Abstract
Quantum efficiencies and ultrafast dynamics of the ring-closure and ring-opening reaction of a trifluorinated dicyclopropyl indolylfulgide with improved photostability are investigated by stationary and ultrafast absorption spectroscopy. The ring-closure reaction occurs on the time scale of 200 fs and is found to be temperature independent (T = 287 – 333 K). However, an activated behaviour is observed for the ring-opening reaction. A comparison with the corresponding non-substituted indolylfulgide shows that the dicyclopropyl group favours the open isomer via lower cyclisation and higher cycloreversion quantum efficiencies and faster dynamics of the ring-opening reaction.
Keywords: photochromism, fluorinated indolylfulgide, ultrafast dynamics, chemical substitution
1. Introduction
Fulgides are photochromic switches, which can undergo ring-opening, ring-closure and E/Z-isomerisation reactions after photoexcitation [1-4]. Therefore fulgides are possible candidates for applications as optical switches and memories for data storage [5-12]. Fluorinated indolylfulgides stand out due to well separated absorption spectra for the Z- and C-form in the UV and visible spectral range and high thermal and photochemical stability [13].
Recently, reaction dynamics and efficiencies of the ring-opening and ring-closure reactions of a trifluorinated indolylfulgide (fulgide 1 in Scheme) were studied in several investigations and the following reaction model was proposed [5,14-17] (values for reaction times and quantum efficiencies were obtained for fulgide 1 dissolved in 1,4-dioxane at room temperature):
Ring-closure reaction: The Z-form isomers exist in two pairs of enantiomers [2,18]. One pair can perform the ring-closure reaction and the other may not. After photoexcitation at 400 nm Z-form molecules are excited from the electronic ground state S0 to the first excited state S1. From the S1 state (lifetime about 0.3 ps) ring-closure to the C-form ground state (quantum efficiency about 15%) and internal conversion to the Z-form ground state (about 84%) occur. A small fraction (about 1%) can switch into the E-isomer, which will be neglected in the further examinations. Additional cooling dynamics of the newly formed hot ground state C- and Z-form molecules are observed in the 10 ps time range.
Ring-opening reaction: Photoexcitation of the closed C-form by visible light populates the Franck-Condon state. With a time constant of several hundred fs wave packet motion, vibrational re-arrangement and solvent dynamics are observed and a relaxed S1 state is formed. The depopulation of the first excited state to the ground state of the product, the Z-form (yield about 4%), and back to the original C-form (yield about 96%) proceeds on the time scale of about 9 ps. Subsequent cooling dynamics are observed in the same time range.
Scheme.
Structure of the investigated fulgides. The numbers denote the atom numbering used in the description of DFT calculations.
The ring-opening reaction was found to be thermally activated with barriers located on the S1 potential energy surface. It was shown that two different reaction channels exist in the S1 state of the closed C-isomer. One channel, the so-called nonreactive pathway, has a small barrier (375 cm−1) and leads exclusively back to the ground state of the C-form. The other, the reactive channel, which allows also access to the open Z-form, exhibits a higher barrier (1055 cm−1), see Table 1 [15].
Table 1.
Quantum efficiency η and excited state lifetime τS1 of both pericyclic reactions and energy barriers Ea,PC and Ea,IC of the ring-opening reaction of fulgide 1 [15] and fulgide 2 (solvent 1,4-dioxane).
| fulgide 1 [15] | fulgide 2 | ||||
|---|---|---|---|---|---|
| T (°C) | η (%) | τS1 (ps) | η (%) | τS1 (ps) | |
| ring-closure | 14 to 60 | 15 | 0.3 | 7.9 | 0.2 |
| ring-opening | 14 | 3.1 (12 °C) | 10.3 (12 °C) | 10.8 | 4.0 |
| 24 | --- | --- | 11.6 | 3.9 | |
| 36 | 4.0 | 8.7 | 12.8 | 3.7 | |
| 60 | 5.0 | 7.6 | 14.5 | 3.4 | |
| barriers ring-opening | Ea,PC (cm−1) | 1055 | 675 | ||
| Ea,IC (cm−1) | 375 | 180 | |||
Recently a new trifluorinated indolylfulgide with two cyclopropyl rings was synthesised (fulgide 2 in Scheme) exhibiting very high resistance against photochemical degradation [19]. Former investigations on other fulgides let assume that this substitution can influence the quantum yield and the dynamics of the photoreactions of fulgide 2 in comparison to fulgide 1 [20,21]. In this study we investigate the photophysics of this new fulgide 2 by temperature dependent stationary and transient absorption spectroscopy. Geometries of the C- and Z-forms of both indolylfulgides were determined by DFT calculations. A comparison of the properties of fulgide 2 with the well-studied fulgide 1 reveals interesting correlations between the molecular structure and resulting dynamics of the pericyclic reactions.
