Abstract
Human diseases associated with exposure to asbestos fibers include pleural fibrosis and plaques, pulmonary fibrosis (asbestosis), lung cancer, and diffuse malignant mesothelioma. The critical determinants of fiber bioactivity and toxicity include not only fiber dimensions, but also shape, surface reactivity, crystallinity, chemical composition, and presence of transition metals. Depending on their size and dimensions, inhaled fibers can penetrate the respiratory tract to the distal airways and into the alveolar spaces. Fibers can be cleared by several mechanisms, including the mucociliary escalator, engulfment, and removal by macrophages, or through splitting and chemical modification. Biopersistence of long asbestos fibers can lead to inflammation, granuloma formation, fibrosis, and cancer. Exposure to synthetic carbon nanomaterials, including carbon nanofibers and carbon nanotubes (CNTs), is considered a potential health hazard because of their physical similarities with asbestos fibers. Respiratory exposure to CNTs can produce an inflammatory response, diffuse interstitial fibrosis, and formation of fibrotic granulomas similar to that observed in asbestos-exposed animals and humans. Given the known cytotoxic and carcinogenic properties of asbestos fibers, toxicity of fibrous nanomaterials is a topic of intense study. The mechanisms of nanomaterial toxicity remain to be fully elucidated, but recent evidence suggests points of similarity with asbestos fibers, including a role for generation of reactive oxygen species, oxidative stress, and genotoxicity. Considering the rapid increase in production and use of fibrous nanomaterials, it is imperative to gain a thorough understanding of their biologic activity to avoid the human health catastrophe that has resulted from widespread use of asbestos fibers.
The manufacturing of engineered nanomaterials for both consumer and industrial applications is undergoing exponential growth. Among the many nanomaterials produced since the discovery of fullerenes in 1985,1 carbon nanofibers (CNFs) and carbon nanotubes (CNTs) are exceptionally attractive, because of their high strength-to-weight ratio, high surface area, thermal stability, and resistance to chemicals.2 Their unique physicochemical characteristics are expected to be useful in a wide range of applications, including reinforcement of biomaterials, optical devices, drug delivery, biosensors, as well as conducting and reinforcing fillers in polymer composites.
Inhalation exposure in occupational environments can occur during the synthesis, collection, purification, handling, and packing of nanomaterials. Because of their shape and dimensions, in particular their high aspect ratio, graphenic CNTs may have similar pathogenic potential as naturally occurring asbestiform fibers. Their structural resemblance to asbestos fibers3 and potential biopersistence make fibrous carbon nanomaterials a potential human health hazard.4 The aim of this review is to address the similarities between asbestos fibers and high aspect ratio nanoparticles with respect to their physicochemical and toxicologic properties.
FIBROUS MATERIALS
Naturally Occurring
Asbestos fibers are naturally occurring silicates, with the following properties: fibrous shape, high tensile strength and flexibility, low thermal and electrical conductivity, high absorbency, high mechanical and thermal stability, and resistance to acids and bases.5 They can be divided into two groups: serpentines and amphiboles (Figure 1). Chrysotile is the only member of the serpentine group. Actinolite, amosite, anthophyllite, crocidolite, and tremolite belong to the amphibole group. Chrysotile is comprised of an octahedral magnesium hydroxide layer intercalated between silicate tetrahedral layers which form tightly rolled sheets ranging in diameter from 25 to 100 nm.6 As the brucite layer of chrysotile is acid-sensitive, fibers that reach the lung can undergo focal fiber disintegration, either in the acidic (pH ~4.5) phagolysosomes of macrophages, or if cleared from the lung and swallowed, by hydrochloric acid in the lumen of the stomach.
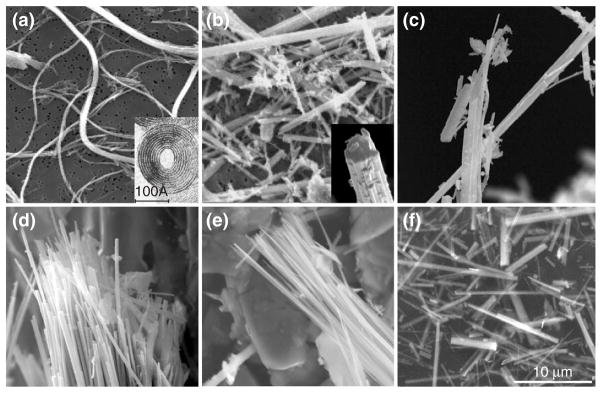
FIGURE 1.
Structure of asbestos fibers by transmission electron microscopy (TEM): (a) serpentine and (b–f) amphiboles. (a) International Union Against Cancer (UICC) asbestos chrysotile ‘A’ standard, (b) UICC asbestos crocidolite standard, Death Valley, California, (c) UICC asbestos anthophyllite standard, (d) winchite-richterite asbestos, Libby, Montana, (e) tremolite asbestos and (f) UICC asbestos amosite standard. Chrysolite is the only member of the serpentine group. Because of the mismatch in the spacing between the magnesium ions and the silica ions, chrysotile curls into a thin-rolled, flexible sheet while amphibole fibers are more rigid. Scale bar = 10 μm. (Reprinted with permission from Denver Microbeam Laboratory at the U.S. Geological Survey).
Amphiboles exhibit prismatic cleavage and diameters in the range of 100–200 nm and their chemical composition is complex (Table 1). Amphibole fibers can split longitudinally into thinner fibers that are extremely biopersistent.7
TABLE 1.
Classification and Chemical Composition of Asbestos Fibers
| Mineral group | Mineral species | Asbestiform variety | Ideal chemical composition |
|---|---|---|---|
| Serpentine | Clinochrysotile | Chrysotile | Mg3Si2O5(OH)4 |
| Serpentine | Orthochrysotile | Chrysotile | Mg3Si2O5(OH)4 |
| Serpentine | Parachrysotile | Chrysotile | Mg3Si2O5(OH)4 |
| Amphibole | Riebeckite | Crocidolite | Na2Fe5Si8O22(OH)2 |
| Amphibole | Grunerite | Amosite | (FeMg)7Si8O22(OH)2 |
| Amphibole | Cummingtonite | Amosite | (MgFe)7Si8O22(OH)2 |
| Amphibole | Gedrite | Amosite | (MgFe)5Al2(Si6Al2)O22(OH)2 |
| Amphibole | Anthophyllite | Asbestiform anthophyllite | (MgFe)7(Si)8O22(OH)2 |
| Amphibole | Tremolite | Asbestiform tremolite | Ca2 Mg5Si8O22(OH)2 |
| Amphibole | Actinolite | Asbestiform actinolite | Ca2(MgFe)5Si8O22(OH)2 |
| Amphibole | Richterite | Asbestiform actinolite | Na2Ca(MgFe)5Si8O22(OH)2 |
| Amphibole | (Alumino) winchite | Asbestiform winchite | CaNa (MgFe)4AlSi8O22(OH)2 |
| Amphibole | Ferriwinchite | Asbestiform winchite | CaNa (MgFe)4Fe3+ Si8O22(OH)2 |
Source: IOM, 2006.6
Fiber toxicity is related to the physicochemical characteristics of the particular fiber and sample, including geometry, surface reactivity, crystallinity, and chemical composition, particularly the presence of redox-active transition metals. Other naturally occurring asbestiform fibers include, but are not limited to, wollastonite and erionite, the latter being a highly carcinogenic fibrous zeolite. In contrast to asbestos and other asbestiform fibers, wollastonite is highly-soluble and has minimal toxicity.8
Synthetic Fibers—CNFs and CNTs
Classification
Although synthetic fibers encompass a wide variety of products with variable chemistries,7 for the purpose of this review we will focus on CNFs and CNTs. CNF is a generic term used to describe filaments comprised of graphene layers stacked at an angle to the fiber axis, where the angle of the graphene layers determines the fiber type9 (Figure 2).
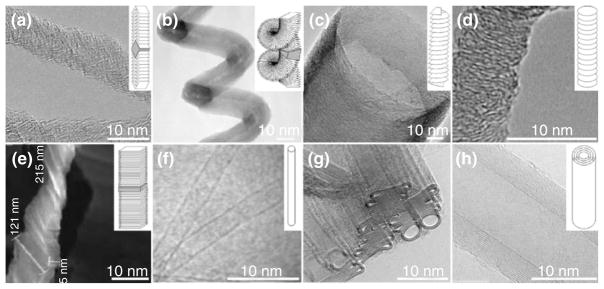
FIGURE 2.
Structure of some carbon fibrous nanomaterials by TEM: (a–e) carbon nanofibers (CNFs) and (f–h) Carbon nanotubes (CNTs); (a) Fishbone solid CNFs, (b) Platelet spiral, (c) Stacked-cup, (d) Platelet-symmetry, (e) Platelet, (f) single-wall CNTs, (g) double-walled CNTs, and (h) multiwalled CNTs. Scale bar= 10 nm. TEM sources: (a) Chen, 2005,15 (b) Bandaru, 2007,16 (c) Vera-Agullo, 2007,17 (d) Jian, 2006,18 (e) Zheng, 2006,19 (f) www.rsc.org, (g) www.msm.cam.ac.uk, and (h) www.nccr-nano.org. Inset sources: (a–c, e, f, and h) Martin-Gullon, 200620 and (d) Jian, 2006.18
CNFs are used commercially as polymer additives, gas storage materials, catalyst supports, and in biomedical devices.10 CNTs are either single walled (SWCNTs) or multi-walled (MWCNTs). SWCNTs are comprised of a single cylindrical graphene sheet with diameters ranging from 0.4 to 3 nm and are often closed at one end. MWCNTs contain several concentric, coaxial graphene cylinders with diameters ranging from 2 to 200 nm,11,12 and bear a structural resemblance to chrysotile asbestos (vida infra). CNTs can coalesce to form ropes or bundles up to 500 nm or 3 μm in diameter, for SWCNT and MWCNT13 respectively.14
Synthesis
Two components are needed for carbon nanomaterial synthesis, a carbon source and an energy source. Depending on the material and method of synthesis, a metal nanoparticle catalyst may be used to increase yield and sample homogeneity, and to reduce the synthesis temperature.21 Carbon nanomaterials can be synthesized by several distinct methods, most commonly used are chemical vapor deposition (CVD), arc discharge, laser ablation, and nanochannel templating methods (Table 2).
TABLE 2.
