Abstract
One-second-long increases in prefrontal cholinergic activity (“transients”) were demonstrated previously to be necessary for the incorporation of cues into ongoing cognitive processes (“cue detection”). Nicotine and, more robustly, selective agonists at α4β2* nicotinic acetylcholine receptors (nAChRs) enhance cue detection and attentional performance by augmenting prefrontal cholinergic activity. The present experiments determined the role of β2-containing and α7 nAChRs in the generation of prefrontal cholinergic and glutamatergic transients in vivo. Transients were evoked by nicotine, the α4β2* nAChR agonist ABT-089 [2-methyl-3-(2-(S)-pyrrolindinylmethoxy) pyridine dihydrochloride], or the α7 nAChR agonist A-582941 [2-methyl-5-(6-phenyl-pyridazin-3-yl)-octahydro-pyrrolo[3,4-c]pyrrole]. Transients were recorded in mice lacking β2 or α7 nAChRs and in rats after removal of thalamic glutamatergic or midbrain dopaminergic inputs to prefrontal cortex. The main results indicate that stimulation of α4β2* nAChRs evokes glutamate release and that the presence of thalamic afferents is necessary for the generation of cholinergic transients. ABT-089-evoked transients were completely abolished in mice lacking β2* nAChRs. The amplitude, but not the decay rate, of nicotine-evoked transients was reduced by β2* knock-out. Conversely, in mice lacking the α7 nAChR, the decay rate, but not the amplitude, of nicotine-evoked cholinergic and glutamatergic transients was attenuated. Substantiating the role of α7 nAChR in controlling the duration of release events, stimulation of α7 nAChR produced cholinergic transients that lasted 10- to 15-fold longer than those evoked by nicotine. α7 nAChR-evoked cholinergic transients are mediated in part by dopaminergic activity. Prefrontal α4β2* nAChRs play a key role in evoking and facilitating the transient glutamatergic–cholinergic interactions that are necessary for cue detection and attentional performance.
Introduction
The cortical cholinergic input system is a critical component of forebrain circuitry mediating attention processes. Removal of cholinergic inputs to the cortex permanently impairs attentional performance. Attentional performance increases the release of acetylcholine (ACh) in the cortex. Cholinergic drugs modulate attentional performance and the activity of brain regions mediating attention (McGaughy et al., 1996; Turchi and Sarter 1997; Arnold et al., 2002; Sarter et al., 2005; Kozak et al., 2006, 2007; Bentley et al., 2008; Furey et al., 2008; Deco and Thiele, 2009).
The specific cognitive operations that depend on cholinergic activity in the prefrontal cortex (PFC) were determined recently. One-second-based increases in PFC cholinergic activity (“transients”) mediate the detection of cues. Cues that failed to evoke such cholinergic transients were missed. Likewise, preventing the generation of transients resulted in misses (Parikh et al., 2007; Parikh and Sarter, 2008).
In animals and humans, the administration of nicotine has been demonstrated to enhance, albeit with limited efficacy and dependent on specific task conditions, cue detection processes and attentional performance (Hahn et al., 2003, 2007; Newhouse et al., 2004; Disney et al., 2007). Accumulating evidence from studies in animals, healthy humans, and patient groups suggests that selective agonists at α4β2* nicotinic ACh receptors (nAChRs) may more efficaciously enhance attention and attention-dependent cognitive performance (Buccafusco et al., 1995; McGaughy et al., 1999; Potter et al., 1999; Wilens et al., 1999, 2006; Hahn et al., 2003; Dunbar et al., 2007; Wilens and Decker, 2007; Howe et al., 2010).
nAChR agonists enhance attentional performance primarily by increasing the probability and efficacy of cue detection. Such enhancement of cue detection is mediated via augmentation of cue-evoked cholinergic transients (Sarter et al., 2009a). The more robust attentional effects of α4β2* nAChR agonists when compared with nicotine appear to be attributable to a more potent positive modulation of the amplitude, but not the duration, of these transients (Howe et al., 2009, 2010). In contrast to the “sharpening” of transients by stimulation of α4β2* nAChRs, longer release events, such as those evoked by nicotine, are hypothesized to insert “noise” into the detection mechanisms, thereby constraining the beneficial effects of larger amplitudes (Sarter et al., 2009a,b).
Our previous studies in rats demonstrated that stimulation of prefrontal α4β2* nAChR, using the selective α4β2* nAChR agonist ABT-089 (Decker et al., 1997; Sullivan et al., 1997; Rueter et al., 2004; Marks et al., 2009), evokes cholinergic transients that mirror the sharp, spike-like rise time and decay rates of transients mediating cue detection in performing animals (Parikh et al., 2008). The present experiments used mice lacking *β2* or α7 nAChRs to test the hypotheses that the generation of fast cholinergic transients requires stimulation of α4β2* nAChRs, that α4β2* nAChR stimulation evokes glutamate release, that the presence of thalamocortical inputs is necessary for generating cholinergic transients, and that the slow decay rate of cholinergic signals evoked by nicotine is primarily attributable to stimulation of α7 nAChRs. Additional experiments addressed the potential role of dopaminergic inputs in the lasting ACh release evoked by stimulation of α7 nAChRs. The results form the basis for a prefrontal network model that describes how PFC glutamatergic–cholinergic interactions mediate fundamental cognitive operations (Couey et al., 2007; Poorthuis et al., 2009).
Materials and Methods
Mice.
C57BL/6J mice were used to generate dose–response information on the effects of nicotine and ABT-089 on the amplitude and decay rate of cholinergic and glutamatergic transients. C57BL/6J mice of either sex were obtained from Harlan. The wild-type mice used for experiments involving knock-out (KO) animals were the respective wild types for each strain.
Chimeric mice with a targeted deletion of the β2 or α7 subunit of the nAChR gene were originally generated at the Baylor College of Medicine (Houston, TX) (Orr-Urtreger et al., 1997; Xu et al., 1999) and maintained on a C57BL/6J background for >10 generations. Mice were bred at Charles River Laboratories for Abbott Laboratories under a license to Abbott Laboratories from Baylor College of Medicine. Heterozygous mice were intercrossed for eight generations to obtain β2 or α7 mutants and wild types at Charles River Laboratories before being shipped to the University of Michigan. The genotype of the mutated gene was confirmed using PCR-based genotyping as described previously (Orr-Urtreger et al., 1997; Xu et al., 1999). Mice weighing 20–30 g were used for all studies.
Rats.
Adult male Fisher/Brown Norway hybrid rats (FBNF1; Harlan, weighing 200–250 g at the beginning of the experiments, were used for the following studies: (1) to investigate whether cholinergic transients evoked by α4β2* nAChR agonist require the presence of thalamocortical glutamatergic afferents, and (2) to test the hypothesis that the long-lasting release of ACh evoked by stimulation of prefrontal α7 nAChRs requires the presence of dopaminergic projections from the midbrain.
After arrival at the University of Michigan, animals were allowed to habituate for 2–3 weeks before the onset of experiments. Animals were individually housed in a temperature-controlled (23°C) and humidity-controlled (45%) environment and on a 12 h light/dark cycle (lights on at 6:30 A.M.). Animals were extensively handled before the beginning of experiments to minimize potential confounds resulting from the effects of novelty or stress. Food and water were available ad libitum (Rodent Chow; Harlan Teklad). All procedures were conducted in adherence with protocols approved by the University Committee on Use and Care of Animals.
Drugs and chemicals.
Choline oxidase (ChOase) (EC 1.1.3.17), bovine serum albumin (BSA), glutaraldehyde, ascorbic acid (AA), dopamine (DA), choline, l-glutamic acid, nicotine, tetrodotoxin (TTX), ibotenic acid, desipramine, 6-hydroxy-dopamine (6-OHDA), methylycaconitine citrate (MLA), and urethane were obtained from Sigma. ABT-089 [2-methyl-3-(2-(S)-pyrrolindinylmethoxy) pyridine dihydrochloride] and A-582941 [2-methyl-5-(6-phenyl-pyridazin-3-yl)-octahydro-pyrrolo [3,4-c]pyrrole] were provided by Abbott Laboratories. Glutamate oxidase (GO) (EC 1.4.3.11) was procured from Seikagaku America. Meta-phenylenediamine (m-PD) was obtained from Fluka Biochemika. Anti-tyrosine hydroxylase (TH) monoclonal antibody (clone LNCL; MAB 318), prediluted biotinylated goat anti-mouse IgG, streptavidin–HRP, and goat serum were purchased from Millipore Corporation. HPLC grade water (Thermo Fisher Scientific) was used to prepare all solutions. Solutions used for intracranial injections were prepared in 0.9% NaCl, pH 7.4, and filtered through 0.22 μm sterile nonpyrogenic filters (Corning Life Sciences) before use.
