Abstract
Objective
To assess the transactive response DNA-binding protein 43 (TDP-43) burden in familial forms of Alzheimer disease (FAD) and Down syndrome (DS) to determine whether TDP-43 inclusions are also present.
Design
Using standard immunohistochemical techniques, we examined brain tissue samples from 42 subjects with FAD and 14 with DS.
Results
We found pathological TDP-43 aggregates in 14.0% of participants (6 of 42 and 2 of 14 participants with FAD and DS, respectively). In both FAD and DS, TDP-43 immunoreactivity did not colocalize with neurofibrillary tangles. Occasionally participants with FAD or DS had TDP-43–positive neuropil threads or dots. Overall, the amygdala was most commonly affected, followed by the hippocampus, with no TDP-43 pathology in neocortical regions. A similar distribution of TDP-43 inclusions is seen in sporadic Alzheimer disease, but it differs from that seen in amyotrophic lateral sclerosis and frontotemporal dementia.
Conclusions
Transactive response DNA-binding protein 43 pathology occurs in FAD and DS, similar to that observed in sporadic Alzheimer disease. Thus, pathological TDP-43 may contribute the cognitive impairments in familial and sporadic forms of Alzheimer disease.
Transactive response DNA-binding protein 43 (TDP-43) is a nuclear ribonucleo-protein that plays a role in a variety of cellular functions including protein processing, particularly, modulating transcription and exon splicing.1 It is also a major pathological hallmark of ubiquitinated inclusions in amyotrophic lateral sclerosis and forms of frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U,2,3 more recently abbreviated FTLD-TDP by Mackenzie et al4). In these conditions, both sporadic and familial cases show abnormal accumulation of hyper-phosphorylated, ubiquitinated, and truncated TDP-43 fragments.2,3 Recently, we developed phosphorylation-specific TDP-43 monoclonal antibodies that recognize only pathological TDP-43, to study the altered phosphorylation patterns of abnormal TDP-43 in amyotrophic lateral sclerosis, FTLD-TDP, and other neurode-generative diseases.5 These and other studies with previously reported anti–TDP-43 antibodies have shown that phosphorylation of S409/410 in TDP-43 is detected in neuronal and glial inclusions of a variety of central nervous system (CNS)–degenerative diseases including Alzheimer disease (AD) as well as in other older individuals with cognitive impairment.5–8 There is also an association between cognitive status and the intensity of TDP-43 pathology in subjects with age-related cognitive impairment.7 Interestingly, in the Nelson et al study,7 there was no association between cognitive status and other pathology, including AD neuropathological features such as amyloid plaque or neurofibrillary tangle burden. Together, these data raise the possibility that TDP-43 plays a significant role in the pathogenic mechanisms underlying cognitive impairment in AD and other age-related brain disorders.
Alzheimer disease is the most common neurodegenerative disease affecting cognition, found in increasing numbers in our elderly population. However, only limited study has been performed to investigate TDP-43 pathology and AD.5–7,9 Caspase-cleaved TDP-43 has been variably described in Hirano bodies, neurofibrillary tangles, reactive glia, and dystrophic plaque-associated neurites,10 but further studies are needed to confirm these findings. Taken together, these studies support the notion that TDP-43 may contribute to the pathobiology of AD. However, no studies have been done to look at the presence of TDP-43 inclusions in genetic forms of AD such as familial AD (FAD) and Down syndrome (DS) to determine whether participants with genetic etiologies have similar TDP-43 pathologies. Hence, the current study was undertaken to assess the presence of TDP-43 immunopathology in a series of well-characterized participants with FAD and DS to determine whether this pathobiological feature differs from that of sporadic forms of AD or other types of CNS degeneration.
