Abstract
Objective
To determine prospectively whether physical activity can prevent age-related weight gain and whether changing levels of activity affect body weight.
Design/subjects
The study consisted of 8080 male and 4871 female runners who completed two questionnaires an average (±standard deviation (s.d.)) of 3.20±2.30 and 2.59±2.17 years apart, respectively, as part of the National Runners’ Health Study.
Results
Changes in running distance were inversely related to changes in men’s and women’s body mass indices (BMIs) (slope±standard error (s.e.): −0.015±0.001 and −0.009±0.001 kg/m2 per Δkm/week, respectively), waist circumferences (−0.030±0.002 and −0.022±0.005 cm per Δkm/week, respectively) and percent changes in body weight (−0.062±0.003 and −0.041±0.003% per Δkm/week, respectively, all P<0.0001). The regression slopes were significantly steeper (more negative) in men than women for ΔBMI and Δ%body weight (P<0.0001). A longer history of running diminished the impact of changing running distance on men’s weights. When adjusted for Δkm/week, years of aging in men and years of aging in women were associated with increases of 0.066±0.005 and 0.056±0.006 kg/m2 in BMI, respectively, increases of 0.294±0.019 and 0.279±0.028% in Δ%body weight, respectively, and increases of 0.203±0.016 and 0.271±0.033 cm in waist circumference, respectively (all P<0.0001). These regression slopes suggest that vigorous exercise may need to increase 4.4 km/week annually in men and 6.2 km/week annually in women to compensate for the expected gain in weight associated with aging (2.7 and 3.9 km/week annually when correct for the attenuation due to measurement error).
Conclusions
Age-related weight gain occurs even among the most active individuals when exercise is constant. Theoretically, vigorous exercise must increase significantly with age to compensate for the expected gain in weight associated with aging.
Keywords: exercise, running, aging, body mass index, regional adiposity
INTRODUCTION
Over half of all adults in the United States are classified as obese [1]. Westernized societies demand relatively little physical activity at work or home while providing ready access to energy dense foods. Most physical activity of moderate or vigorous intensity is voluntary and recreational. About 60% of adults choose to be sedentary and engage in little recreational activity [2]. Thus, there is ample opportunity for weight gain to occur as energy intake exceeds expenditure [3].
Cross-sectional and prospective cohort studies of predominantly sedentary populations show that men and women gain weight as they age. There are concomitant declines in energy expenditure and increases in adiposity with age [4]; however, it is not known whether age-related increases in adiposity are the cause or the consequence of declining energy expenditure with age [5]. The Institute of Medicine (IOM) and others recommend adding exercise to usual daily activity sufficient to raise total energy expenditure to 160 to 180% of basal energy expenditure, which in most adults could be achieved through 60 min day of brisk walking [6,7].
We have proposed that weight maintenance may require progressive increases in exercise with age, rather than the maintenance of a static threshold [8]. Cross-sectional analyses originally presented by us suggest that middle-age weight gain is expected if physical activity remains constant, even if the activity is substantial [8].
The IOM energy requirements to maintain healthy weight, and our own previously published estimates of the exercise required to prevent age-related weight gain were speculative, because cross-sectional data by themselves do not distinguish age-related weight gain from cohort effects, and do not distinguish exercise-induced weight loss from self-selection. In addition, our estimates of the exercise required to prevent age-related weight gain may not apply to women, who are reported to lose less weight than men with exercise [9–11]. This report uses longitudinal data to strengthen the evidence for a causal relationship between exercise and weight maintenance. The demonstration prospectively of weight gain at any sustained activity level may provide insights into the physiological process of aging and shift public health recommendations from static goals to dynamic recommendations for greater investment in physical activity with age.
Methods
A two-page questionnaire, distributed nationally at races and to subscribers of the nation’s largest running magazine (Runners’ World, Emmaus, PA, USA) between 1991 and 2000, solicited information on demographics (age, race, education), running, weight and waist circumference [12]. All participants signed a written consent form that had been approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley, CA, USA.
From the tables by Ainsworth et al. [13], we calculated the caloric cost of running exclusive of the resting metabolic rate as 1.51 kcal/kg/mi. The Institute of Medicine report recommends calculating total exercise energy expenditure by increasing the direct energy expenditure during exercise by 15% for excess postexercise oxygen consumption and by 10% for the thermic effects of the additional food energy required to supply the energy required [6]. These two factors increase the energy cost of running by 27% to 1.91 kcal/kg/mi. Physical activity levels (PAL) were estimated using the equations from the IOM report for basal energy expenditure (kcal/day) in normal weight men and women (Chapter 5) and the impact of physical activity on PAL (Chapter 13) assuming a PAL of 1.39 for sedentary lifestyle [6].
Change in body mass index (BMI) was calculated as the change in weight in kilograms between the first and second questionnaire divided by the square of the average height from the two questionnaires in meters. Self-reported waist circumference was in response to the question ‘Please provide, to the best of your ability, your body circumference in inches’ without further instruction. Self-reported height and weight from the questionnaire have been found previously to correlate strongly with their clinic measurements (unpublished correlation in 110 men were r=0.96 for both). Self-reported waist circumferences are somewhat less precise as indicated by their correlations with self-reported circumferences on a second questionnaire (r=0.84) and with their clinic measurements (r=0.68).
Statistical analyses
The significance of the relationships of Δrunning distance and Δage to Δweight were assessed by multiple linear regression using both variables and average age ((questionnaire 2 age+questionnaire 1 age)/2) as independent variables. Annual weight change was estimated by dividing the mean, standard deviation (s.d.) and standard error (s.e.) for weight change by the mean duration between surveys. The annual mean changes in BMI by age groups after adjustment for changes in running distances were calculated using multiple linear regression using the nine age groups (18–25, 25–29, 30–34…55–59, 60–75 years old) and Δkm/week as independent variables and Δweight as the dependent variable. In these analyses, the contribution of an individual i, i=1…N to the age class j, j=1…9, was zero if the individual was never in the age group j between surveys, and was calculated as (minimum (bi − cj, dj − cj) − maximum (ai − cj,0))/(dj − cj) if they were, where cj and dj are the lower and upper limits of age class j and ai and bi are participant’s i ages on their first and second survey. Simply stated, the contribution of age interval j to the average weight gain of individuals between surveys is proportional to the amount of time spent within the age interval.