Preparation and calibration of enzyme-coated microelectrodes.
Ceramic-based biosensors (Quanteon LLC), featuring an array of four (15 × 333 μm) platinum recording sites arranged in pairs (upper or lower) were coated with ChOase or GO as described previously (Parikh et al., 2004, 2007; Parikh and Sarter, 2006). Briefly, ChOase or GO was cross–linked with the BSA–glutaraldehyde mixture and immobilized onto the bottom pair of recording sites. The upper two recording sites were coated only with the BSA–glutaraldehyde solution and served to record background activity. Before use for in vivo recordings, m-PD was electropolymerized onto the microelectrodes to enhance the selectivity for detecting analyte relative to currents produced by potential electroactive interferents, including AA and catecholamines. The m-PD plated, enzyme-coated microelectrodes were soaked in 0.05 m PBS for 30 min before calibration. Calibrations were performed using fixed potential amperometry by applying a constant voltage of 0.7 V versus Ag/AgCl reference electrode (Bioanalytical Systems) in a beaker containing a stirred solution of 0.05 m PBS (40 ml) maintained at 37°C using a FAST-16 electrochemical system (LLC; Quanteon). Amperometric currents were digitized at a frequency of 5 Hz. After achieving a stable baseline current, aliquots of stock solutions of AA (20 mm), choline or glutamate (20 mm), and DA (2 mm) were added to the calibration beaker such that the final concentrations were 250 μm AA, 20, 40, 60, and 80 μm for choline or glutamate, and 2 μm DA. The slope (sensitivity), limit of detection (LOD), and linearity (R2) for choline/glutamate, as well as selectivity ratio for AA, were calculated for individual recording sites. To be used in subsequent in vivo experiments, electrodes were required to meet the following criteria: >3 pA/μm sensitivity for detecting choline/glutamate on enzyme-coated channels, with a background current of <200 pA on all recording sites; LOD <300 nm choline; ratio of selectivity for choline (or glutamate) and AA, >80:1; detection of increasing analyte concentrations (20–80 μm) on enzyme-coated platinum recording sites, R2 > 0.98; negligible changes in current on all recording channels after DA addition (<3 pA).
In vivo recordings of cholinergic and glutamatergic transients in mice.
Amperometric recordings of cholinergic or glutamatergic transients were obtained from the medial PFC (mPFC) of mice of either sex (n = 3–7 per drug amount and strain). The mouse mPFC is homologous to the rat's prefrontal regions in the medial wall of the frontal cortex (Guldin et al., 1981; Irle et al., 1984). Our recordings were conducted in the thalamic input layers III/V of the prelimbic and infralimbic region and the ventral portion of the cingulate cortex. This region spans ∼1.5 mm in the dorsoventral dimension, and thus all four platinum recording sites could be placed within prefrontal cortex.
Animals were anesthetized with urethane (1.25–1.5 g/kg, i.p) and placed in a stereotaxic frame. The body temperature of animals was maintained at 37°C using an isothermal pad. A miniature (200 μm diameter) Ag/AgCl reference electrode was implanted in the brain at a site remote to the recording electrode. Single-barrel glass capillaries (1.0 × 0.58 mm, 6 in; A-M Systems) were pulled using a micropipette puller (model 51210; Stoelting) and then bumped until the diameter of the inner tip was ∼15 μm. The micropipette was attached to the microelectrode with the tip placed between the lower and upper pairs of recording sites, at a distance of ∼70 μm from the electrode surface. The micropipette was loaded with one of the test solutions before microelectrode implantation. The microelectrode/micropipette assembly was lowered into the third cortical layer (agranular layer) of either the right or left mPFC [anteroposterior (AP), +1.9 mm; mediolateral (ML), ±0.5 mm; dorsoventral (DV), −2.0 mm from bregma] (Franklin and Paxinos, 2008) using a microdrive. Amperometric recordings were conducted in both hemispheres (with side counterbalanced for each drug amount across animals within group), and responses for one concentration per drug per hemisphere from each animal were recorded. Drug solutions were pressure ejected from the micropipettes using a Picospritzer (Picospritzer III; Parker Hannifin) by applying a pressure of 2–10 psi for 1–2 s; ejection volumes were monitored using a stereomicroscope fitted with a reticule. Amperometric recordings were made at 1 Hz by applying a fixed potential of +0.7 V to the electrode, and data were digitized using a FAST-16 recording system (Quanteon). Experiments began after stabilization of the baseline current for 45–60 min in each hemisphere.
Experimental design.
The effects of nicotine and ABT-089 on prefrontal cholinergic activity were determined on the basis of cholinergic signal levels produced by a series of intracranial pressure ejections of either drug (10 pmol, 50 pmol, 1 nmol, and 2.5 nmol; drug was a between-subject variable) in C57BL/6J mice. The amounts of drug that were pressure ejected were calculated on the basis of the volume of ejections (50 nl) of 200 μm, 1 mm, 20 mm, and 50 mm solutions. To determine whether nAChR-evoked cholinergic signals reflect impulse-dependent release of ACh, the effects of TTX on nicotine-evoked ACh release in the mPFC were assessed by copressure ejecting TTX (50 pmol) with nicotine (1 nmol). To determine nAChR agonist-evoked cholinergic transients in the mPFC of mice lacking the β2 and α7 nAChR subunit, the effects of local application of nicotine (1 nmol) or ABT-089 (1 nmol) were determined in these mice and compared with effects in their respective wild-type mice. The effect of α7 nAChR stimulation on prefrontal cholinergic transients was assessed in wild-type mice and mice lacking the α7 nAChR subunit by pressure ejecting A-582941 (500 pmol), a specific α7 nAChR agonist.
In a separate series of experiments, the effects of nAChR agonists on prefrontal glutamate release were assessed using GO-coated microelectrodes. The effects of nicotine and ABT-089 were tested for a series of doses (10 pmol, 50 pmol, and 1 nmol) in different groups of C57BL/6J mice. To determine the neuronal origin of nAChR agonist-induced increases in glutamate release, the effects of voltage-regulated Na+ channel blocker TTX (50 pmol) were pressure ejected together with nicotine (1 nmol). Finally, to determine the contribution of β2 and α7 nAChR to nAChR agonist-evoked glutamatergic transients, the effects of intracortical administration of nicotine (1 nmol) and ABT-089 (1 nmol) were tested in β2 KO or α7 KO animals.
Lesion surgeries in rats.
All surgeries were performed under aseptic conditions. Anesthesia was induced with 4–5% isoflurane using anesthesia machine (Anesco/Surgivet). Rats were placed in stereotaxic instrument (model 962; David Kopf Instruments), and anesthesia was maintained with 2% isoflurane along with oxygen at a flow rate of 1 ml/min. Rats (n = 5) received bilateral infusions of ibotenic acid (10 μg/μl in 0.9% sodium chloride; 0.5 μl/hemisphere) into the mediodorsal thalamic nucleus (MD) using the following coordinates: AP, −2.8 mm; ML, ±0.7 mm relative to bregma; DV, −5.4 mm relative to dura) (Paxinos and Watson, 1988). Sham-lesioned control animals (n = 5) were infused with sterile saline (0.5 μl/hemisphere) into MD. Infusions were made at a rate of 0.25 μl/min using a 1 μl Hamilton microsyringe; the needle remained in place for an additional 4 min after the infusion. Amperometric recording sessions (see below) were performed 3 weeks after lesion surgeries.
Dopaminergic inputs to mPFC were removed by infusing 6-OHDA into the ventral tegmental area (VTA) as described previously (Pioli et al., 2008). Rats (n = 6) received bilateral infusions of 6-OHDA (2 μg/μl in 0.9% sodium chloride solution containing 0.01% ascorbic acid; 1.0 μl/hemisphere) into VTA using the following coordinates: AP, −5.2 mm; ML, ±0.8 mm relative to bregma; DV, −7.6 mm. Sham-lesioned control animals (n = 6) were infused with sterile saline containing 0.01% ascorbic acid (1.0 μl/hemisphere) into the VTA. Infusions were made at a rate of 0.25 μl/min using a 1 μl Hamilton microsyringe. The needle was left in position for 4 min to allow proper absorption of the toxin in the target region. All animals undergoing VTA lesion surgeries were pretreated with desipramine (25 mg/kg, i.p.) 30 min before surgery to protect noradrenergic neurons. Amperometric recording sessions were performed 3 weeks after lesion surgeries.