Common Methods for Carbon Nanomaterial Synthesis
| Synthesis method | Carbon precursor | Catalyst | Energy source | CNFs Symmetry | CNTs |
|---|---|---|---|---|---|
| CVD/PECVD HipCO | Gas-phase precursors (methane, carbon monoxide, synthesis gas (H2/CO), ethyne, and ethane) | Fe, Co, Ni Cr, V, and Mo (alone or in combination, as powder or supported) | Heat/radio frequency, direct current glow discharge, inductively coupled plasma or microwave (500 to 1000 °C) | Platelet (iron powder) Tubular (iron supported on silica) Stacked-cup | Single wall/Multiwall |
| Nanochannel templating | Gas-phase CCVD precursors (as above; discotic liquid crystals | CVD catalysts as above, or non-catalytic | Heat (from 300 to 1000 °C) | Platelet Carbon/carbon composite Nanoribbons | Multiwall |
| Electric arc discharge | Graphite rod electrodes in inert gas (Ar or He) | Ni, Y | Electric discharge (2000 to 3000 °C) | Single wall (metal-doped electrodes)/Multi wall (pure graphite electrodes) | |
| Laser ablation (vaporization) | Graphite in Ar flow | Co, Ni | High intensity laser (Nd:YAG) pulses in a high temperature atmosphere (1200 °C) | Single wall (metal-doped electrodes)/Multi wall (pure graphite electrodes |
PECVD, plasma-enhanced chemical vapor deposition; CCVD, catalytic chemical vapor deposition; Nd:YAG, neodumium-doped yttrium aluminum garnet
The diameter of the fibers depends on the dimensions of the metal nanoparticle used as a catalyst, and the orientation of the graphene layers can be steered by the growth temperature and/or the nature of the metal. Iron catalysts preferentially generate parallel fibers, whereas nickel generates fishbone-like fibers. The fiber strength depends on the graphene layer arrangement and the rate of growth. Fast growth of thin fibers generates weak products, while slow growth of thick fibers leads to strong products. The mechanical strength at the macroscopic scale is also strongly influenced by the structure of CNF agglomerates.20,22
SWCNTs and MWCNTs exhibit unique characteristics depending on the method and conditions of synthesis. The shape, symmetry, dimensions, growth rate, yield, and crystallinity of the materials are influenced by the selection of the catalyst, carbon source, temperature, and time of the reaction, thereby leading to significant heterogeneity among the final products. Moreover, batch-to-batch differences in the amounts of residual catalyst, (typically partially encapsulated inside the graphenic tube or covered by a carbon shell), disordered graphenic or fullerenic carbon, support materials, and other impurities have been observed.23 Consequently, post-synthesis treatments are used to increase the purity of the product. The most common purification approach involves selective oxidation of the amorphous carbon and/or carbon shells at a controlled temperature followed by washing or sonicating the sample in acid (HCl, HNO3, H2SO4) or base (NaOH) to partially remove the catalyst and/or support. Breakage can occur during purification, resulting in defective or shorter tubes. In addition, surface functionalization by air24,25 or oxidizing acids can occur as a side reaction.23 Removing imbedded metal without damaging the desired tube structure is difficult, so even purified materials typically have measurable and often significant metal content. As there are many types of purification processes, the purified materials will exhibit differences in the content of trace elements and residual materials.26,27
FIBER CHARACTERISTICS THAT DETERMINE BIOACTIVITY
The toxicity, fibrogenicity, and carcinogenicity of asbestos are related to its physical and chemical properties, some of which are also shared by fibrous carbon nanomaterials. The following parameters are important in governing the lung burden and subsequent development of fibrosis or cancer following fiber inhalation.
Size and Shape
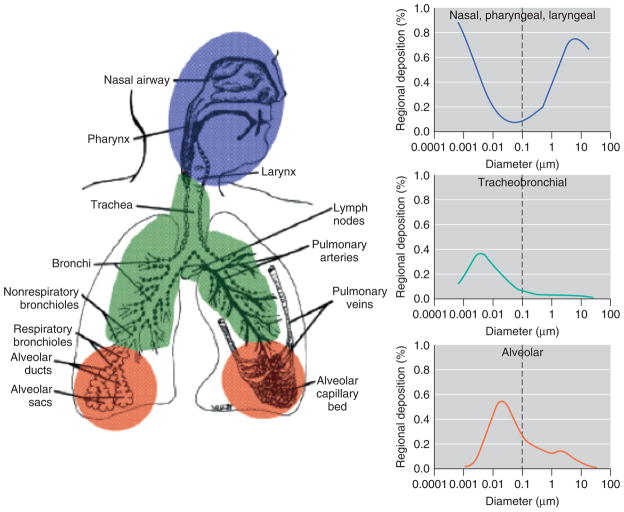
Fiber size and geometry determine the extent of fiber deposition into the lung and are well known to influence carcinogenicity.7 The deposition of inhaled fibers in the lung is determined by their length, width, shape, density, and by the anatomy of the respiratory tract. These parameters determine the aerodynamic behavior of fibers in the conducting airways, as well as their probability of deposition and retention in the distal lung (Figure 3). Aerodynamic diameter (AD) impacts the movement and deposition efficiency of inhaled fibers and particulates in the respiratory tract. Most of the mathematical models used to determine the AD of fibers are extrapolated from studies of the AD of particles, which describe a direct correlation between increased particle density and/or diameter (leading to an increase in the AD) and reduced deposition in the respirable region of the lung. Mechanisms of fiber deposition include impaction, sedimentation, and diffusion. For larger particles with an AD > 5 μm, deposition occurs by impaction as a result of an abrupt directional change in the airways, and sedimentation or settling by gravitational forces, while diffusion is the predominant mechanism for deposition of smaller fibers (AD < 5 μm).7
FIGURE 3.
Relationship between aerodynamic diameters of particles and lung deposition. (Reprinted with permission from Ref 28. Copyright 2005 National Institute of Environmental Health Sciences).
Biopersistence
The ability of fibers to persist in the body following inhalation determines the retained dose. The dose of fibers retained in the respiratory tract is expressed as the initial number of deposited fibers minus the number of fibers subject to clearance, which can occur by both physicochemical and physiological processes. Fibers that cannot be cleared by any of these processes are considered to be biopersistent and are predicted to accumulate during chronic exposure. Physicochemical processes leading to increased clearance include leaching of elements from the fiber matrix, which can alter fiber composition, dissolution in surfactant or physiological fluids, and transverse breakage or splitting into shorter or thinner fibers that are more readily cleared.29 Clearance is believed to occur by the following four major mechanisms.
Clearance via the Mucociliary Escalator in the Nose and Tracheobronchial region
The trachea, bronchi, and larger bronchioles are lined with ciliated epithelial cells, which are covered with a thin layer of mucous. The mucous traps foreign material, which the cilia rhythmically beat and move toward the throat where it can be swallowed or expelled from the body.29
Phagocytosis by Alveolar Macrophages
A limiting factor for complete phagocytosis of fibers is the diameter of the alveolar macrophages. As macrophages range in diameter from ~10 to 20 μm, shorter fibers are more likely to be completely phagocytosed by alveolar macrophages than longer fibers. This leads to incomplete or ‘frustrated’ phagocytosis, which is characterized by prolonged production of reactive oxygen species (ROS).29
Dissolution
Fiber durability is typically assessed by dissolution in vitro. Studies using mathematical models in both static and flow-through methods have been described.30,31 As the pH of the extracellular lung fluid is 7.2, the pH in the phagolysosome of the rat alveolar macrophage is 4.5–5.0, and the intracellular (cytoplasmic) pH of lung tissue has been measured as 6.5, dissolution studies are performed in buffer solutions resembling the pH and composition of these different milieus.29,30,32,33
Translocation
Fibers can also leave the lung by migration, passing across the alveolar wall and into the lung interstitium, where they can reach the lymphatic system. Chrysotile fibers may split into thinner fibrils over time. Compared to amphiboles, chrysotile asbestos is more effectively cleared, in part because of its brucite layer, which dissolves in the acidic milieu of alveolar macrophages, leading to focal fiber disintegration. Because of the carbonaceous nature of CNFs and CNTs, it has been suggested that they may be as biopersistent as amphiboles. Using an in vitro flow-through assay with phagolysosomal simulant fluid at pH 4.5, SWCNTs have been shown to persist for two months.27 It is unknown whether the biopersistence of CNFs and CNTs more closely resembles that of serpentines or amphiboles, and whether uncleared nanomaterials can translocate to the pleura.34
Surface Properties
Surface properties of fibers can affect their biological activity. Unfunctionalized carbon nanomaterials tend to be hydrophobic and are difficult to disperse in physiological solutions. The wide variety of surface functionalization schemes now available for nanotubes, coupled with their high intrinsic surface area, gives these materials the potential to adsorb a wide range of small molecules and macromolecules in biological environments. Characteristics such as pore volume and surface structure can impact the total area over which physical adsorption can occur, while charge and chemical modification can determine the location and degree of adsorption.14,35–37 These phenomena may selectively impact interactions with biological systems, thus altering their potential health risk throughout the product life-cycle.