Results
Multiple baseline questionnaires were submitted by 12.8% of men and 11.4% of women who joined the National Runners’ Health Study between 1991 and 2000. We excluded runners who reported taking thyroid (N=539) or diabetic medications (N=71), smoked (N=274) or consumed strict vegetarian diets (N=288) on their first or second questionnaire. Of the remaining 8080 male and 4871 female runners, 7771 males (96.2%) and 4797 females (98.5%) reported weights and heights to allow the calculation of change in BMI and body weight, and 7060 males (90.9%) and 4071 (83.6%) females reported their waist circumferences at both visits. The male (female) runners who submitted multiple questionnaires had a mean ±s.d. age of 46.4±10.3 years (39.8±9.9 years), average of 16.6±2.5 (16.2±2.4) years of education, a BMI of 23.5±2.3 kg/m2 (22.1±2.2 kg/m2) and had run 19 or more km/week for an average of 13.0±8.2 years (9.5±6.8 years).
Weekly running distance declined an average (±s.d.) of 2.87±16.37 km during the 3.20±2.30 years between surveys in men, and declined 1.65±15.99 km during the 2.59±2.17 years between surveys in women. Although the average changes in weekly running distance between visits were small, individual changes were often substantial. In all, 1% of men (1.4% of women) increased their running distance over 40 km/week between surveys, 3.9% of men (4.1% of women) increased their distance between 24 and 40 km/week, 18.2% of men (20.7% of women) increased their distance between 8 and 24 km/week, 39.9% of men (40.2% of women) remained within 8 km/week of their baseline distance, 27.5% of men (25.3% of women) reduced their distance between 8 and 24 km/week, 6.6% of men (6.1% of women) reduced distance between 24 and 40 km/week, and 2.8% of men (2.2% of women) reduced their weekly running distance by over 40 km/week.
Table 1 presents the annual mean changes in BMI, Δ%body weight and waist circumference by weekly running distance on the first (rows) and second surveys (columns). The cells that lie on the diagonal from the lower left corner to the upper right corner represent individuals who remained within the same running distance category, cells above this diagonal represent decreases in weekly running distance and those below this diagonal represent increases in distance. Table 1 shows that all of the mean changes in men’s BMI, waist circumferences and percent changes in weight on or above the diagonal are significantly positive, representing significant weight gain in men who maintained or reduced their running distance between surveys. There were only a few isolated cases of significant weight loss below the diagonal, and the mean changes suggest that weight loss in men was only achieved when the increase in exercise was substantial. The significance levels at the end of the rows and bottom of the columns test for significant trends within the row or column. Thus, the significance level for the first column (P<0.0001) shows that in men who were running under 16 km/week on the second questionnaire, the annual average weight gain was associated with the amount of decrease in running distance. The significance level for the first row shows that among runners who initially ran over 64 km/week, the annual weight gain was related to their decrease in running distance. Thus, regardless of the starting or ending distances, the mean changes in BMI, Δ%body weight and waist circumference were related to the changes in running distance.
Table 1.
Annual change in men’s adiposity (mean ± s.e.) by reported running distance
| Weekly km run, 1st survey | Weekly km run on 2nd survey | Trend across columns within row, P | ||||
|---|---|---|---|---|---|---|
| 0–16 | 16–32 | 32–48 | 48–64 | ≥ 64 | ||
| BMI (kg/m2) | ||||||
| ≥ 64 | 0.23 ± 0.06§ | 0.26 ± 0.04§ | 0.19 ± 0.02§ | 0.13 ± 0.02§ | 0.06 ± 0.01§ | < 0.0001 |
| 48–64 | 0.33 ± 0.07§ | 0.23 ± 0.03§ | 0.14 ± 0.01§ | 0.06 ± 0.01§ | 0.02 ± 0.02 | < 0.0001 |
| 32–48 | 0.29 ± 0.03§ | 0.15 ± 0.01§ | 0.07 ± 0.01§ | 0.01 ± 0.02 | −0.06 ± 0.04 | < 0.0001 |
| 16–32 | 0.19 ± 0.02§ | 0.09 ± 0.01§ | 0.05 ± 0.01§ | −0.06 ± 0.03* | −0.02 ± 0.06 | < 0.0001 |
| 0–16 | 0.09 ± 0.02§ | 0.03 ± 0.02 | −0.01 ± 0.04 | −0.15 ± 0.08 | −0.44 ± 0.47 | < 0.0001 |
| Trend across rows within columns, P | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Δ% weight | ||||||
| ≥ | 1.03 ± 0.25§ | 1.15 ± 0.19§ | 0.84 ± 0.10§ | 0.58 ± 0.07§ | 0.28 ± 0.05§ | < 0.0001 |
| 48–64 | 1.36 ± 0.30§ | 1.02 ± 0.11§ | 0.63 ± 0.05§ | 0.29 ± 0.05§ | 0.11 ± 0.09 | < 0.0001 |
| 32–48 | 1.23 ± 0.12§ | 0.66 ± 0.05§ | 0.32 ± 0.03§ | 0.05 ± 0.07 | −0.24 ± 0.18 | < 0.0001 |
| 16–32 | 0.79 ± 0.06§ | 0.39 ± 0.03§ | 0.21 ± 0.05§ | −0.23 ± 0.10* | −0.09 ± 0.25 | < 0.0001 |
| 0–16 | 0.38 ± 0.07§ | 0.14 ± 0.09 | −0.05 ± 0.15 | −0.51 ± 0.28 | −1.22 ± 1.46 | < 0.0001 |
| Trend across rows within columns, P | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | |
| Waist circumference (cm) | ||||||
| ≥ 64 | 0.66 ± 0.22† | 0.63 ± 0.12§ | 0.35 ± 0.08§ | 0.27 ± 0.05§ | 0.09 ± 0.04* | < 0.0001 |
| 48–64 | 0.67 ± 0.21‡ | 0.42 ± 0.09§ | 0.34 ± 0.04§ | 0.21 ± 0.04§ | 0.17 ± 0.07† | < 0.0001 |
| 32–48 | 0.57 ± 0.12§ | 0.34 ± 0.04§ | 0.18 ± 0.03§ | 0.13 ± 0.06* | 0.00 ± 0.11 | < 0.0001 |
| 16–32 | 0.48 ± 0.05§ | 0.24 ± 0.03§ | 0.11 ± 0.05* | 0.12 ± 0.08 | −0.39 ± 0.32 | < 0.0001 |
| 0–16 | 0.17 ± 0.07* | 0.06 ± 0.08 | 0.10 ± 0.10 | −0.08 ± 0.33 | −1.29 ± 0.96 | = 0.02 |
| Trend across rows within columns, P | < 0.0001 | < 0.0001 | < 0.0001 | = 0.001 | < 0.0001 | |
Significantly different from zero for cells are coded:
P<0.05;
P<0.01;
P<0.001
P<0.0001.
