Abstract
The decarboxylation product of arginine, agmatine, has effectively reduced or prevented opioid-induced tolerance and dependence when given either systemically (intraperitoneally or subcutaneously) or centrally (intrathecally or intracerebroventricularly). Systemically administered agmatine also reduces the escalation phase of intravenous fentanyl self-administration in rats. The present study assessed whether centrally (intracerebroventricular, i.c.v.) delivered agmatine could prevent the development of fentanyl self-administration in mice. Mice were trained to respond under a fixed-ratio 1 (FR1) schedule for either fentanyl (0.7 μg/70 μl, p.o.) or food reinforcement. Agmatine (10 nmol/5 μl), injected i.c.v. 12-14h before the first session and every other evening (12-14h before session) for 2 weeks, completely attenuated oral fentanyl self-administration (but not food-maintained responding) compared to saline-injected controls. When agmatine was administered after fentanyl self-administration had been established (day 8) it had no attenuating effects on bar pressing. This dose of agmatine does not decrease locomotor activity as assessed by rotarod. The present findings significantly extend the previous observation that agmatine prevents opioid-maintained behavior to a chronic model of oral fentanyl self-administration as well as identifying a supraspinal site of action for agmatine inhibition of drug addiction.
Keywords: NMDA, NOS, neuroprotection, intracerebroventricular
1. Introduction
The organic cation agmatine (decarboxylated L-arginine) was first identified in the mammalian central nervous system (CNS) in 1994 (Li et al., 1994). Since then, we and others have confirmed its presence in CNS through immunohistochemistry and other bioanalytical methods such as high pressure liquid chromatography (HPLC) and mass spectrometry. Agmatine meets many of the criteria of a central neuromodulator. Agmatine is synthesized, stored, and released from specific networks of neurons, is inactivated by energy-dependent reuptake mechanisms and is enzymatically degraded (Reis and Regunathan, 1998). The presence of its synthetic (Li et al., 1994) and degradative (Iyer et al., 2002; Mistry et al., 2002) enzymes in CNS supports the proposal that endogenous agmatine serves a modulatory role in CNS function. In addition, we have recently also shown that [3H]-agmatine is transported into and released from synaptosomes (purified nerve terminals)(Goracke-Postle et al., 2006) under conditions of K+ stimulation in a Ca2+-dependent manner (Goracke-Postle et al., 2007).
Agmatine is unique in that it both antagonizes N-methyl D-aspartate (NMDA) receptors (Fairbanks et al., 2000; Yang and Reis, 1999) and inhibits (Auguet et al., 1995; Galea et al., 1996) or inactivates (Demady et al., 2001) nitric oxide synthase (NOS), a property that distinguishes it from most exogenously applied NMDA receptor antagonists and NOS inhibitors, which usually act at just one site. Taken together with the role of NMDA receptors and NOS in neuroplasticity (Haley et al. 1992), these actions suggest that agmatine may serve as an endogenous modulator of glutamatergic neuroplasticity.
When given exogenously, agmatine inhibits several requisite processes in glutamatergic neuroplasticity: it is neuroprotective in models of spinal cord injury and brain ischemia (Gilad and Gilad, 1996), permanently interrupts neuropathic pain, and blocks opioid tolerance and dependence. It was also found that agmatine, given by repeated intravenous (i.v.) infusions, reduces escalation of intravenous fentanyl self-administration (Morgan et al., 2002). When given i.v. ter in die (t.i.d.) during the self-administration session, agmatine attenuated (but did not completely ablate) the escalation phase of i.v. fentanyl self-administration, indicating that agmatine’s effects extend beyond CNS adaptation to chronic opioid treatment and includes opioid-driven reward. Despite agmatine’s short (<10 min) plasma half-life (Piletz et al., 2003; Raasch et al., 2002; Roberts et al., 2005), its systemic administration consistently affects a wide variety of CNS-mediated processes (Nguyen et al., 2003). Based on the 12h CNS half-life reported by our group (Roberts et. al., 2005; (Roberts, 2007), we hypothesized that intermittent i.c.v. administration would yield prolonged activity; in fact, agmatine given once daily or once every other day completely prevented the development of supraspinal opioid-induced tolerance (Kitto and Fairbanks, 2006). In order to determine whether agmatine could similarly exert a complete inhibition of fentanyl-self administration, the present study evaluated the effects of i.c.v.-administered agmatine in a mouse model of oral fentanyl self-administration.
2. Methods
2.1 Animals
Experimental subjects were Institute of Cancer Research (ICR) male mice (21-24 g, Harlan, Madison). Subjects were housed in groups of eight in a temperature- and humidity-controlled environment, maintained on a 12h light/dark cycle. Water was given ad libitum and mice were fed on a restricted diet of 3 grams per day throughout the duration of all experiments. Each mouse was used in only one experimental group. These experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee.
