Abstract
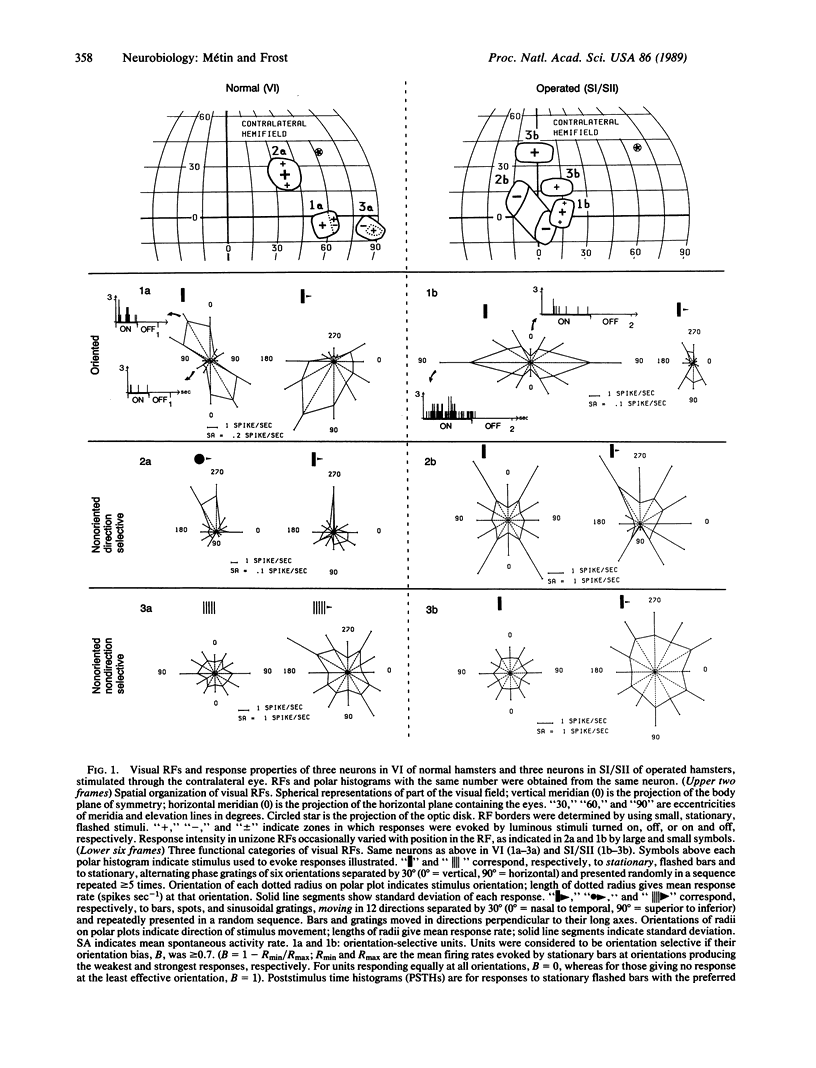
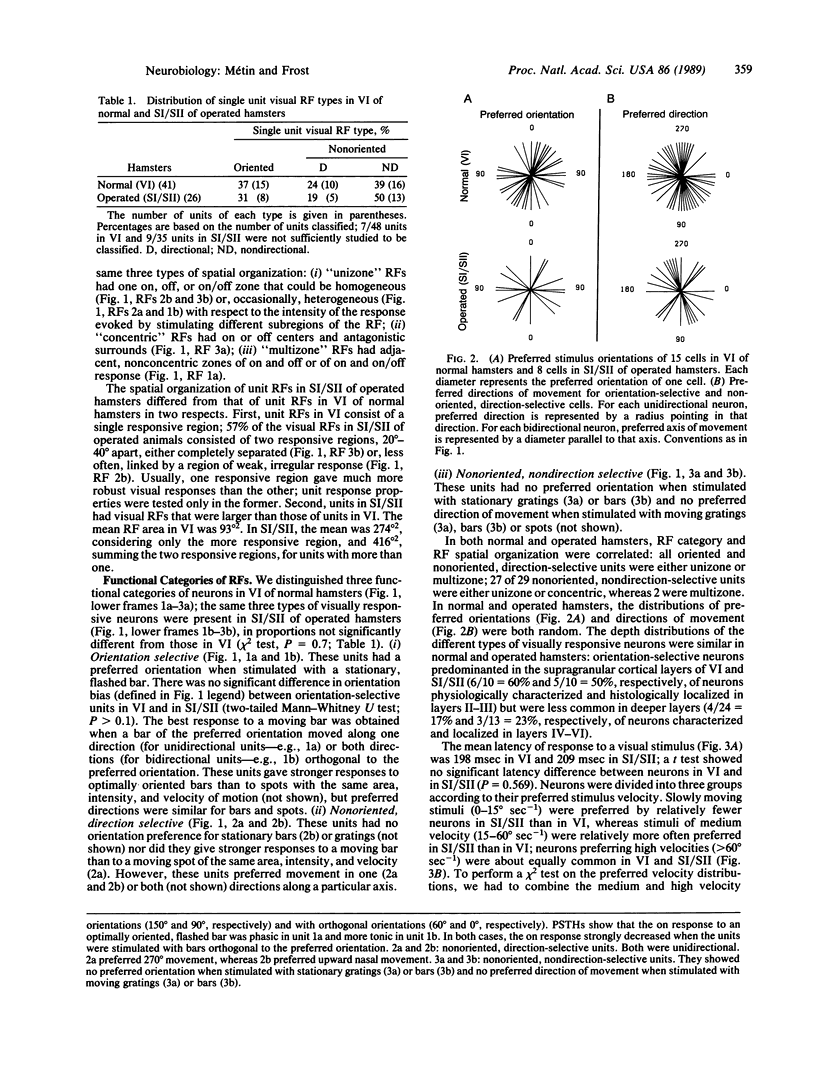
These experiments investigate the capacity of thalamic and cortical structures in a sensory system to process information of a modality normally associated with another system. Retinal ganglion cells in newborn Syrian hamsters were made to project permanently to the main thalamic somatosensory (ventrobasal) nucleus. When the animals were adults, single unit recordings were made in the somatosensory cortices, the principal targets of the ventrobasal nucleus. The somatosensory neurons responded to visual stimulation of distinct receptive fields, and their response properties resembled, in several characteristic features, those of normal visual cortical neurons. In the visual cortex of normal animals and the somatosensory cortex of operated animals, the same functional categories of neurons occurred in similar proportions, and the neurons' selectivity for the orientation or direction of movement of visual stimuli was comparable. These results suggest that thalamic nuclei or cortical areas at corresponding levels in the visual and somatosensory pathways perform similar transformations on their inputs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell G., Frost D. O. Synaptic organization of anomalous retinal projections to the somatosensory and auditory thalamus: target-controlled morphogenesis of axon terminals and synaptic glomeruli. J Comp Neurol. 1988 Jun 15;272(3):383–408. doi: 10.1002/cne.902720308. [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr Architectonic map of neocortex of the normal mouse. J Comp Neurol. 1975 Nov 15;164(2):247–263. doi: 10.1002/cne.901640207. [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Frost D. O. Tangential organization of thalamic projections to the neocortex in the mouse. J Comp Neurol. 1980 Nov 15;194(2):335–367. doi: 10.1002/cne.901940205. [DOI] [PubMed] [Google Scholar]
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essick G. K., Whitsel B. L. Factors influencing cutaneous directional sensitivity: a correlative psychophysical and neurophysiological investigation. Brain Res. 1985 Dec;357(3):213–230. doi: 10.1016/0165-0173(85)90025-6. [DOI] [PubMed] [Google Scholar]
- Frost D. O. Anomalous visual connections to somatosensory and auditory systems following brain lesions in early life. Brain Res. 1982 Apr;255(4):627–635. doi: 10.1016/0165-3806(82)90058-x. [DOI] [PubMed] [Google Scholar]
- Frost D. O. Axonal growth and target selection during development: retinal projections to the ventrobasal complex and other "nonvisual" structures in neonatal Syrian hamsters. J Comp Neurol. 1984 Dec 20;230(4):576–592. doi: 10.1002/cne.902300407. [DOI] [PubMed] [Google Scholar]
- Frost D. O., Caviness V. S., Jr Radial organization of thalamic projections to the neocortex in the mouse. J Comp Neurol. 1980 Nov 15;194(2):369–393. doi: 10.1002/cne.901940206. [DOI] [PubMed] [Google Scholar]
