Abstract
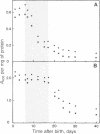
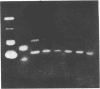
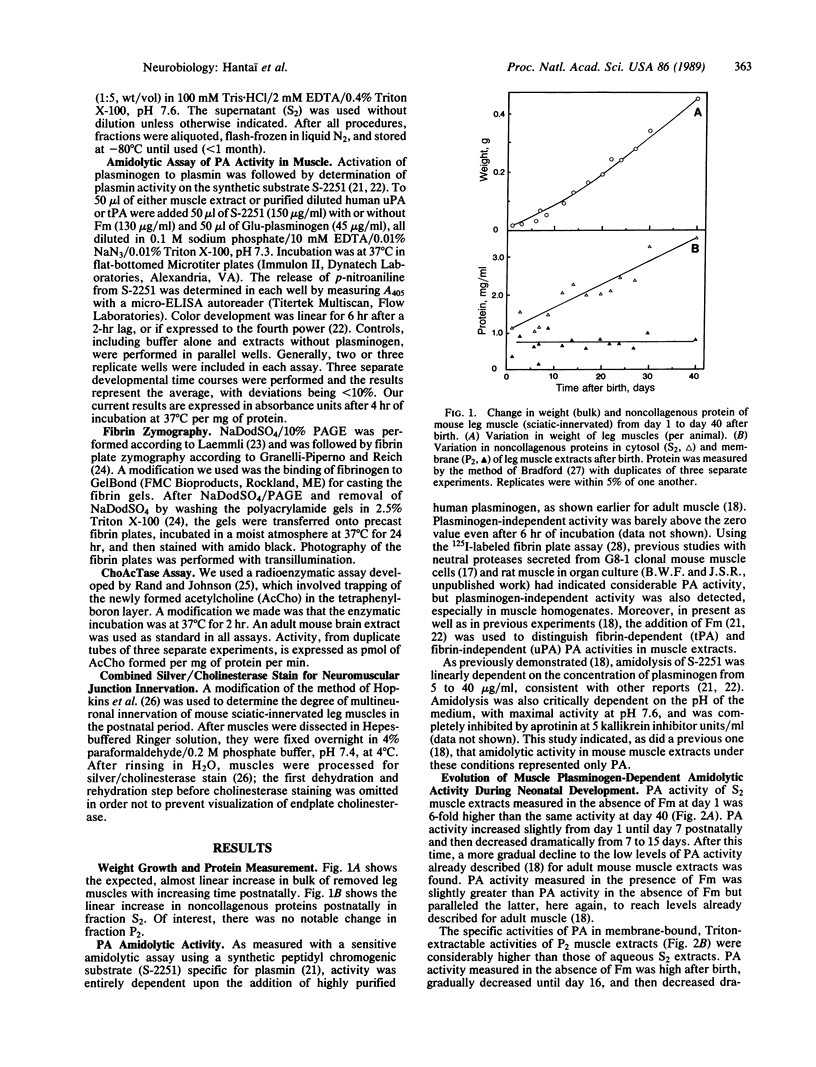
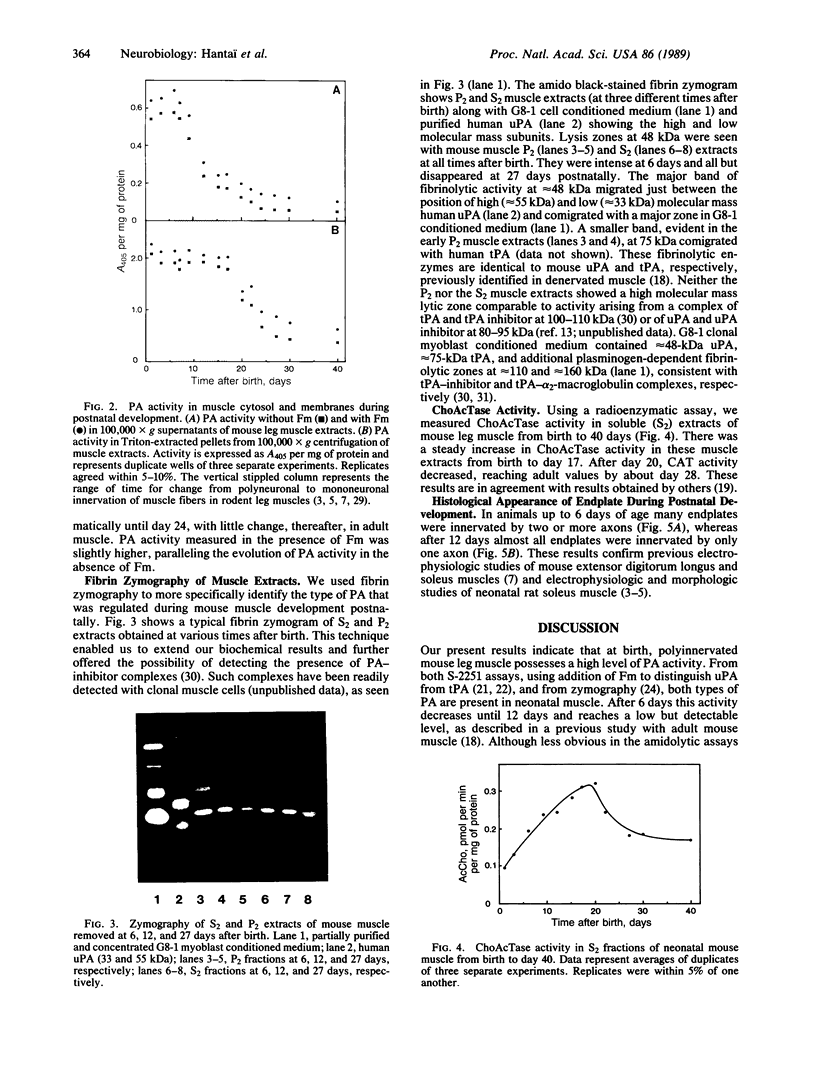
Previous studies have implicated proteases, acting extracellularly, in the mechanism of polyneuronal synapse elimination. Most studies have focused on mammalian, especially rodent, skeletal muscle, where retraction of subordinate nerve terminals occurs during a narrow time window 2-3 weeks after birth. To date no specific protease(s) has been detected that (i) coincides in time with maximal synapse elimination and (ii) is known to act extracellularly on specific extracellular matrix proteins. In previous studies of denervation in adult mouse muscle, rapid activation of urokinase-type plasminogen activator, a neutral serine protease, was detected. This enzyme, by activation of plasminogen to plasmin, specifically degrades matrix components such as fibronectin, type IV collagen, and laminin in muscle. We now present evidence for an initial increase and subsequent decrease in soluble urokinase-type PA--and, to a lesser extent, tissue PA--in developing muscle, suggesting postnatal developmental regulation of these enzymes during the period of maximal synapse elimination. Although considerably higher in specific activity, membrane-bound PA activity followed the wave of synapse elimination, possibly indicating a longer half-life of membrane-bound enzyme(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagust J., Lewis D. M., Westerman R. A. Polyneuronal innervation of kitten skeletal muscle. J Physiol. 1973 Feb;229(1):241–255. doi: 10.1113/jphysiol.1973.sp010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai A., Baker J. B. Urokinase binding sites on human foreskin cells. Evidence for occupancy with endogenous urokinase. Biochem Biophys Res Commun. 1985 Dec 31;133(3):994–1000. doi: 10.1016/0006-291x(85)91234-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown A., Mallett M., Fiser D., Arnold W. C. Acute isoniazid intoxication: reversal of CNS symptoms with large doses of pyridoxine. Pediatr Pharmacol (New York) 1984;4(3):199–202. [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carry M. R., Morita M., Nornes H. O. Morphogenesis of motor endplates along the proximodistal axis of the mouse hindlimb. Anat Rec. 1983 Nov;207(3):473–485. doi: 10.1002/ar.1092070309. [DOI] [PubMed] [Google Scholar]