2. Materials and Methods
2.1 Sample preparation
Details of the synthesis of the investigated indolylfulgides have been published previously [7,10,19,22]. For spectroscopic experiments fulgide 2 was dissolved in 1,4-dioxane (Sigma Aldrich Chemie GmbH) without further purification. The experiments monitoring the ring-opening reaction were performed on a sample in the photostationary state PSS-435, which was prepared by steady-state illumination at 435 nm with a Hg(Xe)-lamp (Hamamatsu, 8251) and optical filters BG3 (1 mm, Schott) and GG420 (3 mm, Schott). The ratio between the isomers C:Z:E of fulgide 2 is 87:12:1 for the PSS-435 [19]. The ring-closure reaction was carried out on a sample in the PSS-570, which was obtained by steady-state illumination of a PSS-435 sample with a cold light source (Schott, KL 1500 electronic) and optical filter OG570 (3 mm, Schott). Here the ratio between the isomers C:Z:E of fulgide 2 is 0:99:1 [19].
2.2 Stationary spectroscopy
Steady-state absorption spectra were recorded by a spectrophotometer (Perkin Elmer, Lambda 19). For quantum efficiency measurements 3 ml of the sample were placed into a quartz cuvette with optical pathlength of 1 cm (Hellma). The sample temperature was set in the range between 287 and 333 K with a precision of ±1 K. The sample concentration was adjusted to an optical transmission of 10% at the respective excitation wavelength (optical pathlength 1 cm). The sample was permanently stirred by a magnetic stirrer. For the ring-opening reaction the sample was illuminated from the top with an optical pathlength of 3 cm at 594 nm by a HeNe laser (JDSU, 1677P, 2 mW). For the ring-closure reaction a GaN laser at 414 nm (Roithner, VLMA-1, 0.3 mW) was used. The power of the lasers was measured by a powermeter (Coherent, fieldmaster, type LM-2-VIS). During illumination (10-60 minutes) every second the actual absorption for a distinct wavelength (ring-closure: 550 nm; ring-opening: 570 nm) of the sample was detected by a spectrophotometer (Perkin Elmer, Lambda 19). The absorption change was used to determine the quantum efficiency η of the respective photoreaction: The quantum efficiency η is defined as the ratio of the number of molecules Nrea, which underwent photoreaction, to the number of excited molecules Nexc. In this setup 99.9 % of the emitted photons are absorbed by the sample, so the number of excited molecules is approximately given by the number of emitted photons Npho = P·t·λ/(h·c). Npho can be determined by the irradiation power P, irradiation time t, irradiation wavelength λ, Planck constant h and speed of light c. The number of reacted molecules Nrea = ΔC·V·NA can be calculated with the Avogadro constant NA, the sample volume V and the concentration difference ΔC of the fulgide isomers. The concentration difference is determined experimentally by steady-state absorption and calculated via Lambert-Beer law to ΔC = ΔOD/(ε·d). Here ΔOD is the change of the optical density, ε the extinction coefficient at the detection wavelength and d the optical pathlength. Thus the quantum efficiency η is given by:
| (1) |
2.3 Transient absorption measurements
For transient absorption measurements a home-built laser system was used. It consists of a Ti:Sapphire oscillator (100 MHz repetition rate, 25 fs pulse duration, 5 nJ pulse energy, 792 nm central wavelength). Pulses were amplified in a CPA (1 kHz repetition rate, 80 fs pulse duration, 400 μJ pulse energy, 796 nm central wavelength) and split into two parts to obtain pump and probe pulses.
For probe pulses a white light continuum was generated in a sapphire crystal (3 mm thickness). A NOPA [23,24] delivered pump pulses centred at 480 nm for the ring-closure reaction and centred at 560 nm for the ring-opening reaction. The band width (FWHM) was about 30 nm for both pump pulses. The pump pulses were compressed in a prism compressor, which leads to a cross-corrleation function of about 80 fs for both experiments. The ring-closure reaction was measured under magic angle polarisation, for the ring-opening reaction parallel polarisation was used. The temporal delay between pump and probe pulses was controlled by a mechanical delay stage. A chopper blocked every second pump pulse for referencing.
