Abstract
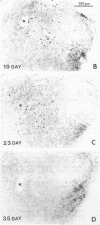
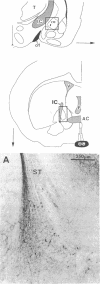
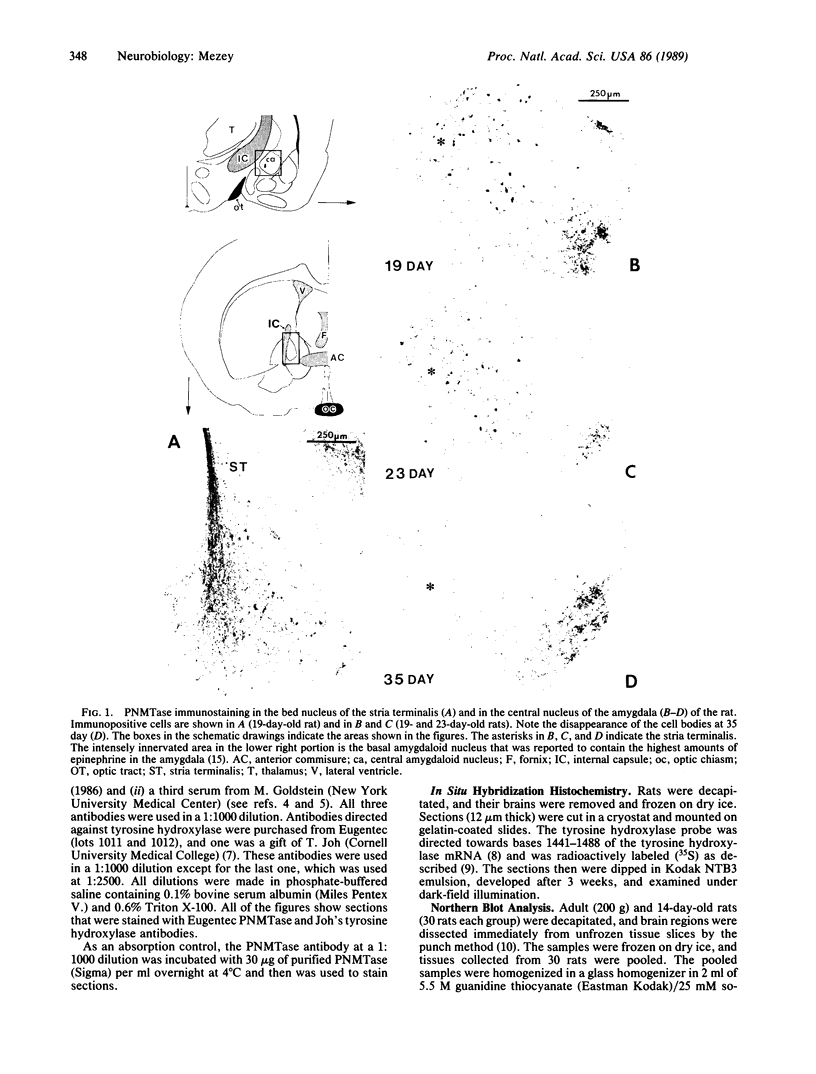
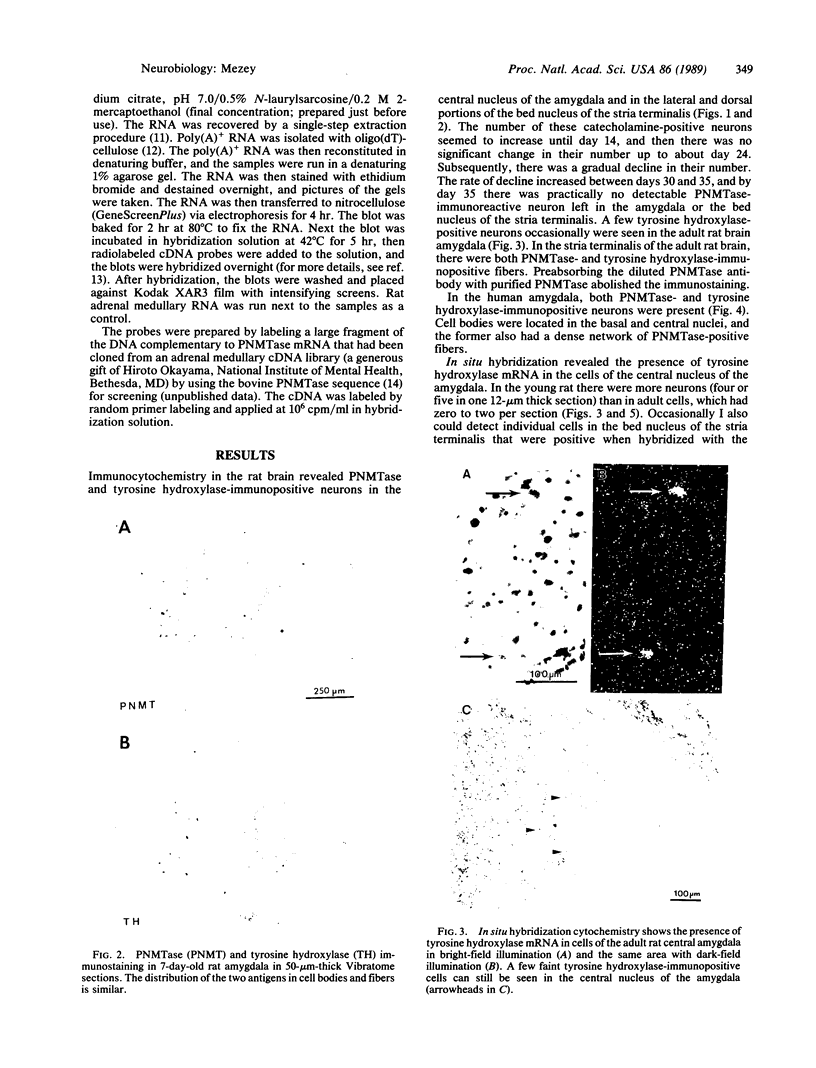
Fifteen years ago epinephrine cells were shown to be present in the medulla oblongata of the rat. These cell groups (C1 and C2) were thought to supply the epinephrine innervation in the rest of the central nervous system. In this study I demonstrate the presence of epinephrine-producing neurons in the forebrain of the young rat. Neurons that are immunopositive for phenylethanolamine N-methyltransferase (S-adenosyl-L-methionine:phenylethanolamine N-methyltransferase, EC 2.1.1.29) are present in the central nucleus of the amygdala as well as in the bed nucleus of the stria terminalis. Neurons in the same location are also immunopositive for tyrosine hydroxylase [tyrosine 3-monooxygenase; L-tyrosine, tetrahydrobiopterine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2]. The phenylethanolamine N-methyltransferase immunopositivity disappears by day 35, while a small amount of tyrosine hydroxylase-positive cells still can be found in the adult. In situ hybridization reveals tyrosine hydroxylase mRNA in the above nuclei in both young and adult animals. The number of the positive cells decreases in adulthood. RNA blot-hybridization analysis showed the presence of phenylethanolamine N-methyltransferase mRNA in the amygdala and the bed nucleus of the stria terminalis in the young and in the adult rat brain. Neurons that are immunopositive for phenylethanolamine N-methyltransferase are also present in the human amygdala.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962 May;237:1657–1660. [PubMed] [Google Scholar]
- Armstrong D. M., Ross C. A., Pickel V. M., Joh T. H., Reis D. J. Distribution of dopamine-, noradrenaline-, and adrenaline-containing cell bodies in the rat medulla oblongata: demonstrated by the immunocytochemical localization of catecholamine biosynthetic enzymes. J Comp Neurol. 1982 Dec 1;212(2):173–187. doi: 10.1002/cne.902120207. [DOI] [PubMed] [Google Scholar]
