Abstract
Background
The benzodiazepine receptor antagonist flumazenil reduces anxiety-like behavior and sensitization of anxiety-like behavior in various models of ethanol withdrawal in rodents. The mechanism and brain region(s) that account for this action of flumazenil remain unknown. This investigation explored the potential role of several brain regions (amygdala, raphe, inferior colliculus, nucleus accumbens, and paraventricular hypothalamus) for these actions of flumazenil.
Methods
Rats were surgically implanted with guide cannulae directed over the brain region of interest and then treated with an ethanol diet for three 7-day dietary cycles (5 days on ethanol diet followed by 2 days on control diet). At approximately 4 hours, flumazenil was administered intracranially into each of the first 2 withdrawals. Examinations of anxiety-like behavior followed 1 week later during a third withdrawal. In other animals, restraint stress sessions or intra-amygdala DMCM (methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate) injections, preceded by intraperitoneal flumazenil injections, were substituted for the first 2 ethanol treatment cycles to assess the potential anxiety-sensitizing action of stress or a benzodiazepine receptor inverse agonist, respectively.
Results
Flumazenil treatment of the amygdala during the first 2 withdrawals blocked the development of sensitized anxiety seen during a third withdrawal. Similar actions of flumazenil were found when stress sessions substituted for the first 2 cycles of ethanol exposure and withdrawal. Amygdala treatment with DMCM magnified the anxiety response to the single subthreshold chronic ethanol treatment, and prophylactic flumazenil blocked this effect.
Conclusions
Intra-amygdala flumazenil inhibits the development of anxiety sensitized by repeated ethanol withdrawal, stress/ethanol withdrawal, or DMCM/ethanol withdrawal. These actions suggest that site-specific and persistent effects of flumazenil on γ-aminobutyric acid-modulatory processes in this brain region are relevant to sensitized behavioral effects seen in alcoholism.
Keywords: Flumazenil, Repeated Ethanol Withdrawal, Amygdala, Dorsal Raphe, Accumbens, Paraventricular Nucleus of the Hypothalamus, Inferior Colliculus, Anxiety
Withdrawal from alcohol (ethanol) induces anxiety-like states across various animal models and probably mimics an analogous state seen in a large proportion of alcoholics (Brady and Lydiard, 1993; Kosten and O’Connor, 2003; Regier et al., 1990). This negative emotional state may be a precipitant of excessive drinking and increases the risk of relapse in alcoholics (e.g., reviewed in Bradizza et al., 2006). In animal models, one strategy used to block both the acute expression of anxiety seen during ethanol withdrawal and the development of anxiety over repeated cycles of chronic ethanol exposure is to administer the benzodiazepine receptor antagonist flumazenil (e.g., Criswell and Breese, 1993; File et al., 1989; Knapp et al., 2004, 2005, 2007; Little, 1991; Moy et al., 1997, 2000). That a benzodiazepine antagonist with unquestionably clear actions against benzodiazepine agonists shares anxiolytic actions with a benzodiazepine agonist like diazepam seems paradoxical and uncertainty remains as to how flumazenil exerts these effects (Adinoff et al., 1996; Barnard et al., 1998; Britton et al., 1988; Buck et al., 1991; Criswell and Breese, 1993; Little et al., 1985; Moy et al., 1997, 2000). Discussions have focused on γ-aminobutyric acid (GABA)-modulatory neuroactive steroid effects, antagonism of endogenous benzodiazepine receptor inverse agonists (which may vary as a function of chronic ethanol exposure) and differential intrinsic activity at benzodiazepine receptor subtypes (see Barnard et al., 1998 for summary), which are known to change their relative abundance as a function of chronic alcohol exposure (e.g., Cagetti et al., 2003; Devaud et al., 1995; Follesa et al., 2003; Grobin et al., 2000). Although the half-life of flumazenil is exceedingly short (16 minutes, Lister et al., 1984; 12 to 20 minutes in human brain, Lassen et al., 1995), its actions appear to far outlast its presence (e.g., Knapp et al., 2005). This profile is consistent with the possibility that flumazenil’s effects include long-term modification of an adaptive process that is central to anxiety and ethanol withdrawal. This action has been described as “resetting the state” of the GABA-receptor complex (Buck et al., 1991; Gonsalves and Gallager, 1988; Knapp et al., 2005; Little, 1991; Moy et al., 2000; Potokar et al., 1997), but further details of this potential mechanism have not emerged.