The sample concentration was adjusted to an optical transmission of about 10% at λ = 560 nm for the ring-opening reaction and about 30% at λ = 480 nm for the ring-closure reaction in a fused silica flow cuvette with optical pathlength of 200 μm (Hellma). The excited volume of the sample was exchanged by a peristaltic pump from shot to shot and the sample reservoir was permanently illuminated to preserve the respective PSS conditions. The cuvette and the reservoir were placed inside copper blocks, which were temperature controlled in the range between 287 and 333 K. The transmission of the probe pulses was detected between 470 and 740 nm by a system of monochromator, 42-channel photodiode array and ADC, which allows single pulse detection with a repetition rate of 1 kHz [25]. Transient absorption data was fitted in a global fitting procedure including three exponentials and an offset for both experiments.
2.4 Theoretical calculations
DFT calculations for the Z- and C-form of both fulgides in vacuum were carried out using the Gaussian 98 program package [26]. The molecular geometry was optimised employing the density functional Becke's three-parameter hybrid method using the LYP correlation function (B3LYP) with the 6-31G* basis set [27-29].
3. Experimental Results
3.1 Steady-state absorption spectra
In Figure 1 the chemical structure and the steady-state absorption spectra in the PSS-435 (mainly C-form) and PSS-570 (mainly Z-form) of fulgide 2 in the solvent 1,4-dioxane are shown. The closed C-isomer of fulgide 2 exhibits a broad absorption band in the visible spectral range with its maximum at about 573 nm while the absorption band of the Z-isomer is centred at 440 nm. The absorption bands of fulgide 2 are slightly red-shifted and broadened in comparison to fulgide 1 without cyclopropyl rings, where the C-form peaks at 563 nm and the Z-form at 424 nm.
Figure 1.
Steady-state absorption spectra of PSS-570 (mainly Z-form) and PSS-435 (mainly C-form) of the same fulgide 2 sample in the solvent 1,4-dioxane. The structures of both isomers, which can be converted into each other by illumination with adequate wavelengths, are depicted in the upper part.
3.2 Results of the calculations
The structural difference between the two investigated indolylfulgides is determined by the cyclopropyl rings at the photochromic hexatriene/cyclohexadiene motif (carbon atoms 1 to 6, see Scheme) of fulgide 2, which replace the methyl groups of fulgide 1. The results of the DFT calculations are summarised in Table 2. These quantum chemical calculations show that the difference between ground state energy of C- and Z-isomer is 665 cm−1 for fulgide 1 and 4925 cm−1 for fulgide 2. This means that the C-form lies above the Z-form and that this difference is considerably larger in fulgide 2. This finding is supported by a closer inspection of the bond distances and angles of the photochromic cyclohexadiene motif near the cyclopropyl rings. The single bonds between carbon atoms 1 and 6 and between carbon atoms 5 and 6 of the closed C-isomer are enlarged for fulgide 2 by about 0.02 Å (see Scheme and Figure 1). Accordingly the dihedral angles defined by the carbon atoms 1, 2, 4, 5 and 1, 2, 3, 4 are about 2 degrees larger for fulgide 2.
Table 2.
Results of the DFT calculations of the structure of fulgides 1 and 2. The carbon atoms 1 to 6 are denoted in the Scheme.
| fulgide 1 | fulgide 2 | |||
|---|---|---|---|---|
| C-isomer | Z-isomer | C-isomer | Z-isomer | |
| ground state energy (cm−1) | −288304056 | −288304721 | −322265397 | −322270322 |
| energy difference ΔEC-Z = EC – EZ (cm−1) | 665 | 4925 | ||
| distance between atoms 1 and 6 (Å) | 1.58 | 3.60 | 1.60 | 3.65 |
| angle 1,2,4,5 (°) | −19.77 | −49.34 | −21.57 | −48.31 |
| distance between atoms 5 and 6 (Å) | 1.52 | 1.36 | 1.54 | 1.38 |
| angle 1,2,3,4 (°) | −5.70 | −53.78 | −6.97 | −54.90 |
The described differences in molecular properties can be explained by the steric interactions of the bulky cyclopropyl rings, which distort the cyclohexadiene motif. For the Z-isomer, which is more flexible the steric interactions by the cyclopropyl rings have a smaller effect on the molecular properties.
3.3 Ring-closure reaction
All experiments investigating the cyclisation reaction of fulgide 2 in the solvent 1,4-dioxane were performed using PSS-570. Quantum efficiency η was measured (steady-state illumination at 414 nm) at four different temperatures (287, 297, 309, and 333 K) and was found to be independent on temperature with a value of 7.9% ± 0.9%.