In vivo recordings of cholinergic transients in rats.
Amperometric recordings of cholinergic transients were conducted in mPFC of urethane-anesthetized rats using FAST-16 recording system as described above for mice. Briefly, the microelectrode/micropipette assembly was lowered into the right mPFC (AP, +3.0 mm; ML, ±0.7 mm from bregma; DV, −3.0 mm from dura) using a microdrive. All recordings were made at a 1 Hz sampling rate, and currents were digitized using FAST-16 software. Experiments began after stabilization of the baseline current for 60 min by pressure ejecting the drug solutions into the mPFC using a Picospritzer.
Experimental design.
To test the hypothesis that prefrontal cholinergic transients evoked by stimulation of α4β2* nAChRs require the presence of glutamatergic projections from the thalamus, the effect of local application of ABT-089 (4 nmol) on cholinergic transients was assessed in MD-lesioned and control rats. To study the involvement of α7 nAChRs in the mediation of long-lasting increases in ACh release in the mPFC, we determined the amplitudes and decay rate of cholinergic transients evoked by pressure ejections of two doses of A-582941 (200 pmol and 2 nmol). Furthermore, to determine the receptor specificity of A-582941-mediated effects on cholinergic signals, cholinergic transients evoked by A-582941 (2 nmol) were recorded in the presence of the α7 nAChR antagonist MLA (200 pmol) (Dwoskin and Crooks, 2001). Finally, to investigate whether the α7 nAChR-mediated long-lasting release of ACh requires dopaminergic inputs from VTA; we tested the effects of locally applied A-582941 (2 nmol) on evoked cholinergic signals in 6-OHDA–VTA-lesioned animals.
Histology and immunohistochemistry.
After completion of amperometric recording sessions, animals were transcardially perfused with 30 ml (mice) or 100 ml (rats) of ice-cold heparinized saline, followed by 50 ml (mice) or 250 ml (rats) of 4% paraformaldehyde in 0.1 m PBS, pH 7.4. Brains were removed and postfixed overnight at 4°C and stored in 30% sucrose in 0.1 m PBS for 72 h. Coronal sections (50 μm) from mPFC were sliced using a freezing microtome (CM 2000R; Leica) and stored in cryoprotectant solution (15% glucose, 30% ethylene glycol, and 0.04% sodium azide in 0.05 m PBS, pH 7.4) at −20°C until additional processing.
For verification of intracortical placement of microelectrodes, sections from the recording regions were thawed, washed in 0.1 m PBS, and stained with cresyl violet solution. Ibotenic acid-induced MD lesions in rats were confirmed by morphological inspection of the Nissl-stained sections for neuronal cell loss and the presence of gliosis. Serial sections from the VTA (6-OHDA infusion sites) and mPFC (recording regions) were processed for visualization of TH-stained immunopositive cells and immunoreactive (IR) fibers. Briefly, free-floating sections were thawed, washed twice in 0.05 m Tris-buffered saline (TBS), pH 7.4, for 10 min and incubated with 0.3% H2O2 for 30 min to block endogenous peroxidase. After washing with TBS, the sections were incubated with blocking solution (10% goat serum in 0.05 mm TBS) with constant shaking for 1 h, followed by overnight incubation with anti-TH antibody (diluted 1:400 in 0.05 m TBS containing 1% donkey serum and 0.1% Triton X-100) at 4°C. After primary antibody incubation, the sections were washed in TBS-T (0.05 m TBS containing 0.1% Triton X-100) three times for 5 min and incubated with prediluted biotinylated goat anti-mouse IgG for 2 h at room temperature. After washing in TBS-T, the sections were incubated with streptavidin–HRP (1:1000) for 45 min at room temperature. Sections were washed again in TBS-T, and staining was developed with 3-3′-diaminobenzidine. Stained sections were mounted onto gelatin-coated slides and air dried. Slides were then dehydrated and coverslipped with DPX. All sections were analyzed using a Leica DM4000B microscope equipped with a SPOT Digital Camera and SPOT software (Diagnostics Inc.). Loss of dopaminergic afferents to mPFC was verified using a semiquantitative analysis of TH-IR fibers using NIH ImageJ software (http://rsb.info.nih.gov/ij). The density of TH-IR fibers in the mPFC was expressed as the percentage of TH-positive pixels in the analyzed area (0.16 mm2). The average fiber pixel density count was based on three sections per animal.
Analyses of cholinergic and glutamatergic transients.
If background noise levels on enzyme-coated channels exceeded 10 pA (equivalent to ∼2 μm choline or glutamate) or artifacts occurred (instantaneous electrostatic buildup of >10 pA), such as in response to pressure-ejection procedures, currents recorded via enzyme-coated sites were corrected by subtracting currents recorded on sentinel sites [“self-referencing” (Parikh et al., 2008)]. Transient signals were analyzed with respect to peak amplitudes and signal decay rate (t50, time required for the signal to decline by 50% from peak amplitude). The averages of two responses per drug manipulation and per animal were used for statistical analyses.
Statistical analyses.
Statistical analyses were conducted using SPSS/PC+ V.13.0 (SPSS). Mixed-factor ANOVAs were used to analyze the effects of drug (nicotinic agonists; two levels), dose (four levels), hemispheric laterality (two levels), and sex (two levels) on amplitudes and decay rates of cholinergic transients. Likewise, the effects of nAChR agonists (two levels) and doses (three levels) on glutamatergic transients were analyzed using mixed-factor ANOVAs. Mixed-factor ANOVAs were also used to assess the effects of genotype (two levels) and drugs (two levels). Post hoc multiple comparisons of within-subject data were conducted using the least significant difference (LSD) test. To compare the effects of nicotine, ABT-089 and A-582941 on cholinergic or glutamatergic transients recorded in both genotypes, sham and lesioned rats, and for other pharmacological manipulations in mice and rats, planned two-tailed unpaired Student's t tests were used. Exact p values are reported as recommended by Greenwald et al. (1996). α was set at 0.05.
Results
Electrode properties
Table 1 summarizes the electrochemical properties of choline- and glutamate-sensitive electrodes that were used in these experiments.
Table 1.
Properties of choline-selective and glutamate-selective microelectrodes (calibration data)
| Sensitivity (pA/μm) | LOD (nm) | R2 | Selectivity |
|---|---|---|---|
| Choline-selective | |||
| 10.67 ± 0.56 | 307.16 ± 28.71 | 0.986 ± 0.002 | 707.76 ± 154.41 |
| Glutamate-selective | |||
| 5.89 ± 0.36 | 365.45 ± 24.62 | 0.990 ± 0.004 | 249.16 ± 56.05 |
Data are based on 90 choline-sensitive and 30 glutamate-sensitive microelectrodes (mean ± SEM). R2 depicts the linearity of the response of the microelectrode to increasing concentrations of choline or glutamate, respectively. Selectivity refers to the ratio of the electrode response to choline or glutamate relative to ascorbic acid.
Intracortical placement of microelectrodes
Microelectrode placement in rats and mice were verified on the basis of Nissl-stained sections. The presence of a tract produced by insertion of the ceramic wafer into the prelimbic region of mPFC was determined as described previously (Parikh et al., 2006, 2007). Examination of serial sections near the recording region indicated that choline and glutamatergic transients were recorded in the thalamic input layers III/V of the prelimbic cortex.
Sensitivity of choline transients recorded in mice to TTX
We demonstrated previously that choline transients recorded in the cortex of rats are almost completely attenuated by blocking voltage-regulated sodium channels with TTX (Parikh et al., 2004). Choline transients evoked in the prefrontal cortex of mice by local stimulation of nAChR likewise were almost completely attenuated by TTX (Fig. 1A) (mean ± SEM amplitudes: nicotine, 3.23 ± 0.46 μm; nicotine + TTX, 0.52 ± 0.11 μm; t(6) = 4.87, p = 0.003).
Figure 1.
