Abstract
Benzenoid carboxyl methyltransferases synthesize methyl esters (e.g., methyl benzoate and methyl salicylate), which are constituents of aromas and scents of many plant species and play important roles in plant communication with the surrounding environment. Within the past five years, eleven such carboxyl methyltransferases were isolated and most of them were comprehensively investigated at the biochemical, molecular and structural level. Two types of enzymes can be distinguished according to their substrate preferences: the SAMT-type enzymes isolated from Clarkia breweri, Stephanotis floribunda, Antirrhinum majus, Hoya carnosa, and Petunia hybrida, which have a higher catalytic efficiency and preference for salicylic acid, while BAMT-type enzymes from A. majus, Arabidopsis thaliana, Arabidopsis lyrata, and Nicotiana suaveolens prefer benzoic acid. The elucidation of C. breweri SAMT’s three-dimensional structure allowed a detailed modelling of the active sites of the carboxyl methyltransferases and revealed that the SAM binding pocket is highly conserved among these enzymes while the methyl acceptor binding site exhibits some variability, allowing a classification into SAMT-type and BAMT-type enzymes. The analysis of expression patterns coupled with biochemical characterization showed that these carboxyl methyltransferases are involved either in floral scent biosynthesis or in plant defense responses. While the latter can be induced by biotic or abiotic stress, the genes responsible for floral scent synthesis exhibit developmental and rhythmic expression pattern. The nature of the product and efficiency of its formation in planta depend on the availability of substrates, the catalytic efficiency of the enzyme toward benzoic acid and/or salicylic acid, and the transcriptional, translational, and post-translational regulation at the enzyme level. The biochemical properties of benzenoid carboxyl methyltransferases suggest that the genes involved in plant defenses might represent the ancestor for the presently existing floral genes which during evolution gained different expression profiles and encoded enzymes with the ability to accept structurally similar substrates.
1. Introduction
Plants produce more than 1000 different volatile secondary metabolites, many of which enable them to interact with their surroundings. Such compounds are released not only from flowers and vegetative tissues into the atmosphere but also from roots into the soil (Steeghs et al., 2004). The primary functions of airborne metabolites are to defend plants against herbivores and pathogens or to provide a reproductive advantage by attracting pollinators and seed dispersers. Plants produce scents, which can vary in number and amount of constituents in relation to different plant species, the stage of plant development, the time during the light/dark cycle, and the pollination status. Since only a few major biochemical pathways (phenylpropanoid/benzenoid, isoprenoid, and lipoxygenase pathway) are involved in the synthesis of plant volatiles, their large diversity originates from specific enzymatic derivatizations which sometimes in addition increase the volatility of compounds at the final step of their formation. It could be predicted that the synthesis of this wealth of volatiles will require almost as many speciffic enzymatic reactions as there are volatile metabolites. However, there are several mechanisms providing multiple mRNAs from one gene, multiple proteins from one mRNA, and multiple products from one enzyme (summarized in Schwab, 2003). Another way to limit the number of individual enzymes required for all these reactions is to broaden their substrate range so that they can accept several related substrates rather than favouring a high substrate specificity. This strategy is particularly applicable for reactions with similar or identical reaction mechanisms such as hydroxylations, acetylations or methylations (Pott et al., 2004; Dudareva et al., 2004).
The process of methylation that is catalyzed by S-adenosyl-L-methionine-dependent methyltransferases (EC 2.1.1.-), is an ubiquitous reaction that takes place in bacteria, fungi, plants and mammals. The reaction involves the transfer of the methyl group of S-adenosyl-L-methionine (SAM) to either nitrogen, sulfur, oxygen or carbon atoms and modifies DNA, RNA, proteins or small molecules with the formation of corresponding methylated product and S-adenosyl-L-homocysteine (Attieh et al., 2002; Ibrahim and Muzac, 2000). Enzymatic methylation of hydroxyl and carboxyl moieties is catalyzed by O-methyltransferases (O-MTs) (EC 2.1.1.6.-). Although the chemical mechanisms of methyl transfer are identical, O-MTs differ in their structure, their acceptance of substrates with different hydroxylation patterns at the benzene ring. Recent elucidation of the three dimensional structure of several plant O-MTs through protein X-ray crystallography defined three types of small molecule O-MTs (Zubieta et al., 2001, 2002; Noel et al., 2003). Type 1 O-MTs exclusively methylate oxygen atoms of hydroxyl moieties of phenylpropanoid-based compounds. The examples include chalcone O-methyltransferase (ChOMT) which methylates the 2′-OH of 2′,4,4′-trihydroxychalcone; isoflavone O-methyltransferase (IOMT) which acts on isoflavanone compounds in vivo, and multifunctional caffeic acid/5-hydroxyferulic acid 3′/5′-O-methyltransferase (COMT) that methylates 3′- and 5′-hydroxylated phenylpropanoids (Noel et al., 2003). Type 2 O-MTs, e.g., caffeoyl-CoA O-MT (CCoAOMT) are specific for phenylpropanoid esters of the coenzyme A which are found in all lignin-producing plants. Type 3 O-MTs specifically methylate carboxyl groups of small molecules and also nitrogen atoms of some alkaloids such as theobromine and caffeine, for which they are collectively named SABATH MTs based on the first three identified genes belonging to this family, SAMT, BAMT, and Theobromine synthase (D’Auria et al., 2003). The first member of the latter type of methyltransferases was shown to be involved in the synthesis of the volatile methyl salicylate in flowers of Clarkia breweri (Ross et al., 1999) and recently its crystal structure was solved (Zubieta et al., 2003; Noel et al., 2003). To date 11 type 3 carboxyl methyltransferases can be found in the data bank, many of those were biochemically characterized. They include the SAM:benzoic acid carboxyl methyltransferase (BAMT) from Antirrhinum majus (Murfitt et al., 2000); the SAM:jasmonic acid carboxyl methyltransferase (JMT) from Arabidopsis thaliana (Seo et al., 2001), the SAM:benzoic acid/salicylic acid carboxyl methyltransferases (BSMT) from A. thaliana and Arabidopsis lyrata (Chen et al., 2003), Petunia hybrida (Negre et al., 2003) and Nicotiana suaveolens (Pott et al., 2004), the SAM:salicylic acid carboxyl methyltransferases (SAMT) from C. breweri (Ross et al., 1999), Atropa belladonna (Fukami et al., 2002), A. majus (Negre et al., 2002), and Stephanotis floribunda (Pott et al., 2002).
The resulting reaction products of the carboxyl MTs are methyl esters, which often contribute to the plants’ characteristic scents and flavors and renders them appealing to humans, animals or insects. Several plant varieties therefore have been selected or bred for high content of methyl esters including methyl salicylate, methyl benzoate, methyl cinnamate, and methyl jasmonate. The latter, first identified in the floral scent of Jasminum grandiflorum (Demole et al., 1962) and broadly used in perfume industry, was also found to be involved in the regulation of diverse developmental processes, such as root growth, seed germination, flower and fruit development, leaf abscission and scenescence (Creelman and Mullet, 1997; Wasternack and Hause, 2002). Methyl salicylate, on the other hand, is recognized as a flavor ingredient and is often produced synthetically to flavor many types of candy, food and medicine (Cauthen and Hester, 1989; Das Gupta, 1974; Howrie et al., 1985). Methyl cinnamate is present in some basil varieties selected for this trait (Simon et al., 1990) while methyl benzoate is one of the major components of ylang-ylang oil (Adam et al., 2000).
Methyl salicylate and methyl benzoate are plant volatiles with importance in inter- and intra-organismic communication. This review focuses on floral enzymes synthesizing these benzenoids and summarizes the recent results obtained for this new class of O-methyltransferases five years after their discovery. The review covers the biological functions of their products, provides a comparison of the biochemical characteristics of these enzymes and also ties in aspects of their evolutionary origin.
2. Methyl salicylate and methyl benzoate emission is widespread in the plant kingdom
Benzoic and salicylic (2-hydroxyl benzoic) acids are synthesized from phenylalanine via the benzenoid branch of the phenylpropanoid pathway (Boatright et al., 2004), although an alternative pathway for salicylic acid biosynthesis through isochorismate synthase was recently found in Arabidopsis (Wildermuth et al., 2001). Benzoic and salicylic acids are precursors for the synthesis of methyl benzoate and methyl salicylate in reactions catalyzed by carboxyl methyltransferases. Methyl salicylate naturally occurs in leaves and flowers of wintergreen (Cauthen and Hester, 1989) and in the vegetative tissue of a wide range of species including strawberry, walnut, fig, tobacco and oat leaves (Buttery et al., 1982a,b, 1986; Andersen et al., 1988; Hamilton-Kemp et al., 1988). It is also a constituent of the flavor of such fruits as plum, strawberry, black cherry, and tomato (Herrmann, 1990). Methyl salicylate is involved in tritrophic interactions by attracting natural enemies of the herbivores upon herbivore damage (Takabayashi et al., 1994; Kessler and Baldwin, 2001) or in interplant communication between damaged and undamaged tissues (Shulaev et al., 1997; Dicke and Bruin, 2001). In strawberry leaves, the level of methyl salicylate increases by 10-fold after fruit harvest, making the plants more resistant to strawberry spider mites (Tetranychus urticae) (Hamilton-Kemp et al., 1988). Similarly, methyl benzoate has been found not only in the scent of flowers, but also in the aroma and flavor of some tropical fruits like kiwi (Young et al., 1983), starfruit (Fröhlich and Schreier, 1989), and feijoa fruit (Shaw et al., 1983). Analysis of moth and hawkmoth (Hyles lineata and Sphinx perelegans) electroantennogram responses to these two volatile esters (Raguso et al., 1996; Raguso and Light, 1998), as well as, their attractiveness for silver Y moth (Autographa gamma L.) in flight tunnel tests (Plepys et al., 2002) suggested their possible involvement in the attraction and guidance of pollinators.
Methyl salicylate and methyl benzoate are present in the floral scents of approximately 100 species of 30 different plant families (Knudsen et al., 1993; Knudsen pers. communication) (Table 1). Some plant species emit only one of these compounds, while many other plant species emit both compounds from their flowers. A detailed analysis revealed that even in closely related species there is a high diversity in emission of these methyl esters. For example, C. breweri emits methyl salicylate, while C. concinna, a member of the same genus, is scentless (Raguso and Pichersky, 1995). S. floribunda emits primarily methyl benzoate and little methyl salicylate (Pott et al., 2002) while Hoya carnosa, a member of the same family Asclepiadaceae, emits only methyl salicylate (Altenburger and Matile, 1988). In night-flowering Silene species (Caryophyllaceae) methyl benzoate is dominated in Silene saxifraga (96% of the total scent output) while in S. chlorantha or S. nutans flowers does not reach a 0.5% level (Jürgens et al., 2002). Predominance of methyl benzoate was also found in the other three members of Caryophyllaceae family, Dianthus arenarius, D. sylvestris, and Saponaria officinalis (42.1%, 85.7%, and 68.7% of the total scent output, respectively) while five Dianthus species (D. armeria, D. barbatus, D. deltoides, D. monspessulanus, and D. superbus) produce very little methyl benzoate (0.1–4.5%) (Jürgens et al., 2003). The emission of methyl salicylate and/or methyl benzoate also varies in the different Nicotiana species: N. suaveolens emits both methyl benzoate and methyl salicylate, N. sylvestris and N. alata only methyl benzoate, N. langsdorffii only methyl salicylate, while neither compound is found in the scents of N. rustica and N. tomentosiformis, N. longiflora, N. plumbaginifolia, N. forgetiana (Loughrin et al., 1990; Raguso et al., 2003).