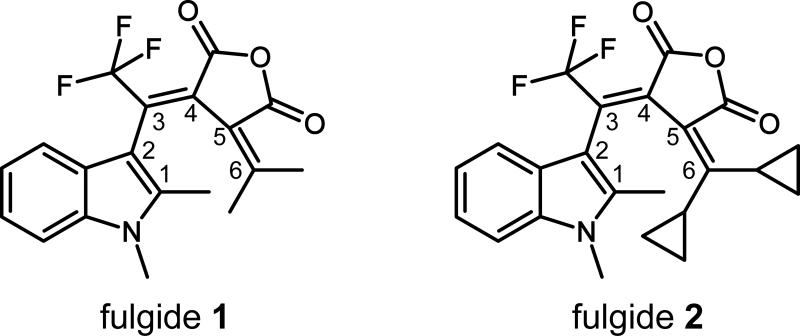
In Figure 2 transient absorption data of the ring-closure reaction of fulgide 2 at 300 K after excitation at 480 nm are shown. At time zero an instantaneous rise of a positive signal can be monitored in the whole detection range between 510 and 720 nm, which decays multi-exponentially to a constant positive offset. A global fitting procedure yields three time constants τ1 = 0.2 ps, τ2 = 1.0 ps and τ3 = 4.3 ps. Thereby the dominant component τ1 is due to the very fast decay of the excited state absorption (ESA) of the S1 state of the Z-isomer. The shape of the decay associated spectra (not shown here) of the longer time constants τ2 and τ3 indicates cooling processes of hot ground state Z- and C-isomers. The weak positive offset at delay times > 100 ps agrees with the steady-state difference spectra of the ring-closure reaction and represents the formation of the photoproduct, the C-isomer. Experiments with excitation at 400 nm were performed on the ring-closure reaction upon varying the sample temperature (data not shown here). No distinct temperature dependence was found in the range between 287 and 333 K.
Figure 2.
Transient absorption data of the ring-closure reaction of fulgide 2 in 1,4-dioxane. The sample was excited at 480 nm and absorption was detected in the range between 510 and 720 nm. Please note that the timescale is linear up to 1 ps and logarithmic afterwards.
3.4 Ring-opening reaction
The experiments on the ring-opening reaction of fulgide 2 dissolved in 1,4-dioxane were performed on samples at PSS-435. Quantum efficiency η (steady-state excitation at 594 nm) was determined between 287 and 333 K and was found to be temperature dependent; it rises from 10.8% at 287 K up to 14.5% at 333 K (see Table 1).
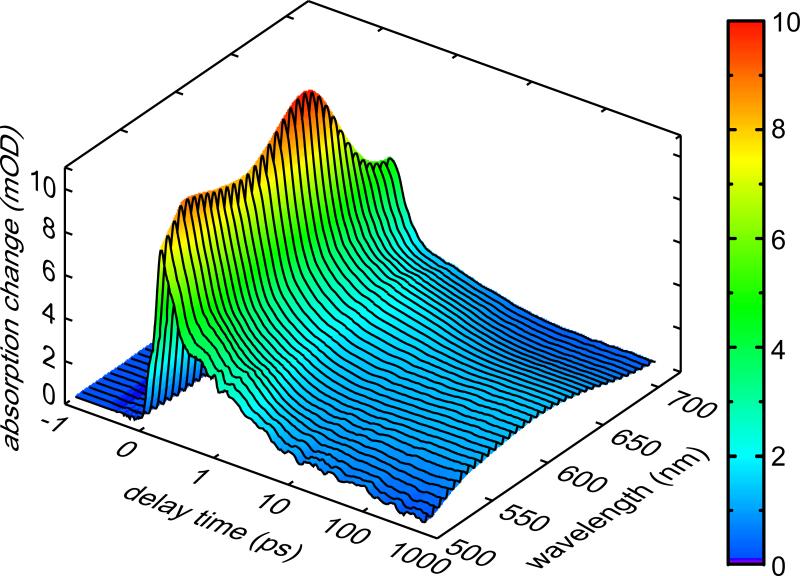
In Figure 3 an overview of the transient absorption data (480 – 720 nm) at 297 K for the ring-opening reaction of fulgide 2 excited at 560 nm is shown. In the wavelength range between 480 and 600 nm a strong positive signal due to ESA can be observed at early delay times. At wavelengths around 630 nm the reduced absorption due to ground state bleach (GSB) and stimulated emission (SE) can be seen. Here also cooling dynamics occur on the 5 ps time scale. A global fitting procedure leads to three time constants: τ1 = 1.2 ps, τ2 = 3.9 ps and τ3 = 6.4 ps. The short time constant τ1 can be related to solvation dynamics in the excited state [30]. The ESA, GSB and SE signals decay with the dominant time constant τ2. Hence this time constant can be interpreted as the ring-opening reaction time. The spectral shape of the time constant τ3 indicates that it is related to cooling of hot C-form ground state molecules (not shown here). The oscillatory component superimposed to the ESA at early delay times is due to wave packet motion, which will not be further discussed in this paper. The weak negative offset at late delay times > 100 ps corresponds to the loss of C-form molecules (reactant) after the ring-opening reaction. The spectral shape of this offset is in agreement with the steady-state difference spectra of the ring-opening reaction.