Choline transients evoked by nicotine and ABT-089 in the prefrontal cortex of C57BL/6J mice. A, Examples of choline transients (self-referenced traces) evoked by pressure ejections of nicotine (1 nmol) and in the presence of TTX (50 pmol; n = 4 per condition). TTX almost completely attenuated the amplitudes of choline transients that were evoked by nicotine (B; mean ± SEM; **p < 0.01). B, Examples of self-referenced traces evoked by pressure ejections of 50 pmol of nicotine or ABT-089. The traces illustrate the greater potency, in terms of amplitude, and the faster decay rate of transients evoked by ABT-089 when compared with nicotine. C, Dose–response curve for the effects of nicotine and ABT-089 on transient amplitudes. Compared with nicotine, ABT-089 was more potent but not more efficacious in increasing cholinergic activity. D, Dose–response curve for the effects of nicotine and ABT-089 on the decay of choline transients. Compared with nicotine, choline transients evoked by ABT-089 returned faster to basal current levels. Nicotine-evoked cholinergic activity lasted longer and more slowly returned to baseline when compared with the more rapid attenuation of ACh release evoked by the α4β2* nAChR agonist (*p < 0.05, **p < 0.01, ***p < 0.001, based on multiple comparisons performed using the LSD for within-subject data and t tests for between-subject data; for significant ANOVA results justifying these multiple comparisons, see Results).
Dose–response in mice
Prefrontal administration of nicotine and ABT-089 (10 pmol to 2.5 nmol) resulted in transient increases in cholinergic activity. Representative traces illustrating choline transients evoked by the two nAChR agonists are shown in Figure 1, A and B. The amplitudes of the choline transients evoked by both compounds were dose dependent (main effect of dose, F(3,28) = 4.42, p = 0.01) (Fig. 1C). The statistical analysis did not indicate a significant interaction between the effects of drug and dose (F(3,28) = 0.78, p = 0.51), reflecting that both drugs dose dependently increased the amplitudes of choline transients, as well as the finding that the efficacy of nicotine and ABT-089, in terms of the amplitude of choline transients, did not differ. However, post hoc one-way ANOVAs and multiple comparisons only indicated significant differences between the amplitudes evoked by the two lower versus the two higher doses of nicotine (F(3,14) = 19.20, p < 0.001, LSD; all four tests comparing the two lower against the two higher doses, all p values <0.002) (Fig. 1C).
Compared with the amplitudes of choline transients evoked by nicotine, ABT-089 was more potent (main effect of drug, F(1,28) = 5.38, p = 0.02). After pressure ejections of the two lower doses (10 and 50 pmol), ABT-089 produced significantly larger choline signal amplitudes than nicotine (10 pmol, t(6) = 2.42, p = 0.05; 50 pmol, t(6) = 3.74, p = 0.01). Higher concentrations of nicotine and ABT-089 (1 and 2.5 nmol) resulted in equivalent peak amplitudes (both p > 0.05) (Fig. 1C).
The decay of choline transients primarily reflects ongoing and slowly diminishing ACh release (Parikh and Sarter, 2006). Choline transients evoked by ABT-089 exhibited significantly faster decay rates than nicotine (main effect of drug, F(1,28) = 29.55, p < 0.001) (Fig. 1C). Multiple comparisons indicated that, after the administration of all but the smallest dose of ABT-089, choline transients decayed significantly faster when compared with nicotine (10 pmol, t(6) = 1.46, p = 0.19; all other three p values <0.04). As illustrated in Figure 1C, the decay rate for ABT-089 was virtually flat across doses (7.76 ± 0.82 s, mean ± SEM). Although inspection of Figure 1C suggests an increase in t50 after 50 pmol of nicotine when compared with the lower dose, the effects of nAChR agonists on decay rate remained unaffected by dose (main effect of dose, F(3,28) = 0.54, p = 0.65; dose × drug, F(3,28) = 1.04, p = 0.39; 14.18 ± 0.85 s).
Choline transients evoked by nicotine or ABT-089 were recorded from both hemispheres. The amplitudes and decay rates did not differ between the two hemispheres (drug, dose, hemisphere; all p values >0.05). Likewise, choline transients did not differ by sex (drug, dose, sex; all p values >0.05).
Choline transients in β2 and α7-KO mice
β2+/+ versus β2−/−
Choline transients in KO animals were recorded after the administration of 1 nmol of nicotine and ABT-089 (Fig. 2). This dose was selected because it is the lowest dose for either drug that evoked maximum and equivalent amplitudes of choline transients in C57BL/6J mice (Fig. 1C). Compared with choline transients recorded in wild-type mice, the amplitudes of nicotine-evoked transients were partly attenuated by β2 KOs; those evoked by ABT-089 were completely abolished in β2-KO mice.
Figure 2.
Choline transients evoked by nicotine (1 nmol) and ABT-089 (1 nmol) in the prefrontal cortex of wild-type and β2 KO mice (the 1 nmol dose for either compound was selected for the assessment of genotype effects because these doses evoke transients with identical amplitudes in the background strain; see Fig. 1D). A, C, Self-referenced traces exemplifying the attenuation of nicotine-evoked (A) or ABT-089-evoked (C) choline transients in β2 KO mice (note that the trace evoked by ABT-089 in β2 KO mice; in C) did not differ from that evoked by vehicle in wild-type mice). B, β2 KO partly attenuated the amplitudes of nicotine-evoked transients and almost completely abolished ABT-089-evoked cholinergic activity. D, β2 KO did not affect the decay rate of nicotine-evoked transients (ABT-089-evoked transients recorded in β2-KO mice were too small to permit reliable determination of t50 values; *p < 0.05, **p <0.01, based on multiple comparisons performed on the basis of significant results of ANOVA; see Results).
The statistical analysis of choline signal amplitudes in β2+/+ and β2−/− mice indicated a main effect of genotype (F(1,18) = 28.79, p < 0.001), as well as a significant interaction between genotype and drug (F(1,18) = 5.50, p = 0.03; main effect of drug, F(1,18) = 0.50, p = 0.49). Post hoc comparisons confirmed that nicotine-evoked choline signal amplitudes were attenuated in β2 KO mice (t(10) = 2.32, p = 0.04) (Fig. 2A,B). ABT-089-evoked choline transients were completely abolished in β2-KO mice (t(9) = 4.28, p = 0.002; nicotine vs ABT-089 in β2 KO mice, t(8) = 2.06, p = 0.07) (Fig. 2C,D).
Because of the absence of significant choline transients evoked by ABT-089 in β2 KO mice, decay rates of transients evoked by this compound in β2 KO mice could not be determined. The decay rates of nicotine-evoked transients did not differ by genotype (t(10) = 0.49, p = 0.63) (Fig. 2D).
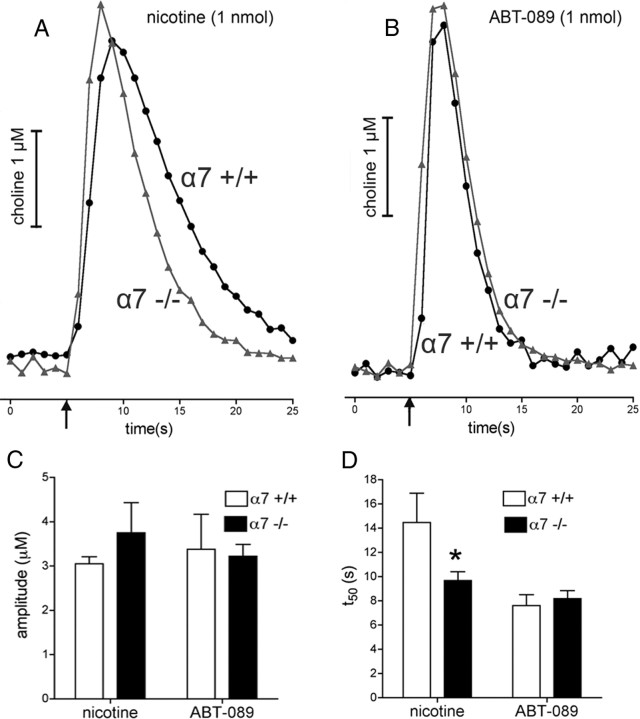
α7+/+ versus α7−/−
The amplitudes of nicotine- and ABT-089-evoked choline transients did not differ between wild-type and α7-KO mice (genotype, F(1,21) = 0.27, p = 0.61; drug, F(1,21) = 0.07, p = 0.79; genotype × drug, F(1,21) = 1.01, p = 0.33) (Fig. 3). Nicotine-evoked choline transients recorded in α7 KO mice decayed faster compared with those obtained from wild-type animals, as indicated by a significant interaction between genotype and drug (F(1,21) = 5.52, p = 0.02; main effect of genotype, F(1,21) = 3.44, p = 0.08). Post hoc multiple comparisons confirmed that the decay rate of nicotine-evoked, but not ABT-089-evoked, transients was attenuated in α7 KO mice (nicotine α7+/+ vs α7−/−, t(8) = 2.35, p = 0.04; ABT-089 α7+/+ vs α7−/−, t(14) = 0.72, p = 0.48) (Fig. 3D). Finally, the main effect of drug on t50 values (F(1,21) = 12.74, p = 0.002) reflected the generally faster decay rate of ABT-089 when compared with nicotine (see above).