Table 1.
Methyl benzoate and methyl salicylate in floral scents
| Plant | MeBA | MeSA | Reference |
|---|---|---|---|
| Agavaceae | |||
| Polyanthes tuberosa | − | 12.0b | (21) |
| Annonaceae | |||
| Cananga odorata | 0.2–0.7b | − | (20) |
| Araceae | |||
| Spathiphyllum wallisii | 2b | 20b | (6) |
| Asclepiadaceae | |||
| Hoya carnosa | − | + | (1) |
| Stephanotis floribundae | 14.7a | 5.9a | (28) |
| Asteraceae | |||
| Cirsium arvense | − | + | (3) |
| Bromeliaceae | |||
| Tillandsia crocata | − | 2b | (6) |
| Cactaceae | |||
| Dolichothele | + | + | (15) |
| Selenicereus | + | + | (15) |
| Sulcorebutia | + | + | (15) |
| Calycanthaceae | |||
| Chimonanthus praecox | − | + | (15) |
| Caryophyllaceae | |||
| Dianthus arenarius | 42.1b | 14.5b | (12) |
| Dianthus armeria | 0.1b | − | (12) |
| Dianthus barbatus | 0.4b | − | (12) |
| Dianthus deltoides | 4.2b | − | (12) |
| Dianthus monspessulanus | 4.5b | 9.1b | (12) |
| Dianthus superbus | 1.8b | 1.7b | (12) |
| Dianthus sylvestris | 85.7 | − | (12) |
| Saponaria officinalis | 68.7b | 0.4b | (12) |
| Silene chlorantha | 0.1b | − | (11) |
| Silene dichotoma | 1.0b | 0.2b | (11) |
| Silene italica | 3.0b | 0.6b | (11) |
| Silene latifolia | 0.5b | 0.3b | (11) |
| Silene nutans | 0.2b | tr | (11) |
| Silene otites | 0.4b | − | (11) |
| Silene saxifraga | 96.1b | 0.4b | (11) |
| Silene sericea | 0.9b | 0.2b | (11) |
| Silene subconica | 1.3b | 0.1b | (11) |
| Silene succulenta | 15.2b | 0.7b | (11) |
| Silene vallesia | 0.3b | 0.4b | (11) |
| Silene viscosa | 0.5b | 0.1b | (11) |
| Silene vulgaris | 0.5b | − | (11) |
| Dipsacaceae | |||
| Knautia arvensis | − | + | (24) |
| Eupomatiaceae | |||
| Eupomatia | + | + | (15) |
| Fabaceae | |||
| Coronilla | + | − | (15) |
| Medicago sativa | − | 2–3b | (2) |
| Gesneriaceae | |||
| Gloxinia perennis | − | 9b | (6) |
| Liliaceae | |||
| Hyacinthus | + | + | (15) |
| Nyctaginaceae | |||
| Acleisanthes crassifolia | 44.4b | 0.19b | (17) |
| Acleisanthes obtusa | 8.0b | 0.8b | (17) |
| Acleisanthes wrightii | − | 0.1b | (17) |
| Mirabilis greenei | − | − | (17) |
| Mirabilis multiflora | 44.5b | 2.8b | (17) |
| Selinocarpus angustifolius | 1.0b | 1.6b | (17) |
| Selinocarpus chenopodioides | 35.9b | 2.8b | (17) |
| Nymphaeaceae | |||
| Victoria | tr | − | (13) |
| Oleaceae | |||
| Jasminum | + | + | (15) |
| Onagraceae | |||
| Clarkia breweri | − | 4.4b | (29) |
| Clarkia concinna | − | − | (29) |
| Orchidaceae | |||
| Angreacum eichlerianum | 77.5b | − | (23) |
| Angreacum girymae | 11.1b | − | (23) |
| Angreacum sesquipedale | 7.3b | 13.5b | (23) |
| Brassavola | + | + | (33) |
| Catasetum collare | + | + | (8) |
| Catasetum dilectum | 0.7b | 98.8b | (9) |
| Catasetum gnomus | + | + | (8) |
| Catasetum luridum | − | − | (8) |
| Catasetum roseum | 0.7b | − | (9) |
| Chaubardia heteroclita | − | 9b | (6) |
| Cochleanthes aromatica | − | 5b | (6) |
| Coryanthes alborosea | + | − | (5) |
| Coryanthes leferenzorium | 3b | − | (6) |
| Coryanthes leucocorys | −; 10b | 76–95b | (6) |
| Coryanthes macrantha | − | 1b | (6) |
| Coryanthes trifoliata | − [+] | 2b | [(5)], (6) |
| Cycnoches | + | + | (15) |
| Dressleria | + | + | (15) |
| Epidendrum ciliare | + | + | (22) |
| Galeottia negrensis | − | 4b | (6) |
| Gongora | + | + | (15) |
| Houlletia tigrina | 2b | 98b [100b] | (35), [(6)] |
| Houlletia wallisii | 1b | (6) | |
| Huntleya lucida | − | 97b | (6) |
| Kefersteinia pellita | − | 3b | (6) |
| Lycaste aromatica | − | 0.55b | (34) |
| Lycaste ciliata | − | 6.3b | (34) |
| Neofinetia | + | − | (10) |
| Pescatorea dayana | − | 3b | (6) |
| Platanthera bifolia | 32.0–62.9b | 2.3–1.4b | (25), (32) |
| Platanthera chlorantha | 25.2b | 0.1b | (25), (32) |
| Stanhopea anfracta | 1–3.5b | 1.9–6.6b | (36) |
| Stanhopea candida | 0.9b | 88.5–93.2b | (36) |
| Stanhopea connata | 3.9–9.3b | − | (36) |
| Stanhopea grandiflora | 2b | − | (6) |
| Stanhopea graveolens | − | − | (36) |
| Trevoria lehmannii | − | 40b | (6) |
| Vandofinetia | + | − | (10) |
| Zygopetalum crinitum | 7b | 3b | (6) |
| Zygopetalum mackayi | − | 1.4b | (31) |
| Papaveraceae | |||
| Hypecoum fragrantissimum | + | + | (4) |
| Hypecoum imberbe | − | + | (4) |
| Hypecoum procumbens | + | + | (4) |
| Pinaceae | |||
| Larix sibirica | − | + | (15) |
| Picea abies | + | + | (15) |
| Pinus sylvestris | − | + | (15) |
| Pyrolaceae | |||
| Moneses uniflora | 0.1b | − | (14) |
| Pyrola media | − | − | (14) |
| Pyrola norvegica | − | − | (14) |
| Pyrola rotundifolia | − | − | (14) |
| Ranunculaceae | |||
| Cimicifuga japonica | 0.4b | − | (7) |
| Cimicifuga simplex | tr-8.5b | 0.2–6.6b | (7) |
| Rosaceae | |||
| Fragaria | − | + | (15) |
| Rubiaceae | |||
| Gardenia | + | + | (15) |
| Saliaceae | |||
| Salix | − | + | (15) |
| Scrophulariaceae | |||
| Antirrhinum majuse | 2.85d | − | (16) |
| Solanaceae | |||
| Cestrum nocturnum | 6.1b | 0.3b | (18) |
| Nicotiana alatae | 2.0b | 0.1b | (30) |
| Nicotiana bonariensise | − | tr | (30) |
| Nicotiana forgetiana | − | − | (30) |
| Nicotiana langsdorffie | − | 1.0b | (30) |
| Nicotiana longiflora | − | − | (30) |
| Nicotiana plumbaginifolia | − | − | (30) |
| Nicotiana suaveolense | 0.71a | 0.19a | (28) |
| Nicotiana sylvestrise | 6.8b [49.1]c | − | (30), [(19)] |
| Nicotiana rustica | − | − | (30) |
| Nicotiana tomentosiformis | − | − | (19) |
| Petunia hybridae | 11.5d | − | (16) |
| Theaceae | |||
| Camellia | tr-27.2b | tr-27.1b | (26) |
| Zamiaceae | |||
| Encephalartos altensteinii | − | 1.5b | (27) |
| Zamia pumila | − | 55.2b | (15) |
MeBA, methyl benzoate; MeSA, methyl salicylate.
+, compound detected in scent, not quantified;
−, compound not detected; tr, traces.
μg per g FW flower per hour.
Relative amounts [% of overall scent components].
ng per g flower.
3. Plant benzenoid carboxyl methyltransferases: sequence comparison, three-dimensional modelling, and biochemical characterization
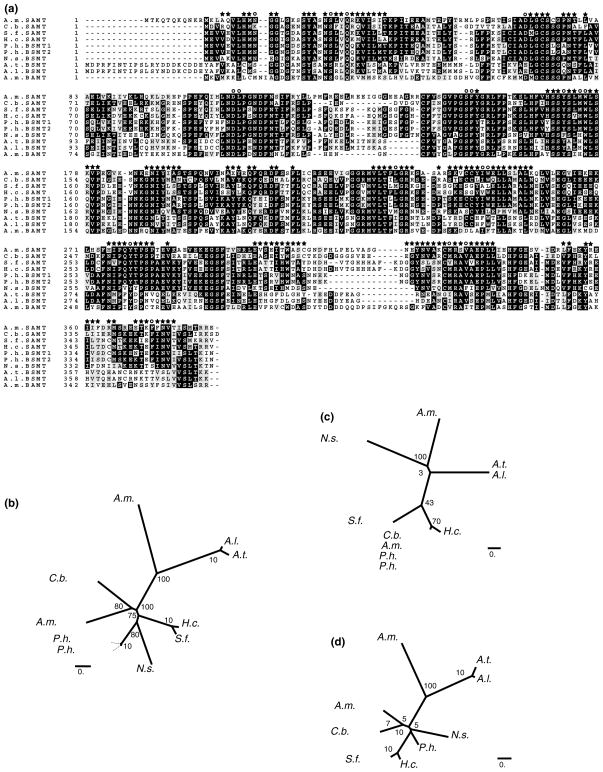
The first gene and enzyme involved in floral methyl salicylate synthesis was isolated from C. breweri (Ross et al., 1999), followed by the benzoic acid methyltransferase from A. majus (Dudareva et al., 2000). Within the past five years eleven related proteins were deposited into the sequence data bank. Sequence alignment (Clustal W) of presently known benzoic and salicylic acid carboxyl methyltransferases revealed 38% to 99% sequence identity at the amino acid level (Table 2). Determination of the molecular mass of the majority of the holoenzymes revealed that the active methyltransferases exist as homodimers with the molecular mass of the subunits varying between 39.9 and 43.7 kD (Table 3). The existence of enzyme dimers was supported by the determination of the protein crystal structure for the C. breweri SAMT, showing that the N-terminal sequence is involved in dimer formation, while the C-terminal domain is primarily involved in substrate binding (Zubieta et al., 2003; Noel et al., 2003). The polypeptide sequences lack encoded signal peptides in their 5′ end suggesting that the carboxyl methyltransferases are cytoplasmic enzymes. Indeed, immunogold localization of the BAMT protein in snapdragon petals provided in vivo evidence for its cytosolic location (Kolosova et al., 2001b).
Table 2.
