). Solid red boxes compare the steely ACP–PKS III interface to DEBS intramodular (ACP-TE) and intermodular (ACP-KS) domain interactions. Dashed box highlights functional equivalence of steely PKS III with an entire PKS I module.
These latter observations suggest the broad suitability of the Steely1 and Steely2 protein templates for constructing combinatorial assemblies of hybrid type I and type III PKS enzymes. The extensive genetic diversity of known modular and iterative type I PKSs represents tremendous functional diversity13,14, and rational genetic manipulation of domains and modules in intensely studied modular type I PKS systems such as 6-deoxyerythronolide B synthase (DEBS) can lead to predictable functional diversification of small-molecule end products30. In marked contrast, type III PKSs are single homodimeric domains but nonetheless recognize a diverse collection of substrates, vary in the number of iterative polyketide extension steps catalyzed and access divergent cyclization pathways to offload not only substituted phloroglucinols or 2-pyrone rings such as those featured in this study, but also substituted resorcinols, naphthalenes and alkaloids8,31,32. This extensive, complementary and distinct functional diversity of CHS-like enzymes8,24,31–33, combined with their small size and simple homodimeric architecture, makes the type III PKS family of enzymes seemingly ideal modules for use in expanding the functional diversity of type I systems.
From the inverse perspective, many demonstrated (or putative) type III PKS reaction pathways leading to desirable natural products proceed from linear or branched acyl starters8, including the biosynthesis of sorgoleone and related plant allelopaths, the polyketide core of Cannabis psychoactive natural products and the hops antimicrobial bitter acids that flavor and preserve beer. Given the promiscuous substrate specificity of type III PKS enzymes, a steely-like direct covalent transfer of specialized acyl thioester substrates from N-terminal FAS-like domains could neatly sidestep several heterologous pathway bioengineering issues regarding enzyme coexpression, colocalization and efficient substrate channeling.
In conclusion, our in vitro and in vivo analyses of these two D. discoideum hybrid FAS-PKS enzymes lay a foundation for further advances on several fronts. First, our definitive identification of Steely2 as the missing DIF-pathway PCP synthase (DAPS) facilitates increasingly sophisticated in vivo analyses of the orchestration of cellular differentiation during the life cycle of this organism. Though our results conversely show that Steely1 does not function as a DAPS, clues generated in this initial characterization will stimulate investigation into the role of other specialized metabolites in the life cycle of D. discoideum. Finally, the catalytically efficient domain structure of the D. discoideum steely proteins, which facilitates direct transfer of N-terminal–derived acyl products to the C-terminal active site, represents an untapped but evolutionarily refined template for the combinatorial construction of a plethora of new fusion enzymes for metabolic engineering.
METHODS
Cloning, expression and purification
We designed single or didomain C-terminal constructs of varying length for each D. discoideum steely fusion protein. We amplified sequences from genomic DNA (a gift from S. Merlot and R. Firtel) using complimentary oligonucleotides with restriction sites for direct cloning into the pHIS-8 expression vector, as previously described34, and we confirmed constructs by automated nucleotide sequencing (Salk Institute DNA sequencing facility). After overexpression in E. coli BL21(DE3) and CodonPlus (Stratagene) cells, we purified recombinant proteins to near homogeneity (with persistent contamination by E. coli chaperones, as confirmed by N-terminal sequencing of PAGE protein bands), concentrated them to between 0.5 and 15 mg ml−1 and stored them at −80 °C, after buffer exchange into 12 mM sodium HEPES (pH 7.5) or Bis-Tris Propane-HCl (pH 7.0), 25 mM NaCl and 5 mM DTT, as described previously34.
In vitro type III PKS assays and HPLC-MS-MS product identification
We probed relative starter specificity using reverse-phase TLC analyses of ethyl acetate–extracted in vitro assays of purified heterologously expressed C-terminal type III PKS domains incubated with [2-14C]malonyl-CoA and various CoA-tethered starters, as previously reported22 (see Supplementary Methods online for additional details). For HPLC-MS-MS analyses we used 25 μl injections of similarly prepared overnight reactions (but without organic extraction) buffered with 100 mM Bis-Tris Propane (pH 7.0), using unlabeled malonyl-CoA. We carried out LC-MS-MS analyses on an Agilent 1100 HPLC with an integrated Agilent LC/MSD Trap XCT ion trap mass spectrometer, using a reversed-phase C18 column (4.6 × 150 mm; Gemini) maintained at 30 °C. A gradient mobile phase ramped from 5% to 100% acetonitrile in water (with each solvent containing 0.1% v/v formic acid) between minutes 3 and 13 of a 25-min run using a flow rate of 0.5 ml min−1 and a 0.1 ml min−1 postcolumn injection of 20 mM ammonium acetate in water. We monitored UV absorbance at 286 nm.