Figure 3.
Choline transients evoked by nicotine (1 nmol) and ABT-089 (1 nmol) in the mPFC of wild-type and α7-KO mice. A, B, Representative self-referenced traces depicting choline transients evoked by nicotine (A) or ABT-089 (B) in wild-type and α7 KO mice. The duration but not the amplitudes of nicotine-evoked choline transients was attenuated in α7 KO mice. C, D, ABT-089-evoked transients were not affected by the absence of α7 nAChR (C, D; *p < 0.05, based on multiple comparisons performed on the basis of significant results of ANOVA; see Results).
nAChR agonist-evoked glutamatergic transients in mouse prefrontal cortex
Effects of TTX on evoked glutamate release
The measurement of glutamate release using more traditional techniques, such as microdialysis, indicated that a significant proportion of glutamate is not released by depolarization-dependent mechanisms (Timmerman et al., 1999). In contrast, prefrontal, nicotine-evoked glutamatergic transients measured by using GO-coated microelectrodes were robustly attenuated by local administration of TTX (by 77%; t(6) = 7.20, p < 0.001) (Fig. 4A,B; see Fig. 2C for a trace evoked by vehicle). This degree of TTX sensitivity of glutamatergic transients is consistent with our previous demonstration of TTX attenuation of evoked glutamatergic transients in rats (Parikh et al., 2008) and may reflect the closer placement of the small recording sites to presynaptic terminals (Day et al., 2006; Oldenziel et al., 2006).
Figure 4.
nAChR agonist-evoked glutamatergic transients in the mPFC of wild-type and β2 KO mice. A, B, In contrast to glutamate released measured by using conventional in vivo microdialysis methods, blockade of voltage-regulated sodium channels by TTX almost completely abolished nicotine-evoked increases in glutamate release. C, With respect to the amplitudes of glutamatergic transients, ABT-089 was more potent but not more efficacious than nicotine. D, The decay rate of glutamatergic transients evoked by ABT-089 remained flat over the entire dose range; in contrast, increasing doses of nicotine produced transients that required increasingly more time to decay, yielding a significantly slower decay of nicotine-evoked glutamatergic transients in response to the highest dose of drug. E, In β2 KO mice, the amplitudes of glutamatergic transients evoked by nicotine (1 nmol) were partly attenuated, whereas ABT-089 (1 nmol)-evoked transients were completely abolished. F, The decay of nicotine-evoked transients appeared to be slower in β2 KO mice, but this difference did not reach significance (ABT-089-evoked glutamatergic transients remained too small to allow a reliable determination of t50 values; *p < 0.05, **p < 0.01, ***p < 0.001, respectively, based on multiple comparisons performed using the LSD for within-subject data and t tests for between-subject data; for significant ANOVA results justifying these multiple comparisons, see Results).
Nicotine- and ABT-089-evoked glutamatergic transients
For these experiments, we tested the effects of only three doses of nicotine and ABT-089 (10 pmol, 50 pmol, and 1 nmol) because, similar to the amplitude of choline transients (above), preliminary evidence indicated that 1 nmol of either compound produces glutamatergic transients with maximum and equivalent amplitudes (Fig. 4C). Administration of nAChR agonists dose dependently increased the amplitudes of glutamatergic transients (main effect of dose, F(2,20) = 10.24, p = 0.001) (Fig. 4C). Furthermore, the amplitudes of glutamatergic transients evoked by the two agonists differed significantly (main effect of drug, F(1,20) = 17.52, p < 0.001), and the effects of drug and dose interacted significantly (F(1,20) = 4.75, p = 0.02). Post hoc analyses indicated that these main effects and the interaction reflected increasing amplitudes of transients evoked by increasing doses of nicotine, but not ABT-089, and that larger amplitudes were evoked by the two lower doses of ABT-089 when compared with these doses of nicotine (Fig. 4C).
The decay rates of glutamatergic transients remained rather flat after the administration of ABT-089. In contrast, increasing doses of nicotine resulted in longer decay rates, yielding main effects of dose (F(2,20) = 16.44, p < 0.001), drug (F(1,20) = 18.95, p < 0.001) and an interaction between the effects of the two factors on t50 values (F(1,20) = 8.98, p = 0.002). Post hoc comparisons (Fig. 4D) indicated that these main effects and the interaction were attributable to a dose-dependent slowing of the decay of nicotine-evoked transients only. Furthermore, after the administration of the highest dose of nicotine (1 nmol), decay rates of glutamatergic transients were significantly longer when compared with transients evoked by ABT-089 (Fig. 4D).
As observed with respect to choline transients, no hemispheric or sex-based differences in the amplitudes of nAChR-evoked glutamate release were found (main effect of hemisphere, F(1,22) = 0.08, p = 0.78; main effect of sex, F(1,22) = 0.85, p = 0.37).
Glutamatergic transients in β2 and α7-KO mice
β2+/+ versus β2−/−
Glutamatergic transients in KO animals were recorded after the administration of 1 nmol of nicotine and ABT-089. As indicated in Figure 4C, this dose was selected because it evoked maximum and equivalent amplitudes of choline transients in C57BL/6J mice.
Analyses of peak amplitudes of glutamatergic transients recorded in β2 KO and wild-type animals revealed significant main effects of genotype (F(1,11) = 49.3, p < 0.001) and drug (F(1,11) = 9.77, p = 0.01), as well as a significant interaction between these factors (F(1,11) = 4.93, p = 0.04). As illustrated in Figure 4E, nicotine-evoked glutamatergic transients in β2 KO mice, albeit with smaller amplitudes than in wild-type animals. In contrast, ABT-089-evoked glutamatergic transients were nearly eliminated in β2 KO mice (nicotine, t(6) = 2.92, p = 0.02; ABT-089, t(6) = 8.63, p < 0.001; nicotine vs ABT in β2 KO mice, t(8) = 5.27, p = 0.001).
Similar to the analysis of decay rates of ABT-089-evoked choline transients, ABT-089-evoked glutamatergic transients recorded in β2 KO mice were too small to allow determination of t50 values. Concerning nicotine-evoked glutamatergic transients in β2 KO mice, there was a trend for slower decay rates of when compared with wild-type animals (t(6) = 1.71, p = 0.1) (Fig. 4F). The β2 KO-induced slower decay rate (higher t50 values) of nicotine-evoked glutamatergic transients was associated with lower amplitudes, further substantiating that amplitude and decay rate are controlled by different neuronal mechanisms.
α7+/+ versus α7−/−
The amplitudes of glutamatergic transients evoked by the two compounds did not differ between wild-type and α7 KO mice (genotype, F(1,13) = 0.89, p = 0.36; drug, F(1,13) = 0.02, p = 0.88; genotype × drug, F(1,13) = 0.02, p = 0.88) (Fig. 5). However, nicotine-evoked glutamatergic transients decayed faster in α7 KO mice (genotype, F(1,13) = 7.34, p = 0.02; drug, F(1,13) = 14.33, p = 0.002; drug × genotype, F(1,13) = 5.13, p = 0.04). Multiple comparisons confirmed that the nicotine-evoked glutamatergic transients decayed faster in α7 KO animals (t(7) = 2.83, p = 0.02) and that the decay rates of glutamatergic transients evoked by ABT-089 did not contribute to the main effect of genotype (t(7) = 2.13, p = 0.07).
Figure 5.
nAChR agonist-evoked glutamatergic transients in the mPFC of wild-type and α7 KO mice. A, B, Examples of glutamatergic transients evoked by nicotine (A) or ABT-089 (B) in wild-type and α7 KO mice. Knock-out of α7 nAChRs reduced the duration, but did not affect the amplitude, of transients evoked by nicotine. C, D, ABT-089-evoked transients were not affected by α7 KO (C, D; *p < 0.05, based on multiple comparisons performed on the basis of significant results of ANOVA; see Results).
Effects of mediodorsal thalamic lesions on choline transients evoked by stimulation of α4β2* nAChRs
Previous studies indicated that prefrontal α4β2* nAChRs are situated on thalamocortical inputs (Gioanni et al., 1999; Lambe et al., 2003; Dickinson et al., 2008) and that stimulation of prefrontal ionotropic glutamate receptors is required for the demonstration of nAChR agonist-evoked choline transients (Parikh et al., 2008). This experiment was designed to demonstrate that thalamocortical inputs are necessary for the generation of α4β2* nAChRs agonist-evoked choline transients. The prefrontal cortex is defined in part based on the area innervated by the MD (Sarter and Markowitsch, 1984).