Amino acid sequence identities (%) of floral benzenoid carboxyl methyltransferases (left to right: highest to lowest identity)
| H. c. SAMT 368 aa | S. f. SAMT 366 aa | P. h. BSMT2 357 aa | P. h. BSMT1 357 aa | A. b. SAMT 357 aa | A. m. SAMT 383 aa | N. s. BSMT 355 aa | C. b. SAMT 359 aa | B. c. NTR1 392 aa | A. m. BAMT 364 aa | A. l. BSMT 380 aa | A. t. BSMT 379 aa | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoya carnosa SAMT | 100 | 90 | 64 | 63 | 63 | 58 | 56 | 56 | 49 | 43 | 43 | 43 |
| Stephanotis floribunda SAMT | 100 | 88 | 88 | 63 | 57 | 56 | 56 | 48 | 43 | 42 | 42 | |
| Petunia hybrida BSMT2 | 100 | 99 | 85 | 61 | 64 | 58 | 48 | 45 | 43 | 42 | ||
| Petunia hybrida BSMT1 | 100 | 84 | 61 | 64 | 57 | 48 | 45 | 44 | 42 | |||
| Atropa belladonna SAMT | 100 | 62 | 61 | 58 | 48 | 44 | 44 | 42 | ||||
| Antirrhinum majus SAMT | 100 | 50 | 55 | 46 | 40 | 40 | 41 | |||||
| Nicotiana suaveolens BSMT | 100 | 52 | 45 | 42 | 42 | 41 | ||||||
| Clarkia breweri SAMT | 100 | 46 | 44 | 42 | 43 | |||||||
| Brassica campestris NTR1 | 100 | 42 | 38 | 38 | ||||||||
| Antirrhinum majus BAMT | 100 | 45 | 44 | |||||||||
| Arabidopsis lyrata BSMT | 100 | 92 | ||||||||||
| Arabidopsis thaliana BSMT | 100 |
Table 3.
Biochemical comparison of benzenoid carboxyl methyltransferases
| SAMT-type |
BAMT-type |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H. c. SAMT | P. h. BSMT1 | P. h. BSMT2 | S. f. SAMT | A. m. SAMT | C. b. SAMT | A. t. BSMT | A. l. BSMT | N. s. BSMT | A. m. BAMT | |
| Calculated subunit mass (D) | 41,360 | 40,662 | 40,676 | 41,316 | 43,690 | 40,289 | 43,365 | 43,234 | 39,944 | 41,011 |
| Holoenzyme mass (D) | nd | 78,430 | 77,100 | 70,000 | 107,000 | 74,500 | 91,000 | 91,000 | nd | 100,000 |
| pH optimum | 7.2–7.6 | 7–8 | 6.5–7.5 | 7.0–7.8 | 7–7.5 | 7.5 | 7–8 | 7–8 | 6.8 | 7.5 |
| Temperature optimum (°C) | nd | nd | nd | 10–20 | nd | 20 | nd | nd | 15–20 | nd |
| Salicylic acid (%) | 100 | 100 | 100 | 100 | 100 | 100 | 46 | 16 | 22.5 | 0 |
| Benzoic acid (%) | 10 | 16.3 | 27.3 | 31.9 | 45 | 69 | 100 | 100 | 100 | 100 |
| 3-OH-BA (%) | 0 | 0.65 | 1.32 | 75.6 | 0 | <2 | 54 | 0 | 15.3 | 0 |
| 4-OH-BA (%) | 0 | nd | nd | 34 | 0 | 0 | 0 | 0 | 76.2 | 0 |
| Cinnamic acid (%) | 0 | 0.41 | 0.69 | 34.5 | 0 | <2 | 0 | 0 | 16.5 | 0 |
| o-Coumaric acid (%) | nd | 0.14 | 0.25 | 25.0 | 0 | nd | nd | nd | 21 | 0 |
| m-Coumaric acid (%) | nd | 0.24 | 0.5 | 23.8 | 0 | nd | nd | nd | 4.9 | 0 |
| 2,3-OH-BA (%) | nd | nd | nd | 99.5 | nd | nd | nd | nd | 14.9 | nd |
| 2,4-OH-BA (%) | nd | nd | nd | 98.5 | nd | nd | nd | nd | 18.2 | nd |
| Km (SAM) μM | nd | 1.9–15.8 | 2.8–30.8 | 63 | 3–4 | 9 | 67 | 87 | 2.25 | 28–87 |
| Km (SA) μM | nd | 51.6 | 69.6 | 250 | 83 | 24 | 16 | 127 | 162.2 | no subst |
| Km (BA) μM | nd | 1273 | 2356 | 2900 | 1720 | 190 | 65 | 131 | 148.6 | 1100–1500 |
| Kcat/Km (SA) s−1 M−1 | nd | 230 | 57 | 16.3 | 132 | 116.7 | 4300 | 400 | 160.3 | no subst. |
| Kcat/Km (BA) s−1 M−1 | nd | 3 | 1.4 | 14.6 | 3.37 | nd | 2900 | 9600 | 625.8 | 19–27 |
nd: not determined; no subst: no substrate.
Analysis of substrate specificity of native or recombinant carboxyl methyltransferases revealed a high specificity of these enzymes for SAM with Km values ranging between 2 and 90 μM. On the other hand, variation of the Km values for the methyl acceptors is much broader. Based on methyl acceptor preferences, the subgroup of benzoate specific enzymes can be divided in two categories: SAMT- and BAMT-types (Table 3). The generation of a relationship tree using the PHYLIP software package (Felsenstein, 1985) also clearly distinguished the SAMT-type group of enzymes from the BAMT-type group with the exception of BSMT from N. suaveolens, which maintains the highest level of amino acid sequence identity with BSMT from P. hybrida, a member of the same Solanaceae family (Fig. 1(a)). The SAMT-type includes the enzymes from C. breweri, S. floribunda, H. carnosa, A. majus, and P. hybrida. This latter group of enzymes favor salicylic acid over benzoic acid. For these enzymes, the Km values for salicylic acid are in the micromolar range, from 24 to 250 μM, while the Km values for benzoic acid are in the millimolar range, from 1.3 to 2.9 mM, with the exception of C. breweri SAMT. This enzyme has a Km value of 190 μM for benzoic acid. The activities of these enzymes towards benzoic acid constitute 10–69% of their activity levels with salicylic acid. Where determined, the catalytic efficiencies of these enzymes for salicylic acid are 39 to 76-fold higher than for benzoic acid. The SAMT from S. floribunda represents the exception and exhibits almost equal catalytic efficiencies for salicylic and benzoic acids, but has about a 12-fold higher specificity for salicylic acid than for benzoic acid based on determined Km values.
Fig. 1.
Phylogenetic trees of carboxyl methyltransferases based on computer modelling. (a) Alignment of amino acid sequences of 10 carboxyl methyltransferase. Alignment was performed using AlignX. The circle symbol indicates amino acids of the binding pocket (see Table 4), and the star symbol indicates amino acids of the 2nd tier. (b) Unrooted neighbor-joining phylogenetic tree based on amino acid sequence similarity between plant benzenoid carboxyl methyltransferases complete amino acid sequences (355 to 392 amino acids). (c) Unrooted neighbor-joining tree based on amino acids involved in the methyl acceptor binding pocket of plant benzenoid carboxyl methyltransferases (amino acids presented in Table 4). (d) Unrooted neighbor-joining tree based on 2nd tier amino acids which were found in clusters within a 10 Å radius around each of the residues in the methyl acceptor binding pocket. The unrooted neighbor joining trees and associated bootstrap values were generated using the PHYLIP software package (Felsenstein, 1985). TreeView was used to visualize the resulting trees. The accession No. of the Hoya carnosa SAMT is AJ 863118.
The BAMT-type group of enzymes comprises BSMTs from N. suaveolens, A. thaliana and A. lyrata, and BAMT from A. majus (Table 3). These enzymes have similar Km values for both salicylic and benzoic acids in the micromolar range (from 16 to 162 μM), with the exception of BAMT from A. majus, which only methylates benzoic acid and has an unexpectedly high Kmvalue towards this substrate (1.1–1.5 mM). However, the catalytic efficiencies of N. suaveolens and A. lyrata BSMTs for benzoic acid are from 4 to 24-fold higher than with salicylic acid, indicating that benzoic acid is the preferred substrate. The Km value of the BSMT from A. thaliana for salicylic acid is only slightly smaller than with benzoic acid (4 fold) and the catalytic efficiency also indicates salicylic acid as a slightly better substrate. However, in in vitro assays in the presence of 1 mM of benzoic or salicylic acids due to its higher turnover value (Kcat) with benzoic acid compared with salicylic acid (0.19 versus 0.07 s−1, respectively) it produces more methyl benzoate (Chen et al., 2003).
Within these two groups of enzymes, two patterns of substrate preferences emerge: H. carnosa, A. majus, C. breweri, P. hybrida, A. thaliana and A. lyrata SAMTs and BSMTs can accept primarily two substrates, benzoic acid and salicylic acid, while substrate acceptance is significantly extended in the S. floribunda and N. suaveolens enzymes, since several benzoic and cinnamic acid derivatives can also be methylated.
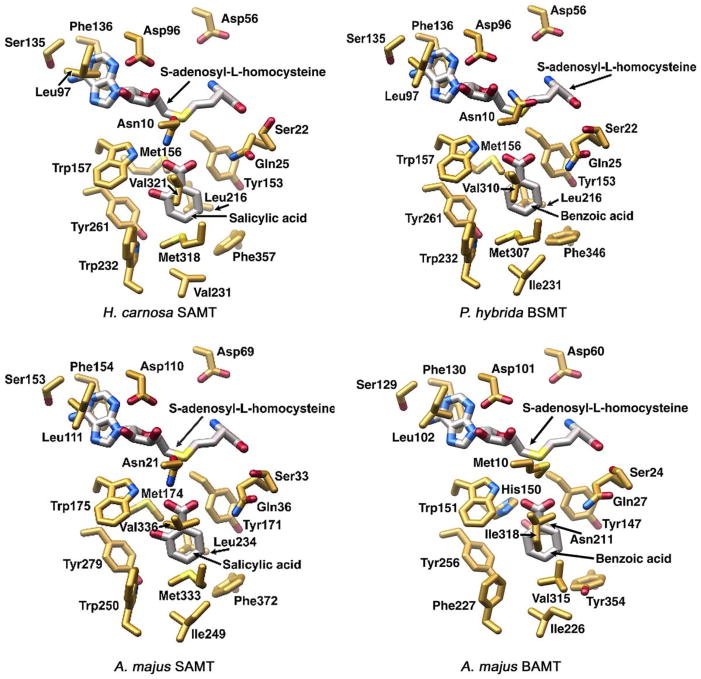
For enzymes that utilize very chemically distinct substrates, substrate binding and product formation are often intimately related to the complementary shape and chemical features of the constellation of amino acids found in the enzymes’ active sites. For the family of SAMTs, which demonstrate a preference for salicylic acid and the BAMTs which, in majority demonstrate activity with both benzoic acid and salicylic acid, structural complementarity of the active sites to the substrates is not obvious. Within the binding sites of the BAMT-type and the SAMT-type enzymes, there is a high degree of overall conservation. Comparative analysis of amino acid composition within the substrate-binding sites shows that the SAM binding pocket is highly conserved among the known benzenoid carboxyl methyltransferases while the methyl acceptor binding sites exhibit more variability between the different species, but do not possess signature sequences that correlate with distinct specificities for benzoate or salicylate (Table 4, Fig. 2).