Asbestos fibers can adsorb xenobiotics from the environment, including polycyclic aromatic hydrocarbons and iron.38–40 Upon contact with the lining fluid and alveolar macrophages in the lung, asbestos fibers adsorb endogenous proteins including ferritin,38,41 immunoglobulin G42,43 and vitronectin.44,45 Formation of ferruginous or asbestos bodies is the consequence of endogenous iron, protein, and mucopolysaccharide deposition on biopersistent fibers in the lungs.46
Carbon nanomaterials can also adsorb biomolecules. Post-synthesis treatments of CNFs and nanotubes can modify their surface, leading to alterations in the selective adhesion of biomolecules, opsonization by complement,47 and adsorption of small solutes, such as folic acid or riboflavin, from biological fluids.36 Unmodified graphene surfaces are hydrophobic, but many types of surface modifications commonly performed on CNTs and CNFs can increase hydrophilicity and improve their dispersion in medium or biological fluids37 As hydrophilic materials interact more readily with cell receptors, surface modifications that enhance hydrophilicity can modify target cell interactions and subsequent biological responses.37
Surface Reactivity: Role of Transition Metals
Redox-active transition metals are an important determinant of fiber toxicity and carcinogenicity, and have been proposed to mediate, in part, the genotoxic, mitogenic, and cytotoxic effects of amphibole asbestos fibers.7 The ability of asbestos fibers to generate reactive species is associated with the presence of surface redox-active iron, which can be present in both ferrous (Fe2+) and ferric (Fe3+) forms. Among commercially used asbestos fibers, crocidolite and amosite asbestos are particularly iron-rich, containing 20–30% iron by weight.39
Reactive species can be generated in the presence of transition metals, whether these metals are present at the fiber surface in a poor coordination state,37 or as a result of mobilization from the crystalline lattice.48 In cell-free systems, iron can be mobilized from asbestos fibers using chelators such as ferrozine, or reductants such as ascorbate or citrate.39 Iron mobilization and redox cycling lead to production of hydroxyl radicals, DNA breaks, and formation of premutagenic DNA adducts such as 8-OHdG.39 Redox-active iron has been proposed to contribute to the development of human cancer.49 Iron saccharate induces sarcomas in rats at the site of injection,50 and iron nitriloacetic acid (Fe-NTA) induces renal tumors and mesotheliomas when injected intraperitoneally.51
Adsorption of endogenous iron onto the fiber surface or into pores may also contribute to the toxic and carcinogenic effects of fibers. This mechanism has been suggested for erionite, a fibrous zeolite that does not contain iron within its molecular structure. The surface area of erionite is 10–100 times greater than that of asbestos and erionite has been shown to accumulate iron in its porous structure.39,40 Erionite is highly carcinogenic in rodents, inducing mesotheliomas more efficiently than crocidolite or amosite asbestos,52 and has been linked to a mesothelioma epidemic in central Turkey, where the incidence exceeds 50% in select villages.53
Metal catalysts are used in the synthesis of CNTs, primarily iron, nickel, yttrium, cobalt, and molybdenum, which are known to raise toxicity concerns. Of primary concern is whether the metal catalysts present in synthetic nanomaterials are bioavailable, especially considering their apparent encapsulation by carbon observed by TEM. Using buffers that model physiological fluids (e.g., phagolysosomal simulant fluid) or biologically relevant fluids, mobilization of iron and nickel from CNTs has been demonstrated.26,54 Although purification methods can be devised to remove the majority of contaminating metals, no purification process is 100% efficient; therefore, even purified samples are not completely metal-free. Recent work has shown that soluble iron and nickel can be mobilized even from vendor-purified CNT samples.26,54 Importantly, the amount of contaminating, and presumably bioavailable, metal varies widely among commercially produced CNTs, which may confound interpretation of toxiciological studies.
In addition to iron, contaminating nickel catalysts may also have toxic and carcinogenic potential in the respiratory tract. Inhalation exposure to nickel induces acute respiratory symptoms in nickel workers, and increases respiratory tract cancers in chronically exposed workers.55,56 Nickel induces acute lung injury and tumorigenesis in rodent models, including mesotheliomas in rats when injected intrapleurally or intraperitoneally with metallic nickel powder.57,58 Carcinogenic nickel compounds, which are generally non-mutagenic, are hypothesized to transform target cells via epigenetic mechanisms.59 Altered DNA methylation within regulatory regions of genes may result in transcriptional silencing of tumor suppressor genes. Epigenetic control of gene expression can also be mediated through altered modifications of histone proteins that are responsible for the degree of DNA condensation. Deacetylation or methylation of histones can induce gene silencing by turning transcriptionally active euchromatin into transcriptionally repressive heterochromatin.60 Both water-soluble and water-insoluble nickel compounds have been shown to induce transgene silencing in the absence of mutation, but in association with increased DNA methylation, decreased histone acetylation, and increased histone methylation.61,62
Tendency to Agglomerate
The degree of fiber dispersion influences physiological responses, tissue distribution, clearance, and translocation. In this review, we use the term agglomerates to describe clusters of high-aspect ratio nanomaterials held together by non-covalent interactions.63 Agglomerates of microscale diameter conserve most of the surface properties of the constituent nanoscale materials.14 Agglomeration introduces an additional level of complexity to their behavior in the lungs. In vivo and in vitro studies correlating the degree of agglomeration with the toxicological outcome are still controversial. Several groups have shown that micron-sized CNT agglomerates are more toxic than well-dispersed nanotubes,64 and in some cases are as toxic as asbestos.65,66 It is important to note the high degree of sample heterogeneity in these studies and to avoid premature conclusions regarding the primary cause for the observed toxicity. To prevent agglomeration, functionalization via covalent and non-covalent methods is used to introduce positive or negative charges onto nanomaterial surfaces. Functionalization is also useful for the attachment of biomolecules or drugs, making carbon nanomaterials attractive as carrier systems for therapeutic agents.67 In addition to improving dispersion, the nature of the functional group and the degree of functionalization can fundamentally change the interaction of fibrous materials with target cells, which itself can lead to different physiological or toxicological outcomes.
BIOLOGICAL RESPONSES TO FIBROUS NANOMATERIALS
There is concern that exposure to fibrous carbon nanomaterials will produce pathological reactions similar to that of asbestos fibers. The most probable route of exposure to fibrous carbon nanomaterials, because of their very light weight, is inhalation.28 With a global production capacity of CNTs in excess of 300 tons per year,68 inhalation exposure may be occurring in workers providing the impetus to assess the potential pathogenicity of these materials.
Studies examining the biological reactions to fibrous carbon nanomaterials have encountered problems with routes of exposure. Rodent models of asbestos-induced disease administer fibers by either inhalation or direct intratracheal instillation.69 Delivery of fibrous carbon nanomaterials using similar techniques has been complicated by their tendency to agglomerate. Inhalation studies have been hindered by electrostatic interaction of nanomaterials which stimulates the formation of large, nonrespirable particles.66 Intratracheal instillation is widely used, but it delivers a bolus of nanomaterials that clump together, potentially limiting distribution to the distal lung parenchyma and translocation to the interstitial space.7,66,70
Acute and Chronic Reactions to Fibrous Nanomaterials
Macrophages attempt to phagocytize fibers that are not removed by mucociliary clearance. Biopersistent fibers stimulate macrophage activation characterized by production of cytokines and ROS.71 This inflammatory environment produces injury and proliferation of lung epithelial cells.71
The acute inflammatory response to fibrous carbon nanomaterials depends on surface properties, fiber length, and chemical composition of the fibers.72,73 Following intratracheal instillation or intraperitoneal injection, CNTs have been shown to produce an inflammatory response characterized by the recruitment of neutrophils and macrophages, and formation of multinucleated giant cells.66,73,74 In contrast, exposure to CNFs produces an inflammatory response consisting predominantly of macrophages.75,76 The rougher, prismatic edges and lower metal content of CNFs have been proposed to facilitate uptake by macrophages, preventing the development of frustrated phagocytosis, and eliciting a different inflammatory response than that of CNTs.7,77,78
Long, biopersistent asbestos fibers trigger persistent inflammation characterized by activated macrophages and multinucleated giant cells, leading to fibrosis of the lungs and pleura.79 Similarly, mice instilled intratracheally with CNTs develop granulomas in the bronchioles, surrounded by hyperplastic epithelial cells and containing multinucleated giant cells, macrophages, and other inflammatory cells.66 The reaction to biopersistent CNTs in rodents parallels the response to asbestos showing recruitment of macrophages, fibroblasts, lymphocytes, neutrophils, and eosinophils,70,74,80 as well as an active fibrotic response.66,80 The pulmonary response may be influenced by degree of CNT agglomeration, as well-dispersed SWCNTs evoke a more potent interstitial fibrotic reaction, in the absence of granuloma formation.81 Asbestos fibers82,83 and CNTs66,70,84 induce the formation of mature granulomas, composed of macrophages and multinucleated giant cells in a more acellular and fibrotic microenvironment. The proximity between the persistent inflammatory microenvironment within granulomas and the hyperplastic surface epithelial or mesothelial cells suggests that chronic macrophage activation could stimulate persistent cell proliferation, possibly facilitating malignant transformation.80,85
Lung Diseases as a Result of Chronic Exposure to Fibers
The most common benign pathologic response to asbestos exposure is development of pleural plaques, which appear on the parietal pleura as raised white calcified lesions or nodules with a smooth surface covered by mesothelium. Pleural plaques occur in approximately 50% of individuals with a history of heavy and prolonged exposure to asbestos, and are considered to be a clinical marker of asbestos exposure whose extent correlates with the degree of asbestos exposure.86 However, the presence of pleural plaques is not associated with an increased risk of malignancy,87 nor are plaques considered to be a precursor lesion in the development of mesothelioma,88 although pleural plaques and mesothelioma may be present simultaneously. Plaques are believed to develop as a result of collagen deposition by submesothelial fibroblasts, which are stimulated by macrophages that have phagocytosed asbestos fibers and are translocated, via the lymphatics, to the pleura.89
Asbestosis refers to the progressive fibrosis of the lung parenchyma as a result of asbestos exposure,90 involving epithelial cell hyperplasia, infiltration of inflammatory cells, and excessive deposition of collagen by fibroblasts. Individuals with asbestosis may remain asymptomatic for 10–30 years, although cigarette smoke and asbestos fibers may act additively to accelerate its progression.91 It is unclear at present whether asbestosis increases the risk for lung cancer, or whether asbestosis is a prerequisite for developing asbestos-related lung cancer or mesothelioma. It is similarly unclear whether exposure to carbon nanomaterials will induce pulmonary fibrosis in humans.
The mechanisms of asbestos carcinogenicity remain unclear, although fibrosis, chronic inflammation, production of reactive species, and interference with the mitotic spindle may each have a role.7 In contrast to asbestos-induced lung disease, graphite-containing dusts accumulate in the lungs of workers but produce minimal fibrosis and no increased risk of cancer.92 In rodent models, all carcinogenic mineral fibers induce fibrosis7; therefore, there is concern that some CNTs that induce lung fibrosis may also have the potential to induce lung cancer.
TOXICOLOGIC ASSESSMENT OF CARBON NANOMATERIALS
Given the similarities in high aspect ratio between manufactured carbon nanomaterials and asbestos fibers and their projected widespread use, considerable effort is being devoted to identify the physical and chemical parameters responsible for their potential toxicity. Although there is an increasing body of evidence concerning toxicity of fibrous nanomaterials (Table 3), results are sometimes conflicting and inconclusive. In vitro studies have reported toxicity of CNTs in a variety of relevant cell types, including macrophages, lung epithelial cell lines, and normal and malignant mesothelial cell lines,65,66,93–97 while other studies have found CNTs to be nontoxic.98–101 It has been reported that CNF and CNT, like asbestos, are capable of inducing ROS and oxidative stress,23,78 while another study indicates that highly purified MWCNT can act as radical scavengers in a cell-free system.102 The apparently conflicting results obtained from current studies is likely because of inherent sample variation among nanomaterials, including structure (SWCNTs vs. MWCNTs), dimensions, extent of purification, degree of agglomeration, and accessibility of metal catalyst residues. Nevertheless, in vivo data have demonstrated the potential for CNTs to produce acute lung injury, inflammation, and fibrosis (Table 4).