METHODS
BRAIN TISSUE AND NEUROPATHOLOGICAL ASSESSMENT
Brain tissues from a total of 42 participants with FAD and 14 with DS were examined using previously described histological methods.5,11,12 These tissues were obtained from Drexel University School of Medicine (36 participants) and the University of Pennsylvania AD Center (20 participants). All participants with FAD except 1 (2.4%) met National Institute for Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorder Association clinical criteria for AD,13 and most had advanced dementia at death. The remaining case14 was a preclinical disease carrier who died aged 51 years, 1 year before anticipated symptom onset in his kindred. Thirty subjects with FAD had the mutations in the genes that encode presenilin 1 (PS-1); 3, presenilin 2 (PS-2); 3, amyloid precursor protein (APP); and 6 were unlinked but had a clear autosomal-dominant history of FAD. Clinical histories regarding cognitive decline were limited for the subjects with DS, but 9 of these 14 individuals were noted to have evidence of cognitive decline consistent with dementia on medical record review, as well as clinical features of DS. Demographic information is summarized in Table 1.
Table 1.
Clinical Characteristics of the 42 Participants With Familial Alzheimer Disease and 14 With Down Syndrome by Institution
| Patient No./Sex/Age at Death, y | Clinical Diagnosis | Mutation and/or Genetic Etiology | Dementia Duration, y |
|---|---|---|---|
| University of Pennsylvania | |||
| 1/M/68 | DS | Trisomy 21 | 4 |
| 2/F/58 | DS | Trisomy 21 | 3 |
| 3/M/62 | DS | Trisomy 21 | 1 |
| 4/M/54 | DS | Trisomy 21 | 3 |
| 5/M/58 | DS | Trisomy 21 | 10 |
| 6/F/57 | DS | Trisomy 21 | 2 |
| 7/F/53 | DS | Trisomy 21 | NA |
| 8/F/67 | DS | Trisomy 21 | 5 |
| 9/F/61 | DS | Trisomy 21 | 4 |
| 10/F/63 | DS | Trisomy 21 | 8 |
| 11/M/61 | DS | Trisomy 21 | NA |
| 12/F/67 | DS | Trisomy 21 | NA |
| 13/M/33 | DS | Trisomy 21 | NA |
| 14/M/23 | DS | Trisomy 21 | NA |
| 42/F/44 | PS-1 | NA | 10 |
| 43/M/NA | PS-1 | NA | 9 |
| 44/F/NA | PS-1 | NA | 20 |
| 45/F/66 | PS-2 | NA | 14 |
| 46/F/41 | PS-2 | NA | 6 |
| 51/M/NA | Unlinked FAD | NA | 3 |
| Drexel University College of Medicine | |||
| 15/M/51 | PS-1 | A246E | 0 |
| 16/M/57 | PS-1 | A246E | 9 |
| 17/F/67 | PS-1 | A246E | 15 |
| 18/F/66 | PS-1 | A246E | 13 |
| 19/M/61 | PS-1 | A246E | 9 |
| 20/M/60 | PS-1 | A246E | 10 |
| 21/M/56 | PS-1 | A246E | 6 |
| 22/M/49 | PS-1 | C410Y | 8 |
| 23/F/60 | PS-1 | C410Y | 16 |
| 24/F/81 | PS-1 | C410Y | 22 |
| 25/M/55 | PS-1 | C410Y | 8 |
| 26/M/59 | PS-1 | C410Y | 9 |
| 27/M/60 | PS-1 | C410Y | 6 |
| 28/F/50 | PS-1 | C410Y | 5 |
| 29/M/59 | PS-1 | C410Y | 6 |
| 30/M/57 | PS-1 | C410Y | 7 |
| 31/M/57 | PS-1 | C410Y | 6 |
| 32/M/57 | PS-1 | C410Y | 6 |
| 33/F/70 | PS-1 | C410Y | 14 |
| 34/F/NA | PS-1 | C410Y | 8 |
| 35/NA/42 | PS-1 | L286V | NA |
| 36/NA/47 | PS-1 | L286V | NA |
| 37/M/61 | PS-1 | L286V | 13 |
| 38/M/60 | PS-1 | M146L | 6 |
| 39/M/60 | PS-1 | M146L | 12 |
| 40/F/NA | PS-1 | M146L | 3 |
| 41/F/53 | PS-1 | M146L | 13 |
| 47/M/64 | PS-2 | Asn141Ile | 9 |
| 48/F/63 | APP | V717Ile | 10 |
| 49/F/58 | APP | V717Ile | 4 |
| 50/F/63 | APP | V717Ile | 14 |
| 52/F/78 | Unlinked FAD | NA | 19 |
| 53/F/62 | Unlinked FAD | NA | 12 |
| 54/F/71 | Unlinked FAD | NA | 9 |
| 55/M/75 | Unlinked FAD | NA | 16 |
| 56/M/70 | Unlinked FAD | NA | 10 |
Abbreviations: APP, gene that encodes amyloid precursor protein; DS, Down syndrome; FAD, familial Alzheimer disease; NA, not available; PS-1 and -2, genes that encode presenilin 1 and 2.