Table 4.
Amino acid alterations of the SAM (A) and substrate binding sites (B) of floral benzenoid carboxyl methyltransferases
| SAMT-type |
BAMT-type |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. b. SAMT | A. m. SAMT | P. h. BSMT1 | P. h. BSMT2 | H. c. SAMT | S. f. SAMT | A. t. BSMT | A. l. BSMT | N. s. BSMT | A. m. BAMT | |
| A | Lys 10 | Asn | Asn | Asn | Asn | Asn | Ser | Ser | Asn | Met |
| Ser 22 | Ser | Ser | Ser | Ser | Ser | Ser | Ser | Ser | Ser | |
| Asp 57 | Asp | Asp | Asp | Asp | Asp | Glu | Glu | Asp | Asp | |
| Asp 98 | Asp | Asp | Asp | Asp | Asp | Asp | Asp | Asp | Asp | |
| Leu 99 | Leu | Leu | Leu | Leu | Leu | Leu | Leu | Leu | Leu | |
| Ser 129 | Ser | Ser | Ser | Ser | Ser | Ser | Ser | Ser | Ser | |
| Phe 130 | Phe | Phe | Phe | Phe | Phe | Phe | Phe | Phe | Phe | |
| B | Gln 25 | Gln | Gln | Gln | Gln | Gln | Gln | Gln | Gln | Gln |
| Tyr 147 | Tyr | Tyr | Tyr | Tyr | Tyr | Tyr | Tyr | Phe | Tyr | |
| Met 150 | Met | Met | Met | Met | Met | His | His | His | His | |
| Trp 151 | Trp | Trp | Trp | Trp | Trp | Trp | Trp | Trp | Trp | |
| Leu 210 | Leu | Leu | Leu | Leu | Met | Ile | Ile | Met | Asn | |
| Ile 225 | Ile | Ile | Ile | Val | Ala | Phe | Phe | Ile | Ile | |
| Trp 226 | Trp | Trp | Trp | Trp | Leu | Trp | Trp | Leu | Phe | |
| Tyr 255 | Tyr | Tyr | Tyr | Tyr | Tyr | Tyr | Tyr | Tyr | Tyr | |
| Met 308 | Met | Met | Met | Met | Val | Ile | Ile | Met | Val | |
| Val 311 | Val | Val | Val | Val | Val | Val | Val | Phe | Ile | |
| Phe 347 | Phe | Phe | Phe | Phe | Phe | Thr | Thr | Ser | Tyr | |
Fig. 2.
Computer modelling of the active site of four floral benzenoid carboxyl methyltransferases. Three-dimensional view of the active sites of the methyltransferases from Hoya carnosa (SAMT, upper left pannel, accession No. AJ 863118), Petunia hybrida (BSMT, upper right pannel), Antirrhinum majus (SAMT, lower left pannel, BAMT, lower right pannel). The side chains are depicted as half-colored sticks. The modelling was performed as described in Zubieta et al. (2003).
For the SAMT-type enzymes, differences in the breadth of substrate tolerance must be explained by amino acid differences outside the active site, which may ultimately exert an influence on binding pocket conformation and catalysis. For the S. floribunda SAMT it is reasonable to hypothesize that residue substitutions in the methyl acceptor binding pocket may contribute to this enzyme’s broader tolerance for diverse carboxyl-bearing substrates. The majority of the substrate binding site changes in S. floribunda SAMT (Ala 231 for Ile, Leu 232 for Trp, and Val 316 for Met) may enhance the active site volume to allow sequesteration of both benzoate-derived substrates with increased hydroxylation and bulkier substrates such as cinnamic and coumaric acid (Table 3).
One of the distinctive structural differences between both enzyme types is the occurrence of a His residue in the BAMT-type, which is replaced by a Met residue in the SAMT-type (Met 150 in C. breweri SAMT). However, this substitution alone cannot account for any preference forx benzoic acid versus salicylic acid, as the His residue at this position is also found in the Arabidopsis enzymes that show substrate specificity for jasmonic acid (A. thaliana JMT, Seo et al., 2001), and for auxin (A. thaliana IAMT, Zubieta et al., 2003).
Two other notable substitutions may be significant to the enzymes’ ability to accept salicylic acid or benzoic acid as substrates. One is the substitution of a Leu residue (Leu 210 in C. breweri SAMT), which is conserved throughout most of the SAMT-type enzymes, by an Asn 211 in the A. majus BAMT. Asn, unlike Leu, has an amide side chain with both a potential hydrogen bond donor (carbonyl oxygen) and a hydrogen bond acceptor (amino nitrogen). Interestingly, the A. majus BAMT accepts benzoic acid as a substrate, but with a Km value significantly higher than that of all other SAMTs or BAMTs, whereas the other enzymes in the BAMT group, which instead have conservative Ile or Met substitutions at the position equivalent to Leu 210 in C. breweri SAMT, show efficient activity with both benzoic and salicylic acid (Tables 3 and 4). Therefore, the Asn substitution may perturb the active site, resulting in the decreased affinity for benzoic acid seen in A. majus BAMT. Another substrate binding site distinction is the conservation of a Phe residue throughout the SAMTs (Phe 347 in C. breweri SAMT), and the substitution of a hydroxyl bearing residue throughout the BAMT-type enzymes (Tyr 354 in A. majus BAMT, Thr 369 and Thr 370 in the A. thaliana and A. lyrata BSMTs, respectively, and Ser 344 in N. suaveolens BSMT). How these substitutions allow the enzyme to make the subtle distinction between the non-hydroxylated benzene ring of benzoic acid versus that of the hydroxylated salicylic acid may best be explained by influences of residues one tier removed from the active site. The dynamics of forces exerted on active site residues cannot be appreciated by inspection of the static models generated based on the experimentally determined structure of the Clarkia SAMT enzyme (Fig. 2).
Perhaps the single most important determinant of an enzyme’s ability to turn over benzoate versus salicylate is revealed by comparison of the structures of the substrates themselves. In salicylic acid, the presence of the hydroxyl substitution on the benzene ring allows for the formation of a very stable, high energy intramolecular hydrogen bond between this hydroxyl moiety and the nearby carboxyl group, constraining the ring to remain in the same plane as this carboxyl. Such an intramolecular hydrogen bond would prevent rotation of the benzene ring around the axis of the bond bearing the carboxyl (Zubieta et al., 2003). In benzoic acid, the lack of the hydroxyl substitution allows for free rotation of the aromatic ring around the axis of the carboxyl-bearing bond. The internal rigidity of salicylic acid allows for active site residues to better constrain this substrate for methylation, thus permitting the substrate binding pocket itself to be more narrow and to fit more tightly around the substrate with little need to adapt to the increased dynamics associated with benzoate. Benzoic acid, in contrast, is a more “slippery” substrate, and presents more of a challenge for an enzyme to sequester in a catalytically competent form with high specificity. To turn over benzoic acid, the enzyme’s active site must either be more spacious, providing an ellipsoidal environment in which the benzene ring can rotate, or its active site must efficiently adapt to this substrate’s increased rotational freedom. If neither case exists, then only a portion of the benzoate substrate will be in a productive conformation for recognition, binding and subsequent transmethylation when encountering the enzyme’s active site. A comparison of the Km data of the SAMT-type enzymes for salicylic acid versus benzoic acid, clearly demonstrates that the enzymes in this group show an increased ability to use salicylic acid over benzoic acid. Within the BAMT-type, the Km data show that three out of four of these enzymes can efficiently turn over both salicylic acid and benzoic acid, and have improved ability in turnover of benzoic acid as compared to the SAMT-type, suggesting that the BAMT-type enzymes must have either a more spacious active site or a more dynamic active site architecture which enables them to accomodate the mobility of benzoic acid during methylation.
The S. floribunda SAMT and the N. suaveolens BSMT exhibit the most promiscuous substrate tolerances (Table 3). The N. suaveolens BSMT shares five out of eleven substrate binding residues with the S. floribunda SAMT. Both enzymes share a conserved Met (position 210) at the position occupied by an Asn residue in the A. majus BAMT, and by Ile in both the A. thaliana and A. lyrata BSMTs. The Ile present in the A. thaliana and A. lyrata BSMTs is a conservative substitution for the Leu residue found generally at this position throughout the SAMT-type enzymes (Leu 210 in C. breweri SAMT), and hence would not appear to confer increased ability to turn over benzoic acid. The Met residue (position 210) might contribute to the broader substrate tolerances of these two enzymes, as its sulfur-containing side chain would allow for increased hydrophobic interactions with other active site residues, aiding in the exclusion of water necessary for binding and turnover of aromatic carboxyl-bearing substrates (Zubieta et al., 2003). Additionally, both enzymes share a conserved Leu at a position occupied by a Trp residue (position 226) in the other SAMT-type enzymes, and in the A. thaliana and A. lyrata BSMTs. This conserved Trp is substituted with another hydrophobic residue, Phe 227, in A. majus BAMT. The substitution of the much smaller Leu for the bulkier aromatic rings of Trp or Phe, could serve to enlarge the active site, accounting for its broader substrate tolerances as compared to the other SAMTs and BAMTs.
The generation of a neighbor-joining tree based on amino acids involved in methyl acceptor binding sites revealed some correlations with the observed substrate specificities of the analyzed enzymes and clearly separated A. majus BAMT and, to a lesser extent, N. suaveolens BSMT, the BAMT-type of enzymes, from the SAMT-type (Fig. 1(b)). Although A. thaliana and A. lyrata BSMTs also belong to BAMT-type of enzymes based on their biochemical properties, the low bootstrap values of the neighbor-joining tree do not allow these latter enzymes to group together with the A. majus BAMT and the N. suaveolens BSMT.