2.2 Chemicals
Agmatine sulfate was purchased from Sigma Chemical (St. Louis, MO) and dissolved in 0.9% saline. Fentanyl citrate was purchased from Gallipot (St. Paul, MN) and dissolved in distilled water (dH2O). Quinine hydrochloride (Sigma) (30 μg/ml) was included in both the fentanyl and the control water to reduce the potential for taste preferences for one fluid over the other. This approach has been used in other studies of oral fentanyl self-administration (Colpaert et al., 2001; Kupers and Gybels, 1995).
2.3 Intracerebroventricular injection
All drug- or saline-treated controls were administered i.c.v. in a 5 μl volume in conscious mice according to the method of Haley and McCormick (Haley, 1957). All injections were performed by one experimenter (KFK) who has over fifteen years of experience with the procedure.
The procedure for the injection was as follows: conscious mice were covered with a cloth to expose just the top of the head. The subject was then restrained at the base of the skull with the experimenter’s thumb and forefinger so that the neck and jaw of the mouse were firmly, but gently, pressed against a firm flat level surface. A 50 μl Hamilton syringe was fitted to a 27-gauge needle with a rubber stopper positioned to expose 1.5 mm of the needle tip. The exposed tip was then inserted into the right lateral cerebral ventricle, through the scalp and the skull, 1 mm to the right of the skull’s midline and level with the external auditory meatus; the skull was sufficiently soft to permit this insertion with minimal force. Once the needle was positioned, 5 μl of solution was injected and the needle removed. This procedure takes less than a minute and requires no anesthetic, surgery, or incision.
2.4 Self-Administration apparatus
Experimental chambers were Modular Mouse Test Chambers (Med-Associates, ENV-307CT, St. Albans, VT). Each chamber was housed in a sound-attenuating cubicle (Med-Associates, ENV-021M), and equipped with a 3.33 RPM syringe pump (Med-Associates, PHM-100) for drug delivery, 20 mg food pellet delivery system (Med-Associates, ENV-203-20), 2 ultra sensitive mouse levers (Med-Associates, ENV-310M) and 2 stimulus lights (Med-Associates, ENV-321M). A 4.8 W house light located at the top of the cage was illuminated during experimental sessions.
2.5 Behavioral procedure
The FR1 reward schedule coupled an active lever press with a delivery of 70 μl drug solution to the receptacle, and illumination of the stimulus light directly above the lever. After each reward, there was a 5 second time-out period during which no reward was possible, regardless of additional lever presses (which will also be recorded). Responding on the control lever resulted only in illumination of the stimulus light above it. Animals in the non-fentanyl control groups received dH20 (+ quinine) instead of fentanyl, which controlled for the possibility that motivation for fluid (rather than drug) is the reinforcer. Responses were monitored for both the active lever (the lever that drives delivery of fluid) and the control lever (the lever for which there was no associated reward provided in response to being pushed) and expressed as mean responses for each test day. The control lever controlled for random activity in the operant chamber during which that lever may be pushed. Each mouse was tested once daily (2 hour session) for the duration of the experiment (24 days).
2.6 Experimental Designs
2.6.1 Experiment 1
The objective of this experiment was to evaluate the characteristics of oral fentanyl self-administration. There were two levers in the operant chamber, one that is an active lever the pressing of which resulted in delivery of 70 μl of either fentanyl or water (the reward). The second lever was a control lever the pressing of which resulted in no reward. Pressing of both levers was tracked to determine the subjects’ discrimination between the two levers. This schedule of reinforcement was applied to varying concentrations of oral fentanyl from 1-300 μg/ml and control, which was dH2O (+ 30 μg/ml of quinine).
2.6.2 Experiment 2
The purpose of this experiment was to determine the effects of supraspinal agmatine on oral fentanyl self-administration using the optimal concentrations from experiment 1. The behavioral design was the same as described above, but 10 nmol/5 μl of agmatine was delivered i.c.v. 12-15 hours before the first self-administration session (the evening before) and every 2 days for 16 days (for a total of 8 injections). Lower doses of agmatine (0.1 and 1 nmol) were also tested.
2.6.3 Experiment 3
The purpose of this experiment was to determine the effects of supraspinal agmatine on food maintained responding using the optimal agmatine i.c.v. dose from Experiment 2. To evaluate the effects of agmatine on food self-administration a separate group of mice were injected i.c.v. with agmatine the day before the first self-administration session and every 2 days for 16 days and followed the same behavioral design, however these mice were not exposed to fentanyl and only bar pressed for food pellets.
2.6.4 Experiment 4
The purpose of this experiment was to determine the effects of supraspinal agmatine on oral fentanyl self-administration after self-administration had been established. While the behavioral design was the same as described in Experiment 2, agmatine was administered after a minimum of 8 mice per group (agmatine vs. saline) had established fentanyl self-administration. The same schedule of i.c.v. injection was followed of agmatine administered every 2 days, starting on Day 8, after self-administration had been established. The criteria for inclusion were the following: the mice had to 1) respond for the fentanyl lever at a ratio of 2 or higher when compared to the control lever and 2) respond for the fentanyl at a minimum of 10 lever presses per session for 3 days in a row.