TABLE 3.
Selected Studies of In Vitro Toxicity of Synthetic Fibrous Nanomaterials
| Material | Metal content (%) | Cell type | Toxicity assay | Results | Reference |
|---|---|---|---|---|---|
| SWCNT/MWCNT | Fe, Y, Ni (trace) Ni (0.6) | Guinea pig alveolar macrophages | MTT | SWCNT were more toxic than MWCNT at an early timepoint (6hr) | (93) |
| SWCNT/MWCNT | Fe (≤0.009), Ni (<.005–1.86), Co (≤2.8), Mo (≤4.2) | Rat alveolar macrophages | MTT WST-1 LDH release PI/Annexin V | Neither raw material nor highly purified SWCNT and MWCNT were toxic after 24hr | (101) |
| MWCNT/CNF | N.D. | Human lung epithelial (H596) | MTT | MWCNT and CNF showed dose-dependent toxicity CNF>MWCNT | (94) |
| SWCNT | Ni (1.2), Co (1.3) | Human lung epithelial (A549) Rat alveolar macrophages | MTT | SWCNT were nontoxic up to 96hr | (98) |
| SWCNT | Highly purified | Human monocyte-derived macrophages | PI/nuclear morphology | Highly purified SWCNT devoid of contaminating metal were nontoxic | (99) |
| SWCNT | Fe (<0.013) | Human lung epithelial (A549) Human mesothelioma (MSTO-211H) | MTT Hoechst | Unpurified material showed greater toxicity than purified bundles SWCNT were more toxic to MSTO-211H than A549 | (95) |
| SWCNT | N.D. | Human lung epithelial (A549) | MTT Alamar Blue Coomassie Blue | SWCNT showed low acute toxicity following 24hr exposure | (100) |
| SWCNT | Fe (<0.013), Ni (2.4–13.8), Y (0.5–1.6) | Human mesothelioma (MSTO-211H) | MTT Hoechst | SWCNT nanoropes >crocidolite >dispersed SWCNT | (65) |
| MWCNT | N.D. | Murine macrophages Human macrophages Human lung epithelial (A549) | MTT | MWCNT samples were toxic to all cell lines, with EC50 (SWCNT) ≤ EC50 (chrysotile) | (97) |
| MWCNT | Fe (0.47), Co (0.95), Al (0.05) | Rat peritoneal macrophage | LDH release | Ground, but not intact MWCNT, induced dose-dependent toxicity | (66) |
N.D., not determined
TABLE 4.
In Vivo Toxicity of Fibrous Nanomaterials
| Material | Metal content (%) | Dose | Route of exposure | Results | Reference |
|---|---|---|---|---|---|
| SWCNT | i.t. (mouse) | Induction of granulomas, fibrotic lesions | (70) | ||
| SWCNT | Ni (5), Co (5) | 1 mg/kg, 5 mg/kg | i.t. (rat) | Induction of multifocal granulomas | (84) |
| SWCNT | 0.5mg | i.t. (mouse) | Induction of chronic inflammatory response, with formation of pulmonary granulomas and activation of NF-KB, AP-1 | (74) | |
| SWCNT | Fe (0.23) | Pharyngeal aspiration (mouse) | Dispersed SWCNT produced diffuse interstital fibrosis, while agglomerated SWCNT induced granulomas | (80) | |
| MWCNT | 12.5mg | i.t. (guinea pig) | Induction of granulomatous reponse | (106) | |
| MWCNT | Fe (~ 1) Co (0.95) | 0.5–5mg | i.t. (rat) | Intact material showed greater biopersistence; ground MWCNT were cleared more readily | (66) |
i.t: intratracheal
When evaluating the cytotoxic and genotoxic potential of fibrous nanomaterials in vitro, several biological and technical caveats must be taken into consideration. First, it is critical to evaluate the uptake of nanomaterials by the target cell population; nanomaterials that are poorly engulfed by cells will have limited opportunity to interact with DNA or to generate intracellular ROS, which could lead to an underestimation of fiber toxicity. For example, SWCNTs that were not readily engulfed by a macrophage cell line in vitro did not generate an intracellular oxidative burst or NO production, or induce macrophage apoptosis.80 In addition, it is important to note that in vitro assays evaluate the oxidative potential of nanomaterials themselves, and do not provide information on ROS generated indirectly by target cells involved in an inflammatory response.
A significant technical caveat in assessing the cytotoxic potential of nanomaterials lies in the use of colorimetric or fluorescence-based viability/cytotoxicity assays. Nanomaterials are highly adsorptive, and interactions have been described between nanomaterials and commonly used viability dyes, including WST-1, MTT, Neutral Red, and Alamar Blue.35,98,104,105 It is necessary to demonstrate that the nanomaterials being tested do not interfere with the viability indicator, otherwise the apparent reduction in viability may be erroneously attributed to fiber cytotoxicity and not to quenching of the fluorescent or colorimetric dyes. A recent study used a clonogenic assay as an alternative endpoint of SWCNT toxicity in human lung cell lines.106 By measuring both the number of colonies and surface area of the colonies, it was possible to determine the effects of SWCNT on cell viability and proliferation simultaneously.
MECHANISMS OF FIBER CARCINOGENICITY
The causal association between exposure to asbestos fibers and development of lung cancer and mesothelioma is well documented. The mechanisms by which asbestos fibers induce carcinogenesis are complex, involving pathways related to cell proliferation, genomic integrity, survival, and apoptosis. On the basis of studies using asbestos fibers, several hypotheses6 have been proposed regarding mechanisms of fiber carcinogenicity (Tables 5 and 6).
TABLE 5.
Direct Mechanisms of Fiber Carcinogenicity
| Mechanism | Endpoints | References |
|---|---|---|
| Genotoxic | ||
| Oxidized bases | 107,108 | |
| DNA breaks | 109 | |
| Aneuploidy | 109,110 | |
| Mutations | 111 | |
| Deletions | 112 | |
| Nongenotoxic | ||
| Mitogenic | Target cell proliferation | 113,114 |
| Binding, activation of growth factor receptors | 44,115 | |
| Growth factor expression | 116,117 | |
| Activation of signaling pathways | 85,118 | |
| Cytotoxic | Apoptosis | 114,119,120 |
| Necrosis | 121 | |
TABLE 6.
Indirect Mechanisms of Fiber Carcinogenicity
Free Radical Generation
Asbestos fibers are thought to promote carcinogenicity, in part, through iron-dependent generation of reactive metabolites, including superoxide, hydrogen peroxide, hydroxyl radical, and nitric oxide.127 Superoxide anion is formed by reduction of molecular oxygen (reaction 1), which is then dismutated via the action of superoxide dismutases to hydrogen peroxide (reaction 2). Highly reactive hydroxyl radicals may then be generated from superoxide anion and hydrogen peroxide via the iron-catalyzed Haber-Weiss reaction (reaction 3a), or via the Fenton reaction (reaction 3b).127
| (1) |
| (2) |
| (3a) |
| (3b) |
Antioxidants, including glutathione and N-acetylcysteine, and iron chelators ameliorate many but not all of the effects of asbestos fibers in vitro, suggesting a role for iron-dependent ROS generation in asbestos toxicity and carcinogenesis.128 ROS produced by asbestos can damage cellular macromolecules via lipid peroxidation and oxidative DNA damage.127,129 Alternatively, ROS may act as second messengers to alter signal transduction pathways and gene expression.71 A role for reactive nitrogen species, asbestos-induced inflammation, and pleural injury has also been suggested, including nitric oxide (NO−) and peroxynitrite (ONOO−). Asbestos-treated macrophages and mesothelial cells show increased expression and activation of inducible nitric oxide synthase, and enhanced immunoreactivity for nitrotyrosine, a marker for peroxinitrite formation, which has been observed in the lungs of rats exposed to chrysotile or crocidolite asbestos.130–132 Importantly, peroxinitrite has been shown to activate numerous components of the extracellular-regulated kinase (ERK) signaling pathway,133 whose activity is associated with proliferation of asbestos-exposed mesothelial and lung epithelial cells.
Accumulating evidence suggests that ROS generation can occur upon exposure to manufactured nanomaterials. This can occur directly, in the presence of redox-active transition metals, or indirectly, as a result of ROS production by target cells. Unpurified SWCNTs containing 30% iron have been shown to induce ROS production in human keratinocytes and bronchial epithelial cells.134–136 Exposure to asbestos fibers induces DNA damage, including oxidized bases (8-OHdG), DNA single-strand breaks (SSB) and double-strand breaks (DSB), mutations, and numerical and structural chromosomal aberrations.109,137 Preliminary evidence suggests that fibrous carbon nanomaterials may also be genotoxic. In a cell-free system, mobilization of redox-active iron from CNTs induced SSB in plasmid DNA,54 while in vitro cellular assays suggest that CNTs can induce DNA damage as assessed by COMET assay and micronucleus formation.138,139 Embryonic stem cells exposed to MWCNTs also upregulate DNA damage response proteins.140 Mice deficient in the p53 tumor suppressor gene, a critical regulator of DNA damage responses, have been used as an in vivo model to investigate the role of genotoxicity in fiber carcinogenesis. The p53 deficiency increases susceptibility to asbestos-induced mesotheliomas, and the majority of mesotheliomas that form in p53 heterozygous mice exhibit loss of the remaining wild-type allele.141 Recent work suggests that p53 heterozygous mice are a sensitive model for assessing the carcinogenic properties of fibrous carbon nanomaterials, as peritoneal tumors form following injection of MWCNTs.142
It has been hypothesized that polymorphisms in genes related to xenobiotic metabolism, oxidative stress, or DNA repair could also influence susceptibility to asbestos-related diseases.143 Although no susceptibility gene has been conclusively identified, a recent study of genetic polymorphisms in DNA repair genes among individuals exposed to asbestos indicated an association between polymorphisms in the X-ray repair cross-complementing group 1 (XRCC1) gene and malignant mesothelioma.143 Although no human ‘nanodiseases’ have yet been identified, it is important to consider the possibility that genetic modifiers may contribute to fiber-induced diseases.