As illustrated in Figure 6, infusions of ibotenic acid into the region of the MD produced extensive gliosis and the near complete absence of neurons. In lesioned animals, choline transients evoked by ABT-089 (4 nmol) were almost completely abolished (mean ± SEM amplitudes: sham-operated, 3.91 ± 0.31 μm; lesioned, 0.93 ± 0.13 μm; t(8) = 8.82, p < 0.001). Note that the dose of ABT-089 that was selected for this experiment is higher than the three highest doses that produced transients with maximum amplitudes (Fig. 1D); this high dose was selected to minimize the possibility that the attenuating effects of the lesion reflected a decrease in the affinity of α4β2* nAChRs for ABT-089.
Figure 6.

Effects of lesions of the MD on prefrontal choline transients evoked by the α4β2* nAChR agonist ABT-089. A shows a Nissl-stained coronal section from an intact brain depicting the area of the MD as well as the adjacent nuclei [central medial thalamic nucleus (CM), oral paracentral thalamic nucleus (OPC), posterior paraventricular nucleus (PVP), and habenular]. B, Infusions of ibotenic acid produced extensive gliosis in the area of the MD; arrows depict the borders of the region lacking neurons. C, Self-referenced choline transients evoked by the α4β2* nAChR agonist in control and lesioned animals, respectively.
Long-lasting ACh release evoked by stimulation of α7 nAChR and role of dopamine
The evidence described above suggests that the decay rate of nicotine-evoked transients is attributable to stimulation of α7 nAChR. The first major aim of this experiment was to substantiate this hypothesis by assessing the effects of stimulation of α7 nAChR using a selective agonist, A-582941 (Bitner et al., 2007). The second aim of this experiment was to determine the role of dopaminergic afferents in the mediation of the cholinergic effects of α7 nAChR stimulation (Livingstone et al., 2009).
Choline transients evoked by A-582941 and evidence in support of selectivity for α7 nAChR
Prefrontal administration of the α7 nAChR agonist A-582941 in rats produced increases in ACh release that lasted for more than hundreds of seconds (Fig. 7). The amplitudes of transients evoked by A-582941 were dose dependent (t(6) = 2.53, p = 0.045) (Fig. 7B). Likewise, the higher dose produced transients that decayed more slowly (mean ± SEM, t50: 200 pmol, 63.93 ± 11.50 s; 2 nmol, 144.80 ± 20.90 s; t(6) = 2.46, p = 0.049). Note that the release evoked by α7 nAChR stimulation lasted 10- to 15-fold longer when compared with the release evoked by nicotine and the even shorter release events evoked by stimulation of α4β2* nAChRs.
Figure 7.

Cholinergic transients evoked by prefrontal pressure ejections of the α7 nAChR agonist A-582941 and role of dopaminergic afferents. A, Examples of traces evoked by 200 pmol and 2 nmol of A-582941. Note the extremely long-lasting increases in ACh release evoked by the α7 nAChR agonist; these release events began to decline 100–150 s after the administration of the higher dose, contrasting with 3–5 s for transients evoked by α4β2* nAChR agonists and with tens of seconds for transients evoked by nicotine (see Fig. 1; for t50 values for transients evoked by A-582941, see Results). Coadministration of the α7 nAChR antagonist MLA attenuated transients evoked by A-582941 (see B for effects on peak amplitudes). C, Removal of dopaminergic inputs to the PFC partly attenuated the release evoked by the α7 nAChR agonist. C shows examples of cholinergic transients evoked by α7 nAChR agonist A-582941 (2 nmol) in a sham-operated control animal and after dopaminergic lesions (for histological documentation of these lesions see Fig. 8; *p < 0.05).
We conducted two experiments to demonstrate that the choline transients evoked by A-582941 were attributable to stimulation of α7 nAChR. First, in rats, coadministration of the relatively selective α7 nAChR antagonist MLA almost completely attenuated choline transients evoked by the α7 agonist A-582941 (2 nmol, t(6) = 3.33, p = 0.02) (Fig. 7B). Second, we measured choline transients in wild-type mice as well as mice lacking the α7 nAChR. α7 KOs completely abolished the transients (amplitudes: A-582941 at 500 pmol in wild-type mice, 1.28 ± 0.27 μm; α7 KO, 0.16 ± 0.01 μm; t(6) = 6.98, p < 0.001).
Effects of removal of dopaminergic afferents
The density of TH-immunoreactive fibers in the mPFC was drastically reduced as a result of infusions of 6-OHDA into the midbrain (for determination of pixel densities, see Materials and Methods) (sham-operated controls, 1.64 ± 0.28% TH-positive pixels/0.16 mm2; lesioned, 0.18 ± 0.15%; t(9) = 5.48, p < 0.001) (Fig. 8). In the recording region in the middle layers, residual TH-positive fibers were nearly completely absent. As illustrated in Figure 8, this was associated with an almost complete absence of TH-positive neurons in the midbrain. Removal of dopaminergic innervation of the PFC partly attenuated the amplitudes of choline transients that were evoked by A-582941 (mean ± SEM amplitudes: sham-operated, 1.86 ± 0.24 μm; lesioned, 0.88 ± 0.07 μm; t(9) = 4.30, p = 0.002). However, the duration of release events evoked by the α7 agonist remained unaffected by removal of dopaminergic inputs (t(9) = 0.05, p = 0.96), indicating that this prominent characteristic of cholinergic transients evoked by stimulation of α7 nAChR is not mediated via dopaminergic activity.
Figure 8.

Lesions of the midbrain dopaminergic system. A–D are coronal sections immunostained against TH. A, Prelimbic region from the brain of an intact rat. TH-immunoreactive fibers and varicosities are visible throughout all layers, with vertical fibers being prominent in deep layers and white matter. C, In lesioned animals, TH-immunopositive puncta are almost completely abolished (see Results for quantification); residual TH-positive fibers were observed in deep layers and white matter (arrows). B, Substantia nigra (SN) and VTA dopaminergic neurons in an intact brain. D, The lesions almost completely destroyed TH-positive neurons in the midbrain (see arrows for residual neurons).
Discussion
We used glutamate- and choline-sensitive microelectrodes to characterize nAChR agonist-evoked increases in glutamatergic and cholinergic neurotransmission in the prefrontal cortex of C57BL/6J mice and in mice lacking β2-containing or α7 nAChRs and their respective wild-type animals. In addition, transients were determined in rats after removal of thalamic glutamatergic or midbrain dopaminergic afferent projections. The collective evidence supports the following main conclusions. (1) Stimulation of α4β2* nAChRs is sufficient for the generation of second-based increases in cholinergic neurotransmission. (2) In the absence of β2-containing nAChRs, both cholinergic and glutamatergic transients are partly attenuated if evoked by nicotine and completely attenuated if evoked by ABT-089, consistent with the hypothesis that the amplitude of glutamatergic transients controls the amplitude of cholinergic transients. This finding is also consistent with the classification of ABT-089 as a selective α4β2* nAChR agonist. (3) The presence of mediodorsal thalamic afferents is necessary for the generation of transients evoked by stimulation of α4β2* nAChRs. (4) Stimulation of α7 nAChRs is not a necessary mechanism for generating maximum signal amplitudes of glutamatergic or choline transients. Instead, stimulation of α7 nAChRs is primarily responsible for the longer duration of nicotine-evoked glutamate and ACh release. Choline transients evoked by an α7 nAChR agonist are characterized by ACh release events that are 10- to 15-fold longer than those evoked by nicotine or the α4β2* nAChR agonist. The amplitude, but not the duration, of α7 nAChR-evoked ACh release is partly mediated via prefrontal dopaminergic activity. The discussion below focuses first on important methodological issues and then on the implications of these findings for our understanding of the regulation and function of the prefrontal cholinergic input system, specifically the mechanisms underlying the enhancement of cue detection by nAChR ligands.
Methodological issues: origin of ACh
Based in part on the renewed interest in the functions of cortical cholinergic interneurons, the origin of the cholinergic transients recorded in the present study needs be addressed. Bipolar, cholinergic interneurons in the cortex are extremely sparsely distributed. Their phenotype remains unclear (Oh et al., 1992). Furthermore, their axons innervate superficial layers, and their direct postsynaptic impact is not well understood (von Engelhardt et al., 2007). In addition, and in contrast to cholinergic inputs arising from the basal forebrain, these cholinergic interneurons do not express the p75 nerve growth factor receptor and thus are not lesioned by the immunotoxin 192IgG–saporin. However, such lesions completely abolish the demonstration of potassium-evoked cholinergic transients (Parikh and Sarter, 2006). Thus, our collective evidence indicates that prefrontal choline transients reflect ACh release from cholinergic neurons originating in the basal forebrain.