BRAIN TISSUE PROCESSING AND IMMUNOHISTOCHEMISTRY
In most cases, one hemisphere was immersion fixed in 10% neutral buffered formalin solution for 2 weeks, while in the remaining participants, small biopsy specimens of different regions were obtained from the fresh brain at autopsy, and these samples were fixed overnight in neutral buffered formalin or ethanol with 150mM sodium chloride prior to embedding. Subsequently, tissue blocks from 36 areas were embedded in paraffin and sectioned according to a standardized protocol. Sections cut at 6 μm were used for histochemical studies. To survey these participants for TDP-43–positive inclusions, we used the rabbit anti-TARDBP antibody (1:8000; Proteintech Group, Chicago, Illinois) and a rat antiphosphorylated TDP-43 monoclonal antibody specific to phosphorylated residues 409/410 (clone 1D3, 1:1000) recently described by Neumann et al.4 However, because monoclonal antibody 1D3 stained granulovacuolar degeneration (GVD) in some participants with FAD (see “Results” section), we reexamined sections from these participants with additional antibodies to widely distributed nonphosphorylated epitopes in TDP-43 including 171 (mouse monoclonal antibody), 205 (mouse monoclonal antibody, C-terminal half of TDP-43), 1039 (rabbit polyclonal antibody, extreme C-terminal region of TDP-43), and 1065N (rabbit polyclonal antibody, extreme N-terminal region of TDP-43).15
Slides were deparaffinized in xylene and rehydrated in descending alcohol solutions. A 5% hydrogen peroxide solution in methanol was used to block. Antigen retrieval was achieved with Antigen Unmasking Solution (Vector Laboratories, Burlingame, California) according to manufacturer’s instructions, followed by a blocking with 2% fetal bovine serum in 0.1M Tris. Slides were incubated with the primary antibody overnight at 4°C. Subsequently, slides were treated with biotinylated secondary antibodies to rabbit and rat, respectively (1:1000; Vector) for 1 hour at room temperature. Slides were then incubated with an avidin/biotin complex solution (1:1000; Vector) for another hour at room temperature. This was followed by application of 3,3′-diaminobenzidine (Bio-genex Laboratories, Inc, San Ramon, California). All slides were counterstained with hematoxylin.
STATISTICAL ANALYSES
Comparisons of presence and location of pathology with clinical and demographic features was accomplished through χ2 or Fisher exact tests for categorical variables and analyses of variance for continuous ones.
RESULTS
We observed aggregates of TDP-43 immunoreactivity in the cytoplasm of many patients with FAD and DS (Figure 1) but, as described by Neumann et al,5 nuclei were not stained with the phosphorylation-specific anti–TDP-43 antibody, which only stained pathological aggregates of TDP-43.5 Fourteen percent of participants had some TDP-43 pathology in the temporal lobe, hippocampus, or amygdala. This proportion did not change with the specific genetic etiology (Table 2). In 5 participants with FAD and 3 with DS, the TDP-43 inclusions were restricted to 1 brain region, but in 4 participants with FAD and 2 with DS, cytoplasmic inclusions were more widespread. When isolated to 1 region, TDP-43–positive neuronal inclusions were most frequent in the hippocampus (2 participants with FAD and 3 with DS). For all but 1 case, patient 15, a PS-1–positive participant with FAD with GVD pathology in the temporal region, staining of TDP-43 pathology was limited to the hippocampus, entorhinal cortex, and amygdala. There were no TDP-43 immunoreactive lesions seen in the frontal regions of any case.