Physical Interference with Mitosis
It is hypothesized that long, rigid, biopersistent fibers, may induce carcinogenicity through physical interaction with the mitotic spindle, leading to chromosomal breakage or loss, or improper chromosomal segregation during mitosis. This may contribute to genetic instability, in the form of micronuclei and chromosomal imbalances, or aneuploidy, in daughter cells. Asbestos fibers are known to have aneuploidogenic effects in mesothelial cells and lung epithelial cells in vitro.144
Physical interference with mitosis requires interaction between fibers and mitotic chromosomes or penetration of the nucleus during interphase. While some in vitro studies have shown that asbestos fibers interact with the nucleus or chromosomes,145 other studies have shown that intracellular asbestos fibers can be surrounded by a phagolysosomal membrane, which could sequester the fibers in the cytoplasm.146 It is possible that longer fibers, which cannot be completely engulfed by cellular membranes, are more likely to pierce the nucleus and interact with chromosomes. Internalization of long fibers has been associated with lagging mitotic chromosomes,147 and exposure to chryostile or crocidolite asbestos fibers in vitro increases the percentage of binucleated cells in rat pleural mesothelial cells,148 and in primary and immortalized human mesothelial cells.149 Together with in vivo studies showing formation of micronuclei following asbestos exposure,141 these results suggest that asbestos may interfere with both chromosome segregation and cytokinesis. Although limited data are available on the ability of carbon nanomaterials to interfere with mitosis, two recent studies have suggested that carbon nanomaterials may have the potential to induce aneuploidy. Exposure to SWCNTs containing 0.23% iron induced a slight increase in micronucleus formation in Chinese hamster lung fibroblasts at the highest dose tested,138 although this study did not distinguish between clastogenic and aneugenic micronuclei. Ground, purified MWCNTs containing less than 2% iron produced a dose-dependent increase in micronuclei in rat type II pneumocytes as well as in clastogenic and aneugenic micronuclei in a human lung epithelial cell line.139
Stimulation of Target Cell Proliferation
In order for fiber-induced mutations or chromosomal aberrations to be transmitted to daughter cells, exposed cells must be stimulated to proliferate. Exposure to asbestos fibers in vivo induces proliferation of mesothelial cells and bronchiolar epithelial cells.7 Mesothelial cell proliferation is associated with fiber biopersistence, as crocidolite asbestos induces a more extended proliferative response than wollastonite, which is susceptible to dissolution and clearance in vivo.150 Biopersistent asbestos fibers may induce chronic cellular proliferation directly through upregulated expression of growth factors and activation of growth factor receptors. Asbestos fibers have been shown to bind and activate the epidermal growth factor receptor, and downstream mitogen-activated protein kinases (MAPKs): ERK1 and ERK2.151 ERK1/2 activity leads to expression of c-fos and c-jun,151–153 which are components of the redox-sensitive activator-protein 1 (AP-1) transcription factor. As transcription of AP-1 target genes influences cell proliferation, survival, and apoptosis, persistent activation of MAPKs and AP-1 may drive proliferation and survival of asbestos-exposed cells.153 Recent in vivo studies suggest a critical role for the ERK signaling pathway in asbestos-induced cell proliferation.154 Alternatively, asbestos may induce target cell proliferation indirectly. Release of cytokines and growth factors, including tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-α, potent mitogens for mesothelial and lung epithelial cells, may stimulate proliferation of nearby mesothelial and epithelial cells.155 In addition, asbestos-induced cellular injury can induce compensatory proliferation, as has been shown for mesothelial cells in vivo.82 It has recently been shown that carbon nanomaterials, like asbestos fibers, may activate some of the same stress-response pathways; for example, SWCNT-induced oxidative stress is associated with NF-κB activation via MAPK signaling in human keratinocytes as well as in normal and malignant mesothelial cells.96,135 In human fibroblasts, exposure to MWCNTs induces transcriptional activation of numerous genes involved in the p38/MAPK stress-signaling cascade.156
Persistent Chronic Inflammation
Persistent release of ROS and inflammatory mediators can contribute to establishment of a chronic inflammatory environment, which has been linked to carcinogenesis.157 Activation of macrophages is accompanied by production and release of ROS, cytokines, and inflammatory mediators.158 Cell proliferation in the context of chronic inflammation may result in accumulation of DNA damage and mutations that promote carcinogenesis. Recent work has suggested a direct link between asbestos-induced inflammation and mesothelial cell transformation. Asbestos-induced TNF-α release is associated with upregulation of TNF-α receptor on mesothelial cells, as well as NF-κB activation, suggesting that activation of pro-survival pathways by inflammatory cytokines may prolong the exposure of mesothelial cells to the genotoxic effects of asbestos fibers.126 Chronic inflammation accompanied by oxidant generation has also been associated with epigenetic gene silencing by hypermethylation.159
Exposure to SWCNT in rodent models produces pulmonary inflammation and fibrosis. Fibrous nanomaterials can induce release of TNF-α and from human peripheral blood mononuclear cells, as well as frustrated phagocytosis and impaired macrophage function, although there is considerable variability among commercial CNT and CNF samples.78 The importance of oxidative stress in physiological responses to fibrous nanomaterials is highlighted by recent work showing that mice fed diets deficient in Vitamin E, a potent antioxidant, showed an enhanced inflammatory and fibrotic response to SWCNTs.160 These responses were accompanied by increased recruitment of inflammatory cells and enhanced production of pro-inflammatory (TNF-α and interleukin-6) and profibrotic (TGF -β) cytokines.160
REMEDIATION OF ASBESTOS FIBERS/GREEN NANOTECHNOLOGY
Although its use has been banned or restricted in many countries, asbestos remains a human health hazard throughout the world. Despite the established link between asbestos exposure and human disease, asbestos use continues in developing countries. Even in countries where asbestos use has been discontinued, fibers are still present in homes and schools in the form of insulation, ceiling, and roofing tiles or in the environment as naturally-occurring deposits. Liberation of asbestos fibers during demolition of buildings or disturbance of previously unidentified asbestos deposits could lead to new exposure scenarios. As iron mobilization from asbestos fibers is associated with increased free radical generation and DNA damage,39,128 selective removal of iron has been proposed as a method of asbestos remediation. Exciting recent work has shown that soil fungi161 and lichens162 are capable of detoxifying crocidolite and chyrostile asbestos, respectively, through chelation and removal of redox-active iron.
Unlike naturally-occurring asbestos fibers, fibrous carbon nanomaterials are manufactured and therefore more amenable to modification before use. Removal of redox-active metals through purification may reduce fiber toxicity prior to their use in various downstream applications. However, residual metal catalysts may persist because of incomplete purification or redeposition of mobilized metal onto the surface of the nanotube.27 Acid-based purification may inadvertently result in increased metal bioavailability by damaging the protective carbon shells surrounding the catalysts in cases where the subsequent acid dissolution of the uncovered metal is incomplete. Evidence for all three mechanisms is provided by a recent study of commercial SWCNT, which addresses the source of bioavailable metal in purified SWCNTs and proposes metal removal as a potential detoxification strategy.27 Additionally, tocopheryl polyethylene glycol succinate, a synthetic vitamin E analogue linked to polyethylene glycol, when used in carbon nanomaterial processing, has the potential for simultaneously acting as a surfactant to promote nanomaterial dispersion, and as an antioxidant, to ameliorate oxidative stress.163
SUMMARY
The epidemic of asbestos-related diseases has been an unfortunate consequence of wide-scale use of fibers for industrial and consumer applications in the absence of the recognition and acceptance of their toxicologic and carcinogenic properties. Although an asbestos ban may go a long way toward preventing occupationally-related diseases, it cannot completely eliminate environmental exposures and continuing exposure to asbestos-in-place. Enormous effort has been devoted to identifying the properties of asbestos that determine its bioactivity. Currently, the toxicologic and carcinogenic properties of high aspect ratio nanoparticles await complete investigation, and it is unclear whether they will be equally, more, or less hazardous than asbestos fibers. Given the relative novelty of manufactured carbon nanomaterials, we are now presented with a unique opportunity to evaluate nanomaterial bioactivity prior to wide-scale use, and the inevitable scenarios of human exposure. The goal of nanotoxicology should not be to restrict production and use of nanomaterials, as has been done with asbestos, but to recognize that their use in industrial and consumer applications must be selective and balanced against their potentially harmful inherent properties. On the basis of the current understanding of asbestos pathogenicity, we can use this knowledge as a framework to guide the design and post-processing of carbon nanomaterials in a pre-emptive attempt to reduce the potential for exposure-related ‘nanodiseases’.
Acknowledgments
Financial support was provided by the NIEHS Superfund Basic Research Program P42 ES013660, EPA STAR Grant RD-83171901-0, NIEHS R01 ES016178 and RO1 ES03721 grants, and NSF Nanoscale Interdisciplinary Research Team (NIRT) grant DMI0506661. Although this research was funded in part by the EPA and NIEHS, it does not necessarily reflect the views of either agency. The technical contributions of Xinyuan Liu and Aihui Yan in nanotube purification strategies are gratefully acknowledged.