Methodological issues: TTX sensitivity
The present evidence indicates that nAChR agonist-evoked choline and glutamatergic transients in the PFC of mice are sensitive to blockade of voltage-regulated sodium channels with TTX. These findings are consistent with our previous demonstration of TTX sensitivity of glutamatergic transients recorded in rats (Parikh et al., 2008) to transients recorded in mice. Furthermore, it confirms that 75–80% of the increase in glutamate release that is evoked by nAChR agonists and measured by using this electrochemical method is attributable to neuronal depolarization. This observation, together with the demonstration that this method also reveals TTX-induced decreases in basal glutamate release (Hascup et al., 2008) (see also Oldenziel et al., 2006), suggests that the dimensions, geometric, and surface characteristics of the ceramic wafers and platinum recording sites allow monitoring glutamate release that originates primarily from neuronal terminals.
Prefrontal α4β2* nAChRs, glutamatergic–cholinergic interactions and dual cholinergic roles
The present results indicate that stimulation of α4β2* nAChRs is sufficient to produce maximum amplitudes of cholinergic transients. Furthermore, the results are consistent with the hypothesis that glutamate release from thalamocortical inputs is causally necessary for the generation of cholinergic transients. In conjunction with the previously reported attenuation of cholinergic transients by AMPA and NMDA receptor antagonists in rats (Parikh et al., 2008), the present findings indicate that prefrontal glutamatergic–cholinergic interactions are a key mechanism in the mediation of the fundamental cognitive operations that are evoked and modulated by nAChR agonists (Radcliffe and Dani, 1998; Gioanni et al., 1999; Lambe et al., 2003; Quarta et al., 2007).
The collective evidence indicates that PFC cholinergic transients are required for cue detection (Parikh et al., 2007; Howe et al., 2010) and that α4β2* nAChR stimulation, glutamate release from thalamic inputs, and ionotropic glutamate receptor stimulation are required for the generation of such cholinergic transients. This hypothesis assumes a dual role of ACh that requires comment. A relatively simple scenario suggests that prefrontal glutamatergic–cholinergic interactions are bidirectional: a strong cue, “inserted” into the cortex via increases in glutamate release from thalamic afferents, would drive the glutamatergic generation of cholinergic transients and thus ensures detection. In turn, these increases in cholinergic activity, via stimulation of α4β2* nAChRs, would increase the gain of such interactions, thereby fostering the detection of weaker cues.
An alternative model suggests that the cholinergic innervation of thalamic glutamatergic terminals is separate from the cholinergic neurons that are involved in the generation of detection-mediating cholinergic transients (for anatomical evidence beginning to suggest such a heterogeneous projection system, see Zaborszky et al., 2005). Furthermore, this model uses the finding that, in animals performing attention tasks and in addition to the cholinergic transients mediating cue detection, there is also a more tonic, task session-related increase in cholinergic activity that remains relatively stable over the entire task session (Kozak et al., 2006, 2007; Parikh and Sarter, 2008). This increase in tonic cholinergic activity levels is evoked by the anticipation of performance and/or the initiation of the test session and mediated via prefrontal–basal forebrain (Lemann and Saper, 1985; Zaborszky et al., 1997) and/or prefrontal–mesolimbic–basal forebrain projections (Neigh-McCandless et al., 2002; Neigh et al., 2004; Sarter et al., 2005; Zmarowski et al., 2005; Briand et al., 2007). Such tonic increases in ACh release may facilitate the prefrontal glutamatergic–cholinergic interactions and thus optimize cue detection (Kawai et al., 2007). This appears plausible also because α4β2* nAChRs do not seem to desensitize but in fact upregulate after persistent stimulation (Yates et al., 1995; Buisson and Bertrand, 2001; Walsh et al., 2008; Xiao et al., 2009). Finally, assuming the presence of a cholinergic tone, a relatively straightforward mechanism may underlie the generation of cholinergic transients. Glutamate release could evoke such transients by relieving the tonic inhibition of ACh release that is imposed by tonic cholinergic stimulation of presynaptic M2 muscarinic AChRs (Parnas et al., 2005).
Prefrontal α7 nAChRs
The present results indicate that stimulation of α7 nAChR is primarily responsible for the increased duration of cholinergic as well glutamatergic transients evoked by nicotine. α7 nAChR KO facilitated the return of transients to baseline levels, with t50 values approaching the values of ABT-089-evoked transients in wild-type animals. Because transient amplitudes were not affected in α7 KO mice, this evidence strongly suggest that stimulation of α7 nAChRs controls the duration of both glutamate and ACh release but not the amplitude of the release events. In simplified terms, and with respect to the decay rate of cholinergic and glutamatergic transients, α7 KO alters the effects of nicotine to match those evoked by selective agonists at α4β2* nAChRs.
The characteristics of cholinergic transients evoked by the direct α7 agonist substantiate the conclusion that the duration of release events is controlled by the α7 nAChR. These transients were relatively small in terms of amplitude, consistent with the predominant role of α4β2* nAChR-mediated, glutamatergic control of amplitudes. However, these transients were relatively long lasting. Notably, evidence from another α7 agonist, ABT-107, using both the electrochemical recording technique as well as in vivo microdialysis in awake animals (Paolone et al., 2009), confirmed the long duration of release events evoked by direct stimulation of α7 nAChRs. Such lasting release events are likely to reflect the high permeability of α7 nAChRs for Ca2+ and the subsequent activation of neurotransmitter release mechanisms and Ca2+-dependent signal transduction cascades (Fayuk and Yakel, 2007; Gilbert et al., 2009).
The effects of the lesions of the midbrain dopaminergic system did not support our hypothesis that a dopaminergic mechanism mediates the longer durations of cholinergic release events evoked by nicotine or the even longer-lasting increases in release evoked by α7 AChR stimulation. Rather, such lesions merely attenuated release amplitudes. Given that we postulated above that the amplitude of cholinergic transients is primarily determined by glutamate, this finding would be consistent with evidence indicating α7 nAChR-mediated excitatory amino acid release and the demonstration that ionotropic receptor blockade attenuates α7 nAChR-evoked dopamine release (Girod et al., 2000; Dickinson et al., 2008; Livingstone et al., 2009). The mediator of relatively long duration of release events evoked by α7 nAChR stimulation remains unknown.
Treatment implications
The present results are consistent with the hypothesis that stimulation of prefrontal α4β2* nAChRs evoke glutamate release from thalamic afferents and that such glutamate release causes the generation of fast, cholinergic transients. Thus, α4β2* nAChRs are ideally positioned to amplify the detection of preattentionally processed cues (Crick, 1984; Weese et al., 1999; Pinault, 2004) by prefrontal circuitry. Agonists or positive allosteric modulators at this receptor therefore are expected to enhance potently cue detection and attentional performance (Sarter et al., 2009a). In contrast, stimulation of α7 nAChR primarily increases the duration of the release event, via mechanisms that remain unsettled. The degree to which α7 nAChR-mediated ACh release, which last for tens of seconds, benefits attentional performance remains poorly understood (Grottick and Higgins, 2000; Hahn et al., 2003; Hoyle et al., 2006; Buccafusco et al., 2007; Young et al., 2007).
Conclusions
Research on the role of nAChRs in modulating prefrontal glutamatergic–cholinergic interactions continues to reveal the prefrontal neuronal networks, including their afferent neuromodulator systems, which mediate fundamental cognitive operations, such as cue detection. Afferent neuromodulator systems control the gain of prefrontal glutamatergic–cholinergic interactions and thereby gate the efficacy with which cues control behavior (Kawai et al., 2007). nAChR heteroreceptors, specifically α4β2* nAChRs, are essential components of such networks. Important issues, specifically concerning the presence and functions of multiple modes of cholinergic activity, remain to be resolved.
Footnotes
This research was supported in part by Public Health Service Grant MH080426 and by a contract from Abbott Laboratories.