Significance levels presented on the bottom of each column and ends of each row test whether changes in adiposity were significantly related to changes in running distance (as continuous variables) when stratified by starting (rows) and ending (columns) running distances.
Table 2 presents the corresponding results for women. The significant mean increases in cells lying on or above the diagonal show that as in men, there were significant annual increases in body weight and waist circumferences in women who maintained or reduced their weekly running distance. The significant trend for all rows suggests that the changes in women’s weights were related to changes in running distances regardless of their initial running level. The test for trends at the bottom of the columns suggest that the change in weight was also related to the change in weekly running distance regardless of their ending level (except Δwaist circumference in women running between 16 and 32 km/week at the end of the survey).
Table 2.
Annual change in women’s adiposity (mean ± s.e.) by reported running distance
| Weekly km run, 1st survey | Weekly km run on 2nd survey | Trend across columns within row, P | |||
|---|---|---|---|---|---|
| 0–16 | 16–32 | 32–48 | ≥ 48 | ||
| BMI (kg/m2) | |||||
| ≥ 48 | 0.18 ± 0.05§ | 0.15 ± 0.03§ | 0.12 ± 0.02§ | 0.04 ± 0.01§ | <0.0001 |
| 32–48 | 0.30 ± 0.07§ | 0.12 ± 0.02§ | 0.09 ± 0.01§ | 0.03 ± 0.02 | <0.0001 |
| 16–32 | 0.23 ± 0.03§ | 0.11 ± 0.01§ | 0.05 ± 0.02† | −0.01 ± 0.04 | <0.0001 |
| 0–16 | 0.16 ± 0.03§ | 0.06 ± 0.04 | −0.01 ± 0.06 | 0.01 ± 0.04 | =0.004 |
| Trend across rows within column, P | <0.0001 | <0.0001 | <0.0001 | =0.002 | |
| Δ% weight | |||||
| ≥ 48 | 0.84 ± 0.22§ | 0.75 ± 0.15§ | 0.61 ± 0.08§ | 0.21 ± 0.05§ | <0.0001 |
| 32–48 | 1.48 ± 0.33§ | 0.58 ± 0.08§ | 0.43 ± 0.06§ | 0.19 ± 0.10* | <0.0001 |
| 16–32 | 1.04 ± 0.12§ | 0.51 ± 0.05§ | 0.23 ± 0.08† | 0.00 ± 0.17 | <0.0001 |
| 0–16 | 0.74 ± 0.11§ | 0.32 ± 0.15* | −0.01 ± 0.26 | 0.10 ± 0.20 | <0.003 |
| Trend across rows within column, P | <0.0001 | <0.0001 | <0.0001 | =0.003 | |
| Waist circumference (cm) | |||||
| ≥ 48 | 0.33 ± 0.19 | 0.43 ± 0.12‡ | 0.41 ± 0.09§ | 0.21 ± 0.06‡ | =0.001 |
| 32–48 | 0.93 ± 0.24§ | 0.41 ± 0.10§ | 0.26 ± 0.07§ | 0.08 ± 0.11 | =0.0001 |
| 16–32 | 0.50 ± 0.13§ | 0.41 ± 0.06§ | 0.33 ± 0.10‡ | 0.08 ± 0.18 | =0.02 |
| 0–16 | 0.44 ± 0.15† | 0.43 ± 0.19* | −0.38 ± 0.28 | −0.16 ± 0.55 | =0.006 |
| Trend across rows within column, P | =0.004 | =0.09 | =0.004 | =0.05 | |
Significantly different from zero for cells are coded:
P<0.05;
P<0.01;
P<0.001
P<0.0001.
Significance levels presented on the bottom of each column and ends of each row test whether changes in adiposity were significantly related to changes in running distance (as continuous variables) when stratified by starting (rows) and ending (columns) running distances.
The analyses to follow assess the separate contributions of aging (time) and change in running distance to changes in weight (presumably adiposity). Specifically, we examine the effects of changes in reported weekly running distance on changes in adiposity when adjusted for the time interval between surveys (Δage) and age at the midpoint of the two surveys. To assess the independent effect of aging in these vigorously active men and women, we adjusted for mean age and the change in weekly running distance between surveys.