4. Expression of plant benzenoid carboxyl methyltransferase
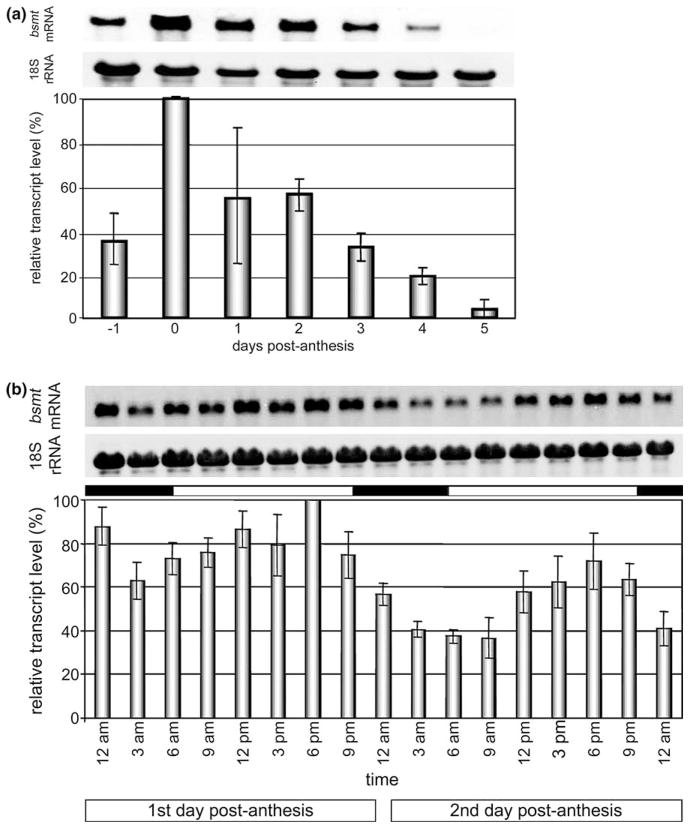
Presently characterized carboxyl methyltransferases are thought to be involved in the production of flower volatiles (A. majus BAMT, P. hybrida and N. suaveolens BSMTs, C. breweri, H. carnosa and S. floribunda SAMTs) or in plant defense responses (A. thaliana and A. lyrata BSMTs, and A. majus and A. belladonna SAMTs). The first group represents floral genes which are highly and specifically expressed in petal tissues, the principal emitters of scent methyl esters (Table 5, Fig. 3). Further dissection of petunia, snapdragon and S. floribunda flowers revealed that tubes of these flowers contribute very little to the expression pattern, thus concentrating scent production to the flower areas facing pollinators and increasing advertising efficiency (Dudareva et al., 2000; Negre et al., 2003; Rohrbeck and Piechulla, unpublished). Expression of C. breweri, S. floribunda SAMT and A. majus BAMT genes is epidermis specific and, in the case of S. floribunda SAMT and A. majus BAMT, two epidermal petal layers, the inner and the outer, are differentially involved in floral scent biosynthesis (Kolosova et al., 2001b; Ross, 2002; Rohrbeck and Piechulla, unpublished). The expression of carboxyl methyltransferases was found to be developmentally regulated over the life span of the flowers and positively correlated with the corresponding methyltransferase activities and the emission of the volatile methyl ester (Dudareva et al., 2000; Pott et al., 2002; Ross, 2002; Fig. 3(a)), supporting the involvement of these genes in scent production.
Table 5.
Expression of the plant benzenoid carboxyl methyltransferases
| Floral genes |
Defense genes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. b. SAMT | A. m. BAMT | S. f. SAMT | P. h. BSMTs | N. s. BSMT | H. c. SAMT | A. b. SAMT | A. m. SAMT | A. t. BSMT | A. l. BSMT | |
| Tissue specific expression (in decreasing order) | Petals, other parts of flower, leaves; | Upper and lower petal lobes | Petals | Limbs, tubes | Petals, stamens, styles and stigmas; | Petals, gynecium | Hairy roots | Petal lobes, sepals, leaves, tubes; | Flowers, leaves, stems | Flowers, leaves |
| Method used Regulation of expression | Northern blot Developmental | Northern blot Developmental, rhythmic (max. 6 pm), circadian | Northern blot Developmental, rhythmic (max. 6 pm–midnight), circadian | Northern blot Developmental, rhythmic (nocturnal) | Northern blot Developmental, rhythmic (max. 6 pm), circadian | Northern blot Developmental, rhythmic (max. 6pm) | Northern blot nd |
RT-PCR nd |
RT-PCR nd |
RT-PCR nd |
| Induction by | nd | nd | nd | nd | nd | nd | SA*, in hairy roots | SA*, JA*, only in flowers | alamethicin, MeJA*, herbivory, uprooting, physical wounding, in flowers and leaves | nd |
| Activity (pkats/g FW) | ||||||||||
| SA* | 28.1 | 0 | 235 | 259.6 | 0.56 | 9.8 | nd | nd | nd | nd |
| BA* | nd | 36 | 29.4 | 58.3 | 2.2 | 0 | nd | nd | nd | nd |
| Suggested function in planta | MeSA* production in flowers | MeBA* production in flowers | MeBA* and MeSA* production in flowers | MeBA* production in flowers | MeBA* and MeSA* production in flowers | MeSA* production in flowers | Detoxification of SA* into MeSA* in hairy roots | Defense through production of MeSA* in flowers | Defense through production of MeSA*, occasionally MeBA* in plant | nd |
| References | Dudareva et al. (1998), Ross (2002) | Dudareva et al. (2000), Kolosova et al. (2001a,b) | Pott et al. (2003, 2004) | Negre et al. (2003); Dudareva and Kish, unpublished | Pott et al. (2004); Fig. 3 | Pott (2003); Piechulla, Heidebrecht and Piechulla, unpublished | Fukami et al. (2002) | Negre et al. (2002) | Chen et al. (2003) | Chen et al. (2003) |
SA: salicylic acid; BA: benzoic acid; MeSA: methyl salicylate; MeBA: methyl benzoate; JA: jasmonic acid; MeJA: methyl jasmonate.
Fig. 3.
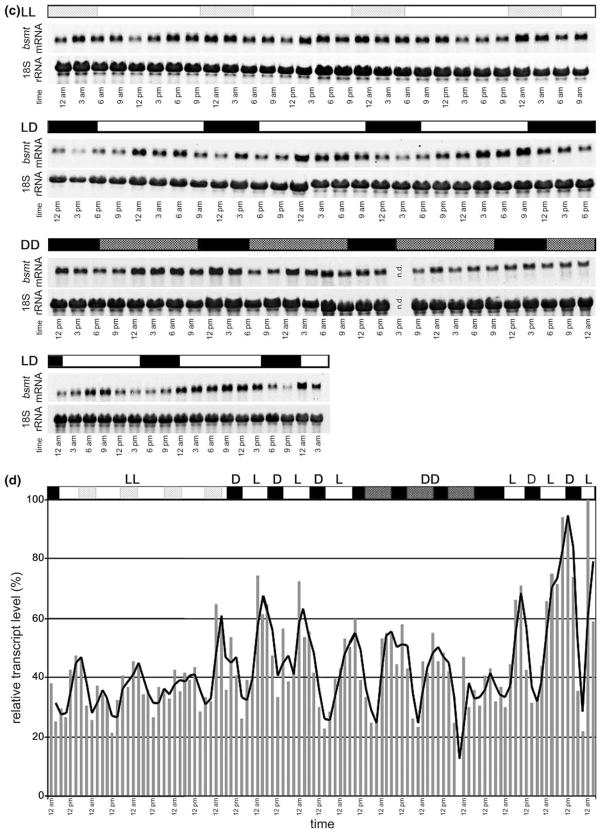
Expression of the N. suaveolens BSMT. (a) BSMT transcript levels at different flower age: Plants were grown under LD cycle (16 h/8 h). Petals of N. suaveolens were harvested at 6 pm, in flower buds (day 1), at the day of flower opening (day 0) and at day 1 to 5 after flower opening. At day 5 flowers show obvious signs of senescence. (b) BSMT transcript levels at different time points during the day: Plants were grown under 16 h light and 8 h darkness (10 pm to 6 am). Petals of N. suaveolens were harvested at indicated time points during the day at the first and second day after flower opening. Black bars represent time of darkness, white bars represent time of illumination. (c) and (d) BSMT transcript levels under varied light regimes: Plants were grown under 16 h light and 8 h darkness (10 pm to 6 am) before they were shifted to continuous light conditions (LL for 4 days). Subjective night is represented by light gray shaded bars. Thereafter, light conditions were altered to 16 h light and 8 h darkness (10 to 6 pm darkness) for 3 days (LD). Black bars represent time of darkness, white bars represent time of illumination. Light was then switched off and plants were kept in complete darkness for 3 days (DD) (n.d. not determined). The subjective day is indicated by the dark gray shaded bar. Light conditions were then changed to 16 h light and 8 h darkness (4 pm to midnight) for 2 days (LD). Petals of N. suaveolens were harvested at indicated time points. RNA was extracted and RNA gels were run with 5 microgramm total RNA. The blots were hybridized with the N. suaveolens BSMT specific probe and rehybridized with 18 S rDNA probe. Relative transcript levels (normalized with rRNA data) were calculated, and the highest value was set as 100% (lowest panel).
Among the six plant species for which floral carboxyl methyltransferases were isolated, five species exhibit rhythmicity in methyl ester emission with a maximum during the day in A. majus and during the night in P. hybrida, N. suaveolens, H. carnosa, and S. floribunda (Kolosova et al., 2001a; Pott et al., 2003; Altenburger and Matile, 1988). In these species, the expression of carboxyl methyltransferase genes or enzyme activities oscillate during a light/dark cycle. These oscillations in the case of A. majus, S. floribunda and N. suaveolens were found to be controlled by a circadian clock (Kolosova et al., 2001a; Pott et al., 2003; Figs. 3(b–d).
The second group represents genes involved in defense reactions since their expression could be induced by biotic and/or abiotic stresses (Fukami et al., 2002; Negre et al., 2002; Chen et al., 2003). These genes are not only expressed in floral tissues but also in leaves (Table 5). Additionally, a high level of SAMT expression was found in A. belladonna hairy roots, however the expression of this gene in other parts of the plant was not analyzed (Fukami et al., 2002). The common feature of genes belonging to this group is that they are responsible primarily for the biosynthesis of methyl salicylate, which is thought to be involved in plant–plant and plant–insect interactions (van Poecke et al., 2001; Shulaev et al., 1997). Indeed, it has been shown that alamethicin, herbivore damage by the larvae of Plutella xylostella, uprooting, mechanical wounding, and methyl jasmonate treatment induce the expression of BSMT gene and, in some of these cases, the emission of methyl salicylate and occasionally methyl benzoate in A. thaliana leaves (Chen et al., 2003). Moreover, when leaves were damaged by thrips of the genus Franklinella, the induction of BSMT expression occurred specifically around lesions. While salicylic acid was not a good inducer of BSMT gene expression in A. thaliana leaves, it induced A. majus SAMT in snapdragon petals 48h after treatment (Negre et al., 2002). Similar results were obtained with jasmonic acid treatment, suggesting the possible role of SAMT in plant defense. Salicylic acid also induced expression of the SAMT gene in A. belladonna hairy roots starting 12 h after the treatment thereby converting the deleterious amount of salicylic acid to methyl salicylate in a detoxification process (Fukami et al., 2002). While the results described above suggest the involvement of this group of genes in plant defense, the exact biological significance of the induction of these carboxyl methyltransferases and the function of their products awaits further investigations.
5. Prediction of in planta function of benzenoid carboxyl methyltransferases
The biochemical characterization of the cloned carboxyl methyltransferases confirms their involvement in the synthesis of methyl benzoate and/or methyl salicylate in plant floral tissues. The question of which product the enzymes synthesize in planta does not always have a straight forward answer. Analysis of the scent profile of various plant species revealed different emission patterns with some species emitting only one methyl ester (A. majus, C. breweri, H. carnosa, P. hybrida) (Dudareva et al., 2000; Raguso and Pichersky, 1995; Verdonk et al., 2003) while the others emitted both (N. suaveolens and S. floribunda) (Pott et al., 2002; Raguso et al., 2003). Obviously, the in vitro specificities of O-methyltransferases do not always correlate with the scent production in vivo, since it also depends on the plant cellular pools of available substrates.
Out of six carboxyl methyltransferase genes involved in scent production, two (C. breweri SAMT and A. majus BAMT) were isolated via a classical biochemical approach through enzyme purification from petal tissues (Ross et al., 1999; Murfitt et al., 2000). While the function of these genes in the biosynthesis of methyl esters in planta was clear, the isolation of A. majus SAMT via a functional genomic approach suggested the possible contribution of this gene to methyl benzoate production in snapdragon flowers. However, the low expression of A. majus SAMT in petal tissue along with the high Km value and low catalytic efficiency of the enzyme for benzoic acid revealed that this gene could not make a significant, if any, contribution to methyl benzoate production and emission in snapdragon flowers (Negre et al., 2002).