Figure 3.
Transient absorption data of the ring-opening reaction of fulgide 2 in 1,4-dioxane. The sample was excited at 560 nm and absorption was detected in the range between 480 and 720 nm. Please note that the timescale is linear up to 1 ps and logarithmic there after.
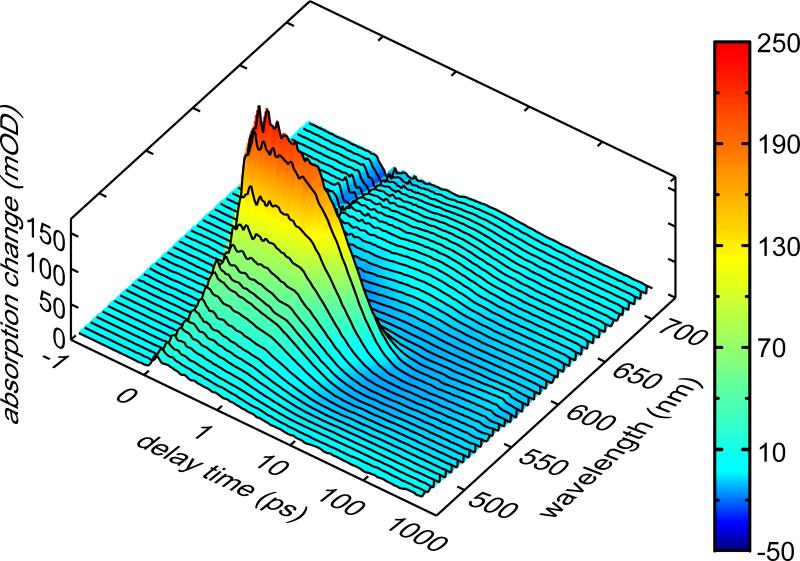
This experiment was also performed at different temperatures (287, 309, 333 K). In Figure 4 the transient absorption at 573 nm (maximum of ESA signal, where the excited electronic state dominates the decay of the signal) is presented for these temperatures. In all traces very similar absorption transients are observed. The exact values for the time constants are temperature dependent as can be seen in Figure 4 and more clearly in its inset. A clear acceleration of the reaction time with increasing temperature is observed. The global fitting procedure reveals a decrease of τ2 from 4.0 ps (287 K) to 3.4 ps (333 K) (see Table 1), whereas the time constants τ1 and τ3 remain nearly unchanged. Thus quantum yield measurements and transient absorption experiments show a temperature dependence, which points to an activated behaviour for the ring-opening reaction [2,31].
Figure 4.
Transient absorption at the probe wavelength of 573 nm of fulgide 2 in 1,4-dioxane at three different temperatures (287, 309, 333 K). With increasing temperature the reaction is accelerated, which can be seen more clearly in the inset, where only the relevant section is shown in detail.
4. Discussion
4.1 Ring-closure reaction
The transient absorption data clearly show that the excited state of the Z-form is short-lived (0.2 ps) and that the photoinduced ring-closure reaction of fulgide 2 occurs on a very short timescale. The further time constants observed in the transient absorption data can be assigned to cooling dynamics of the hot ground state of the reactant and product molecules. This result is in very good agreement with data on the ring-closure reaction of other indolylfulgides and indolylfulgimides, though the quantum efficiency η of fulgide 2 (about 8%) is lower and the reaction time is somewhat faster than in the previous investigated molecules [15,16,32-34]. It is observed that this reaction is not thermally activated, since reaction rates and efficiencies do not vary in the investigated temperature range between 287 and 333 K.
4.2 Ring-opening reaction
The essential features observed for fulgide 2 agree well with those found for other indolylfulgides and indolylfulgimides [15,17,30,33-37]. The experiments on the ring-opening reaction of fulgide 2 indicate that cycloreversion occurs on a time scale of about 4 ps (corresponding to the lifetime τ2 of the excited state), whereas other signal components can be attributed to solvation and cooling dynamics.