We identified PCP by direct HPLC-MS-MS comparison with an authentic synthetic standard, kindly provided by S. Horinouchi and N. Funa. We identified other hexanoyl- and butanoyl-primed enzymatic products by comparing their relative HPLC elution times and negative MS-MS fragmentation patterns with previously published LC-MS-MS analyses of authentic standards35. We used EICs with parent ion masses of plausible polyketide products to detect trace amounts of minor enzymatic products, but we observed only triketide and tetraketide products.
Characterization of hexanoyl-derived products: triketide acylpyrone (4-hydroxy-6-pentyl-pyran-2-one), LC retention time 14.7 min, negative MS 181.4 [M-H]−, negative MS-MS (precursor ion at m/z 181.4) 136.5 [M-H-CO2]−; tetraketide acylpyrone (4-hydroxy-6-(2-oxo-heptyl)-pyran-2-one), LC retention time 14.5 min, negative MS 223.5 [M-H]−, negative MS-MS (precursor ion at m/z 223.5) major 124.5 [C6H5O3]− and minor 178.5 [M-H-CO2]−; tetraketide acylphloroglucinol (1-(2,4,6-trihydroxy-phenyl)-hexan-1-one, PCP), LC retention time 15.9 min, negative MS 222.7 [M-H]−, negative MS-MS (precursor ion at m/z 222.7) major 178.5 [M-H-44]− and minor 124.6 [C6H5O3]−.
Characterization of butanoyl-derived products is described in Supplementary Methods.
In vivo Dictyostelium methods
We maintained D. discoideum strain Ax2 in HL-5 medium at 22 °C and selected transformants with blasticidin (10 μg ml−1); we initiated development by washing cells free of nutrients using differential centrifugation in KK2 (20 mM K1K2PO4, 2 mM MgSO4, pH 6.2)36. We made disruption vectors by in vitro transposition of a blastocidin resistance cassette37 into PCR-amplified genomic fragments of the steely genes cloned into the plasmid pCR2.1-TOPO by topoisomerase cloning (TOPO-TA cloning kit; Invitrogen). We isolated a 2.6-kilobase fragment of the Steely1 gene using the primers I-1, CCAATTCCATTGTAAATGGTAGCC, and I-4, CCATATGCATGA TATACATCCCAAGTG; we isolated a 2.6-kilobase fragment of the the Steely2 gene using the primers II-1, AGCCATTAGATTCATGTCATCCGGT, and II-4, CAGGATGAGTAGCAAAGAATTCGA (Supplementary Fig. 5). We linearized the knockout vectors with KpnI and NotI and transformed them by electro-poration into Ax2 cells38. We identified disruptant clones using PCR (Supplementary Fig. 5); HM1157 is stlA−, HM1154 and HM1156 are stlB−.
We labeled cells with 36Cl− either (i) developing on an agar surface (1.8% electrophoresis-grade agarose) containing 10% DIFlab (100% is 12 mM KH2PO4, 8 mM Na2HPO4), 0.1 mM MgSO4 at pH 6.7, with 0.1 μCi ml−1 36Cl− or (ii) submerged in tissue culture plates containing 50% DIFlab and 4 mM cyclic AMP; at the appropriate times we extracted the labeled compounds, resolved them by TLC (using Whatman LK6D silica developed with hexane/ethyl acetate/acetic acid, 40:60:2 v/v/v) and visualized them using a Phosphorimager, as previously described5 (Supplementary Methods). We synthesized DIF-1, PCP and other standards related to DIF-1 as previously described39. For development, we harvested axenically grown cells and washed them twice with KK2. We allowed the cells to develop at 22 °C on KK2 agar plates with or without 100 nM DIF-1 at a density of 2 × 106 cells cm−2. We took photographs with a digital camera (Olympus HC-300z/OL) attached to a stereomicroscope (Olympus SZX12). We extracted total RNA with RNeasy extraction kit (Qiagen) according to the manufacturer’s instructions; we performed reverse transcription PCR with One Step RNA PCR kit (Takara) according to the manufacturer’s instructions. We examined the expression kinetics of Steely1, Steely2 and dmtA by reverse transcription PCR using the following forward and reverse primers: Steely1, CTCAAACGATCCAGAGTCATTATGG and CAAAGAGTGCATTTACACCACCGCA; Steely2, GGAATCTCACCAAAAGAAGCTCAAC and CTAACATATCCAGATGCACGTGGATC; dmtA, GCGGTACTAGTCAAGGTATGACCA and CAATGACAGACATTCTTTTACTATCTGG. As PCR control, we amplified IG7 using GGCCAATTTAAAGGAGGCGCTGG and GACCCACCTCAGTCCTCTCGTAC.