Whatever its mechanism(s) of action, flumazenil can block ethanol withdrawal-induced anxiety not only acutely (e.g., File et al., 1989, 1992; Knapp et al., 2004, 2005; Moy et al., 1997, 2000) but also prophylactically whereby treatments from 1 to 10 days prior to withdrawal also exert anxiolytic (Breese et al., 2004; Knapp et al., 2005) or anticonvulsant actions (Buck et al., 1991) during withdrawal. The consistent functional effects of flumazenil on emotional responding and similar actions of other drugs injected into the amygdala in related models (Overstreet et al., 2006) strongly suggest that limbic regions including the amygdala and/or extended amygdala (i.e., anxiety-related circuitry) could be involved in flumazenil’s effects.
Cycling of chronic ethanol treatments sensitizes anxiety-like behavior (Holter et al., 1988; Overstreet et al., 2002) in a fashion that mimics kindling effects of such treatments on seizures or withdrawal severity in animals (McCown and Breese, 1990) and in humans (Ballenger and Post, 1978; Brown et al., 1988; Malcolm et al., 2000) (although the sensitized anxiety effect has not been found by all researchers (e.g., Borlikova et al., 2006; Duka et al., 2002). Regardless of whether these two phenotypes develop concurrently, it is likely that ethanol-sensitized anxiety-like behavior and ethanol-kindled seizures arise from the activity of different brain regions. The preponderance of evidence has shown a progressive worsening or “kindling” of various withdrawal symptoms that may arise more readily after repeated ethanol exposure than after continuous exposure (e.g., Becker and Hale, 1993; Booth and Blow, 1993; Lechtenberg and Worner, 1991; McCown and Breese, 1990; Moak and Anton, 1996; Overstreet et al., 2002). In this regard, in a model characterized by cycled withdrawal-induced sensitization of social-interaction deficits, the withdrawal behavior can be antagonized by peripheral administration of drugs that antagonize corticotropin-releasing factor-type 1 or 5-hydroxytryptamine (5-HT)2C receptors or stimulate benzodiazepine, GABA-B, or 5-HT1A receptors (e.g., Knapp et al., 2005, 2007; Overstreet et al., 2003, 2004). Comparable actions do not appear to arise following administration of some agents that modify specific actions of glutamate (Knapp et al., 2007); however, the multiplicity of agents with ameliorative effects points to the relative richness of potential targets that may modify the alcoholic process, at least in its early stages. This investigation was conducted to explore the potential role of several brain regions of relevance to ethanol actions in models of anxiety (Overstreet et al., 2006), stress (Lee and Rivier, 1997), seizures (McCown and Breese, 1990), and reward (Di Chiara and Imperato, 1985; Engel and Liljequist, 1976; Weiss et al., 1993; Woodward et al., 1999) in the action of flumazenil to prevent anxiety sensitization arising after repeated ethanol withdrawals. Because results suggested activity within the amygdala, the potential inhibitory role of flumazenil against stress-sensitized withdrawal and inverse agonist activity in this region was also studied.