The ring-opening reaction (yield η and dynamics τ2) is temperature dependent and this implies an activated behaviour. The differences in temperature dependence of the reaction yield η and the ring-opening time τ2 indicate that a similar reaction scheme to that that used previously for fulgide 1 can be applied [15]. Here two rates are considered: kPC for the photochemical pathway of the ring-opening reaction and kIC for the rate of internal conversion to the reactant ground state, both characterised with an Arrhenius-type behaviour [38]:
| (2) |
ki(T) is the reaction rate for photochemical or internal conversion pathway, Ai the pre-exponential factor and Ea,i the height of the respective energy barrier in the excited state. When we assume that the excited electronic state decays only via these two channels (1/τ2 = kPC + kIC) the rate constants can be calculated from the experimental values for η and τ2:
| (3) |
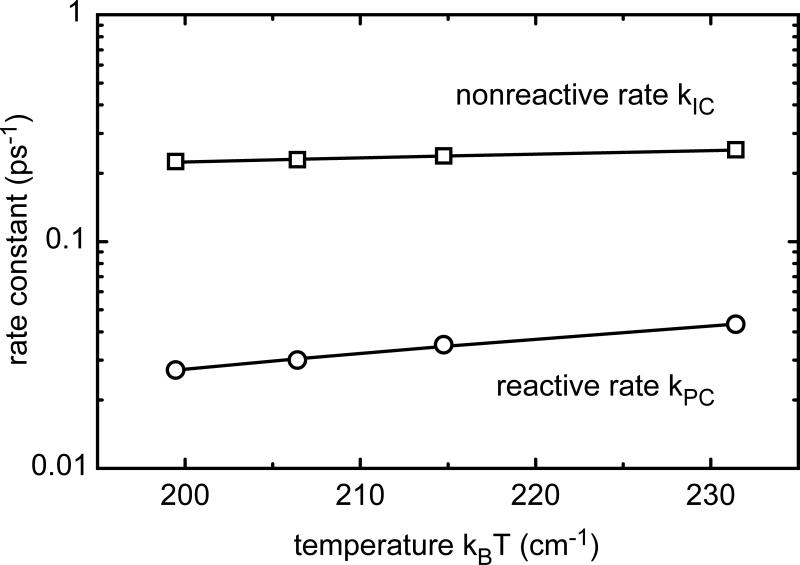
Figure 5 shows the values for kPC(T) and kIC(T) for the different temperatures. The reactive rate kPC is about 10 times smaller than the nonreactive rate kIC. Both rates rise with increasing temperature, but kPC shows a stronger temperature dependence. According to Equation 2 energy barriers Ea,i can be determined to 675 cm−1 for the photochemistry pathway and 180 cm−1 for internal conversion. So the barrier for the ring-opening photoreaction is about 4 times higher than for internal conversion.
Figure 5.
Temperature dependence of the reactive and nonreactive rate of the ring-opening reaction (fulgide 2). This photoreaction is thermally activated. Lines are fits to the data according Equation 2 and the resulting barriers deduced from the analysis are listed in Table 1.
4.3 Comparison of the two fulgides
Fulgide 2, studied in this publication, was initially designed to reduce photochemical degradation of indolylfulgides by substitutions. Indeed photochemical degradation of fulgide 2 was found to be 0.0013% in a photocycle as compared to fulgide 1, which has a 6 time larger photochemical degradation [19]. The improvement in photostability of fulgide 2 is related with a number of changes in the molecular properties.
(i) The thermal stability of the Z-form of fulgide 2 is strongly improved as compared to fulgide 1, whereas the thermal stability of the C-form is reduced. This finding is supported by the change in energy difference ΔEC-Z calculated by DFT (Table 2), which is larger in fulgide 2 by about 4260 cm−1. This change modifies the thermal equilibrium between the two forms and reduces the height of the ground state barrier for the ring-opening reaction.
(ii) The light-induced ring-closure reaction, which is ultrafast (τ = 0.3 ps) in fulgide 1 is further accelerated (τ = 0.2 ps) for fulgide 2. In addition its quantum efficiency ηZ-C is reduced in fulgide 2 by a factor of 2 (ηZ-C = 7.9% versus 15%). These observations show that the photochemical reaction is hindered by substitution of the methyl groups with cyclopropyl rings.
(iii) The most important modifications are observed for the light induced ring-opening reaction. The quantum efficiency ηC-Z rises by a factor of about 3 and the reaction is speeded up by a factor of about 2.5 for fulgide 2. The related energy barriers in the excited electronic state are considerably reduced. These change can be explained by the steric interactions of the cyclopropyl rings in the closed C-form. The related distortion destabilises the C-form of fulgide 2 both in the electronic ground and the excited state; this lowers the reaction barriers and accelerates the reaction.