Changes in adiposity and running distance adjusted for age and aging
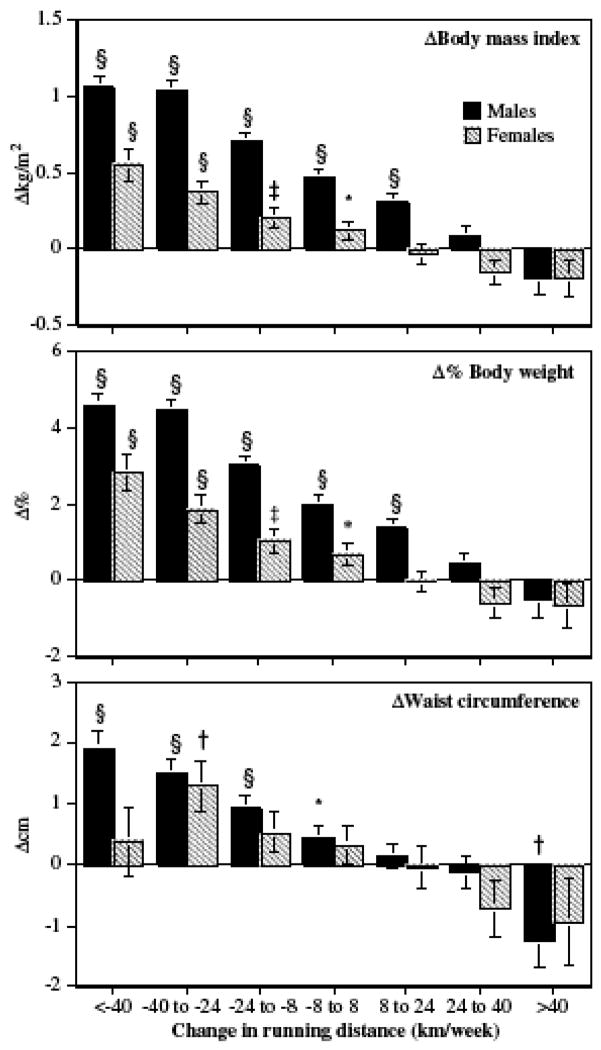
Figure 1 displays the adjusted mean changes in BMI, Δ%body weight and waist circumference when grouped by change in weekly running distance. The bars show that adjusted declines in weekly running distances were associated with significant increases in mean body weight and waist circumference in a dose-dependent manner. This observation is confirmed by the adjusted regression slopes that use changing distances across the continuum of values rather than their categorical division, that is, changes in weekly running distances were inversely related to changes in men’s and women’s BMIs (slope=s.e.: −0.015±0.001 and −0.009±0.001 kg/m2 per km/week, respectively), Δ%body weights (−0.062±0.004% and −0.041±0.003% per km/week, respectively) and waist circumferences (−0.030±0.002 and −0.022±0.005 cm per km/week, all P<0.0001).
Figure 1.
Total mean changes (±s.e. represented by bars) in BMI, %body weight and waist circumference by change in weekly running distance in male and female runners after adjustment for Δ age and mean age. Significance levels are coded *P<0.05; †P<0.01; ‡P<0.001 and §P<0.0001. The trend for an inverse relationship between Δkm/week and changes in BMI, Δ%body weight and waist circumference were all significant at P<0.0001.
The adjusted regression slopes per Δkm/week were significantly steeper (more negative) in men than women for ΔBMI (male minus female difference in slope±s.e.: −0.006±0.001 kg/m2, P<0.0001) and Δ%body weight (−0.022±0.005%, P<0.0001), but not waist circumference (−0.008±0.005 cm, P=0.10). The differences in slopes persist for ΔBMI versus Δkcal from running (P<0.0003, analyses not displayed).
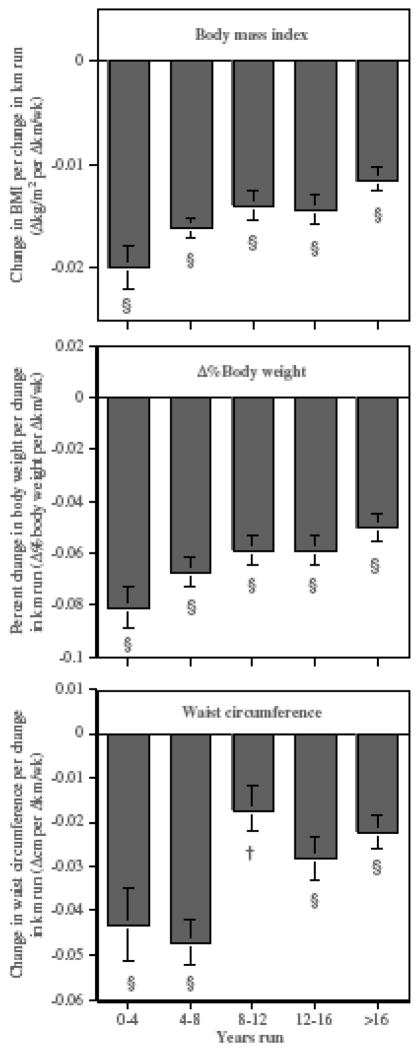
Figure 2 suggests in men a longer history of running 19 or more km/week appeared to diminish the impact of changing running distance on ΔBMI, Δ%body weight and Δwaist circumferences (P<0.0001 for all). For example, in men who ran under 4 years, each 1 km increase (decrease) in weekly running distance was associated with a −0.020±0.002 kg/m2 decrease (increase) in their BMI. This change in BMI was 82% larger than the change in men who had run 16 or more years (−0.011±0.001 kg/m2 per Δkm/week). There was a 62% difference in the percent change in men’s body weight and a two-fold difference in the change in men’s waist circumference per Δkm/week for men who ran 4 years or less compared to those who ran at least 16 years.
Figure 2.
Total change in BMI, %body weight and waist circumference per Δkm/week in male runners by the number of years run at 19 or more kilometers per week after adjustment for Δ age and mean age. Significance levels are coded *P<0.05; †P<0.01; ‡P<0.001 and §P<0.0001. The trend for an inverse relationship between the slopes and the number of years run were all significant at P<0.0001.