- Baetge E. E., Suh Y. H., Joh T. H. Complete nucleotide and deduced amino acid sequence of bovine phenylethanolamine N-methyltransferase: partial amino acid homology with rat tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5454–5458. doi: 10.1073/pnas.83.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crowley W. R., Terry L. C. Effects of an epinephrine synthesis inhibitor, SKF64139, on the secretion of luteinizing hormone in ovariectomized female rats. Brain Res. 1981 Jan 5;204(1):231–235. doi: 10.1016/0006-8993(81)90670-3. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Iacangelo A., Eiden L. E. Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci U S A. 1988 May;85(9):3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. W. Pharmacology of brain epinephrine neurons. Annu Rev Pharmacol Toxicol. 1982;22:31–55. doi: 10.1146/annurev.pa.22.040182.000335. [DOI] [PubMed] [Google Scholar]
- Gold M. S., Donabedian R. K., Redmond D. E., Jr Clonidine-induced increase in serum growth hormone: possible role of epinephrine-mediated synapses. Psychoneuroendocrinology. 1978 Apr;3(2):187–194. doi: 10.1016/0306-4530(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe P. R., Costa M., Furness J. B., Chalmers J. P. Simultaneous demonstration of phenylethanolamine N-methyltransferase immunofluorescent and catecholamine fluorescent nerve cell bodies in the rat medulla oblongata. Neuroscience. 1980;5(12):2229–2238. doi: 10.1016/0306-4522(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fuxe K., Goldstein M., Johansson O. Evidence for adrenaline neurons in the rat brain. Acta Physiol Scand. 1973 Oct;89(2):286–288. doi: 10.1111/j.1748-1716.1973.tb05522.x. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Purification and fractionation of poly(A)+ RNA. Methods Enzymol. 1987;152:254–261. doi: 10.1016/0076-6879(87)52028-6. [DOI] [PubMed] [Google Scholar]