2.6.5 Rotarod assay
Following 2 consecutive training sessions, mice walk for 300 seconds on a rotarod apparatus (Accelerating Rotarod for Mice Ugo Basile Biological Research Apparatus, Varese, Italy) during which time the rotarod undergoes a linear acceleration from 4 to 40 rpm. We compared the latency to fall before and after administration of saline or agmatine.
2.7 Data analysis
The area under the curve (AUC) was determined by the trapezoidal rule using the statistical software package JMP® 6 from SAS. The resulting AUCs were analyzed using analysis of variance (ANOVA) from Prism 4.0. Significance was defined as P < 0.05.
3. Results
3.1. Oral Fentanyl Self-Administration
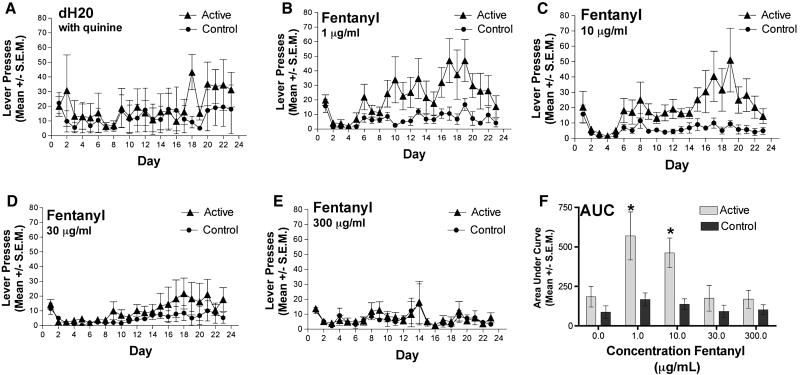
The primary objective of the overall study was to evaluate the impact of supraspinal agmatine treatment on oral self-administration of fentanyl. In order to pursue that aim, a protocol for establishing oral self-administration of fentanyl was needed. An experimental protocol specifically for the study of oral fentanyl intake in mice using an operant conditioning method has not been previously described. Therefore, we first evaluated a range of concentrations of fentanyl to determine the optimal concentration for establishment of apossible taste aversion using 30 μg/ml of quinine, we tested concentrations of fentanyl from 1, 10, 30 and 300 μg/ml and the vehicle, dH2O (Fig. 1A-E).
FIG. 1. Oral fentanyl self-administration.
Mice did not respond for vehicle (dH2O + 30 μg/ml quinine (A). Fentanyl self-administration peaked on Days 17-19 for 1 μg/ml and Day 19 for 10 μg/ml but concentrations (B and C) 30 μg/ml and 300 μg/ml failed to induce opioid self-administration (D and E). Data shown is representative of mean lever presses for the fentanyl response bar (active lever) (triangles; FR1) and the control lever (circles). Analysis of the area under the curve (AUC) across all self-administration sessions indicated that 10 μg/ml of fentanyl (+ 30 μg/ml quinine) was the highest concentration of fentanyl that established self-administration behavior, showing statistically significant separation between the active and control levers (F) (*denotes a difference between active and respective control lever, Student’s paired t test, p< 0.05). N=8 mice per group.
Lever pressing for the vehicle control (dH2O + 30 μg/ml quinine) did not differ between the active and control levers throughout the duration of the experiment (Fig. 1A). Lever pressing on the active lever for delivery of 1 and 10 μg/ml of fentanyl increased across days 8-19 with the maximum mean reaching 50 active lever presses on day 19 (Fig. 1B,C). In this group there was no increase in responding on the control lever; the difference in response on the active and control levers was significant indicating a preference of lever pressing for drug delivery. A concentration of 30 μg/ml of fentanyl showed a maximum mean of 20 fentanyl active lever presses later in the experiment, but which was not significantly different from that of the control lever (Fig. 1D) so discrimination between the levers was not evident. Finally, at the 300 μg/ml concentration of fentanyl mice showed minimal responding and no preference for fentanyl intake between the control and active levers over the duration of the study (Fig. 1E). Analysis of the AUC across the concentration range indicated that 10 μg/ml concentration of fentanyl was the highest concentration of fentanyl that showed statistically significant separation between the active and control levers with the least variability indicating the best representation of motivated self-administration behavior (Fig. 1F).