Crystallization, data collection and structure determination and refinement
We obtained crystals of the heterologously expressed Steely1 C-terminal single-domain construct using vapor diffusion in hanging drops consisting of a 1:1 mixture of protein and crystallization buffer. The crystallization buffer contained 17% (w/v) PEG 17,500, 0.5 M ammonium formate and 100 mM MOPSO−Na+ buffer at pH 7.0. Before freezing in liquid nitrogen, we passed the crystals through a cryogenic buffer identical to the crystallization buffer except for the use of 19% (w/v) PEG 17,500 and the inclusion of 18% (v/v) glycerol.
We collected data at the National Synchrotron Light Source (NSLS) beam-line X8-C. Crystallization and refinement statistics are in Supplementary Table 1, and molecular replacement and model refinement are described in Supplementary Methods. Ramachandran analysis (using Procheck in CCP440) of the final model diagnosed backbone conformations as either core (most favorable), allowed, generally allowed or disallowed. The percentage of Steely1 C-terminal domain residues in each group is 87.6%, 11.3%, 0.8% and 0.3%, respectively. Disallowed residues are in a hairpin turn at the protein surface (distant from the active site), as observed in previous type III PKS structures6,8,15,22.
Other methods
Additional details are provided in Supplementary Methods.
Database accession codes
Steely1 (stlA) is DDB0190208 at dictyBase (http://dictybase.org/) and Steely2 (stlB) is DDB0219613. The atomic coordinates and structure factors of the Steely1 type III PKS domain crystal structure have been deposited in the Protein Data Bank (PDB) under the accession code 2H84.
Supplementary Material
Acknowledgments
D. discoideum genomic DNA was a gift from R. Firtel and S. Merlot (University of California, San Diego). We also thank S. Horinouchi and N. Funa (University of Tokyo) for providing the synthetic PCP authentic standard, R. Schaloske (University of California, San Diego) for discussion of (A+T)-rich PCR and T. Baiga (Salk Institute) and N. Funa for discussion of LC-MS-MS. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. This work was supported by the National Institutes of Health Grant AI52443 (B.S.M. and J.P.N.), the Hayashi Memorial Foundation (T.S.) and core support from the Medical Research Council (R.R.K.). J.P.N. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Note: Supplementary information is available on the Nature Chemical Biology website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
AUTHOR CONTRIBUTIONS
M.B.A., conceptualization of project, in vitro experimental design, bioinformatic analysis from raw sequencing contigs, execution and analysis of the in vitro data including enzyme assays and structural elucidation, and substantial drafting of the manuscript; T.S., conceptualization of project, experimental design, execution and analysis of in vivo data including knockouts and complementation, and drafting of manuscript; M.E.B., expression, purification and crystallization of steely proteins; S.H., genome annotation, data analysis and interpretation; A.K., experimental assistance; B.S.M., funding and manuscript editing; R.R.K. and J.P.N., principal investigators of the project, experimental concept and design, data analysis, manuscript editing and funding.