References
- 1.Kroto H, Heath J, O’Brien S, Curl R, Smalley R. C60:Buckminsterfullerene. Nature. 1985;318(14):162–163. [Google Scholar]
- 2.Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36(3):189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- 3.Murr L, Soto K. TEM comparison of chrysotile (asbestos) nanotubes and carbon nanotubes. J Mater Sci. 2004;39:4941–4947. [Google Scholar]
- 4.Stern S, Mcneil S. Nanotechnology safety concerns revisited. Toxicol Sci. 2008;101(1):4–21. doi: 10.1093/toxsci/kfm169. [DOI] [PubMed] [Google Scholar]
- 5.Mossman BT, Borm PJ, Castranova V, Costa DL, Donaldson K, et al. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part Fibre Toxicol. 2007;4:4. doi: 10.1186/1743-8977-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asbestos: Selected Cancers. Washington, DC: The National Academies Press; 2006. Biological Aspects of Asbestos-Related Diseases; pp. 81–103. [PubMed] [Google Scholar]
- 7.Bernstein D, Castranova V, Donaldson K, Fubini B, Hadley J, et al. Testing of fibrous particles: short-term assays and strategies. Inhal Toxicol. 2005;17(10):497–537. doi: 10.1080/08958370591001121. [DOI] [PubMed] [Google Scholar]
- 8.Maxim LD, McConnell EE. A review of the toxicology and epidemiology of wollastonite. Inhal Toxicol. 2005;17(9):451–466. doi: 10.1080/08958370591002030. [DOI] [PubMed] [Google Scholar]
- 9.De Jong K, Geus J. Carbon nanofibers: Catalytic synthesis and applications. Catal Rev Sci Eng. 2000;42(4):481–510. [Google Scholar]
- 10.Martin CR, Kohli P. The emerging field of nanotube biotechnology. Nat Rev Drug Discov. 2003;2(1):29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- 11.Smart S, Cassady A, Lu G, Martin D. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047. [Google Scholar]
- 12.Tagmatarchis N, Prato M. Functionalization of carbon nanotubes via 1,3-dipolar cycoadditions. J Mater Chem. 2004;14:437–439. [Google Scholar]
- 13.Warheit D. What is currently known about the health risks related to carbon nanotube exposures? Carbon. 2006;44(6):1064–1069. [Google Scholar]
- 14.Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Christensen K, Ochoa-Fernandez E, Yu Z, Totdal B, et al. Synthesis of carbon nanofibers: effects of Ni crystal size during methane decomposition. J Catal. 2005;229:82–96. [Google Scholar]
- 16.Bandaru P, Daraio C, Yang KR, Rao A. A plausible mechanism for the evolution of helical forms in nanostructure growth. J Appl Phys. 2007;101:943071–9430714. [Google Scholar]
- 17.Vera-Agullo J, Varela-Rioz H, Conesa J, Almonsa C, Merino C, et al. Evidence for growth mechanism and helix-spiral cone structure of stacked-cup carbon nanofibers. Carbon. 2007;45:2751–2758. [Google Scholar]
- 18.Jian K, Yan A, Kulaots I, Crawford G, Hurt R. Reconstruction and hydrophobicity of nanocarbon surfaces composed solely of graphene edges. Carbon. 2006;44:2105–2109. [Google Scholar]
- 19.Zheng R, Zhao Y, Liu H, Liang C, Cheng G. Preparation, characterization and growth mechanism of platelet fibers. Carbon. 2006;44:742–746. [Google Scholar]
- 20.Martin-Gullon I, Vera J, Conesa J, Gonzalez J, Merino C. Differences between carbon nanofibers produced using Fe and Ni ctalysts in a floating catalysts reactor. Carbon. 2006;44:1572–1580. [Google Scholar]
- 21.Harris P. Carbon Nanotubes and Related Structures. Cambridge: Press Syndicate of the University of Cambridge; 1999. [Google Scholar]
- 22.Rodriguez N. A review of catalytically grown carbon nanofibers. J Mater Res. 1993;8(12):3233–3250. [Google Scholar]
- 23.Donaldson K, Aitken R, Tran L, Stone V, Duffin R, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 24.Ajayan PM, Ebbesen TW, Ichihashi T, Iijima S, Tanigaki K, et al. Opening carbon nanotubes with oxygen and implications for filling. Nature. 1993;362(6420):522–525. [Google Scholar]
- 25.Tsang SC, Chen YK, Harris PJF, Green MLH. A simple chemical method of opening and filling carbon nanotubes. Nature. 1994;372(6502):159–162. [Google Scholar]
- 26.Liu X, Gurel V, Morris D, Murray D, Zhitkovich A, et al. Bioavailability of nickel in single-wall carbon nanotubes. Adv Mater. 2007;19(19):2790–2796. [Google Scholar]
- 27.Liu X, Guo L, Morris D, Kane A, Hurt R. Targeted removal of bioavailable metal as a detoxification strategy for carbon nanotubes. Carbon. 2008;46:489–500. doi: 10.1016/j.carbon.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moolgavkar SH, Brown RC, Turim J. Biopersistence, fiber length, and cancer risk assessment for inhaled fibers. Inhal Toxicol. 2001;13(9):755–772. doi: 10.1080/089583701316941294. [DOI] [PubMed] [Google Scholar]
- 30.IARC W. Man-Made Vitreous Fibres. Lyon: IARC; 2002. IARC Monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 31.Kogan FM, Nikitina OV. Solubility of chrysotile asbestos and basalt fibers in relation to their fibrogenic and carcinogenic action. Environ Health Perspect. 1994;102(suppl 5):205–206. doi: 10.1289/ehp.94102s5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdett G, Bard D. An Inventory of Fibres to Classify their Potential Hazard and Risk. Sudbury: Health and Safety Executive; 2006. pp. 1–116. [Google Scholar]
- 33.Thelohan S, de Meringo A. In vitro dynamic solubility test: influence of various parameters. Environ Health Perspect. 1994;102(suppl 5):91–96. doi: 10.1289/ehp.102-1567254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki Y, Yuen SR. Asbestos tissue burden study on human malignant mesothelioma. Ind Health. 2001;39(2):150–160. doi: 10.2486/indhealth.39.150. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro-Riviere M, Inman A. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon. 2006;44:1070–1078. [Google Scholar]
- 36.Guo L, von dem Bussche A, Buechner M, Kane A, Hurt R. Adsorption of essential micronutrients by carbon nanotubes and its implications for nanotoxicity testing. Small. 2008;4(6):721–727. doi: 10.1002/smll.200700754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fubini B. Surface reactivity in the pathogenic response to particulates. Environ Health Perspect. 1997;105(suppl 5):1013–1020. doi: 10.1289/ehp.97105s51013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fubini B, Barcelo F, Otero Arean C. Ferritin adsorption on amosite fibers: possible implications in the formation and toxicity of asbestos bodies. J Toxicol Environ Health. 1997;52(4):343–352. doi: 10.1080/00984109708984069. [DOI] [PubMed] [Google Scholar]
- 39.Hardy J, Aust A. Iron in asbestos chemistry and carcinogenicity. Chem Rev. 1995;95:97–118. [Google Scholar]
- 40.Eborn SK, Aust AE. Effect of iron acquisition on induction of DNA single-strand breaks by erionite, a carcinogenic mineral fiber. Arch Biochem Biophys. 1995;316(1):507–514. doi: 10.1006/abbi.1995.1067. [DOI] [PubMed] [Google Scholar]
- 41.Otero Arean C, Barcelo F, Fubini B. Free radical activity of mineral fibres containing adsorbed ferritin: Detection using supercoiled DNA. Res Chem Intermed. 1999;25(2):177–185. [Google Scholar]
- 42.Donaldson K, Hill IM, Beswick PH. Superoxide anion release by alveolar macrophages exposed to respirable industrial fibres: modifying effect of fibre opsonisation. Exp Toxicol Pathol. 1995;47(4):229–231. doi: 10.1016/S0940-2993(11)80253-8. [DOI] [PubMed] [Google Scholar]
- 43.Scheule RK, Holian A. IgG specifically enhances chrysotile asbestos-stimulated superoxide anion production by the alveolar macrophage. Am J Respir Cell Mol Biol. 1989;1(4):313–318. doi: 10.1165/ajrcmb/1.4.313. [DOI] [PubMed] [Google Scholar]
- 44.Boylan AM, Sanan DA, Sheppard D, Broaddus VC. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J Clin Invest. 1995;96(4):1987–2001. doi: 10.1172/JCI118246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Liu W, Koenig K, Idell S, Broaddus VC. Vitronectin adsorption to chrysotile asbestos increases fiber phagocytosis and toxicity for mesothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L916–L923. doi: 10.1152/ajplung.2000.279.5.L916. [DOI] [PubMed] [Google Scholar]
- 46.Ghio AJ, Stonehuerner J, Richards J, Devlin RB. Iron homeostasis in the lung following asbestos exposure. Antioxid Redox Signal. 2008;10(2):371–377. doi: 10.1089/ars.2007.1909. [DOI] [PubMed] [Google Scholar]
- 47.Garnett M, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occup Med. 2006;56:307–311. doi: 10.1093/occmed/kql052. [DOI] [PubMed] [Google Scholar]
- 48.Aust AE, Ball JC, Hu AA, Lighty JS, Smith KR, et al. Particle characteristics responsible for effects on human lung epithelial cells. Res Rep Health Eff Inst. 2002;110:1–65. discussion 67–76. [PubMed] [Google Scholar]
- 49.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533(1–2):153–171. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Bhasin G, Kauser H, Athar M. Iron augments stage-I and stage-II tumor promotion in murine skin. Cancer Lett. 2002;183(2):113–122. doi: 10.1016/s0304-3835(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 51.Nishiyama Y, Suwa H, Okamoto K, Fukumoto M, Hiai H, et al. Low incidence of point mutations in H-, K- and N-ras oncogenes and p53 tumor suppressor gene in renal cell carcinoma and peritoneal mesothelioma of Wistar rats induced by ferric nitrilotriacetate. Jpn J Cancer Res. 1995;86(12):1150–1158. doi: 10.1111/j.1349-7006.1995.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dogan A. Malignant mesothelioma and erionite. In: Pass H, Vogelzang N, Carbone M, editors. Malignant Mesothelioma. New York: Springer; 2005. pp. 242–258. [Google Scholar]
- 53.Baris YI, Saracci R, Simonato L, Skidmore JW, Artvinli M. Malignant mesothelioma and radiological chest abnormalities in two villages in Central Turkey. An epidemiological and environmental investigation. Lancet. 1981;1(8227):984–987. doi: 10.1016/s0140-6736(81)91742-6. [DOI] [PubMed] [Google Scholar]
- 54.Guo L, Morris D, Liu X, Vaslet C, Hurt R, et al. Iron bioavailability and redox activity in diverse carbon nanotube samples. Chem Mater. 2007;19:3472–3478. [Google Scholar]
- 55.Doll R, Morgan L, Speizer F. Cancers of the lung and nasal sinuses in nickel workers. Br J Cancer. 1970;24(4):623–632. doi: 10.1038/bjc.1970.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IARC W. Chromium, Nickel and Welding. Lyon: IARC; 1990. IARC monographs on the evaluation of carcinogenic risks to humans; pp. 257–446. [PMC free article] [PubMed] [Google Scholar]
- 57.Furst A, Cassetta D, Sasmore D. Rapid induction of pleural mesotheliomas in the rat. Proc West Pharmacol Soc. 1973;16:150–153. [Google Scholar]