METHODS
Animals
Male Sprague–Dawley rats (Charles-River, Raleigh, NC) were obtained between 160 and 180 g and housed in groups of 3 or 4 for several days to adapt to the local conditions (at 22°C and 40% humidity; light:dark cycle of 12:12 with lights on between 7 AM and 7 PM. After the rats were adapted to these conditions for 5 days, surgeries were performed as described below. Animals were individually housed and, after a 7-day recovery period, placed on a nutritionally complete lactalbumin–dextrose diet and ultimately microinjected centrally as per the strategies below. All procedures involving animals described herein were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Surgery
Surgery was performed under pentobarbital [50 mg/kg, intraperitoneal (i.p.)] anesthesia with methyl atropine (2 mg/kg, i.p.) to reduce respiratory secretions. Once anesthetized, the rat was placed in a stereotaxic instrument (Kopf Instruments, Tujunga, CA). After exposing the dorsal surface of the skull, holes were drilled in the skull at the appropriate locations, and cannulae were inserted at the appropriate depth (Fig. 1). Jeweler’s screws were implanted into the skull, and dental acrylic was applied to secure the cannulae to the skull. All cannulae were made from 26-gauge stainless steel tubing. Once recovered from anesthesia, the rats were given acetaminophen (children’s Q-Pap, cherry flavor, 6 mg/ml) in the drinking water for 48 hours. After recovery, animals were individually housed and allowed 3 to 4 days to recover before proceeding to experimental treatments.
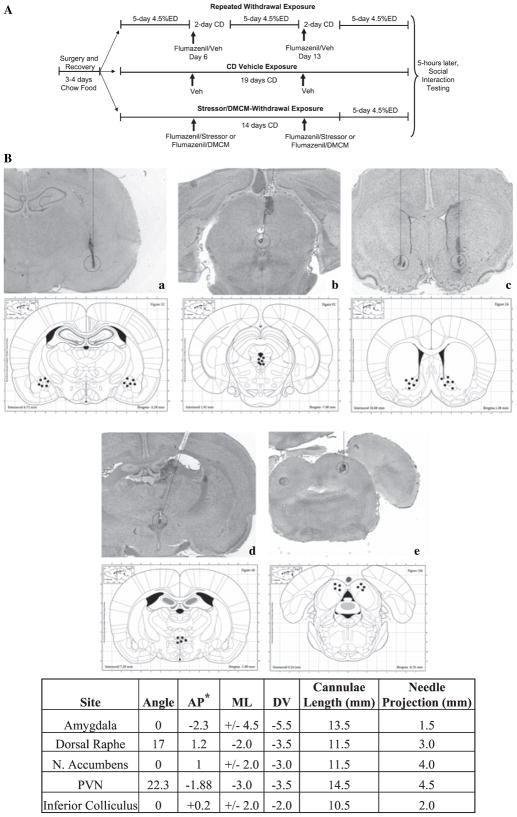
Fig. 1.
Schematic of treatment and testing strategies (A) and digital photomicrographs (top panel) illustrating targeted areas/needle tracks and schematics (bottom panel) showing representative positive locations of injections into (a) amygdala, (b) dorsal raphe, (c) nucleus accumbens, (d) paraventricular nucleus of the hypothalamus (PVN), (e) inferior colliculus (B). For cannula placements, all measurements are in millimeters, except for the angle of approach, which is measured in degrees from vertical.* All coordinates are relative to Bregma, except for the raphe, which is relative to lambda, and the inferior colliculus where the anterior–posterior (AP) location was defined as 0.2 mm anterior to the interaural line. CD, control diet; ED, 4.5% ethanol diet; ML, medial lateral; DV, dorsoventral. Coordinates and anatomical schematics are from Paxinos and Watson (2005).