- Mezey E., Kiss J. Z., Skirboll L. R., Goldstein M., Axelrod J. Increase of corticotropin-releasing factor staining in rat paraventricular nucleus neurones by depletion of hypothalamic adrenaline. Nature. 1984 Jul 12;310(5973):140–141. doi: 10.1038/310140a0. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973 Sep 14;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Petty M. A., Reid J. L. Catecholamine synthesizing enzymes in brain stem and hypothalamus during the development of renovascular hypertension. Brain Res. 1979 Mar 16;163(2):277–288. doi: 10.1016/0006-8993(79)90355-x. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Katz R. J., Sibel M., Mefford I. N., Barchas J. D., Carroll B. J. Central epinergic inhibition of corticosterone release in rat. Life Sci. 1981 May 21;28(21):2389–2394. doi: 10.1016/0024-3205(81)90505-1. [DOI] [PubMed] [Google Scholar]
- Ruggiero D. A., Ross C. A., Anwar M., Park D. H., Joh T. H., Reis D. J. Distribution of neurons containing phenylethanolamine N-methyltransferase in medulla and hypothalamus of rat. J Comp Neurol. 1985 Sep 8;239(2):127–154. doi: 10.1002/cne.902390202. [DOI] [PubMed] [Google Scholar]
- Schwaber J. S., Kapp B. S., Higgins G. A., Rapp P. R. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982 Oct;2(10):1424–1438. doi: 10.1523/JNEUROSCI.02-10-01424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J Physiol. 1954 Mar 29;123(3):451–481. doi: 10.1113/jphysiol.1954.sp005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gugten J., Palkovits M., Wijnen H. L., Versteeg D. H. Regional distribution of adrenaline in rat brain. Brain Res. 1976 Apr 30;107(1):171–175. doi: 10.1016/0006-8993(76)90107-4. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Warden M., Mezey E. Tyrosine hydroxylase mRNA is increased by hyperosmotic stimuli in the paraventricular and supraoptic nuclei. Neuroendocrinology. 1987 Nov;46(5):439–444. doi: 10.1159/000124858. [DOI] [PubMed] [Google Scholar]