References
- 1.Kessin RH. Dictyostelium. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- 2.Morris HR, Taylor GW, Masento MS, Jermyn KA, Kay RR. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature. 1987;328:811–814. doi: 10.1038/328811a0. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CR, Kay RR. The role of DIF-1 signaling in Dictyostelium development. Mol Cell. 2000;6:1509–1514. doi: 10.1016/s1097-2765(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 4.Gokan N, et al. Structural requirements of Dictyostelium differentiation-inducing factors for their stalk-cell-inducing activity in Dictyostelium cells and anti-proliferative activity in K562 human leukemic cells. Biochem Pharmacol. 2005;70:676–685. doi: 10.1016/j.bcp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Kay RR. The biosynthesis of differentiation-inducing factor, a chlorinated signal molecule regulating Dictyostelium development. J Biol Chem. 1998;273:2669–2675. doi: 10.1074/jbc.273.5.2669. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer JL, Jez JM, Bowman ME, Dixon RA, Noel JP. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Biol. 1999;6:775–784. doi: 10.1038/11553. [DOI] [PubMed] [Google Scholar]
- 7.Schröder J. The family of chalcone synthase-related proteins: functional diversity and evolution. Recent Adv Phytochem. 2000;34:55–89. [Google Scholar]
- 8.Austin MB, Noel JP. The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep. 2003;20:79–110. doi: 10.1039/b100917f. [DOI] [PubMed] [Google Scholar]
- 9.Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirala SS, Wakil SJ. Structure and function of animal fatty acid synthase. Lipids. 2004;39:1045–1053. doi: 10.1007/s11745-004-1329-9. [DOI] [PubMed] [Google Scholar]
- 11.Asturias FJ, et al. Structure and molecular organization of mammalian fatty acid synthase. Nat Struct Mol Biol. 2005;12:225–232. doi: 10.1038/nsmb899. [DOI] [PubMed] [Google Scholar]
- 12.Maier T, Jenni S, Ban N. Architecture of mammalian fatty synthase at 4.5 A resolution. Science. 2006;311:1258–1262. doi: 10.1126/science.1123248. [DOI] [PubMed] [Google Scholar]
- 13.Khosla C, Gokhale RS, Jacobsen JR, Cane DE. Tolerance and specificity of polyketide synthases. Annu Rev Biochem. 1999;68:219–253. doi: 10.1146/annurev.biochem.68.1.219. [DOI] [PubMed] [Google Scholar]
- 14.Staunton J, Weissman KJ. Polyketide biosynthesis: a millennium review. Nat Prod Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- 15.Jez JM, et al. Structural control of polyketide formation in plant-specific polyketide synthases. Chem Biol. 2000;7:919–930. doi: 10.1016/s1074-5521(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 16.Jez JM, Bowman ME, Noel JP. Structure-guided programming of polyketide chain-length determination in chalcone synthase. Biochemistry. 2001;40:14829–14838. doi: 10.1021/bi015621z. [DOI] [PubMed] [Google Scholar]
- 17.Rangan VS, Joshi AK, Smith S. Mapping the functional topology of the animal fatty acid synthase by mutant complementation in vitro. Biochemistry. 2001;40:10792–10799. doi: 10.1021/bi015535z. [DOI] [PubMed] [Google Scholar]
- 18.Tillett D, et al. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806: an integrated peptide-polyketide synthetase system. Chem Biol. 2000;7:753–764. doi: 10.1016/s1074-5521(00)00021-1. [DOI] [PubMed] [Google Scholar]
- 19.Tsai SC, et al. Crystal structure of the macrocycle-forming thioesterase domain of the erythromycin polyketide synthase: versatility from a unique substrate channel. Proc Natl Acad Sci USA. 2001;98:14808–14813. doi: 10.1073/pnas.011399198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookman JJ, Town CD, Jermyn KA, Kay RR. Developmental regulation of stalk cell differentiation-inducing factor in Dictyostelium discoideum. Dev Biol. 1982;91:191–196. doi: 10.1016/0012-1606(82)90022-7. [DOI] [PubMed] [Google Scholar]
- 21.Morandini P, et al. The proximal pathway of metabolism of the chlorinated signal molecule differentiation-inductin factor-1 (DIF-1) in the cellular slime mould Dictyostelium. Biochem J. 1995;306:735–743. doi: 10.1042/bj3060735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin MB, Bowman ME, Ferrer J, Schröder J, Noel JP. An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketides synthases. Chem Biol. 2004;11:1179–1194. doi: 10.1016/j.chembiol.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Austin MB, et al. Crystal structure of a bacterial type III polyketide synthase and enzymatic control of reactive polyketide intermediates. J Biol Chem. 2004;279:45162–45174. doi: 10.1074/jbc.M406567200. [DOI] [PubMed] [Google Scholar]