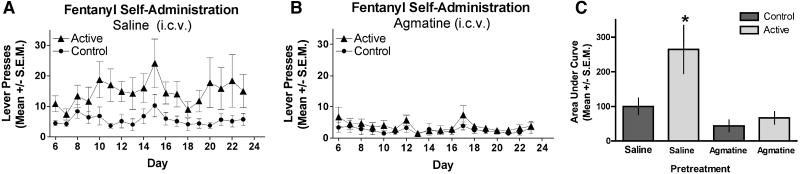
3.2. Agmatine attenuates oral fentanyl self-administration
We next evaluated the effect of supraspinally-administered agmatine (i.c.v) on oral self-administration of 10 μg/ml of fentanyl. Agmatine (10 nmol) or saline was injected i.c.v. every other day until day 16 as described in the methods. Mice that received saline injections developed increased active lever pressing for fentanyl over time distinct from that of control lever pressing, as expected (Fig. 2A); the injection protocol did not impact the acquisition of the self-administration behavior. In contrast, agmatine-treatment completely inhibited the responding of mice on the active lever, which did not differ from that of control levers for the duration of the experiment well beyond cessation of the drug delivery protocol (Fig. 2B). Delivery of two lower agmatine doses (1 and 0.1 nmol) also completely inhibited fentanyl self-administration (data not shown).
FIG. 2. Agmatine-mediated attenuation of fentanyl self-administration.
Time course of fentanyl self-administration for mice receiving i.c.v. treatments of saline (A) or agmatine (10 nmol/ 5 μL) (B) given the day before the first self-administration sessions and every 2 days for 16 days. Responses represent lever presses on one of two bars. The first bar (active lever) delivers 70 μL of fentanyl (10 μg/ml) (triangles). Pressing the control lever results in no reward and is indicative of non-specific activity (circles). Mice did not respond differently for food-maintained responding under either the saline or agmatine conditions (C). Analysis of the AUC for the groups in A and B show that animals that received repeated ICV saline discriminated between the control (1st bar, left to right) and the active (2nd bar) levers whereas the mice that received repeated agmatine did not discriminate between control (3rd bar) and active (4th bar) levers suggesting that agmatine inhibited the development of fentanyl self-administration behavior. (*significance was determined by ANOVA, F(3,26); p < 0.05).
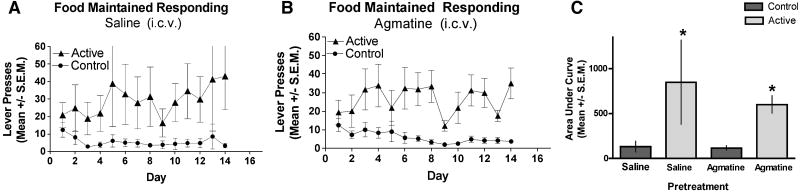
3.3. Agmatine does not affect food maintained-responding
We next examined the effects of i.c.v. agmatine (10 nmol) on food-maintained responding to evaluate the possibility that supraspinal agmatine treatment inhibited lever pressing in a non-specific manner. In contrast to the fentanyl self-administration experiment, agmatine pretreatment had no effect on the acquisition of food-maintained responding; mice responded for food pellet delivery comparably both in mice treated with supraspinal saline (Fig. 3A) or agmatine (Fig. 3B) with significant discrimination between the active and control levers in both treatment groups. This result argues against the possibility that the agmatine inhibition of fentanyl self-administration is a result or reduction of either learning or motor ability to perform the dependent measure. Consistent with that assertion, the ability of mice to stay on an accelerating rotarod device did not differ between mice pre-treated i.c.v. with saline (saline-treated: 238 ± 12 s) or agmatine (agmatine-treated: 245 ± 13 s). That these same doses did not impair lever presses in food-restricted mice or rotarod performance illustrate that the agmatine does not have an impact on satiety or motor function, excluding such effects as possible explanations for the reduction in lever presses in Fig. 2B.
FIG. 3. Agmatine does not affect food-maintained responding.
Time course of food-maintained responding for mice receiving i.c.v. treatments of saline (A) or agmatine (10 nmol/ 5 μL) (B) given the day before the first self-administration sessions and every 2 days for 16 days. Responses represent lever presses on one of two bars. The first bar (active lever) delivers 1 pellet of food (triangles). Pressing the control lever results in no reward and is indicative of non-specific activity (circles). Mice did not respond differently for food-maintained responding in either the saline (A) or agmatine (B) group indicating that agmatine has no effect on food reward. (C) Analysis of the AUC for the groups in A and B show that animals that received repeated ICV saline discriminated between the control (1st bar, left to right) and the active (2nd bar) levers. The mice that received repeated agmatine also discriminated between control (3rd bar) and active (4th bar) levers suggesting that agmatine does not inhibit the response for food reward (*indicates significant difference in responding between the respective control and active lever within experimental group was determined by ANOVA, F(3,28); p < 0.05).