The other four carboxyl methyltransferase genes involved in scent production were isolated using a functional genomic approach (P. hybrida) or reverse transcriptase (RT)-PCR (H. carnosa, N. suaveolens, S. floribunda) based on sequence information available from previously isolated carboxyl methyltransferases. Considering that the O-MTs catalyze equivalent reactions and exhibiting diverse biochemical properties, their physiological contribution is under debate and therefore should be discussed case by case. The most straight forward situation was found in H. carnosa which emits only methyl salicylate (Table 1) (Altenburger and Matile, 1988; Heidebrecht and Piechulla, unpublished results). In vitro, the isolated enzyme (SAMT) has a 10-fold higher activity with salicylic acid than with benzoic acid (Table 3) and only salicylic acid was detected in petal tissue (Table 6). Taken together with the expression data (Table 5), these results suggest that this enzyme synthesizes methyl salicylate in planta.
Table 6.
Levels of benzoic acid and salicylic acid in petals of methyl ester emitting flowers
| Substrate (nmol/g FW) | H. carnosa | S. floribunda | N. suaveolens | P. hybrida | A. majus |
|---|---|---|---|---|---|
| Free SA | 0.19a | 0.35 ± 0.02b | nd | 0.38 ± 0.05e | nd |
| Total SA | 3.75 ± 0.5a | 1.54 ± 0.37b | udlc 0.97 ± 0.06d | nd | nd |
| Free BA | udl | udl | nd | 518 ± 72f | 49. 2 ± 1.3g |
| Total BA | udl | 2863 ± 424b | 2568 ± 874c | nd | nd |
udl: under detection limit.
nd: not determined.
Level at 6 am on day 3 after flower opening (Pott, 2003).
Level at midnight on day 1 after flower opening (Pott, 2003).
Level at 9 pm on day after flower opening (Slusarenko, Piechulla, pers. communication).
Level at 9 am on day 1 after flower opening (Slusarenko, Piechulla, pers. communication).
Level at 9 pm on day 1 postanthesis (Negre et al., 2003).
Level at the maximum of methyl benzoate emission (12 am) on day 1 postanthesis (Kolosova et al., 2001a).
Level at the maximum of methyl benzoate emission (3–6 pm) on day 3 postanthesis (Kolosova et al., 2001a).
In petunia flowers, which emit only methyl benzoate, the apparent catalytic efficiencies (kcat/Km ratio) of the isolated BSMTs were higher with salicylic acid than with benzoic acid (Table 3), indicating that salicylic acid was the preferred substrate. Although a high carboxyl methyltransferase activity towards salicylic acid was also detected in petunia petals, there was a very small internal pool of free salicylic acid (~10 times lower than the apparent Km values of these enzymes for salicylic acid) indicating that the enzymes could not produce methyl salicylate in planta due to the lack of substrate (Table 6). On the other hand, the level of benzoic acid (~7 mM) was in the range of Km values for benzoic acid, suggesting that these enzymes are involved in methyl benzoate emission (Negre et al., 2003).
In S. floribunda, the isolated SAMT has similar catalytic efficiencies towards both benzoic and salicylic acid (Table 3). However, S. floribunda flowers emit 5 times as much methyl benzoate as methyl salicylate (Table 1). Analysis of internal pools of benzoic acid and salicylic acid revealed that the level of salicylic acid (~12.3 μM) is 20-fold lower than the Km value of this enzyme for salicylic acid, while the level of benzoic acid (~9 mM) is in the range of Km value for benzoic acid, suggesting that this enzyme in planta is primarily involved in methyl benzoate and to a lesser extent in methyl salicylate formation (Pott et al., 2004).
In the case of N. suaveolens, which emits both methyl benzoate and methyl salicylate, the isolated enzyme exhibits a higher catalytic efficiency with benzoic acid than with salicylic acid, partially reflecting the ratio of the two methyl esters in the floral bouquet. However, with the benzoic acid level exceeding that of salicylic acid in petal tissue, it is likely that the isolated enzyme from N. suaveolens flowers is primarily involved in the synthesis of methyl benzoate (Pott et al., 2004).
In summary, the examples described above show that sequence annotations and/or the elucidation of the biochemical properties of isolated enzymes cannot always predict their function(s) in planta. The nature of the product and the efficiency of its formation depend on several parameters, such as the availability of substrates, the catalytic efficiency of the enzyme towards each substrate, and the transcriptional, translational, and posttranslational regulation at the enzyme level.
6. Evolution of plant benzenoid carboxyl methyltransferases
Analysis of substrate specificity of carboxyl methyltransferases catalyzing the final step in the biosynthesis of volatile benzenoid esters revealed that they display a wide range of substrate preferences. The C. breweri SAMT and A. majus BAMT and SAMT are examples of enzymes with high substrate specificity while the N. suaveolens BSMT and S. floribunda SAMT can accept several benzoic and cinnamic acid derivatives. Such a broad range in substrate specificity does not reflect the differences found in the amino acid sequences nor does it mirror plant ancestry (Tables 2, 4, Fig. 1(a)). For example, both S. floribunda and H. carnosa belong to the Asclepiadaceae family, but S. floribunda SAMT exhibits a wide substrate spectrum while H. carnosa SAMT is highly specific and accepts predominantly salicylic acid. Opposite examples are the BSMT enzymes from the related species A. thaliana and A. lyrata, which posses a very similar substrate selectivity (Chen et al., 2003). Further detailed investigations of enzymes from related species have to be performed to determine whether the evolutionary process proceeded in the direction of gain or loss of substrate selectivity.
The substrate selectivity of these enzymes can be put into a broader biological context of specialization and generalization. Both processes can be advantageous and disadvantageous for plants. Specialized enzymes synthesize a single product and the level of product formation can be regulated at the level of enzyme or substrates for the reaction. Enzymes with a broader substrate acceptance have the advantage that several substrates can be used and several products can be simultaneously synthesized. Such a scenario is found in S. floribunda flowers emitting both methyl benzoate and methyl salicylate, both of which are synthesized by SAMT (Pott et al., 2002). However, the regulation of the formation of a single product out of the array of possible products will require multiple levels of regulation to make available only one of the multiple substrates.
The carboxyl methyltransferases known to date have lower Km values for salicylic acid than for benzoic acid (Table 3). This finding might be important regarding the ancestry of these enzymes. It is likely that they originated from salicylic acid-specific enzymes, which during evolution adapted the capability to accept other related substrates such as benzoic acid derivatives and cinnamic acid derivatives. The development/evolution of the enzymes might have started from a previously existing salicylic acid specific enzyme operating in vegetative tissue (leaves) during pathogen defense. This ancestor enzyme was then subsequently recruited in petals to synthesize structurally related products attractive to pollinators (e.g., methyl benzoate). The comparison of substrate preferences of characterized benzenoid carboxyl methyltransferases (Table 3) and the amino acid sequences in the substrate binding pocket (Table 4) supports this hypothesis. The ancestral enzyme might have been similar to the salicylic acid-specific enzymes now present in the floral tissue of H. carnosa, P. hybrida, C. breweri, and A. majus. More generalized enzymes could have evolved from these salicylic acid specific enzymes to become similar to enzymes found in S. floribunda and N. suaveolens which exhibit a broad substrate specificity. These generalized enzymes might in turn be templates for the generation of enzymes with new substrate preference (like A. thaliana and A. lyrata BSMTs which prefer benzoic acid over salicylic acid) and ultimately for the generation of a benzoic acid specialized enzyme (e.g., BAMT A. majus). As summarized in this review, specialized and transitional enzymes presently exist and can be isolated from plants. It is possible that some carboxyl methyltransferases evolved independently multiple times during plant evolution. This might be the case for N. suaveolens BSMT which is more genetically related to the SAMT-type of enzymes than to the BAMT-type and represents a new diverging event in evolution (Fig. 1(a)). To further support our hypothesis of generalization followed by a process of specialization will require the characterization of enzymes from related species which vary in methyl benzoate and methyl salicylate emission patterns and for which a phylogenetic tree is available.
Acknowledgments
The authors are grateful to Claudia Dinse (University of Rostock) for technical assistance, to Marcella B. Pott (Carnegie Institution of Washington, Stanford), Daniela Heidebrecht and Diana Rohrbeck (University of Rostock) and A. Slusarenko (RWTH, Aachen) for providing unpublished data, Eran Pichersky (University of Michigan, Ann Arbor) for his critical reading of the manuscript. Work in ND laboratory is supported by the US Department of Agriculture (Grant No. 2003-35318-13619), the US Israel Binational Agriculture Research and Development funds (Grant No. US-3437-03) and Fred Gloeckner Foundation, Inc. Work in JPN laboratory is supported by the National Science Foundation Arabidopsis 2010 Project MCB-0312449 and MCB-0312466. The work was further supported by grants from the DFG to BP (Pi153/17-1, 17-2, 17-3).
Biographies

Natalia Dudareva is presently a professor at Purdue University in West Lafayette, Indiana. She received her B.Sc and M.Sc. in Biology and Biochemistry at the Novosibirsk State University (Russia) and PhD in Molecular Biology at the Institute of Biochemistry (Kiev, Ukraine) in 1982. From 1982 to 1991 she worked as a senior scientist in the Institute of Cytology and Genetics of the USSR Academy of Sciences in Novosibirsk and her research was focused on the structural organization and transcription of the plant mitochondrial genome. Natalia then completed her postdoctoral training in the Institut de Biologie Moléculaire des Plantes (Strasbourg, France) (1991–1993) and in the Department of Biological Science (Windsor University, Canada) (1993–1995) with emphasis on isolation and characterization of pollen-specific genes in sunflower. As a postdoctoral research fellow in the laboratory of Prof. Eran Pichersky at the University of Michigan, Ann Arbor, she became interested in plant secondary metabolism and biosynthesis of plant volatile compounds. Using Anthirrinum majus and Petunia hybrida as model systems, she continued the investigation of the regulation of floral volatiles’ production at Purdue University, which she joined in 1997 as an assistant professor and was appointed an associate professor in 2001. Natalia’s laboratory is now combining the power of biochemical and genetic engineering approaches with metabolic modelling to gain new insights in the metabolic network leading to volatile secondary metabolites and to obtain a comprehensive understanding of the regulation of their production and emission in planta.

Uta Effmert studied Pharmacy at the University of Greifswald (Germany). She received her PhD in 1990, which focussed on isolation and characterization of immunomodulatory substances from cyanobacteria. As a postdoc she worked in the lab of Prof. N. Erdmann (Plant Physiology, University of Rostock) and in the lab of Yuzuru Shimizu at the University of Rhode Island investigating toxins of microalgae. Since 2000 Uta switched subjects and joined the lab of Prof. B. Piechulla at the University of Rostock, where she focussed her work on the molecular and biochemical elucidation of floral volatile production and emission in various plant species.

Chris Fraser is a PhD candidate in the Genetics Program at Purdue University. He is a member Clint Chapple’s lab, and is currently working on the characterization of an Arabidopsis gene family encoding serine carboxy-peptidase-like proteins. In addition to plant secondary metabolism, Chris’ research interests include molecular modelling and bioinformatics. He received his B.Sc. from the University of California, Santa Cruz and holds an M.Sc. in Statistics from the California State University, Hayward.