The changed reaction dynamics can also be related to the improved photochemical stability of fulgide 2. Based on the structural similarity of fulgide 1 and 2 one can assume that the photochemical degradation pathways for both molecules are the same. Besides, the high thermal stability of the isomers in the electronic ground state indicates that photochemical degradation should be proportional to the time the molecules stay in the excited state in a complete photocycle. So the yield of photochemical degradation is closely related to the time the molecules remain after illumination in an excited electronic state and to the quantum efficiencies of the reaction steps in the photocycle. For the studied fulgides the excited state lifetime is much shorter for the ring-closure reaction than for the ring-opening reaction. As a consequence photochemical degradation upon starting the ring-opening reaction should be most important. Here fulgide 2 with its accelerated reaction speed (2.5 times) and improved reaction efficiency (3 times) should be less susceptible to photochemical degradation by a factor of 2.5 · 3 = 7.5. This number is close to the improvement of photostability seen above.
5. Conclusion
In this study a recently synthesised dicyclopropyl indolylfulgide of improved photochemical stability was investigated. Temperature dependent quantum efficiency and transient absorption measurements of the ring-closure and ring-opening reaction were presented. It was found that the ring-closure reaction is not activated. However, the ring-opening reaction is a thermally activated reaction incorporating two energy barriers in the excited electronic state. In comparison with a very similar indolylfulgide without cyclopropyl rings a preference of the open Z-isomer was found, which explains the differences in reaction rates, efficiencies and the improved photochemical stability.
6. Acknowledgements
Supported by Deutsche Forschungsgemeinschaft through the DFG-Cluster of Excellence Munich-Centre for Advanced Photonics and the SFB 749. Financial support to W.J.L. from the NIH/NIGMS program (SC3GM084752) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stobbe H. Ber. Dtsch. Chem. Ges. 1905;38:3673–3685. [Google Scholar]
- 2.Yokoyama Y. Chem. Rev. 2000;100:1717–1739. doi: 10.1021/cr980070c. [DOI] [PubMed] [Google Scholar]
- 3.Bouas-Laurent H, Dürr H. Pure Appl. Chem. 2001;73:639–665. [Google Scholar]
- 4.Renth F, Foca M, Petter A, Temps F. Chem. Phys. Lett. 2006;428:62–67. [Google Scholar]
- 5.Malkmus S, Koller F, Draxler S, Schrader TE, Schreier WJ, Brust T, DiGirolamo JA, Lees WJ, Zinth W, Braun M. Adv. Funct. Mat. 2007;17:3657–3662. [Google Scholar]
- 6.Ramsteiner IB, Hartschuh A, Port H. Chem. Phys. Lett. 2001;343:83–90. [Google Scholar]
- 7.Wolak MA, Thomas CJ, Gillespie NB, Birge RR, Lees WJ. J. Org. Chem. 2003;68:319–326. doi: 10.1021/jo026374n. [DOI] [PubMed] [Google Scholar]
- 8.Inada T, Uchida S, Yokoyama Y. Chem. Lett. 1997:321–322. [Google Scholar]
- 9.Otto B, Rück-Braun K. Eur. J. Org. Chem. 2003:2409–2417. [Google Scholar]
- 10.Wolak MA, Gillespie NB, Thomas CJ, Birge RR, Lees WJ. J. Photochem. Photobiol. A-Chem. 2001;144:83–91. [Google Scholar]
- 11.Geppert D, Seyfarth L, de Vivie-Riedle R. Appl. Phys. B-Lasers Opt. 2004;79:987–992. [Google Scholar]
- 12.Geppert D, de Vivie-Riedle R. J. Photochem. Photobiol. A-Chem. 2006;180:282–288. [Google Scholar]
- 13.Yokoyama Y, Takahashi K. Chem. Lett. 1996:1037–1038. [Google Scholar]