- 24.Sankaranarayanan R, et al. A novel tunnel in mycobacterial type III polyketide synthase reveals the structural basis for generating diverse metabolites. Nat Struct Mol Biol. 2004;11:894–900. doi: 10.1038/nsmb809. [DOI] [PubMed] [Google Scholar]
- 25.Seshime Y, Juvvadi PR, Fujii I, Kitamoto K. Discovery of a novel superfamily of type III polyketide synthases in Aspergillus oryzae. Biochem Biophys Res Commun. 2005;331:253–260. doi: 10.1016/j.bbrc.2005.03.160. [DOI] [PubMed] [Google Scholar]
- 26.Morris HR, Masento MS, Taylor GW, Jermyn KA, Kay RR. Structure elucidation of two differentiation inducing factors (DIF-2 and DIF-3) from the cellular slime mould Dictyostelium discoideum. Biochem J. 1988;249:903–906. doi: 10.1042/bj2490903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serafimidis I, Kay RR. New prestalk and prespore inducing signals in Dictyostelium. Dev Biol. 2005;282:432–441. doi: 10.1016/j.ydbio.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Takaya Y, et al. Novel acyl alpha-pyronoids, dictyopyrone A, B, and C, from Dictyostelium cellular slime molds. J Org Chem. 2000;65:985–989. doi: 10.1021/jo991338i. [DOI] [PubMed] [Google Scholar]
- 29.Witkowski A, et al. Head-to-head coiled arrangement of the subunits of the animal fatty acid synthase. Chem Biol. 2004;11:1667–1676. doi: 10.1016/j.chembiol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Menzella HG, et al. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat Biotechnol. 2005;23:1171–1176. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 31.Abe I, Utsumi Y, Oguro S, Noguchi H. The first plant type III polyketide synthase that catalyzes formation of aromatic heptaketide. FEBS Lett. 2004;562:171–176. doi: 10.1016/S0014-5793(04)00230-3. [DOI] [PubMed] [Google Scholar]
- 32.Abe I, et al. A plant type III polyketide synthase that produces pentaketide chromone. J Am Chem Soc. 2005;127:1362–1363. doi: 10.1021/ja0431206. [DOI] [PubMed] [Google Scholar]
- 33.Abe I, Watanabe T, Noguchi H. Enzymatic formation of long-chain polyketide pyrones by plant type III polyketide synthases. Phytochemistry. 2004;65:2447–2453. doi: 10.1016/j.phytochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Jez JM, Ferrer JL, Bowman ME, Dixon RA, Noel JP. Dissection of malonyl-coenzyme A decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry. 2000;39:890–902. doi: 10.1021/bi991489f. [DOI] [PubMed] [Google Scholar]
- 35.Funa N, Ohnishi Y, Ebizuka Y, Horinouchi S. Properties and substrate specificity of RppA, a chalcone synthase-related polyketide synthase in Streptomyces griseus. J Biol Chem. 2002;277:4628–4635. doi: 10.1074/jbc.M110357200. [DOI] [PubMed] [Google Scholar]
- 36.Kay RR. Cell differentiation in monolayers and the investigation of slime mold morphogens. Methods Cell Biol. 1987;28:433–448. doi: 10.1016/s0091-679x(08)61661-1. [DOI] [PubMed] [Google Scholar]
- 37.Abe T, Langenick J, Williams JG. Rapid generation of gene disruption constructs by in vitro transposition and identification of a Dictyostelium protein kinase that regulates its rate of growth and development. Nucleic Acids Res. 2003;31:e107. doi: 10.1093/nar/gng095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito T, Ochiai H. Identification of delta5-fatty acid desaturase from the cellular slime mold Dictyostelium discoideum. Eur J Biochem. 1999;265:809–814. doi: 10.1046/j.1432-1327.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- 39.Masento MS, et al. Differentiation-inducing factor from the slime mould Dictyostelium discoideum and its analogues. Biochem J. 1988;256:23–28. doi: 10.1042/bj2560023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodson EJ, Winn M, Ralph A. Collaborative Computational Project, Number 4: providing programs for protein crystallography. Methods Enzymol. 1997;277:620–633. doi: 10.1016/s0076-6879(97)77034-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.