- 58.Pott F, Ziem U, Reiffer FJ, Huth F, Ernst H, et al. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp Pathol. 1987;32(3):129–152. doi: 10.1016/s0232-1513(87)80044-0. [DOI] [PubMed] [Google Scholar]
- 59.Lee YW, Klein CB, Kargacin B, Salnikow K, Kitahara J, et al. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Mol Cell Biol. 1995;15(5):2547–2557. doi: 10.1128/mcb.15.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gronbaek K, Hother C, Jones PA. Epigenetic changes in cancer. APMIS. 2007;115(10):1039–1059. doi: 10.1111/j.1600-0463.2007.apm_636.xml.x. [DOI] [PubMed] [Google Scholar]
- 61.Klein CB, Costa M. DNA methylation, heterochromatin and epigenetic carcinogens. Mutat Res. 1997;386(2):163–180. doi: 10.1016/s1383-5742(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 62.Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol Cell Biol. 2006;26(10):3728–3737. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oberdorster G, Oberdorster E, Oberdorster J. Concepts of nanoparticle dose metric and response metric. Environ Health Perspect. 2007;115(6):A290. doi: 10.1289/ehp.115-1892118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sayes C, Liang F, Hudson J, Mendez J, Guo W, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168(2):121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Muller J, Huaux F, Moreau N, Misson P, Heilier JF, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y, Taylor S, Li H, Fernando K, Qu L, et al. Advances toward bioapplications of carbon nanotubes. J Mater Chem. 2004;14:527–541. [Google Scholar]
- 68.Eklund P, Ajayan P, Blackmon R, Hart A, Kong J, et al. In: International Assessment of Research and Development of Carbon Nanotube Manufacturing and Applications. Eklund P, Ajayan P, Blackmon R, Hart A, Kong J, et al., editors. Baltimore: World Technology Evaluation Center, Inc; 2007. pp. 1–138. [Google Scholar]
- 69.Kane A. In: Animal Models of Malignant Mesothelioma. Rom W, editor. Philadelphia, PA: Lippincot-Raven Publishers; 1998. pp. 377–386. [Google Scholar]
- 70.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77(1):126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 71.Shukla A, Gulumian M, Hei T, Kamp D, Rahman Q. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med. 2003;34(9):1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 72.Dobrovolskaia M, Mcneil S. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 73.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 74.Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, et al. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8(2):437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 75.Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, et al. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol Sci. 2007;100(1):203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- 76.Lison D, Muller J. Lung and systemic responses to carbon nanotubes (CNT) in mice. Toxicol Sci. 2008;101(1):179–180. doi: 10.1093/toxsci/kfm249. (Author reply 181–172) [DOI] [PubMed] [Google Scholar]
- 77.Grabinski C, Hussain S, Lafdi K, Braydich L, Schlager J. Effect of particle dimension on biocompatibility of carbon nanomaterials. Carbon. 2007;45(14):2828–2835. [Google Scholar]
- 78.Brown D, Kinloch I, Bangert U, Windle A, Walter D, et al. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon. 2007;45:1743–1756. [Google Scholar]
- 79.Roggli V, Oury T, Sporn T. Pathology of Asbestos-Associated Diseases. New York: Springer; 2004. [Google Scholar]
- 80.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 81.Mercer R, Scabillon J, Wang L, Kisin K, Murray A, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 82.Moalli P, MacDonald J, Goodglick L, Kane A. Acute injury and regeneration of the mesothelium in response to asbestos fibers. Am J Pathol. 1987;128(3):426–445. [PMC free article] [PubMed] [Google Scholar]
- 83.Kane AB, Macdonald J. Mechanisms of mesothelial cell injury, proliferation, and neoplasia induced by asbestos fibers. In: Warheit D, editor. Fiber Toxicology. San Diego, CA: Academic Press; 1993. [Google Scholar]
- 84.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77(1):117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 85.Manning CB, Vallyathan V, Mossman BT. Diseases caused by asbestos: mechanisms of injury and disease development. Int Immunopharmacol. 2002;2(2–3):191–200. doi: 10.1016/s1567-5769(01)00172-2. [DOI] [PubMed] [Google Scholar]
- 86.Gibbs A. Determination of asbestos exposure by pathology and clinical history. In: Pass HI, Vogelzang NJ, Carbone MC, editors. Malignant Mesothelioma. New York: Springer Science+Business Media, INC; 2005. pp. 259–266. [Google Scholar]
- 87.Weiss W. Asbestos-related pleural plaques and lung cancer. Chest. 1993;103(6):1854–1859. doi: 10.1378/chest.103.6.1854. [DOI] [PubMed] [Google Scholar]
- 88.Greenberg S. Benign asbestos-related pleural disease. In: Roggli VL, Greenberg S, Pratt PC, editors. Pathology of Asbestos-Associated Diseases. Boston, MA: Little, Brown; 1992. pp. 165–187. [Google Scholar]
- 89.Flores R. Management of benign variants of mesothelioma. In: Pass HI, Vogelzang NJ, Carbone MC, editors. Malignant Mesothelioma. New York: Springer Science+Business Media, INC; 2005. pp. 581–592. [Google Scholar]
- 90.Kamp D, Weitzman S. Asbestosis: clinical spectrum and pathogenic mechnisms. Proc Soc Exp Biol Med. 1997;214(1):12–26. doi: 10.3181/00379727-214-44065. [DOI] [PubMed] [Google Scholar]
- 91.Weiss W. Cigarette smoke, asbestos, and small irregular opacities. Am Rev Respir Dis. 1984;130(2):293–301. doi: 10.1164/arrd.1984.130.2.293. [DOI] [PubMed] [Google Scholar]
- 92.Green F, Vallyathan V. Coal workers’ pneumoconiosis and pneumoconiosis due to other carbonaceous dusts. In: Mitchell C, editor. Pathology of Occupational Lung Disease. 2. Baltimore: Williams & Wilkins; 1998. pp. 129–197. [Google Scholar]
- 93.Jia G, Wang H, Yan L, Wang X, Pei R, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39(5):1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 94.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6(6):1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 95.Kaiser J, Wick P, Manswer P, Spohn P, Bruinink A. Single walled carbon nanotubes (SWCNT) affect cell physiology and cell architecture. J Mater Sci Mater Med. 2008;19:1523–1527. doi: 10.1007/s10856-007-3296-y. [DOI] [PubMed] [Google Scholar]
- 96.Pacurari M, Yin X, Zhao J, Ding M, Leonard S, et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;11(9):1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soto K, Garza KM, Murr LE. Cytotoxic effects of aggregated nanomaterials. Acta Biomater. 2007;3(3):351–358. doi: 10.1016/j.actbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6(6):1261–1268. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 99.Fiorito S, Serafino A, Andreola F, Bernier P. Effects of fullerences and single-wall carbon nanotubes on murine and human macrophages. Carbon. 2006;44:1100–1105. [Google Scholar]
- 100.Davoren M, Herzog E, Casey A, Cottineau B, Chambers G, et al. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol In Vitro. 2007;21(3):438–448. doi: 10.1016/j.tiv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Fenoglio I, Tomatis M, Lison D, Muller J, Fonseca A, et al. Reactivity of carbon nanotubes: free radical generation or scavenging activity? Free Radic Biol Med. 2006;40(7):1227–1233. doi: 10.1016/j.freeradbiomed.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 103.Grubek-Jaworska H, Nejman P, Czuminska K, Przybylowski T, Huczko A, et al. Preliminary results on the pathogenic effects of intratracheal exposure to one-dimensional nanocarbons. Carbon. 2006;44:1057–1063. [Google Scholar]
- 104.Casey A, Herzog E, Davoren M, Lyng FM, Byrne HJ, et al. Spectroscopic analysis confirms the interactions between single walled carbon nanotubes and various dyes commonly used to assess cytotoxicity. Carbon. 2007;45(7):1425–1432. [Google Scholar]
- 105.Hurt R, Monthioux M, Kane A. Toxicology of carbon nanomaterials: status, trends, and perspectives on the special issue. Carbon. 2006;44:1028–1033. [Google Scholar]
- 106.Herzog E, Casey A, Lyng F, Chambers G, Byrne H, et al. A new approach to the toxicity testing of carbon-based nanomaterials-The clonogneic assay. Toxicol Lett. 2007;174:49–60. doi: 10.1016/j.toxlet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Chao CC, Park SH, Aust AE. Participation of nitric oxide and iron in the oxidation of DNA in asbestos-treated human lung epithelial cells. Arch Biochem Biophys. 1996;326(1):152–157. doi: 10.1006/abbi.1996.0059. [DOI] [PubMed] [Google Scholar]
- 108.Fung H, Kow Y, Van Houten B, Mossman B. Patterns of 8-hydroxydeoxyguanosine formation in DNA and indications of oxidative stress in rat and human pleurlal mesothelial cells after exposure to crocidolite asbestos. Carcinogenesis. 1997;18(4):825–832. doi: 10.1093/carcin/18.4.825. [DOI] [PubMed] [Google Scholar]
- 109.Jaurand M. Use of in-vitro genotoxicity and cell transformation assays to evaluate the potential carcinogenicity of fibres. IARC Sci Publ. 1996;140:55–72. [PubMed] [Google Scholar]
- 110.Jensen CG, Jensen LC, Rieder CL, Cole RW, Ault JG. Long crocidolite asbestos fibers cause polyploidy by sterically blocking cytokinesis. Carcinogenesis. 1996;17(9):2013–2021. doi: 10.1093/carcin/17.9.2013. [DOI] [PubMed] [Google Scholar]
- 111.Park SH, Aust AE. Participation of iron and nitric oxide in the mutagenicity of asbestos in hgprt−, gpt+ Chinese hamster V79 cells. Cancer Res. 1998;58(6):1144–1148. [PubMed] [Google Scholar]
- 112.Hei T, Xu A, Louie D, Zhou Y. Genotoxicity verses carcinogenicity: Implications from fiber toxicity studies. Inhal Toxicol. 2000;12(suppl 3):141–147. doi: 10.1080/08958378.2000.11463207. [DOI] [PubMed] [Google Scholar]
- 113.BeruBe K, Quinlan T, Fung H, Magae J, Vacek P. Apoptosis is observed in mesothelial cells after exposure to crocidolite. asbestos Am J Respir Cell Mol Biol. 1996;15(1):141–147. doi: 10.1165/ajrcmb.15.1.8679218. [DOI] [PubMed] [Google Scholar]
- 114.Goldberg JL, Zanella CL, Janssen YM, Timblin CR, Jimenez LA, et al. Novel cell imaging techniques show induction of apoptosis and proliferation in mesothelial cells by asbestos. Am J Respir Cell Mol Biol. 1997;17(3):265–271. doi: 10.1165/ajrcmb.17.3.2991. [DOI] [PubMed] [Google Scholar]
- 115.Pache JC, Janssen YM, Walsh ES, Quinlan TR, Zanella CL, et al. Increased epidermal growth factor-receptor protein in a human mesothelial cell line in response to long asbestos fibers. Am J Pathol. 1998;152(2):333–340. [PMC free article] [PubMed] [Google Scholar]