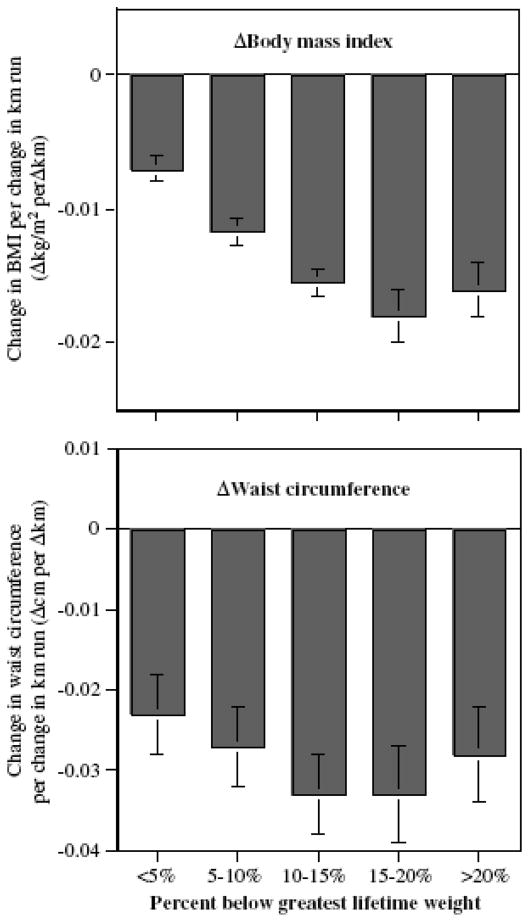
Figure 3 suggests that weight change during exercise reduction also appears to be affected by whether the men are proximal or far away from their greatest lifetime weight. Men who were more than 10% below their greatest lifetime weight on their first survey experienced changes in BMI per Δkm/week (−0.017±0.001 kg/m2) that were significantly greater than experienced by men 5–10% below their maximum weight (−0.012±0.002 kg/m2, P=0.0003 for difference) or within 5% of their maximum weight (−0.007±0.001, P<0.0001 for difference). The men who were at least 10% below their greatest lifetime weight also experienced a greater percent reduction in body weight (−0.069±0.004% per Δkm/week) than men who were 5–10% below (−0.049±0.004% per Δkm/week, P=0.0003) or within 5% of their maximum weight (−0.031±0.005% per Δkm/week, P<0.0001 for difference). Change in waist circumference did not achieve significance in these comparisons.
Figure 3.
Change in BMI and waist circumference per Δkm/week in male runners by the their percentage below greatest lifetime weight on the first survey. Slopes all significantly different from zero at P<0.0001.
Changes in adiposity with aging
When adjusted for changes in weekly running distances and age, each year of follow-up was associated with increases of 0.066±0.005 and 0.056±0.006 kg/m2 in men’s and women’s BMI, respectively, (P<0.0001), increases of 0.294±0.019 and 0.279±0.028% in men’s and women’s Δ%body weight, respectively, (P<0.0001), and increases of 0.203±0.016 and 0.271±0.033 cm in waist circumference (P<0.0001). The effects of aging were not significantly different between men and women for ΔBMI (P=0.18) or Δ%body weight (P=0.65), but were slightly greater for women than men for Δwaist circumference (difference in slope±s.e.: 0.068±0.033 cm/year, P=0.04).
Table 3 displays the annual increases in BMI, body weight and waist circumference by age. The increases in weight and waist circumference with age were generally significant between 18 and 59 years old. Increasing age was significantly related to increases in waist circumference but not increases in BMI or body weight in men and women between 60 and 75 years old, suggesting age-related increases in visceral fat that may not be reflected in body mass due to a loss of lean body mass in older individuals.
Table 3.
Annual increases (mean ± s.e.) in adiposity in vigorously active men and women
| Age interval | Male runners | Female runners | ||||
|---|---|---|---|---|---|---|
| Δ BMI (kg/m2) | Body weight (Δ %kg) | Δ Waist circumference (cm) | Δ BMI (kg/m2) | Body weight (Δ %kg) | Δ Waist circumference (cm) | |
| 18–24 | 0.17 ± 0.03§ | 0.83 ± 0.14§ | 0.26 ± 0.13§ | 0.06 ± 0.03* | 0.39 ± 0.13† | 0.07 ± 0.16 |
| 25–29 | 0.02 ± 0.03 | 0.10 ± 0.12 | 0.24 ± 0.10§ | 0.06 ± 0.02† | 0.28 ± 0.10† | 0.01 ± 0.11 |
| 30–34 | 0.11 ± 0.02§ | 0.48 ± 0.07§ | 0.29 ± 0.06§ | 0.03 ± 0.02 | 0.14 ± 0.07* | 0.47 ± 0.08§ |
| 35–39 | 0.09 ± 0.01§ | 0.38 ± 0.05§ | 0.20 ± 0.04§ | 0.07 ± 0.01§ | 0.33 ± 0.06§ | 0.23 ± 0.07‡ |
| 40–44 | 0.09 ± 0.01§ | 0.41 ± 0.04§ | 0.23 ± 0.03§ | 0.09 ± 0.01§ | 0.41 ± 0.06§ | 0.24 ± 0.07‡ |
| 45–49 | 0.08 ± 0.01§ | 0.36 ± 0.04§ | 0.20 ± 0.03§ | 0.05 ± 0.01‡ | 0.24 ± 0.07‡ | 0.30 ± 0.08§ |
| 50–54 | 0.04 ± 0.01§ | 0.19 ± 0.04§ | 0.17 ± 0.03§ | 0.04 ± 0.02* | 0.19 ± 0.08* | 0.13 ± 0.09 |
| 55–59 | 0.05 ± 0.01§ | 0.21 ± 0.05§ | 0.17 ± 0.04§ | 0.08 ± 0.02‡ | 0.37 ± 0.11‡ | 0.49 ± 0.13§ |
| 60–75 | 0.00 ± 0.01 | 0.01 ± 0.04 | 0.15 ± 0.03§ | 0.01 ± 0.02 | 0.03 ± 0.10 | 0.34 ± 0.12† |
Significance levels coded:
P<0.05;
P<0.01;
P<0.001
P<0.0001.