- Connold A. L., Evers J. V., Vrbová G. Effect of low calcium and protease inhibitors on synapse elimination during postnatal development in the rat soleus muscle. Brain Res. 1986 Jul;393(1):99–107. doi: 10.1016/0165-3806(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Dangain J., Vrbová G. Elimination of polyneuronal innervation in a fast muscle of normal and dystrophic mice. J Physiol. 1983 Sep;342:267–275. doi: 10.1113/jphysiol.1983.sp014850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Danø K., Nielsen L. S., Møller V., Engelhart M. Inhibition of a plasminogen activator from oncogenic virus-transformed mouse cells by rabbit antibodies against the enzyme. Biochim Biophys Acta. 1980 Jun 5;630(1):146–151. doi: 10.1016/0304-4165(80)90146-4. [DOI] [PubMed] [Google Scholar]
- Dennis M. J. Development of the neuromuscular junction: inductive interactions between cells. Annu Rev Neurosci. 1981;4:43–68. doi: 10.1146/annurev.ne.04.030181.000355. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Baker J. B. Evidence that a variety of cultured cells secrete protease nexin and produce a distinct cytoplasmic serine protease-binding factor. J Cell Physiol. 1983 Nov;117(2):175–182. doi: 10.1002/jcp.1041170207. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Hantaï D., Soria J., Thomaïdis A., Soria C. Plasminogen activator in mammalian skeletal muscle: characteristics of effect of denervation on urokinase-like and tissue activator. J Cell Biol. 1986 Oct;103(4):1415–1421. doi: 10.1083/jcb.103.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festoff B. W. Neuromuscular junction macromolecules in the pathogenesis of amyotrophic leteral sclerosis. Med Hypotheses. 1980 Feb;6(2):121–131. doi: 10.1016/0306-9877(80)90078-x. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Patterson M. R., Romstedt K. Plasminogen activator: the major secreted neutral protease of cultured skeletal muscle cells. J Cell Physiol. 1982 Feb;110(2):190–195. doi: 10.1002/jcp.1041100213. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantaï D., Festoff B. W. Degradation of muscle basement membrane zone by locally generated plasmin. Exp Neurol. 1987 Jan;95(1):44–55. doi: 10.1016/0014-4886(87)90005-7. [DOI] [PubMed] [Google Scholar]
- Hantaï D., Rao J. S., Festoff B. W. Serine proteases and serpins: their possible roles in the motor system. Rev Neurol (Paris) 1988;144(11):680–687. [PubMed] [Google Scholar]
- Hopkins W. G., Brown M. C., Keynes R. J. Postnatal growth of motor nerve terminals in muscles of the mouse. J Neurocytol. 1985 Aug;14(4):525–540. doi: 10.1007/BF01200795. [DOI] [PubMed] [Google Scholar]
- Kielberg V., Andreasen P. A., Grøndahl-Hansen J., Nielsen L. S., Skriver L., Danø K. Proenzyme to urokinase-type plasminogen activator in the mouse in vivo. FEBS Lett. 1985 Mar 25;182(2):441–445. doi: 10.1016/0014-5793(85)80350-1. [DOI] [PubMed] [Google Scholar]