Ethanol and Control Diets
At the start of dietary treatments, standard chow was taken away and a calorically balanced and nutritionally complete liquid diet containing 4.5% ethanol (e.g., Frye et al., 1981; Knapp et al., 2005; Moy et al., 1997, 2000; Overstreet et al., 2002) was administered in 1 of 2 protocols depending on the experiment (Fig. 1). In the first protocol (cycled), ethanol diet was administered for a period of 5 days followed by 2 days of withdrawal during which all animals received control liquid diet. This procotol was then repeated twice for a total of 3 cycles. Four hours after the ethanol was withdrawn during the first and second cycles (days 6 and 13), animals were treated with drug or vehicle (see below). In the second protocol (noncycled), rats received control diet during the period in which the cycled rats had experienced 2 cycles—i.e., the first 2 weeks. Rats were injected twice with methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM) at the same time (days 6 and 13) as animals in the first protocol experienced the onset of ethanol withdrawal. However, at the end of this 2-week period, animals in protocol 2 received a single 5-day cycle of ethanol diet for the first time. The target withdrawal for behavioral assessments for both protocols was this third (protocol 1) or first and only (protocol 2) withdrawal at the end of the 3-week period. Behavioral assessments were made between 5 and 6 hours after the removal of the ethanol based on the consistent expression of anxiety-like behavior and zero blood ethanol levels seen at this time (e.g., Breese et al., 2004; Knapp et al., 1998; Moy et al., 2000; Overstreet et al., 2002). In both protocols, groups of rats maintained on control diet were given a volume of diet equivalent to the average volume consumed the previous day by the rats maintained on the ethanol diet. The rats were weighed at weekly intervals, and volumes of diet were adjusted to ensure that the groups had similar body weight gains.
Intra-brain Injections
Rats maintained on ethanol diet were injected with vehicle or flumazenil into relevant brain sites at approximately 4 hours after the ethanol was withdrawn during the first and second cycles. Some groups were injected with DMCM (0.3 μg μl) into the amygdala while being maintained on control diet. For central drug injections, all injection needles were made from 32-gauge stainless steel tubing, and injections were made over 2 minutes with an additional 30 seconds before the removal of the needle from the site. Before and after injections, cannulae were kept closed with stainless steel plugs (32-gauge wire) which were inserted into the cannulae to a point 0.5 mm beyond the cannulae tips. To prevent damage to the targeted brain regions, the cannulae were placed from 1.5 to 4.5 mm above the injection site, depending on the target, and the smaller gauge injection needles projected past the cannulae tips to the areas of interest. Injections were directed at 1 of 5 brain sites depending upon the experiment (see below). Figure 1 provides further details of the specific cannulae coordinates for each site. For the amygdala, nucleus accumbens, and inferior colliculus, injections were bilateral with two cannulae inserted vertically into the brain. Injections were 1 μl per side (2.5–10 μg μl or 2.5–10 μg per injection). Because the dorsal raphe and paraventricular nucleus sites are small midline structures, bilateral injections were not needed and smaller volumes were used to limit drug spreading to the surrounding brain regions. These cannulae were angled to the midline location from a drill hole on the right side of the skull to avoid the superior sagittal sinus. Injections for these sites were 0.5 μl (5 μg μl or 2.5 μg per injection). Overall, choices of concentration ranges and volume ranges were based on the literature (e.g., Da Cunha et al., 1999; Mazarati and Wasterlain, 2002; McCown et al., 1987; Pesold and Treit, 1994, 1995) and based on our previous experience with successful central injections in the amygdala (e.g., Overstreet et al., 2006) and other regions.