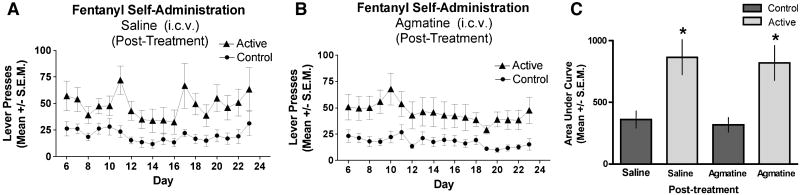
3.4. Agmatine post-treatment does not impact fentanyl self-administration
In this experiment mice were trained to self-administer prior to the first agmatine injection. Those that reliably self-administered fentanyl by Day 8 were divided into two groups: 9 mice receiving 0.9% NaCl and 9 mice receiving 10 nmol/5 μl agmatine i.c.v. In order to be included mice had to respond for fentanyl at a ratio of 2 or greater compared to control lever presses and lever press for fentanyl a minimum of 10 times per session for 3 days; 75% of the mice met the criteria. Agmatine or saline was administered 12-14h prior to the ninth day of sessions and every other day thereafter as in the previous experiments. Mice that received saline injections maintained active lever pressing for fentanyl over time distinct from that of control lever pressing, as expected (Fig. 4A). As in Experiments 2 and 3, the i.c.v. injection schedule did not impact the acquisition of the self-administration behavior. However, in contrast to the result in Fig. 2B, agmatine-treatment after established fentanyl responding did not affect the responding of mice on the active lever; in other words, the agmatine-treated mice continued to discriminate between the active and control levers for the duration of the experiment (Fig. 4B). Therefore, this experiment shows that supraspinal agmatine treatment, when given after fentanyl self-administration was established, has no effect on fentanyl self-administration in contrast to the inhibitory effect of pre-treatment and continued treatment of agmatine during the acquisition phase of fentanyl self-administration (Fig. 2).
FIG. 4. Delivery of agmatine after the establishment of fentanyl self-administration.
Time course of fentanyl self-administration for mice receiving i.c.v. treatments of saline (A) or agmatine (10 nmol/ 5 μL) (B) given after fentanyl self-administration has been established on day 8 and every 2 days for 14 days. The arrow on the graph denotes the first injection. Responses represent lever presses on one of two bars. The first bar (active lever) delivers 70 μL of fentanyl (10 μg/mL) (triangles). Pressing the control lever results in no reward and is indicative of non-specific activity (circles). Only mice meeting inclusion criteria were tested (ratio of 2 or greater compared to control lever presses and that the mice had to lever press for fentanyl a minimum of 10 times per session for 3 days; 75% of mice met this criteria). Mice did not alter active lever responding in response to agmatine i.c.v. injections. (C) Analysis of the AUC for the groups in A and B show that animals that received repeated ICV saline continued to discriminated between the control (1st bar, left to right) and the active (2nd bar) levers. The mice that received repeated agmatine after establishment of self-administration behavior also continued to discriminate between control (3rd bar) and active (4th bar) levers suggesting that this injection schedule of agmatine does not reverse the maintenance of fentanyl self-administration (*signifies difference in responding between the respective control and active lever within experimental group ANOVA, F(3,32); p < 0.05). N=9 mice per group.
4. Discussion
The present study demonstrates that supraspinally-administered (i.c.v.) agmatine prevents the acquisition of fentanyl self-administration. First, mice readily self-administered oral fentanyl at 10 μg/ml. Using that concentration of fentanyl it was observed that agmatine (0.1-10 nmol, i.c.v.) pretreatment completely inhibited fentanyl self-administration. That i.c.v. agmatine did not alter food-maintained responding or rotarod performance indicates that agmatine’s action is specific to drug-reinforcing behavior and does not affect the ability of the mice to acquire the lever-pressing response. Further, the observation that agmatine (10 nmol, i.c.v.) post-treatment after fentanyl self-administration had been established had no effect, suggests the importance of timing of the delivery of agmatine in the acquisition phase of the behavior.
It has been previously shown that agmatine, whether given systemically (Kolesnikov, 1996) or centrally, prevents morphine tolerance (Fairbanks and Wilcox, 1997; Kitto and Fairbanks, 2006) and dependence (Aricioglu et al., 2003; Aricioglu et al., 2004; Aricioglu-Kartal and Uzbay, 1997). Morgan and colleagues (Morgan et al., 2002) demonstrated that systemically administered agmatine (i.v.) reduced the escalation of fentanyl (but not cocaine) self-administration (i.v.). While this study did not specifically evaluate agmatine against cocaine self-administration, together, these data suggests that the relationship of agmatine may be specific to opioidergic systems. Further, prior evidence has linked agmatine to the glutamatergic system, specifically as an NMDA receptor antagonist (Yang and Reis, 1999) and NOS inhibitor (Demady et al., 2001; Galea et al., 1996). Such linkage is significant since NMDA receptor antagonism and NOS inhibition are both well-established mechanisms for inhibition of opioid-evoked analgesic tolerance.