- Frost D. O. Development of anomalous retinal projections to nonvisual thalamic nuclei in Syrian hamsters: a quantitative study. J Comp Neurol. 1986 Oct 1;252(1):95–105. doi: 10.1002/cne.902520106. [DOI] [PubMed] [Google Scholar]
- Frost D. O., Metin C. Induction of functional retinal projections to the somatosensory system. Nature. 1985 Sep 12;317(6033):162–164. doi: 10.1038/317162a0. [DOI] [PubMed] [Google Scholar]
- Frost D. O. Orderly anomalous retinal projections to the medial geniculate, ventrobasal, and lateral posterior nuclei of the hamster. J Comp Neurol. 1981 Dec 1;203(2):227–256. doi: 10.1002/cne.902030206. [DOI] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984 Jan;64(1):325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen J., Poranen A. Movement-sensitive and direction and orientation-selective cutaneous receptive fields in the hand area of the post-central gyrus in monkeys. J Physiol. 1978 Oct;283:523–537. doi: 10.1113/jphysiol.1978.sp012517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. G., Coulter J. D., Hendry S. H. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol. 1978 Sep 15;181(2):291–347. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Wise S. P. Size, laminar and columnar distribution of efferent cells in the sensory-motor cortex of monkeys. J Comp Neurol. 1977 Oct 15;175(4):391–438. doi: 10.1002/cne.901750403. [DOI] [PubMed] [Google Scholar]
- Kaas J. H. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev. 1983 Jan;63(1):206–231. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- Kalil R. E., Schneider G. E. Abnormal synaptic connections of the optic tract in the thalamus after midbrain lesions in newborn hamsters. Brain Res. 1975 Dec 26;100(3):690–698. doi: 10.1016/0006-8993(75)90171-7. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Sur M., Lin C. S. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in "SI" in the owl monkey (Aotus trivirgatus). J Comp Neurol. 1978 Sep 1;181(1):41–73. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Rojas A., Torrealba F. Retinotopic organization of striate and peristriate visual cortex in the albino rat. Brain Res. 1973 Apr 13;53(1):197–201. doi: 10.1016/0006-8993(73)90780-4. [DOI] [PubMed] [Google Scholar]
- Métin C., Godement P., Imbert M. The primary visual cortex in the mouse: receptive field properties and functional organization. Exp Brain Res. 1988;69(3):594–612. doi: 10.1007/BF00247312. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Early lesions of superior colliculus: factors affecting the formation of abnormal retinal projections. Brain Behav Evol. 1973;8(1):73–109. doi: 10.1159/000124348. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Tiao Y. C., Blakemore C. Functional organization in the visual cortex of the golden hamster. J Comp Neurol. 1976 Aug 15;168(4):459–481. doi: 10.1002/cne.901680403. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T. R. A model of striate response properties based on geniculate anisotropies. Biol Cybern. 1987;57(1-2):11–23. doi: 10.1007/BF00318712. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]