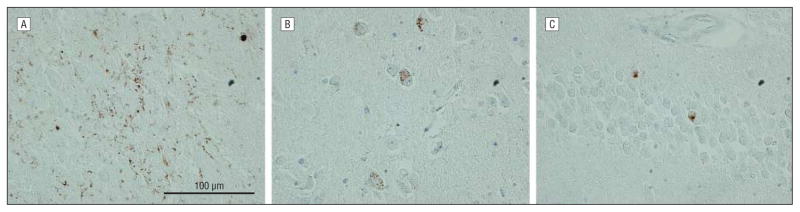
Figure 1.
Photomicrographs of transactive response DNA-binding protein 43 (TDP-43) pathology in the brains of patients with familial Alzheimer disease (FAD) and Down syndrome (DS). A, The TDP-43 inclusions in the amygdala at medium power magnification (original magnification ×20) are shown in a participant with Down syndrome (patient 11). Multiple neurons in the amygdala show immunoreactivity for the phosphorylation-specific TDP-43 monoclonal antibody. B–D, Higher-power magnification (original magnification ×60) of neuronal cytoplasmic staining in amygdala neurons was performed for patients withDS (B, patient 11), PS-1–positive FAD (C, patient 44), andPS-2–positive FAD (patient 45). In all participants, there is intense TDP-43 immunoreactivity in aggregates that extended throughout the cytoplasm. The appearance is similar in PS-1–positive FAD, PS-2–positive FAD, and DS.
Table 2.
Distribution of TDP-43 Immunoreactivity in Down Syndrome and Familial Alzheimer Disease
| No./Total (%) |
|||||
|---|---|---|---|---|---|
| Pathology Present | DS | PS-1 | PS-2 | APP | Unlinked FAD |
| TDP-43a | 2/14 (14.3) | 5/30 (16.7) | 1/3 (33.3) | 0/3 | 0/6 |
| Amygdala TDP-43 | 2/14 (14.3) | 5/18 (27.8)b | 1/2 (50.0)b | 0/3 | 0/6 |
| Hippocampal TDP-43 | 1/14 (7.1) | 2/21 (9.5)b | 0/3 | 0/3 | 0/6 |
Abbreviations: APP, gene that encodes amyloid precursor protein; DS, Down syndrome; FAD, familial Alzheimer disease; PS-1 and -2, genes that encode presenilin 1 and 2; TDP-43, transactive response DNA-binding protein 43.
Includes amygdala and hippocampal regions.
Percentages reflect missing cases for some regions.
In nonhippocampal medial temporal lobe regions, TDP-43 immunoexpression was diffuse within the cytoplasm of neurons, but this did not appear to colocalize with neurofibrillary pathology or β-amyloid plaques. Occasionally, we observed TDP-43–positive threadlike structures in the neuropil (Figure 2A).
Figure 2.
Photomicrograph of transactive response DNA-binding protein 43 (TDP-43) immunostaining of neuropathological features in patients with familial Alzheimer disease and Down syndrome using monoclonal antibody 1D3. A, In patient 11, TDP-43–positive threadlike structures are demonstrated in the neuropil. B, Granules in granulovacuolar degeneration (GVD) are immunoreactive to TDP-43 (patient 4). C, Diffuse and granular cytoplasmic staining for TDP-43 is shown in dentate granule cells (patient 11). However, none of the other TDP antibodies produced similar GVD or granular staining, so we infer that the granular and GVD staining seen here with the 1D3 monoclonal antibody does not reflect the presence of TDP-43 itself in these structures. Original magnification is ×60 for all images.
Within the hippocampus, we most commonly observed neuronal cytoplasmic TDP-43 immunoreactivity within the granules of GVD using the monoclonal antibody 1D3 (Figure 2B). Interestingly, this often had a predilection for the CA4 region, though it was also seen in other hippocampal sectors. Further, in 1 case (patient 11), diffuse and granular TDP-43 immunoreactivity was observed in the cytoplasm of dentate granule cell neurons (Figure 2C). Within the hippocampus, 4 participants with FAD and 4 with DS also had TDP-43 staining with our monoclonal antibody 1D3 in the granules of GVD inclusions. However, when we probed adjacent sections with the other TDP antibodies that recognize nonphosphorylated epitopes, none of them produced similar GVD or any granular staining. Thus, we infer that the 1D3 monoclonal antibody cross-reacts with a phosphorylated epitope that is an immunological mimic of the TDP-43 epitope recognized by the monoclonal antibody, so TDP-43 is most likely not present in GVD in our participants with AD. Also, TDP-43–positive neuropil threads were noted in 2 participants with FAD (patients 42 and 45) and 2 with DS (patients 10 and 11).