Figure 4 shows that among men and women whose running distance remained relatively constant between surveys (a difference no greater than 8 km/week between surveys), weight and waist circumference increased annually regardless of running distance, although the annual increase was smaller among longer distance runners.
Figure 4.
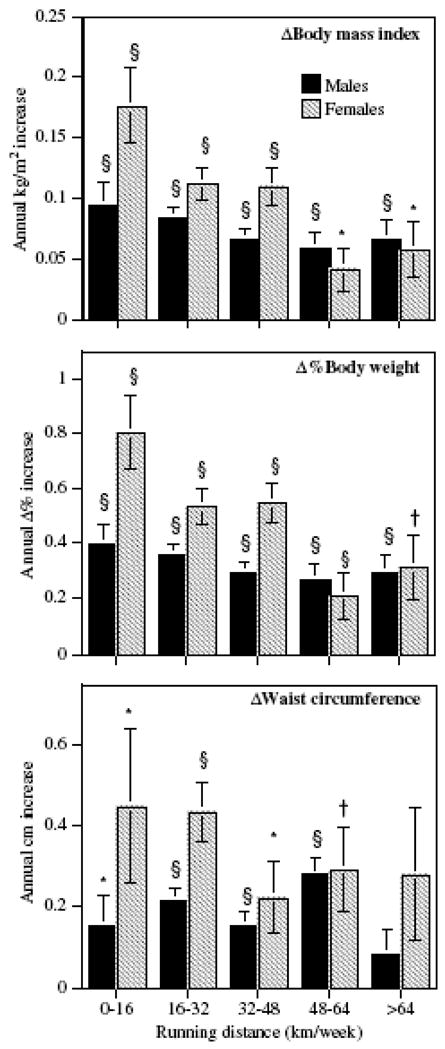
Annual increases in BMI, Δ%body weight and waist circumference in men and women who remained within ±8 km/week of their baseline running distance by average running distance. Bars represent ± one s.e. Significance levels are coded *P<0.05; †P<0.01; ‡P<0.001 and §P<0.0001.
It has been suggested that maintenance of healthy weight (BMI<25 kg/m2) can be achieved by maintaining total energy expenditure that is at least 70% higher than basal energy expenditure.[6] Among runners who we estimated maintained this minimum physical activity level at both surveys, the men increased their body weight by 0.185±0.021 kg/year and decreased their body weight by −0.0415±0.0033 per Δkm/week, and women increased their body weight by 0.069±0.025 kg/year and decreased their body weight by −0.0228±0.0039 kg per Δkm/week.
DISCUSSION
Our three primary findings are: (1) even among the most vigorously active populations, age-related weight gain occurs through middle-age; (2) changes in vigorous activity are associated with changes in weight in a dose-dependent manner; (3) changes in vigorous activity are associated with significantly greater changes in weight in men than in women. Prior observational studies of physical activity and adiposity have been criticized for the low prevalence of higher intensity physical activity, the measurement error associated with low-intensity activity and the inappropriate time frame of the assessment [14,15]. The men and women studied here nearly all engaged in running, which is a well-quantified activity that had been sustained over many years (Table 1).
Our data lend essential support for the hypothesis that vigorous exercise promotes leanness. As our analyses are based on changing levels of exercise, the associations are unlikely to arise from lean men and women choosing to run (albeit changes in weight could affect exercise participation). Intervention studies would provide stronger evidence for causal relationship between change in weight and change in adiposity than the prospective observations we report. However, it is unlikely that any intervention studies will include the sample size (nearly 13 000 vigorously active men and women), duration (3.2 and 2.6 years of follow-up in men and women, respectively) or amount of activity (running approximately 40 km/week) reported here.
In formulating public health recommendations, there has been little discussion of the inevitability of age-related weight gain, or acknowledgement that gaining weight may be naturally associated with the aging process. Weight gain has been primarily treated as a behavioral inadequacy requiring behavioral interventions. Yet, even among runners who run 64 or more km/week, there is statistically significant weight gain over time. The caloric expenditures of these runners greatly exceed the 3.5–5 h/week of moderate intensity exercise (e.g. brisk walking) recommended by the American College of Sports Medicine to facilitate the maintenance of long-term weight loss [16]. They also exceed other recommendations for achieving weight maintenance (e.g. 35 min of vigorous activity per day [17], 45–60 min [18] or 60 or 80 min of moderate intensity activity [6], or 1500–2000 kcal/week [19]), an unexpected result given that the amount of activity required to maintain large weight losses is purported to be greater than the activity required to prevent incipient weight gain [18].
Our prospective data suggest that an annual change in physical activity equivalent to 1 km/week of running is associated with changes in BMI of −0.015±0.001 and −0.009± 0.001 kg/m2 in men and women, respectively. These estimates are somewhat smaller than the cross-sectional relationships between BMI and km/week of running we have previously reported for men (−0.033±0.001 kg/m2 per km/week) and women (−0.014±0.003 kg/m2 per km/week) [8]. Others also report that physical activity has a stronger relationship to weight cross-sectionally than to change in weight measured prospectively [20]. In part, the larger cross-sectional slope may reflect the contributions of self-selection to the cross-sectional relationship. For example, leanness of physically active older women is reported to reflect their leanness during early adulthood (suggesting a component of self-selection) [21]. In addition, the smaller regression slope of the change data could theoretically be due to greater attenuation of the regression slope by measurement error for change data than cross-sectional data. Specifically, errors in measuring the independent variables are known to bias estimates of the regression slope towards zero. This bias is likely to be greater for change data than cross-sectional data because measurement error is accumulated twice in the calculation of a difference but only once for cross-sectional data. Correcting the regression slope for the apparent measurement error for self-reported running distance would increase the regression slope to −0.024 and −0.015 kg/m2 per Δkm in men and women, respectively, assuming a correlation of 0.89 between repeated measurements [12].