Florence Negre is a Ph.D. candidate in Prof. N. Dudareva’s laboratory, which she joined in 2001. Her research interest is in the biosynthesis of floral volatiles of Antirrhinum majus and Petunia hybrida and the molecular mechanisms involved in the regulation of their production and emission. She received her M.Sc. in Biotechnology and Plant Breeding at the Ecole Nationale Supérieure d’Agronomie de Toulouse (ENSAT), French Graduate School of Agronomy (France, 1997–2000). Before coming to Purdue University she worked in the International Elidia Flower Breeding Company on optimizing the pollen harvest, storage, and utilization for commercial flower seed production.

Joseph P. Noel is a professor in the Jack Skirball Chemical Biology and Proteomics laboratory at The Salk Institute for Biological Studies in La Jolla, California. In addition, he holds adjunct professorships at the University of California, San Diego in both the Chemistry and Biochemistry Department and the Division of Biology. Joe obtained a B.Sc. degree in Natural Sciences with a concentration in Chemistry from the University of Pittsburgh at Johnstown. Joe then entered the graduate program in Chemistry at the Ohio State University in the summer of 1985, and graduated from the Chemistry Department in 1990 with a PhD centered on the examination of the mechanistic enzymology of phospholipases with Professor Ming-Daw Tsai. Joe then completed his postdoctoral training with the late Paul B. Sigler in the Department of Molecular Biophysics and Biochemistry at Yale. While at Yale University, he undertook the x-ray crystallographic examination of heterotrimeric G-proteins. Joe and his group are now utilizing a combination of traditional mechanistic enzymology, molecular biology, plant biology, and tools in structural biology including protein x-ray crystallography and NMR to decipher the structure, function, and evolutionary lineage of a large number of enzymes that act in plant cells and many microorganisms to produce biologically active natural products including terpenes, polyketides, alkaloids and compounds of mixed origin such as terpene polyketides. Using the three dimensional structure of the enzymes in plants and microbes responsible for the creation of this diverse array of bioactive compounds, his group is also rationally engineering new specificities into these pathways to create novel products using a structurally-guided approach.

Birgit Piechulla is professor at the University of Rostock. She studied Biology (Microbiology, Biochemistry and Organic Chemistry) at the University of Oldenburg and Goettingen. Her diploma thesis (1980) had the title ‘Analyse mitochondrialer 4S und 5S RNA aus Aspergillus nidulans’. She worked at the Max Planck Insitute for Experimental Medicine and received her PhD at the University of Göttingen with Prof. G. Gottschalk (PhD thesis: ‘Der mitochondriale Elongationsfaktor EF-Tu aus Saccharomyces cerevisiae’). From 1984 to 1986 she was a postdoc at the University of Berkeley (California) and started to work with plants in the lab of Willi Gruissem. During this period she was interested to understand the molecular mechanisms that lead to the plastid differentiation. Thereafter she joined the lab of Prof. HW. Heldt at the University of Goettingen (1986–1996) and focussed her research on nuclear photosynthetic genes and the molecular mechanisms of the circadian clocks in plants. She habilitated 1992. Since 1996 she is professor for biochemistry at the Universität Rostock focussing her research since 2000 on the plant secondary metabolism. Her research goal is to decipher the mechanisms leading to different floral scent compositions.

Jeannine Ross is presently pursuing postdoctoral training in Structural Biology with Professor Joe Noel in the Jack Skirball Chemical Biology and Proteomics Laboratory at The Salk Institute for Biological Studies in La Jolla, California. Jeannine earned a B.A. in Physical Anthropology (Comparative Anatomy, Evolution, and Development) at the University of California at Berkeley in 1990. She studied Ecology and Evolutionary Biology and received a M.S. in Biology from the University of Michigan in Ann Arbor in 1994. Jeannine began work in the area of Plant Biochemistry as a technician in the lab of Professor Charles Yocum from 1994 to 1996 at the University of Michigan. She entered the Ph.D. program in Biology (Molecular, Cellular, and Developmental Biology) at the University of Michigan in 1996. Jeannine graduated from the University of Michigan in 2002 with a Ph.D. centered on the cloning, and molecular and biochemical characterization of the salicylic acid methyltransferase (SAMT) enzyme involved in floral scent and defense in the flowering plant, Clarkia breweri, with Professor Eran Pichersky. As a postdoc Jeannine is focusing on structural and functional elucidation of a family of methyltransferases in Arabidopsis, elucidating how enzyme active site and overall topology influence the evolution of substrate acceptance and plant natural product diversity.

Sandra Saschenbrecker is presently involved in a PhD program at the Max Planck Institute for Biochemistry (Martinsried, Germany) under the supervision of professor U. Hartl. Sandra was born in Schwerin (Mecklenburg-Vorpommern, Germany), studied biology at the University of Rostock and got her diploma degree 2003. The research of the diploma thesis focussed on the expression of the benzenoid carboxyl methyltransferase from Nicotiana suaveolens.
References
- Adam G, Anke H, Boland W, Breiling M, Donath J, Francke W, Fugmann B, Hansske D, Hartmann T, et al. In: Römpp Encyclopedia Natural Products. Steglich W, Fugmann B, Lang-Fugmann S, editors. Thieme Verlag; Stuttgart, New York: 2000. pp. 701–702. [Google Scholar]
- Andersen RA, Hamilton-Kemp TR, Loughrin JH, Hughes CG, Hildebrandt DF, Sutton TG. Green leaf headspace volatile from Nicotiana tabacum lines of different trichome morphology. J Agric Food Chem. 1988;36:295–299. [Google Scholar]
- Altenburger R, Matile P. Circadian rhythmicity of fragrance emission in flowers of Hoya carnosa R. Br. Planta. 1988;174:248–252. doi: 10.1007/BF00394778. [DOI] [PubMed] [Google Scholar]
- Attieh J, Djiana R, Koonyul P, Etienne C, Sparace SA, Saimi HS. Cloning and functional expression of two plant thiol methyltransferases: a new class of enzymes involved in the biosynthesis of sulfur volatiles. Plant Mol Biol. 2002;50:511–521. doi: 10.1023/a:1019865829534. [DOI] [PubMed] [Google Scholar]
- Boatright J, Negre F, Chen X, Kish CM, Wood B, Peel G, Orlova I, Gang D, Rhodes D, Dudareva N. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 2004;135:1993–2011. doi: 10.1104/pp.104.045468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery RG, Ling LC, Wellso SG. Oat leaf volatiles – possible insect attractants. J Agric Food Chem. 1982a;30:791–792. [Google Scholar]
- Buttery RG, Kamm JA, Ling LC. Volatile components of alfalfa flowers and pods. J Agric Food Chem. 1982b;30:739–742. [Google Scholar]
- Buttery RG, Flath RA, Mon TR, Ling LC. Identification of germacrene-D in walnut and fig leaf volatiles. J Agric Food Chem. 1986;34:820–822. [Google Scholar]
- Cauthen WL, Hester WH. Accidental ingestion of oil of wintergreen. J Fam Pract. 1989;29:680–681. [PubMed] [Google Scholar]
- Chen F, D’Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E. An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J. 2003;36:577–588. doi: 10.1046/j.1365-313x.2003.01902.x. [DOI] [PubMed] [Google Scholar]
- Connick WJ, Jr, French RC. Volatiles emitted during the sexual stage of the Canada thistle rust fungus and by thistle flowers. J Agric Food Chem. 1991;39:185–188. [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Dahl AE, Wassgren AB, Bergström G. Floral scents in Hypecoum (Papaveraceae). Chemical composition and relevance to taxonomy and mating system. Biochem Syst Ecol. 1990;18:157–168. [Google Scholar]
- Das Gupta V. Quantitative-determination of methyl salicylate in a liniment. Am J Hosp Pharm. 1974;31:1001–1002. [PubMed] [Google Scholar]
- D’Auria JC, Chen F, Pichersky E. The SABATH family of MTs in Arabidopsis thaliana and other plant species. In: Romeo JT, editor. Recent Advances in Phytochemistry. Vol. 37. Elsevier Science & Technology; Oxford: 2003. pp. 253–283. [Google Scholar]
- Demole E, Lederer E, Mercier D. Isolement et determination de la structure du jasmonate de methyle, constituant odorant characteristique de lesence de jasmin. Helv Chim Acta. 1962;45:675. [Google Scholar]
- Dicke M, Bruin J. Chemical information transfer between plants: back to the future. Biochem Syst Ecol. 2001;29:981–994. [Google Scholar]
- Dudareva N, Murfitt LM, Mann CJ, Gorenstein N, Kolosova N, Kish CM, Bonham C, Wood K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell. 2000;12:949–961. doi: 10.1105/tpc.12.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Raguso RA, Wang J, Ross JR, Pichersky E. Floral scent production in Clarkia breweri. III. Enzymatic synthesis and emission of benzenoid esters. Plant Physiol. 1998;116:599–604. doi: 10.1104/pp.116.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fröhlich O, Schreier P. Additional volatile constituents of carambola (Averrhoa carambola L.) fruit. Flav Fragr J. 1989;4:177–184. [Google Scholar]
- Fukami H, Asakura T, Hirano H, Abe K, Shimomura K, Yamakawa T. Salicylic acid carboxyl methyltransferase induced in hairy root cultures of Atropa belladonna after treatment with exogenously added salicylic acid. Plant Cell Physiol. 2002;43:1054–1058. doi: 10.1093/pcp/pcf119. [DOI] [PubMed] [Google Scholar]
- Gerlach G, Schill R. Fragrance analyses, an aid to taxonomic relationships of the genus Coryanthes (Orchidceae) Plant Syst Evol. 1989;168:159–165. [Google Scholar]
- Gerlach G, Schill R. Composition of orchid scents attracting euglossine bees. Bot Acta. 1991;104:379–391. [Google Scholar]
- Groth I, Bergström G, Pellmyr O. Floral fragrance in Cimifuga: Chemical polymorphism and incipient speciation in Cimifuga simplex. Biochem Syst Ecol. 1987;15:441–444. [Google Scholar]
- Hamilton-Kemp TR, Andersen RA, Rodriguez JG, Loughrin JH, Patterson CG. Strawberry foliage headspace vapour components at periods of susceptibility and resistance to Tetranychus urticae Koch. J Chem Ecol. 1988;14:789–796. doi: 10.1007/BF01018773. [DOI] [PubMed] [Google Scholar]
- Herrmann K. Salicylsäure und andere verbreitete Hydroxysäuren und deren natürlich vorkommende Verbindungen in Lebensmitteln. Ernährungs-Umschau. 1990;37:108–112. [Google Scholar]
- Hills HG, Williams NH, Dodson CH. Identification of some orchid fragrance components. Am Orchid Soc Bull. 1968;37:967–971. [Google Scholar]
- Hills HG, Williams NH, Dodson CH. Floral fragrances and isolating mechanisms in the genus Catasetum (Orchidaceae) Biotropica. 1972;4:61–76. [Google Scholar]