The TDP-43 inclusions appeared identical in FAD and DS, and had an appearance similar to those described in sporadic AD.6 Inclusions were usually multiple, and were more often present in medium and large neurons in the amygdala and adjacent entorhinal cortex. However, here the TDP-43 immunostaining was usually limited to diffuse staining (22.9% of all participants). There were no significant differences in presence of TDP-43 amygdala pathology by genetic etiology, although no staining was found in the few participants with APP or unlinked mutations (Table 2).
Duration of dementia was associated with the presence of diffuse TDP-43 pathology in the hippocampus (duration for those with no pathology=8.0+5.0 years; those with diffuse pathology=17.5+3.5 years; P=.01). There was no association for either type of pathology with age at death or between GVD TDP-43 immunoreactivity and clinical features.
COMMENT
Abnormally phosphorylated, insoluble, and pathologically truncated TDP-43 forms a variety of neuronal and glial inclusions in TDP-43 proteinopathies, age-related cognitive impairment, AD, and other CNS-degenerative disorders. We extend the current body of data on primary and secondary TDP-43 proteinopathies by characterizing the extent and distribution of TDP-43 lesions in a large 2-center cohort of participants with FAD and DS. These novel data extend the spectrum of disorders with abnormalities involving TDP-43.
Using our recently developed 409/410 phosphorylation-specific anti–TDP-43 monoclonal antibody,5 we detected TDP-43 pathology most commonly in neuronal perikarya in a subset of subjects with FAD and DS. This pathology was most frequent in the amygdala, where it was often intense. Lesions immunoreactive to TDP-43 also were observed in other limbic areas; however, they were not observed in neocortical (frontal and parietal) regions of these participants with FAD and DS. When present in the temporal lobe, TDP-43 pathology was restricted to the hippocampal complex, adjacent entorhinal cortex, and amygdala.
Occasional TDP-43-positive threads were observed in the neuropil of the medial temporal lobe. However, TDP-43 immunoexpression did not cooccur with neurofibrillary tangles or senile plaques, although infrequent colocalization of TDP-43 and tau in neurofibrillary tangles has been reported.3,5–7,9,15
Amador-Ortiz et al9 and Uryu et al6 identified TDP-43 pathology in 25% to 30% of participants with sporadic AD, while Arai et al16 recently observed TDP-43 pathology in more than 50% of participants with sporadic AD. As in our series, others report TDP-43 pathology in AD most commonly in the amygdala, hippocampus (dentate gyrus), and adjacent entorhinal cortex. Few participants showed an extension of this pathology to the neo-cortex. The regional distribution of TDP-43 lesions also parallels data described by Hu et al,17 who suggested that TDP-43 lesions begin in the temporal limbic regions and spread. The regional distribution of TDP-43 pathology in our participants with FAD and DS as well as in participants with sporadic AD described by others contrasts sharply with data from participants with FTLD-TDP that are classified as TDP-43 proteinopathies.2,18 In these participants with dementia, TDP-43 immunoreactivity is common in cortical areas; even in participants with amyotrophic lateral sclerosis, there may be widespread TDP-43 pathology that extends beyond upper and lower motor neuron systems.19 Overall, these studies show that there is a regional predilection for TDP-43 aggregates to occur in brain areas that are susceptible to the primary disease process.
Immunoreactivity to TDP-43 is not present in all types of tauopathy, and it is not limited to this group of CNS degenerations. It has been described in corticobasal degeneration, but it does not occur consistently in Pick disease or progressive supranuclear palsy.6 Correspondingly, TDP-43 immunoreactivity has been described in dementia with Lewy bodies.16 Additionally, the nearly complete absence of TDP-43 colocalizing with tau pathologies and the lack of TDP-43 immunoreactivity in senile plaques in sporadic or familial AD make a direct association between abnormal TDP-43 and these other pathological AD proteins less likely.