Our earlier paper of men studied cross-sectionally suggested that middle-age weight gain is expected if physical activity remains constant, even if the activity is substantial.[8] We originally estimated that the men would need to increase their distance run by 2.24 km/week annually to compensate for the anticipated weight gain during middle age [8]. DiPietro et al. have also reported that men and women gained weight during 7.5 years of follow-up unless treadmill test duration improved [22]. The prospective data presented here suggest that vigorous exercise may need to increase 4.4 km/week annually in men and 6.2 km/week annually in women to compensate for the expected gain in weight due to aging (2.7 and 3.9 km/week annually in men and women, respectively, if we correct for the attenuation due to measurement error associated with self-reported running distance as described above).
The IOM report [6] concluded that the maintenance of healthy weight (i.e., 18.5 kg/m2<BMI<25 kg/m2, NHLBI/NIDDK [23]) requires a level of total energy expenditure that is 160% of basal daily energy expenditure (i.e., a PAL or physical activity index (PAI) of 1.6). Among runners who we estimated to maintain a PAI of 1.7 at both visits, we calculated that the men and women would need to increase their annual weekly running distance by 4.5 and 3.0 km to maintain a constant body weight (analyses not displayed). These estimates are greater than the annual increases of 10 kcal/day in men’s and 7 kcal/day in women’s total energy expenditure that the IOM estimate are required to maintain adult BMIs within the desirable range based on changes in total energy expenditure alone.
We found that changes in weekly running distances had less of an effect on body weight in women than men. Others report that physical activity as measured by doubly labeled water was related to body fat in males but not females [24,9]. This finding is unexpected given that the net energy cost of running at self-selected running speeds is reported to be 11% higher in women than men [10,25]. Some training studies speculate that the same exercise challenge is less likely to cause weight loss in women than men because women have a greater tendency to compensate for energy expenditure through increased energy intake [26,11]. It has also been suggested that training may produce less weight loss in women than men because abdominal fat (generally higher in males) is more responsive to exercise than gluteofemoral fat (generally higher in females) [27]. BMI is a better predictor of differences in body fat in women than men, so it is unlikely that the difference is due to the inadequacy of BMI to reflect body fat changes in women [6]. The sex difference may be less apparent for waist circumference than BMI or Δ%body weight because waist circumference is more weakly related to %body fat in women than men [6].
The majority of the men and women in our study had BMIs that were below the 25 kg/m2 threshold that the National Institutes of Health and other government and nongovernmental organizations have identified as desirable. However, this does not necessarily mean that increases in BMI below this threshold are benign. Willett et al.[28] reported that relative to a BMI of 21 kg/m2, the risk for coronary heart disease (CHD) was 19% higher for women with a BMI of 21–22.9 kg/m2 and 46% higher for a BMI of 23–24.9 kg/m2. They also reported that weight gain after 18 years of age was a strong predictor of CHD risk even among women whose BMI remained below 25 kg/m2 [28]. However, others suggest that weight gain does not increase mortality in middle-aged [29,30] or older men [31], or lean postmenopausal women [32], or that the increased risk primarily restricted to those experiencing the greatest weight gain [33]. Although the health risks associated with weight gain in the vigorously active men and women remains controversial, their mortality risk is known to be less than sedentary physically unfit individuals matched for weight [34].
Our surveys lacked reliable data on changes in energy intake and other sources of energy expenditure that could theoretically account for some of the results reported here. Some of the change in body weight could reflect changes in caloric intake or other activities. Technical limitations of food records and comprehensive activity diaries limit their use in accounting variations in weight over time. Intra-individual variability in daily energy intake is estimated to be ±23% [35], whereas the long-term error in adjusting cumulative energy intake to expenditure is estimated be less than 2% of energy expenditure [36]. Underestimation of food intake by food records is reported to range from 10 to 45% [6]. Between 140 and 700 kcal/day has been attributed to spontaneous physical activities, including fidgeting, which is missed by comprehensive physical activity diaries [37]. We also note that the duration of follow-up was short (3.2 years in men and 2.6 years in women) and extrapolating our calculations to longer time spans may not be warranted. Our questionnaire requested that the participant report their average running mileage for the current and preceding 4 years, and therefore we lack data on variations in distance, intensity or training schedules that varied in response to season, race preparation, illness or injury.
In our opinion, the more demanding physical activity recommendations by the IOM report represent an important improvement over earlier guidelines.[2] Our analyses suggest these guidelines may be further improved by: (1) promoting investments in physical activity that increase with age and (2) acknowledging differences in the expected weight loss for men and women who exercise vigorously.