- Korneliussen H., Jansen J. K. Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibres in newborn rats. J Neurocytol. 1976 Oct;5(8):591–604. doi: 10.1007/BF01175572. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Skriver L., Nielsen L. S., Grøndahl-Hansen J., Kristensen P., Danø K. Distribution of urokinase-type plasminogen activator immunoreactivity in the mouse. J Cell Biol. 1984 Mar;98(3):894–903. doi: 10.1083/jcb.98.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. The effect of acetylcholine on the function and structure of the developing mammalian neuromuscular junction. Neuroscience. 1980;5(7):1367–1379. doi: 10.1016/0306-4522(80)90209-2. [DOI] [PubMed] [Google Scholar]
- Oldfors A., Fardeau M. The permeability of the basal lamina at the neuromuscular junction. An ultrastructural study of rat skeletal muscle using particulate tracers. Neuropathol Appl Neurobiol. 1983 Nov-Dec;9(6):419–432. doi: 10.1111/j.1365-2990.1983.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Poberai M., Sávay G. Time course of proteolytic enzyme alterations in the motor end-plates after stimulation. Acta Histochem. 1976;57(1):44–48. doi: 10.1016/S0065-1281(76)80006-2. [DOI] [PubMed] [Google Scholar]
- Rand J. B., Johnson C. D. A single-vial biphasic liquid extraction assay for choline acetyltransferase using [3H]choline. Anal Biochem. 1981 Sep 15;116(2):361–371. doi: 10.1016/0003-2697(81)90372-9. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rånby M., Norrman B., Wallén P. A sensitive assay for tissue plasminogen activator. Thromb Res. 1982 Sep 15;27(6):743–749. doi: 10.1016/0049-3848(82)90012-3. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R. Plasminogen activator in the developing rat cerebellum: biosynthesis and localization in granular neurons. Brain Res. 1983 Dec;313(2):149–158. doi: 10.1016/0165-3806(83)90212-2. [DOI] [PubMed] [Google Scholar]
- Soreq H., Miskin R., Zutra A., Littauer U. Z. Modulation in the levels and localization of plasminogen activator in differentiating neuroblastoma cells. Brain Res. 1983 Apr;283(2-3):257–269. doi: 10.1016/0165-3806(83)90182-7. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valinsky J. E., Le Douarin N. M. Production of plasminogen activator by migrating cephalic neural crest cells. EMBO J. 1985 Jun;4(6):1403–1406. doi: 10.1002/j.1460-2075.1985.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Hobbs M. M., Pizzo S. V. Microassay for the photometric quantitation of cell-associated plasminogen activator using a chromogenic tripeptide substrate. J Immunol Methods. 1984 Dec 31;75(2):289–294. doi: 10.1016/0022-1759(84)90112-1. [DOI] [PubMed] [Google Scholar]