Arai et al16 and Uryu et al6 showed that the biochemical banding pattern of TDP-43 in immunoblot studies of sarkosyl-insoluble fractions of AD- and dementia with Lewy bodies–affected brain tissue was similar to that of FTLD-TDP due to progranulin mutations. These data imply that, when present, there are biochemical and structural similarities in the pathological features of TDP-43 pathology in CNS-degenerative diseases regardless of whether they are a primary TDP-43 proteinopathy.
Uryu et al6 determined that in sporadic AD, TDP-43 pathology was associated with longer disease duration. Here, we found that limbic pathology correlated with disease duration as well. Our data also show that TDP-43 immunoreactive lesions are independent of age at death. This contrasts with Josephs et al,20 who showed that subjects with AD with TDP-43 immunoreactivity were older at age of disease onset and age at death than individuals lacking these inclusions. However, the study by Josephs et al differed from the present study in that they did not focus on genetic forms of the disease, which are often of early onset.
In conclusion, we describe the prevalence and regional distribution of TDP-43 pathologies in a large cohort of subjects with FAD and DS, providing evidence that genetic forms of AD show frequent TDP-43 lesions similar to sporadic AD, but in a more restricted distribution than that seen in primary TDP-43 proteinopathies. The biological significance of this TDP-43 pathology in genetic forms of AD is unknown; however, our data add to what is known about TDP pathobiology by showing that TDP-43 lesions in FAD and DS occur in neurons that are susceptible to forming cytoplasmic fibrillary tau inclusions, and that pathological TDP-43 aggregates are features of both the sporadic and familial form of AD in addition to DS.
Acknowledgments
Funding/Support: This work was supported by grants AG10124 and AG17586 from the National Institute on Aging, National Institutes of Health.
Footnotes
Author Contributions: Dr Lippa had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Lippa, Stutzbach, Lee, and Trojanowski. Acquisition of data: Rosso, Stutzbach, Lee, and Trojanowski. Analysis and interpretation of data: Lippa, Rosso, Stutzbach, Neumann, Lee, and Trojanowski. Drafting of the manuscript: Lippa, Lee, and Trojanowski. Critical revision of the manuscript for important intellectual content: Rosso, Stutzbach, Neumann, Lee, and Trojanowski. Obtained funding: Lee and Trojanowski. Administrative, technical, and material support: Lippa, Stutzbach, and Neumann. Study supervision: Lippa.
Financial Disclosure: None reported.
Additional Contributions: We also wish to thank Terry Schuck, Linda Kwong, PhD, and Eddie Lee, PhD, for their advice and assistance with this study. We also extend our thanks to the many families of our patients. Without their donations, none of this work would be possible.
References
- 1.Wang HY, Wang IF, Bose J, Shen CK. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83(1):130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in fronto-temporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 3.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117(1):15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117(2):137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67(6):555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons [published online ahead of print November 19, 2008] Brain Pathol. doi: 10.1111/j.1750–3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa M, Arai T, Nonaka T, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64 (1):60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61(5):435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohn TT. Caspase-cleaved TAR DNA-binding protein-43 is a major pathological finding in Alzheimer’s disease. Brain Res. 2008;1228:189–198. doi: 10.1016/j.brainres.2008.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol. 1999;45(3):353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153(5):1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Lippa CF, Nee LE, Mori H, St George-Hyslop P. Abeta-42 deposition precedes other changes in PS-1 Alzheimer’s disease. Lancet. 1998;352(9134):1117–1118. doi: 10.1016/s0140-6736(05)79757-9. [DOI] [PubMed] [Google Scholar]
- 15.Igaz LM, Kwong LK, Xu Y, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117(2):125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 17.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116(2):215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geser F, Martinez-Lage M, Robinson J, et al. The clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66(2):180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geser F, Brandmeir NJ, Kwong LK, et al. Evidence of multiple system disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65(5):636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 20.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70(19 pt 2):1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]