- 116.Liu JY, Morris GF, Lei WH, Corti M, Brody AR. Up-regulated expression of transforming growth factor-alpha in the bronchiolar-alveolar duct regions of asbestos-exposed rats. Am J Pathol. 1996;149(1):205–217. [PMC free article] [PubMed] [Google Scholar]
- 117.Brody AR, Liu JY, Brass D, Corti M. Analyzing the genes and peptide growth factors expressed in lung cells in vivo consequent to asbestos exposure and in vitro. Environ Health Perspect. 1997;105(suppl 5):1165–1171. doi: 10.1289/ehp.97105s51165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mossman BT, Faux S, Janssen Y, Jimenez LA, Timblin C, et al. Cell signaling pathways elicited by asbestos. Environ Health Perspect. 1997;105(suppl 5):1121–1125. doi: 10.1289/ehp.97105s51121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Broaddus V, Yang L, Scavo L, Ernst J, Boylan A. Asbestos induces apoptosis of human and rabbit pleural mesothelial cells via reactive oxygen species. J Clin Invest. 1996;98(9):2050–2059. doi: 10.1172/JCI119010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levresse V, Renier A, Fleury-Feith J, Levy F, Moritz S, et al. Analysis of cell cycle disruptions in cultures of rat pleural mesothelial cells exposed to asbestos fibers. Am J Respir Cell Mol Biol. 1997;17(6):660–671. doi: 10.1165/ajrcmb.17.6.2854. [DOI] [PubMed] [Google Scholar]
- 121.Kane A. Mechanisms of mineral fibre carcinogenesis. In: Kane AB, Boffetta P, Saracci R, Wilbourn JD, editors. Mechanisms of Mineral Fibre Carcinogenesis. Lyon: IARC Scientific Publications; 1996. pp. 11–35. [PubMed] [Google Scholar]
- 122.Lee BW, Wain JC, Kelsey KT, Wiencke JK, Christiani DC. Association between diet and lung cancer location. Am J Respir Crit Care Med. 1998;158(4):1197–1203. doi: 10.1164/ajrccm.158.4.9804089. [DOI] [PubMed] [Google Scholar]
- 123.Nelson HH, Kelsey KT. The molecular epidemiology of asbestos and tobacco in lung cancer. Oncogene. 2002;21(48):7284–7288. doi: 10.1038/sj.onc.1205804. [DOI] [PubMed] [Google Scholar]
- 124.Esteller M. Dormant hypermethylated tumour suppressor genes: questions and answers. J Pathol. 2005;205(2):172–180. doi: 10.1002/path.1707. [DOI] [PubMed] [Google Scholar]
- 125.Vallyathan V, Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ Health Perspect. 1997;105(suppl 1):165–177. doi: 10.1289/ehp.97105s1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci USA. 2006;103(27):10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamp D, Weitzman S. The molecular basis of asbestos induced lung injury. Thorax. 1999;54:638–652. doi: 10.1136/thx.54.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fubini B, Mollo L. Role of iron in the reactivity of mineral fibers. Toxicol Lett. 1995;82–83:951–960. doi: 10.1016/0378-4274(95)03531-1. [DOI] [PubMed] [Google Scholar]
- 129.Mossman B, Marsh J. Evidence supporting a role for active oxygen species in asbestos-induced toxicity and lung disease. Environ Health Perspect. 1989;81:91–94. doi: 10.1289/ehp.898191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choe N, Tanaka S, Kagan E. Asbestos fibers and interleukin-1 upregulate the formation of reactive nitrogen species in rat pleural mesothelial cells. Am J Respir Cell Mol Biol. 1998;19(2):226–236. doi: 10.1165/ajrcmb.19.2.3111. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka S, Choe N, Hemenway D, Zhu S, Matalon S, et al. Asbestos inhalation induces reactive nitrogen species and nitrotyrosine formation in the lungs and pleura of the rat. J Clin Invest. 1998;102(2):445–454. doi: 10.1172/JCI3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Quinlan T, BeruBe K, Hacker M, Taatjes D, Timblin C. Mechanisms of asbestos-induced nitric oxide production by rat alveolar macrophages in inhalation and in vitro models. Free Radic Biol Med. 1998;24(5):778–788. doi: 10.1016/s0891-5849(97)00357-2. [DOI] [PubMed] [Google Scholar]
- 133.Zhang P, Wang YZ, Kagan E, Bonner JC. Peroxynitrite targets the epidermal growth factor receptor, Raf-1, and MEK independently to activate MAPK. J Biol Chem. 2000;275(29):22479–22486. doi: 10.1074/jbc.M910425199. [DOI] [PubMed] [Google Scholar]
- 134.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 135.Manna S, Sarkar S, Barr J, Wise K, Barrera O, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor- K B in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shvedova A, Kisin E, Murray A, Schwegler-Berry D, Gandelsman V, et al. Exposure of human bronchial cells to carbon nanotubes caused oxidative stress and cytotoxicity. Proceedings of the Society for Free Radical Research Meeting, European Section; Ioannina. June 26–29, 2003; pp. 91–103. [Google Scholar]
- 137.Okayasu R, Takahashi S, Yamada S, Hei T, Ullrich R. Asbestos and DNA double strand breaks. Cancer Res. 1999;59:298–300. [PubMed] [Google Scholar]
- 138.Kisin ER, Murray AR, Keane MJ, Shi XC, Schwegler-Berry D, et al. Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health A. 2007;70(24):2071–2079. doi: 10.1080/15287390701601251. [DOI] [PubMed] [Google Scholar]
- 139.Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, et al. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008;29(2):427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- 140.Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7(12):3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 141.Vaslet C, Messier N, Kane A. Accelerated progression of asbestos-induced mesotheliomas in heterozygous p53+/− mice. Toxicol Sci. 2002;68:331–338. doi: 10.1093/toxsci/68.2.331. [DOI] [PubMed] [Google Scholar]
- 142.Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 143.Dianzani I, Gibello L, Biava A, Giordano M, Bertolotti M, et al. Polymorphisms in DNA repair genes as risk factors for asbestos-related malignant mesothelioma in a general population study. Mutat Res. 2006;599(1–2):124–134. doi: 10.1016/j.mrfmmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 144.Apostolou S, Balsara B, Testa J. Cytogenetics of malignant mesothelioma. In: Pass HI, Vogelzang NJ, Carbone MC, editors. Malignant Mesothelioma. New York: Springer Science + Business Media, Inc; 2005. pp. 101–111. [Google Scholar]
- 145.Wang N, Jaurand M, Magne L, Lheuang L, Pinchon M, et al. The interactions between asbestos fibers and metaphase chromosomes of rat pleural mesothelial cells in culture. A scanning and transmission electron microscopic study. Am J Pathol. 1987;126(2):343–349. [PMC free article] [PubMed] [Google Scholar]
- 146.Jensen C, Jensen L, Ault J, Osorio G, Cole R. Time-lapse video light microscopic and electron microscopic observations of vertebrate epithelial cells exposed to crocidolite asbestos. In: Davis JMG, Jaurand MC, editors. Cellular and Molecular Effects of Mineral and Synthetic Dusts and Fibres. Berlin: Springer-Verlag; 1994. pp. 63–78. [Google Scholar]
- 147.Hesterberg T, Barrett J. Induction by asbestos fibers of anaphase abnormalities: mechanism for aneuploidy induction and possibly carcinogenesis. Carcinogenesis. 1985;6(3):473–475. doi: 10.1093/carcin/6.3.473. [DOI] [PubMed] [Google Scholar]
- 148.Jaurand M, Bastie-Sigeac I, Renier A, Bignon J. Comparative toxicities of different forms of asbestos on rat pleural mesothelial cells. Environ Health Perspect. 1983;51:153–158. doi: 10.1289/ehp.8351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pelin K, Hirvonen A, Taavitsainen M, Linnainmaa K. Cytogenetic response to asbestos fibers in cultured primary mesothelial cells. Mutat Res. 1995;334(2):225–233. doi: 10.1016/0165-1161(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 150.Macdonald JL, Kane AB. Mesothelial cell proliferation and biopersistence of wollastonite and crocidolite asbestos fibers. Fundam Appl Toxicol. 1997;38(2):173–183. doi: 10.1006/faat.1997.2344. [DOI] [PubMed] [Google Scholar]
- 151.Zanella C, Posada J, Tritton T, Mossman B. Asbestos causes stimulation of the ERK-1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–5338. [PubMed] [Google Scholar]
- 152.Heintz N, Janssen Y, Mossman B. Persistent induction of c-fos and c-jun by asbestos. Proc Natl Acad Sci USA. 1993;90(8):3299–3303. doi: 10.1073/pnas.90.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Timblin CR, Guthrie GD, Janssen YW, Walsh ES, Vacek P, et al. Patterns of c-fos and c-jun proto-oncogene expression, apoptosis, and proliferation in rat pleural mesothelial cells exposed to erionite or asbestos fibers. Toxicol Appl Pharmacol. 1998;151(1):88–97. doi: 10.1006/taap.1998.8450. [DOI] [PubMed] [Google Scholar]
- 154.Manning CB, Sabo-Attwood T, Robledo RF, Macpherson MB, Rincon M, et al. Targeting the MEK1 cascade in lung epithelium inhibits proliferation and fibrogenesis by asbestos. Am J Respir Cell Mol Biol. 2008;38(5):618–626. doi: 10.1165/rcmb.2007-0382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walker C, Everitt J, Ferriola PC, Stewart W, Mangum J, et al. Autocrine growth stimulation by transforming growth factor alpha in asbestos-transformed rat mesothelial cells. Cancer Res. 1995;55(3):530–536. [PubMed] [Google Scholar]
- 156.Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, et al. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005;5(12):2448–2464. doi: 10.1021/nl051748o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hussain S, Hofseth L, Harris C. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 158.Robledo R, Mossman B. Cellular and molecular mechanisms of asbestos-induced fibrosis. J Cell Physiol. 1999;180(2):158–166. doi: 10.1002/(SICI)1097-4652(199908)180:2<158::AID-JCP3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 159.Govindarajan B, Klafter R, Miller MS, Mansur C, Mizesko M, et al. Reactive oxygen-induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Mol Med. 2002;8(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 160.Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221(3):339–348. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martino E, Prandi L, Fenoglio I, Bonfante P, Perotto S, et al. Soil fungal hyphae bind and attack asbestos fibers. Angew Chem Int Ed Engl. 2003;42(2):219–222. doi: 10.1002/anie.200390083. [DOI] [PubMed] [Google Scholar]
- 162.Favero-Longo SE, Turci F, Tomatis M, Castelli D, Bonfante P, et al. Chrysotile asbestos is progressively converted into a non-fibrous amorphous material by the chelating action of lichen metabolites. J Environ Monit. 2005;7(8):764–766. doi: 10.1039/b507569f. [DOI] [PubMed] [Google Scholar]
- 163.Yan A, von dem Bussche A, Kane A, Hurt R. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon. 2007;45:2463–2470. doi: 10.1016/j.carbon.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]