The role of NMDA receptor antagonists and NOS inhibitors in opioid self-administration studies has been less widely evaluated and is less clear. Evidence to support a role for the NMDA receptor includes the observation that Lewis rats with significantly higher NMDA receptor levels in specific brain regions reach higher breaking points (e.g. the progressive ratio where rats will respond more for a single infusion) and response ratios when compared to Fischer rats (Martin et al., 2003). Semenova and colleagues (Semenova et al., 1999), however, demonstrated seemingly discordant pharmacological results; while the NMDA receptor antagonists, MRZ2/579 (i.p.) and memantine (i.p.), inhibited the acquisition of morphine (i.v.) self-administration, NMDA receptor antagonist, MK-801 (i.p.) did not affect morphine self-administration. The difference between memantine/MRZ2/579 and MK801 is potentially attributable to differences in affinity for the receptor. MK-801 is a high-affinity NMDA receptor antagonist with widely noted motor side effects in rat and mouse including the ICR mouse strain used in the present study (Fairbanks et al., 2000). Memantine and MRZ2/579 are considered to be low affinity NMDA receptor antagonists with Ki values in the low μM range (Parsons et al., 1999a). Like memantine and MRZ2/579, agmatine demonstrates a low affinity for the NMDA receptor in terms of its ability to compete with [3H]-MK801 (Reynolds et al., 1990). Also, like memantine (Parsons et al., 1999b), agmatine demonstrates a significantly improved side-effect profile relative to MK801 in pre-clinical mouse models (accelerating rotarod assay) (Fairbanks et al., 2000).
The role of nitric oxide in opioid self-administration has yet to be fully elucidated. Kivastik and colleagues (Kivastik et al., 1996) demonstrated the effects of N(G)-nitro-L-arginine (L-NOARG), a NOS inhibitor, on morphine-conditioned place preference and found that intraperitoneally delivered L-NOARG decreased the amount of time spent in the drug-paired side. Alternatively, Sarhaei and colleagues (Sahraei et al., 2004) found that the NOS inhibitor N(G)-nitro-L-arginine methyl ester (L-NAME) (i.p.) increased morphine self-administration, the nitric oxide (NO) precursor L-arginine decreased morphine self-administration and supports morphine self-administration. These observations may result from NO-induced release of dopamine from striatal neurons (Kiss, 2000).
It is significant that the most effective agmatine dose (10 nmol, i.c.v.) is within the range of agmatine doses that inhibit NMDA-evoked scratching and biting behavior when given spinally (Fairbanks et al., 2000). It is also a dose that has been shown to inhibit NMDA-evoked thermal tail flick hyperalgesia (Fairbanks et al., 2000), a behavior that is dependent on NOS activation (Kitto et al., 1992). Agmatine’s complete prevention of fentanyl-evoked self-administration is a typical observation of agmatine-mediated attenuation or reversal of glutamate-driven behavioral responses (Nguyen et al., 2003). Similar effects have now been reported in at least ten plasticity-related behaviors (Nguyen et al., 2003).
It is noteworthy that while we observed that pre-treatment of agmatine in the acquisition phase prevented the development of oral fentanyl self-administration, post-treatment with agmatine following establishment of fentanyl self-administration was not effective. This finding is consistent with other opioid related studies demonstrating that other NMDA receptor antagonists are effective in the development but not the maintenance phase of the opioid response specifically in opioid tolerance studies (Herman et al., 1995) and studies of opioid conditioned place preference (Ma et al., 2006; Papp et al., 2002) These outcomes also concur with evidence supporting the NMDA receptor in the induction, but not the maintenance phase of long-term potentiation in spinal cord (Benrath et al., 2005), hippocampus (Ohno et al., 2002), and cortex (Myers et al., 2000). Therefore, the pattern of agmatine inhibition of oral fentanyl self-administration that we have observed is consistent with a general relationship of NMDA receptor antagonists in plasticity related events in a broad spectrum of CNS regions. It has been previously noted that agmatine shares many of the classic characteristics of a modulator of neurotransmission (Reis and Regunathan, 1998). Consequently, agmatine may act as an anti-glutamatergic modulator in vivo, a role largely unexplored in CNS. Further, we have recently shown Ca2+-dependent depolarization-evoked release of [3H]-agmatine from synaptosomes (Goracke-Postle et al., 2006; Goracke-Postle et al., 2007). Additionally, it has been observed that agmatine concurrently inhibits both glutamate release and seizures evoked by pentylenetetrazole in the rat (Feng et al., 2005). Together with the pharmacological data (Nguyen et al., 2003) these observations support the proposal that endogenous agmatine may serve as a neuromodulator of the glutamatergic system (Reis and Regunathan, 1998). Further studies are required to determine whether supraspinal endogenous agmatine prevents induction of opioid-induced self-administration in an NMDA receptor/NOS dependent manner.