- Holman RT, Heimermann WH. Identification of components of orchid fragrances by gas chromatography–mass spectrometry. Am Orchid Soc Bull. 1973;42:678–682. [Google Scholar]
- Howrie DL, Moriarty R, Breit R. Candy flavoring as a source of salicylate poisoning. Pediatrics. 1985;75:869–871. [PubMed] [Google Scholar]
- Ibrahim RK, Muzac I. The methyltransferase gene superfamily: A tree with multiple branches. In: Romeo JT, Ibrahim R, Varin L, De Luca V, editors. Recent Advances in Phytochemistry. Vol. 34. Elsevier Science & Technology; Oxford: 2000. pp. 349–384. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Flower scent composition in night-flowering Silene species (Caryophyllaceae) Biochem Syst Ecol. 2002;30:383–397. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Flower scent composition in Dianthus and Saponaria species (Caryophyllaceae) and its relevance for pollination biology and taxonomy. Biochem Syst Ecol. 2003;31:345–357. [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Kite G, Reynolds T, Prance GT. Potential pollinator-attracting chemicals from Victoria (Nymphaeaceae) Biochem Syst Ecol. 1991;19:535–539. [Google Scholar]
- Knudsen JT, Tollsten L. Floral scent and intrafloral scent differentiation in Moneses and Pyrola (Pyrolaceae) Plant Syst Evol. 1991;177:81–91. [Google Scholar]
- Knudsen JT, Tollsten L, Bergström G. Floral scents–a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Kolosova N, Gorenstein N, Kish CM, Dudareva N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell. 2001a;13:2333–2347. doi: 10.1105/tpc.010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova N, Sherman D, Karlson D, Dudareva N. Cellular and subcellular localization of S-adenosyl-L-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiol. 2001b;126:956–964. doi: 10.1104/pp.126.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RA, Raguso RA, McDade LA. Fragrance chemistry and pollinator affinities in Nyctaginaceae. Phytochemistry. 2001;58:429–440. doi: 10.1016/s0031-9422(01)00257-6. [DOI] [PubMed] [Google Scholar]
- Li C, Zheng YQ, Sun YL, Wu ZP, Liu MX. Studies on the odoriferous volatile constituents of the flower of Cestrum nocturnum L. Youji Huaxue. 1988;8:357–361. [Google Scholar]
- Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF. Headspace compounds from flowers of Nicotiana tabacum and related species. J Agric Food Chem. 1990;38:455–460. [Google Scholar]
- Ma L, Zheng Y, Sun Y, Wu Z, Liu M. Studies on the aroma volatile constituents of ylang-ylang flowers by gas chromatography/mass spectrometry. Sepu. 1988;6:11–18. [Google Scholar]
- Mookherjee BD, Trenkle RW, Wilson RA. The chemistry of flowers, fruits and spices: live vs. dead a new dimension in fragrance research. Pure Appl Chem. 1990;62:1357–1364. [Google Scholar]
- Moya S, Ackermann JD. Variation in the floral fragrance of Epidendrum ciliare (Orchidaceae) Nord J Bot. 1993;13:41–47. [Google Scholar]
- Murfitt LM, Kolosova N, Mann CJ, Dudareva N. Purification and characterization of S-adenosyl-L-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methyl benzoate in flowers of Antirrhinum majus. Arch Biochem Biophys. 2000;382:145–151. doi: 10.1006/abbi.2000.2008. [DOI] [PubMed] [Google Scholar]
- Murrell JT, Williams NH, Pridgeon AM, Dodson CH. Floral fragrances in Angraecum (Orchidaceae) Selbyana. 1981;5:286–290. [Google Scholar]
- Naumann CM, Ockenfels P, Schmitz J, Schmidt F, Francke W. Reactions of Zygaena moths to volatile compounds of Knautia arvensis (Lepidoptera: Zygaenidae) Entomol Ger. 1991;15:255–264. [Google Scholar]
- Negre F, Kolosova N, Knoll J, Kish CM, Dudareva N. Novel S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase, an enzyme responsible for biosynthesis of methyl salicylate and methyl benzoate, is not involved in floral scent production in snapdragon flowers. Arch Biochem Biophys. 2002;406:261–270. doi: 10.1016/s0003-9861(02)00458-7. [DOI] [PubMed] [Google Scholar]
- Negre F, Kish CM, Boatright J, Underwood B, Shibuya K, Wagner C, Clark DG, Dudareva N. Regulation of methyl benzoate emission after pollination in snapdragon and petunia flowers. Plant Cell. 2003;15:2992–3006. doi: 10.1105/tpc.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LA. Characteristics and distribution of intermediates between Platanthera bifolia and P. chlorantha (Orchidaceae) in the Nordic countries. Nord J Bot. 1985;5:407–419. [Google Scholar]
- Noel JP, Dixon RA, Pichersky E, Zubieta C, Ferrer JL. Structural, functional, and evolutionary basis formethylation of plant small molecules. In: Romeo JT, editor. Recent Advances in Phytochemistry. Vol. 37. Elsevier Science&Technology; Oxford: 2003. pp. 37–58. [Google Scholar]
- Omata A, Yomogida K, Nakamura S, Ota T, Izawa Y. Studies on the volatile compounds of Camellia flowers. J Jpn Soc Hortic Sci. 1989;58:429–434. [Google Scholar]
- Pellmyr O, Tang W, Groth I, Bergström G, Thien LB. Cycad cone and angiosperm floral volatiles: Inferences for the evolution of insect pollination. Biochem Syst Ecol. 1991;19:623–627. [Google Scholar]
- Plepys D, Ibarra F, Löfstedt C. Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae) Oikos. 2002;99:69–74. [Google Scholar]
- Pott MB, Pichersky E, Piechulla B. Evening specific oscillations of scent emission, SAMT enzyme activity, and SAMT mRNA in flowers of Stephanotis floribunda. J Plant Physiol. 2002;159:925–934. [Google Scholar]
- Pott MB. PhD Thesis. Universiy of Rostock; 2003. Molekulare und biochemische Regulation der Emission von Blütenduftstoffen. Available from: < www.dissertation.de>. [Google Scholar]
- Pott MB, Effmert U, Piechulla B. Transcriptional and posttranslational regulation of S-adenosyl-L-methionine–salicylic acid carboxyl methyltransferase (SAMT) during Stephanotis floribunda flower development. J Plant Physiol. 2003;160:635–643. doi: 10.1078/0176-1617-00968. [DOI] [PubMed] [Google Scholar]
- Pott M, Hippauf F, Saschenbrecker S, Chen F, Ross J, Kiefer I, Slusarenko A, Noel JP, Pichersky E, Effmert U, Piechulla B. Biochemical and structural characterization of benzenoid carboxyl methyltransferases involved in floral scent production in Stephanotis floribunda and Nicotiana suaveolens. Plant Physiol. 2004;135:1946–1955. doi: 10.1104/pp.104.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso RA, Pichersky E. Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): recent evolution of floral scent and moth pollination. Plant Syst Evol. 1995;194:55–67. [Google Scholar]
- Raguso RA, Light DM, Pichersky E. Electroantennogram responses of Hyles lineata (Sphingidae: Lepidoptera) to volatile compounds from Clarkia breweri (Onagraceae) and other mothpollinated flowers. J Chem Ecol. 1996;22:1735–1766. doi: 10.1007/BF02028502. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Light DM. Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and ‘green-leaf’ volatiles. Entomol Exp Appl. 1998;86:287–293. [Google Scholar]
- Raguso RA, Levin RA, Foose SE, Holmberg MW, Mc Dade LA. Fragrance chemistry, noctural rhythms and pollination syndromes in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Ross JR, Nam KH, D’Auria JC, Pichersky E. S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch Biochem Biophys. 1999;367:9–16. doi: 10.1006/abbi.1999.1255. [DOI] [PubMed] [Google Scholar]
- Ross JR. PhD Thesis. University of Michigan; Ann Arbor: 2002. S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase (SAMT): an enzyme involved in floral scent and plant defense in Clarkia breweri. [Google Scholar]
- Schwab W. Metabolome diversity: Too few genes, too many metabolites. Phytochemistry. 2003;62:837–849. doi: 10.1016/s0031-9422(02)00723-9. [DOI] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc Natl Acad Sci USA. 2001;98:4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GJ, Ellingham PJ, Birch EJ. Volatile constituents of feijoa – headspace analysis of intact fruit. J Sci Food Agric. 1983;34:743–747. [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Simon J, Quinn J, Murray RG. Basil: a source of essential oil. In: Janick J, Simon JE, editors. Advances in New Crops. Timber Press; Portland: 1990. pp. 484–489. [Google Scholar]
- Steeghs M, Bais HP, de Gouw J, Goldan P, Kuster W, Northway M, Fall R, Vivanco JM. Proton-transfer-reaction mass spectrometry (PTR-MS) as a new tool for real time analysis of root-secreted volatile organic compounds (VOCs) in Arabidopsis thaliana. Plant Physiol. 2004;135:47–58. doi: 10.1104/pp.104.038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA. Volatile herbivore-induced terpenoids in plant–mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol. 1994;20:1329–1354. doi: 10.1007/BF02059811. [DOI] [PubMed] [Google Scholar]
- Tatsuka K, Kohama M, Suekane S. Floral fragrance components of Zygopetalum mackayi (Orchidaceae) Agric Biol Chem (Tokyo) 1988;52:1599–1600. [Google Scholar]
- Tollsten L, Bergström J. Variation and post-pollination changes in floral odours released by Platanthera bifolia (Orchidaceae) Nord J Bot. 1989;9:359–362. [Google Scholar]
- van Poecke RP, Posthumus MA, Dicke M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol. 2001;27:1911–1928. doi: 10.1023/a:1012213116515. [DOI] [PubMed] [Google Scholar]
- Verdonk JC, Ric de Vos CH, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry. 2003;62:997–1008. doi: 10.1016/s0031-9422(02)00707-0. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates and octadecanoids: signals in plant stress responses and development. Prog Nucleic Acid Res Mol Biol. 2002;72:165–221. doi: 10.1016/s0079-6603(02)72070-9. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Williams NH. Floral fragrance components of Brassavola (Orchidaceae: Laeliinae) Selbyana. 1981;5:279–285. [Google Scholar]
- Williams NH, Atwood JT, Dodson CH. Floral fragrance analysis in Anguloa, Lycaste and Mendoncella (Orchidaceae) Selbyana. 1981;5:291–295. [Google Scholar]
- Williams NH, Whitten WM, Dodson CH. Preliminary analyses of the floral fragrances of species of Acineta, Houlettia, Luddemannia, Lycomormium, Paphinia, and Sievekingia (Orchadaceae) Selbyana. 1984;7:315–317. [Google Scholar]
- Whitten WM, Williams NH. Floral fragrance of Stanhopea (Orchidaceae) Lindleyana. 1992;7:130–153. [Google Scholar]
- Young H, Paterson VJ, Burns DJW. Volatile aroma constituents of kiwifruit. J Sci Food Agric. 1983;34:81–85. [Google Scholar]
- Zubieta C, He XZ, Dixon RA, Noel JP. Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nat Struct Biol. 2001;8:271–279. doi: 10.1038/85029. [DOI] [PubMed] [Google Scholar]
- Zubieta C, Kota P, Ferrer JL, Dixon RA, Noel JP. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferases. Plant Cell. 2002;14:1265–1277. doi: 10.1105/tpc.001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta C, Ross JR, Koscheski P, Yang Y, Pichersky E, Noel JP. Structural basis for substrate recognition in the salicylic acid carboxyl methyltransferase family. Plant Cell. 2003;15:1704–1716. doi: 10.1105/tpc.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]