References
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer's disease and health. Brain. 2008;131:409–424. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Terry AV, Jr, Marsh KC, Decker MW, Arneric SP. Improvement in performance of a delayed matching-to-sample task by monkeys following ABT-418: a novel cholinergic channel activator for memory enhancement. Psychopharmacology (Berl) 1995;120:256–266. doi: 10.1007/BF02311172. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Terry AV, Jr, Decker MW, Gopalakrishnan M. Profile of nicotinic acetylcholine receptor agonists ABT-594 and A-582941, with differential subtype selectivity, on delayed matching accuracy by young monkeys. Biochem Pharmacol. 2007;74:1202–1211. doi: 10.1016/j.bcp.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of l-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, Lin NH, Li Y, Daanen JF, Buccafusco JJ, Prendergast MA, Jackson WJ, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]. II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther. 1997;283:247–258. [PubMed] [Google Scholar]
- Deco G, Thiele A. Attention: oscillations and neuropharmacology. Eur J Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN, Wonnacott S. Presynaptic α7- and β2 containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI) J Psychopharmacol. 2007;21:171–178. doi: 10.1177/0269881107066855. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. Competitive neuronal nicotinic receptor antagonists: a new direction for drug discovery. J Pharmacol Exp Ther. 2001;298:395–402. [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Dendritic Ca2+ signalling due to activation of α7-containing nicotinic acetylcholine receptors in rat hippocampal neurons. J Physiol. 2007;582:597–611. doi: 10.1113/jphysiol.2007.135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Ed 3. San Diego: Academic; 2008. [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Lecchi M, Arnaudeau S, Bertrand D, Demaurex N. Local and global calcium signals associated with the opening of neuronal α7 nicotinic acetylcholine receptors. Cell Calcium. 2009;45:198–207. doi: 10.1016/j.ceca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepous é C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000;39:2715–2725. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Gonzalez R, Harris RJ, Guthrie D. Effects sizes and p values: what should be reported and what should be replicated? Psychophysiology. 1996;33:175–183. doi: 10.1111/j.1469-8986.1996.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Guldin WO, Pritzel M, Markowitsch HJ. Prefrontal cortex of the mouse defined as cortical projection area of the thalamic mediodorsal nucleus. Brain Behav Evol. 1981;19:93–107. doi: 10.1159/000121636. [DOI] [PubMed] [Google Scholar]
- Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Exp Ther. 2008;324:725–731. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Parikh V, Decker M, Sarter M. Cognition enhancement by nAChR agonists: facilitation of cue detection based on augmented cholinergic transients in prefrontal cortex. Soc Neurosci Abstr. 2009;35:873–20. [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M Advance online publication. Enhanced attentional performance by selective stimulation of α4β2* nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E, Sarter M, Guldin WO, Markowitsch HJ. Afferents to the ventral tegmental nucleus of Gudden in the mouse, rat, and cat. J Comp Neurol. 1984;228:509–541. doi: 10.1002/cne.902280406. [DOI] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. Toward a neuro-cognitive animal model of the cognitive symptoms of schizophrenia: disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing, but not non-performing, rats. Neuropsychopharmacology. 2007;32:2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lemann W, Saper CB. Evidence for a cortical projection to the magnocellular basal nucleus in the rat: an electron microscopic axonal transport study. Brain Res. 1985;334:339–343. doi: 10.1016/0006-8993(85)90228-8. [DOI] [PubMed] [Google Scholar]
- Livingstone PD, Srinivasan J, Kew JN, Dawson LA, Gotti C, Moretti M, Shoaib M, Wonnacott S. alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur J Neurosci. 2009;29:539–550. doi: 10.1111/j.1460-9568.2009.06613.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA. Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain. Biochem Pharmacol. 2009;78:795–802. doi: 10.1016/j.bcp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Arnold HM, Rabenstein RL, Sarter M, Bruno JP. Neuronal activity in the nucleus accumbens is necessary for performance-related increases in cortical acetylcholine release. Neuroscience. 2004;123:635–645. doi: 10.1016/j.neuroscience.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Neigh-McCandless G, Kravitz BA, Sarter M, Bruno JP. Stimulation of cortical acetylcholine release following blockade of ionotropic glutamate receptors in nucleus accumbens. Eur J Neurosci. 2002;16:1259–1266. doi: 10.1046/j.1460-9568.2002.02201.x. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Oh JD, Woolf NJ, Roghani A, Edwards RH, Butcher LL. Cholinergic neurons in rat central nervous system demonstrated by in situ hybridization of choline acetyltransferase mRNA. Neuroscience. 1992;47:807–822. doi: 10.1016/0306-4522(92)90031-v. [DOI] [PubMed] [Google Scholar]
- Oldenziel WH, Dijkstra G, Cremers TI, Westerink BH. In vivo monitoring of extracellular glutamate in the brain with a microsensor. Brain Res. 2006;1118:34–42. doi: 10.1016/j.brainres.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Ji J, Williams S, Howe WM, Ward J, Parikh V, Decker MW, Sarter M. Effects of the selective α7 nAChR agonist ABT-107 on prefrontal glutamatergic and cholinergic activity and attentional performance. Soc Neurosci Abstr. 2009;35:227–5. [Google Scholar]
- Parikh V, Sarter M. Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. J Neurochem. 2006;97:488–503. doi: 10.1111/j.1471-4159.2006.03766.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H, Slutsky I, Rashkovan G, Silman I, Wess J, Parnas I. Depolarization initiates phasic acetylcholine release by relief of a tonic block imposed by presynaptic M2 muscarinic receptors. J Neurophysiol. 2005;93:3257–3269. doi: 10.1152/jn.01131.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. San Diego: Academic; 1988. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Pioli EY, Meissner W, Sohr R, Gross CE, Bezard E, Bioulac BH. Differential behavioral effects of partial bilateral lesions of ventral tegmental area or substantia nigra pars compacta in rats. Neuroscience. 2008;153:1213–1224. doi: 10.1016/j.neuroscience.2008.01.084. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochem Pharmacol. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer's disease. Psychopharmacology (Berl) 1999;142:334–342. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Morris HV, Patel S, Genn RF, Stolerman IP. Different effects of ionotropic and metabotropic glutamate receptor antagonists on attention and the attentional properties of nicotine. Neuropharmacology. 2007;53:421–430. doi: 10.1016/j.neuropharm.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Radcliffe KA, Dani JA. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J Neurosci. 1998;18:7075–7083. doi: 10.1523/JNEUROSCI.18-18-07075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, Lin NH, Osinski MA, Reinhart GA, Buckley MJ, Martin RL, McDermott JS, Preusser LC, Seifert TR, Su Z, Cox BF, Decker MW, Sullivan JP. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev. 2004;10:167–182. doi: 10.1111/j.1527-3458.2004.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Markowitsch HJ. Collateral innervation of the medial and lateral prefrontal cortex by amygdaloid, thalamic, and brain-stem neurons. J Comp Neurol. 1984;224:445–460. doi: 10.1002/cne.902240312. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009a;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009b;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Xue IC, Piattoni-Kaplan M, Molinari E, Campbell JE, McKenna DG, Gunn DE, Lin NH, Ryther KB, He Y, Holladay MW, Wonnacott S, Williams M, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy) pyridine]. I. A potent and selective cholinergic channel modulator with neuroprotective properties. J Pharmacol Exp Ther. 1997;283:235–246. [PubMed] [Google Scholar]
- Timmerman W, Cisci G, Nap A, de Vries JB, Westerink BH. Effects of handling on extracellular levels of glutamate and other amino acids in various areas of the brain measured by microdialysis. Brain Res. 1999;833:150–160. doi: 10.1016/s0006-8993(99)01538-3. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Brain Res Cogn Brain Res. 1997;6:147–158. doi: 10.1016/s0926-6410(97)00027-x. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Eliava M, Meyer AH, Rozov A, Monyer H. Functional characterization of intrinsic cholinergic interneurons in the cortex. J Neurosci. 2007;27:5633–5642. doi: 10.1523/JNEUROSCI.4647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. Upregulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- Weese GD, Phillips JM, Brown VJ. Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. J Neurosci. 1999;19:10135–10139. doi: 10.1523/JNEUROSCI.19-22-10135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74:1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances α4β2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci. 2009;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem Pharmacol. 1995;50:2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Buhl DL, Pobalashingham S, Bjaalie JG, Nadasdy Z. Three-dimensional chemoarchitecture of the basal forebrain: spatially specific association of cholinergic and calcium binding protein-containing neurons. Neuroscience. 2005;136:697–713. doi: 10.1016/j.neuroscience.2005.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M, Bruno JP. NMDA and dopamine interactions in the nucleus accumbens modulate cortical acetylcholine release. Eur J Neurosci. 2005;22:1731–1740. doi: 10.1111/j.1460-9568.2005.04333.x. [DOI] [PubMed] [Google Scholar]