Acknowledgments
The authors appreciate the contributions of Dr. Andrew M. Morgan for assisting in establishing the experimental protocols in our laboratory and of Dr. Marilyn Carroll for useful discussion on experimental design. These studies were made possible by the generous support of the National Institute on Drug Abuse (NIDA) in the form of a K01 award (DA-00509) and an R21 CEBRA (DA-15387) award made to C.A.F. An NRSA pre-doctoral fellowship (F31 DA021054) supports C.L.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aricioglu F, Ercil E, Dulger G. Agmatine inhibits naloxone-induced contractions in morphine-dependent Guinea pig ileum. Ann N Y Acad Sci. 2003;1009:147–151. doi: 10.1196/annals.1304.016. [DOI] [PubMed] [Google Scholar]
- Aricioglu F, Paul IA, Regunathan S. Agmatine reduces only peripheral-related behavioral signs, not the central signs, of morphine withdrawal in nNOS deficient transgenic mice. Neurosci Lett. 2004;354:153–157. doi: 10.1016/j.neulet.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Aricioglu-Kartal F, Uzbay IT. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci. 1997;61:1775–1781. doi: 10.1016/s0024-3205(97)00801-1. [DOI] [PubMed] [Google Scholar]
- Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. 1995;69:285–287. doi: 10.1254/jjp.69.285. [DOI] [PubMed] [Google Scholar]
- Benrath J, Brechtel C, Stark J, Sandkuhler J. Low dose of S+-ketamine prevents long-term potentiation in pain pathways under strong opioid analgesia in the rat spinal cord in vivo. Br J Anaesth. 2005;95:518–523. doi: 10.1093/bja/aei215. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91:33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- Demady DR, Jianmongkol S, Vuletich JL, Bender AT, Osawa Y. Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol. 2001;59:24–29. doi: 10.1124/mol.59.1.24. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, Nguyen HO, Grocholski BM, Shoeman DW, Kehl LJ, Regunathan S, Reis DJ, Yezierski RP, Wilcox GL. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc Nat Acad Sci USA. 2000;97:10584–10589. doi: 10.1073/pnas.97.19.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J Pharmacol Exp Ther. 1997;282:1408–1417. [PubMed] [Google Scholar]
- Feng Y, LeBlanc MH, Regunathan S. Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: a potential mechanism for the anticonvulsive effects. Neurosci Lett. 2005;390:129–133. doi: 10.1016/j.neulet.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J. 1996;316:247–249. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. Brain polyamine stress response: recurrence after repetitive stressor and inhibition by lithium. J Neurochem. 1996;67:1992–1996. doi: 10.1046/j.1471-4159.1996.67051992.x. [DOI] [PubMed] [Google Scholar]
- Goracke-Postle CJ, Nguyen HO, Stone LS, Fairbanks CA. Release of tritiated agmatine from spinal synaptosomes. Neuroreport. 2006;17:13–17. doi: 10.1097/01.wnr.0000192739.38653.aa. [DOI] [PubMed] [Google Scholar]
- Goracke-Postle CJ, Overland AC, Riedl MS, Stone LS, Fairbanks CA. Potassium- and capsaicin-induced release of agmatine from spinal nerve terminals. J Neurochem. 2007;102:1738–1748. doi: 10.1111/j.1471-4159.2007.04647.x. [DOI] [PubMed] [Google Scholar]
- Haley TJ, Mccormick WG. Pharmacological effects produced by intracerebral injections of drugs in the conscious mouse. Br J Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction. Neuropsychopharmacology. 1995;13:269–293. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Iyer RK, Kim HK, Tsoa RW, Grody WW, Cederbaum SD. Cloning and characterization of human agmatinase. Mol Genet Metabol. 2002;75:209–218. doi: 10.1006/mgme.2001.3277. [DOI] [PubMed] [Google Scholar]
- Kiss JP. Role of nitric oxide in the regulation of monoaminergic neurotransmission. Brain Res Bull. 2000;52:459–466. doi: 10.1016/s0361-9230(00)00282-3. [DOI] [PubMed] [Google Scholar]
- Kitto KF, Fairbanks CA. Supraspinally administered agmatine prevents the development of supraspinal morphine analgesic tolerance. Eur J Pharmacol. 2006;536:133–137. doi: 10.1016/j.ejphar.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett. 1992;148:1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- Kivastik T, Rutkauskaite J, Zharkovsky A. Nitric oxide synthesis inhibition attenuates morphine-induced place preference. Pharmacol Biochem Behav. 1996;53:1013–1015. doi: 10.1016/0091-3057(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Pasternak GW. Modulation of opioid analgesia by agmatine. Eur J Pharmacol. 1996;296:17–22. doi: 10.1016/0014-2999(95)00669-9. [DOI] [PubMed] [Google Scholar]