Acknowledgments
This work was supported in part by Grants HL-45652, HL-072110 and DK066738 from the National Heart Lung and Blood Institute, and was conducted at the Lawrence Berkeley Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
References
- 1.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Physical Activity and Health: A report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 3.Miller WC. Introduction: obesity: diet composition, energy expenditure, and the treatment of the obese patient. Med Sci Sports Exerc. 1991;23:273–274. [PubMed] [Google Scholar]
- 4.Roberts SB. Energy requirements of older individuals. Eur J Clin Nutr. 1996;50 (Suppl 1):S112–S117. [PubMed] [Google Scholar]
- 5.Roberts SB, Leibel RL. Excess energy intake and low energy expenditure as predictors of obesity. Int J Obes Relat Metab Disord. 1998;22:385–386. doi: 10.1038/sj.ijo.0800640. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; Washington, DC: 2002. p. 936. [Google Scholar]
- 7.Erlichman J, Kerbey AL, James WP. Physical activity and its impact on health outcomes. Paper 2: prevention of unhealthy weight gain and obesity by physical activity: an analysis of the evidence. Obes Rev. 2002;3:273–287. doi: 10.1046/j.1467-789x.2002.00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams PT. Evidence for the incompatibility of age-neutral overweight and age-neutral physical activity standards from runners. Am J Clin Nutr. 1997;65:1391–1396. doi: 10.1093/ajcn/65.5.1391. [DOI] [PubMed] [Google Scholar]
- 9.Westerterp KR, Goran MI. Relationship between physical activity related energy expenditure and body composition: a gender difference. Int J Obes. 1997;21:184–188. doi: 10.1038/sj.ijo.0800385. [DOI] [PubMed] [Google Scholar]
- 10.Bhambhani Y, Singh M. Metabolic and cinematographic analysis of walking and running in men and women. Med Sci Sports Exerc. 1985;17:131–137. [PubMed] [Google Scholar]
- 11.Tremblay A, Despres JP, Leblanc C, Bouchard C. Sex dimorphism in fat loss in response to exercise-training. J Obes Weight Regul. 1984;3:193–203. [Google Scholar]
- 12.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8,283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–198. [PMC free article] [PubMed] [Google Scholar]
- 13.Larry Kenny W, editor. ACSM’s Guidelines for Exercise Testing and Prescription. 5. Williams and Wilkins; New York: 1995. p. 278. [Google Scholar]
- 14.Stefanick ML. Exercise and weight control. Exerc Sport Sci Rev. 1993;21:363–396. [PubMed] [Google Scholar]
- 15.DiPietro L. Physical activity, body weight, and adiposity: an epidemiologic perspective. Exerc Sport Sci Rev. 1995;23:275–303. [PubMed] [Google Scholar]
- 16.American College of Sports Medicine. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exercise. 2001;33:2145–2156. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66:551–556. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- 18.Saris WH, Blair SN, van Baak MA, Eaton SB, Davies PS, Di Pietro L, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4:101–114. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 19.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain – a systematic review. Obes Rev. 2000;1:95–111. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 20.Ching PLYH, Willett WC, Rimm EB, Colditz GA, Gortmaker SL, Stampfer MJ. Activity level and risk of overweight in male health professionals. Am J Public Health. 1996;86:25–30. doi: 10.2105/ajph.86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voorrips LE, Meijers JHH, Sol P, Seidell JC, van Staveren WA. History of body weight and physical activity of elderly women differing in current physical activity. Int J Obes Relat Metab Disord. 1992;16:199–205. [PubMed] [Google Scholar]
- 22.DiPietro L, Kohl HW, 3rd, Barlow CE, Blair SN. Improvements in cardiorespiratory fitness attenuate age-related weight gain in healthy men and women: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord. 1998;22:55–62. doi: 10.1038/sj.ijo.0800543. [DOI] [PubMed] [Google Scholar]
- 23.NHLBI/NIDDK (National Heart, Lung, and Blood Institute/National Institute of Diabetes and Digestive and Kidney Diseases) The Evidence Report. NIH Publication No. 98–4083. National Institutes of Health; Bethesda, MD: 1998. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. [Google Scholar]
- 24.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 25.Howley ET, Glover ME. The caloric costs of running and walking one mile for men and women. Med Sci Sports. 1974;6:235–237. [PubMed] [Google Scholar]
- 26.Westerterp KR, Meijer GAL, Janssen EME, Saris WHN, Ten Hoor F. Long term effect of physical activity on energy balance and body composition. Br J Nutr. 1992;68:21–30. doi: 10.1079/bjn19920063. [DOI] [PubMed] [Google Scholar]
- 27.Egger G, Bolton A, O’Neill M, Freeman D. Effectiveness of an abdominal obesity reduction programme in men: the GutBuster ‘waist loss’ programme. Int J Obes Relat Metab Disord. 1996;20:227–231. [PubMed] [Google Scholar]
- 28.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk with the ‘normal’ weight range Risk with the ‘normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 29.Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med. 2002;162:2575–2580. doi: 10.1001/archinte.162.22.2575. [DOI] [PubMed] [Google Scholar]
- 30.Jeffreys M, McCarron P, Gunnell D, McEwen J, Smith GD. Body mass index in early and mid-adulthood, and subsequent mortality: a historical cohort study. Int J Obes Relat Metab Disord. 2003;27:1391–1397. doi: 10.1038/sj.ijo.0802414. [DOI] [PubMed] [Google Scholar]
- 31.Yarnell JW, Patterson CC, Thomas HF, Sweetnam PM. Comparison of weight in middle age, weight at 18 years, and weight change between, in predicting subsequent 14 year mortality and coronary events: Caerphilly Prospective Study. J Epidemiol Community Health. 2000;54:344–348. doi: 10.1136/jech.54.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh PN, Haddad E, Knutsen SF, Fraser GE. The effect of menopause on the relation between weight gain and mortality among women. Menopause. 2001;8:314–320. doi: 10.1097/00042192-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Strandberg TE, Strandberg A, Salomaa VV, Pitkala K, Miettinen TA. Impact of midlife weight change on mortality and quality of life in old age. Prospective cohort study. Int J Obes Relat Metab Disord. 2003;27:950–954. doi: 10.1038/sj.ijo.0802313. [DOI] [PubMed] [Google Scholar]
- 34.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 35.Bingham SA, Gill C, Welch A, Day K, Cassidy A, Khaw KT, et al. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br J Nutr. 1994;72:619–643. doi: 10.1079/bjn19940064. [DOI] [PubMed] [Google Scholar]
- 36.Blundell JE, King NA. Effects of exercise on appetite control: loose coupling between energy expenditure and energy intake. Int J Obes Relat Metab Disord. 1998;22 (Suppl 2):S22–S29. [PubMed] [Google Scholar]
- 37.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-h energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]