- 14.Draxler S, Malkmus S, Brust T, DiGirolamo JA, Lees WJ, Braun M, Zinth W. In: Ultrafast Phenomena XVI, Proceedings of the 16th International Conference. Corkum P, et al., editors. Springer; Berlin: 2009. [Google Scholar]
- 15.Draxler S, Brust T, Malkmus S, DiGirolamo JA, Lees WJ, Zinth W, Braun M. Phys. Chem. Chem. Phys. 2009;11:5019–5027. doi: 10.1039/b819585d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordes T, Herzog TT, Malkmus S, Draxler S, Brust T, DiGirolamo JA, Lees WJ, Braun M. Photochem Photobiol. Sci. 2009;8:528–534. doi: 10.1039/b817627b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordes T, Malkmus S, DiGirolamo JA, Lees WJ, Nenov A, de Vivie-Riedle R, Braun M, Zinth W. J. Phys. Chem. A. 2008;112:13364–13371. doi: 10.1021/jp807097w. [DOI] [PubMed] [Google Scholar]
- 18.Wolak MA, Finn RC, Rarig RS, Thomas CJ, Hammond RP, Birge RR, Zubieta J, Lees WJ. Acta Cryst. C. 2002;58:o389–o393. doi: 10.1107/s0108270102008041. [DOI] [PubMed] [Google Scholar]
- 19.Islamova NI, Chen X, Garcia SP, Guez G, Silva Y, Lees WJ. J. Photochem. Photobiol. A-Chem. 2008;195:228–234. doi: 10.1016/j.jphotochem.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaze AP, Heller HG, Whittall J. J. Chem. Soc. Perkin Trans. 1992;2:591–594. [Google Scholar]
- 21.Martin SC, Singh N, Wallace SC. J. Phys. Chem. 1996;100:8066–8069. [Google Scholar]
- 22.Thomas CJ, Wolak MA, Birge RR, Lees WJ. J. Org. Chem. 2001;66:1914–1918. doi: 10.1021/jo005722n. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm T, Piel J, Riedle E. Opt. Lett. 1997;22:1494–1496. doi: 10.1364/ol.22.001494. [DOI] [PubMed] [Google Scholar]
- 24.Riedle E, Beutter M, Lochbrunner S, Piel J, Schenkl S, Spörlein S, Zinth W. Appl. Phys. B-Lasers Opt. 2000;71:457–465. [Google Scholar]
- 25.Seel M, Wildermuth E, Zinth W. Meas. Sci. Technol. 1997;8:449–452. [Google Scholar]
- 26.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, E.S. R, Pople JA. Gaussian 98 (Revision A.7) Gaussian, Inc.; Pittsburgh PA: 1998. [Google Scholar]
- 27.Becke AD. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 28.Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- 29.Young DC. Computational Chemistry. Wiley and Sons, Inc.; New York: 2001. [Google Scholar]
- 30.Brust T, Draxler S, Malkmus S, Schulz C, Zastrow M, Rück-Braun K, Zinth W, Braun M. J. Mol. Liq. 2008;141:137–139. [Google Scholar]
- 31.Heller HG. Spec. Publ. - Royal Society of Chemistry, 60, Fine Chem. Electron. Ind. 1986:120–135. [Google Scholar]
- 32.Koller FO, Schreier WJ, Schrader TE, Malkmus S, Schulz C, Dietrich S, Rück-Braun K, Braun M. J. Phys. Chem. A. 2008;112:210–214. doi: 10.1021/jp073545p. [DOI] [PubMed] [Google Scholar]
- 33.Draxler S, Brust T, Malkmus S, Koller FO, Heinz B, Laimgruber S, Schulz C, Dietrich S, Rück-Braun K, Zinth W, Braun M. J. Mol. Liq. 2008;141:130–136. [Google Scholar]
- 34.Heinz B, Malkmus S, Laimgruber S, Dietrich S, Schulz C, Rück-Braun K, Braun M, Zinth W, Gilch P. J. Am. Chem. Soc. 2007;129:8577–8584. doi: 10.1021/ja071396i. [DOI] [PubMed] [Google Scholar]
- 35.Brust T, Malkmus S, Draxler S, Ahmed SA, Rück-Braun K, Zinth W, Braun M. J. Photochem. Photobiol. A-Chem. 2009 submitted. [Google Scholar]
- 36.Koller FO, Schreier WJ, Schrader TE, Sieg A, Malkmus S, Schulz C, Dietrich S, Rück-Braun K, Zinth W, Braun M. J. Phys. Chem. A. 2006;110:12769–12776. doi: 10.1021/jp0657787. [DOI] [PubMed] [Google Scholar]
- 37.Malkmus S, Koller FO, Heinz B, Schreier WJ, Schrader TE, Zinth W, Schulz C, Dietrich S, Rück-Braun K, Braun M. Chem. Phys. Lett. 2006;417:266–271. doi: 10.1021/jp0657787. [DOI] [PubMed] [Google Scholar]
- 38.Arrhenius S. Z. phys. Chem. 1889;4:226–248. [Google Scholar]