- Kupers R, Gybels J. The consumption of fentanyl is increased in rats with nociceptive but not with neuropathic pain. Pain. 1995;60:137–141. doi: 10.1016/0304-3959(94)00106-O. [DOI] [PubMed] [Google Scholar]
- Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. see comments. [DOI] [PubMed] [Google Scholar]
- Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL. The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol. 2006;200:343–355. doi: 10.1016/j.expneurol.2006.02.117. [DOI] [PubMed] [Google Scholar]
- Martin S, Lyupina Y, Crespo JA, Gonzalez B, Garcia-Lecumberri C, Ambrosio E. Genetic differences in NMDA and D1 receptor levels, and operant responding for food and morphine in Lewis and Fischer 344 rats. Brain Res. 2003;973:205–213. doi: 10.1016/s0006-8993(03)02482-x. [DOI] [PubMed] [Google Scholar]
- Mistry SK, Burwell TJ, Chambers RM, Rudolph-Owen L, Spaltmann F, Cook WJ, Morris SM., Jr Cloning of human agmatinase. An alternate path for polyamine synthesis induced in liver by hepatitis B virus. Am J Phys - Gastrointestinal & Liver Physiology. 2002;282:G375–381. doi: 10.1152/ajpgi.00386.2001. [DOI] [PubMed] [Google Scholar]
- Morgan AD, Campbell UC, Fons RD, Carroll ME. Effects of agmatine on the escalation of i.v. cocaine and fentanyl self-administration in rats. Pharmacol Biochem Behav. 2002;72:873–880. doi: 10.1016/s0091-3057(02)00774-8. [DOI] [PubMed] [Google Scholar]
- Myers WA, Churchill JD, Muja N, Garraghty PE. Role of NMDA receptors in adult primate cortical somatosensory plasticity. J Comp Neurol. 2000;418:373–382. [PubMed] [Google Scholar]
- Nguyen HO, Goracke-Postle CJ, Kaminski LL, Overland AO, Morgan AD, Fairbanks CA. Neuropharmacokinetic and dynamic studies of agmatine. Ann N Y Acad Sci. 2003;1009:82–105. doi: 10.1196/annals.1304.009. [DOI] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Silva AJ. A pharmacogenetic inducible approach to the study of NMDA/alphaCaMKII signaling in synaptic plasticity. Curr Biol. 2002;12:654–656. doi: 10.1016/s0960-9822(02)00767-4. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Willner P. Selective blockade of drug-induced place preference conditioning by ACPC, a functional NDMA-receptor antagonist. Neuropsychopharmacology. 2002;27:727–743. doi: 10.1016/S0893-133X(02)00349-4. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Bartmann A, Spielmanns P, Frankiewicz T, Hesselink M, Eilbacher B, Quack G. Amino-alkyl-cyclohexanes are novel uncompetitive NMDA receptor antagonists with strong voltage-dependency and fast blocking kinetics: in vitro and in vivo characterization. Neuropharmacology. 1999a;38:85–108. doi: 10.1016/s0028-3908(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999b;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Piletz JE, May PJ, Wang G, Zhu H. Agmatine crosses the blood-brain barrier. Ann N Y Acad Sci. 2003;1009:64–74. doi: 10.1196/annals.1304.007. [DOI] [PubMed] [Google Scholar]
- Raasch W, Schafer U, Qadri F, Dominiak P. Agmatine, an endogenous ligand at imidazoline binding sites, does not antagonize the clonidine-mediated blood pressure reaction. Br J Pharmacol. 2002;135:663–672. doi: 10.1038/sj.bjp.0704513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Regunathan S. Agmatine: an endogenous ligand at imidazoline receptors may be a novel neurotransmitter in brain. J Auton Nerv Sys. 1998;72:80–85. doi: 10.1016/s0165-1838(98)00091-5. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Rush EA, Aizenman E. Reduction of NMDA receptors with dithiothreitol increases [3H]-MK-801 binding and NMDA-induced Ca2+ fluxes. Br J Pharmacol. 1990;101:178–182. doi: 10.1111/j.1476-5381.1990.tb12109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JC. Ph D Thesis Dissertation. 2007. Pharmacokinetics, Pharmacodynamics and Transport of Agmatine in the CNS. [Google Scholar]
- Roberts JC, Grocholski BM, Kitto KF, Fairbanks CA. Pharmacodynamic and pharmacokinetic studies of agmatine after spinal administration in the mouse. J Pharmacol Exp Ther. 2005;314:1226–1233. doi: 10.1124/jpet.105.086173. [DOI] [PubMed] [Google Scholar]
- Sahraei H, Poorheidari G, Foadaddini M, Khoshbaten A, Asgari A, Noroozzadeh A, Ghoshooni H, Firoozabadi SH, Zarrindast MR. Effects of nitric oxide on morphine self-administration in rat. Pharmacol Biochem Behav. 2004;77:111–116. doi: 10.1016/j.pbb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Semenova S, Danysz W, Bespalov A. Low-affinity NMDA receptor channel blockers inhibit acquisition of intravenous morphine self-administration in naive mice. Eur J Pharmacol. 1999;378:1–8. doi: 10.1016/s0014-2999(99)00431-8. [DOI] [PubMed] [Google Scholar]
- Yang XC, Reis DL. Agmatine selectively blocks the N-Methyl-D-Aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther. 1999;288:544–549. [PubMed] [Google Scholar]