Social-Interaction Test
At approximately 5 hours after the fifth (experiments 7 to 9) or 15th and last day of ethanol exposure (experiments 1 to 6), pairs of rats were placed in the social-interaction test. The social-interaction test is an efficient and validated index of anxiety-like behavior in the rat (File and Seth, 2003) and is quite sensitive to the consequences of chronic ethanol exposure (Breese et al., 2004; File et al., 1989; Knapp et al., 2007; Overstreet et al., 2002). Briefly, the test involves monitoring the social behavior of 2 weight-matched and similarly treated male rats placed concurrently in an open-field apparatus (60 cm by 60 cm, with 16 equal-sized squares marked out on the floor) for 5 minutes. Statistical analyses of several previous data sets revealed that scoring individual animals in the pair yielded the same statistical outcome as treating the scores of the pair as a unit (Overstreet et al., 2003, 2004). Further, control diet-treated animals that underwent the surgery for cannulae placement were found to have normal levels of social-interaction when compared with nonimplanted controls (Breese et al., 2007; Overstreet et al., 2006); thus, reference animals treated with control diet received comparable handling experiences in lieu of surgeries. Behaviors contributing to the score for each animal in the pair included grooming the partner, closely following, crawling over/under, sniffing, and boxing. Behaviors were scored by observers blind to the experimental condition. A forward locomotion score (i.e., the number of 2 forepaw line crossings) that provides a general activity measure independent of social-interaction was simultaneously recorded (File and Seth, 2003; Overstreet et al., 2002, 2003).
Histology
Within 48 hours of completion of the study, rats were anesthetized with pentobarbital for the determination of the locations of the cannulae, 0.5 μl of 0.5% methyl green dye was injected into the cannulae using the same injectors previously used to deliver the drugs, and animals were euthanized. The brain was removed, and the location of the dye was recorded. Only rats with correct cannulae locations were used in the statistical analyses.
Stressor
A subgroup of rats was stressed twice during the exposure to the control liquid diet. The stress sessions consisted of restraining rats for 60 minutes (6 days apart) in plastic conical decapicones prior to ethanol exposure to a single 5-day cycle of ethanol diet (Breese et al., 2004). In this fashion, the restraint stress substituted for the first 2 withdrawal experiences. A subgroup was treated intra-amygdalarly with flumazenil as described further below.
Drugs
For i.p. injection, the benzodiazepine receptor antagonist flumazenil (RO15-1788; a gift of Hoffman, La Roche, Nutley, NJ) was prepared as a suspension in 0.5% carboxymethylcellulose and injected at a dose of 5 mg/kg (2.5 mg/ml). For intra-amygdala injections, concentrations of 2.5–5 μg μl were prepared as a fine suspension in artificial cerebrospinal fluid (aCSF, pH 7.4). For Experiment 5 (inferior colliculus), an additional, higher dose (10 μg μl) was also included. The benzodiazepine receptor inverse agonist DMCM (RBI, Natick, MA) was prepared as a fine injectable suspension at a concentration of 0.3 μg μl in aCSF using a micro mortar and pestle.
Protocol
The study consisted of eight experiments. Experiments 1 to 6 were designed to test the ability of the benzodiazepine receptor antagonist flumazenil to reduce ethanol withdrawal-induced anxiety by direct injection into specific brain regions. These 6 experiments targeted the amygdala (including dose–response effects; experiments 1 and 2), dorsal raphe (experiment 3), paraventricular nucleus of the hypothalamus (experiment 4), nucleus accumbens (experiment 5), and inferior colliculus (experiment 6), respectively. It was previously shown that the benzodiazepine receptor inverse agonist DMCM potentiated ethanol withdrawal-induced anxiety-like behavior when given systemically (Knapp et al., 2005). This report also showed that flumazenil given systemically can counteract this anxiogenic effect. Thus, in the present study, two additional experiments (7 and 8) were conducted to test whether DMCM injected directly into the amygdala would have the same anxiogenic properties, and whether flumazenil given systemically would counteract these effects. Two injections 1 week apart were given while animals were maintained on control diet. After the second drug administration, animals were placed on one 5-day cycle with 4.5% ethanol diet. Social-interaction testing was performed 5 hours into withdrawal at the end of the fifth day of this cycle. Thus, all behavioral testing was done in a drug-free state. Finally, given that peripherally administered flumazenil antagonized the ability of stress to sensitize ethanol withdrawal-induced anxiety (Breese et al., 2004), experiment 9 assessed the ability of intra-amygdala flumazenil to block this effect of stress.
Data Analysis
Statistical analyses of social-interaction time and locomotor behavior were carried out using the GBStat software package (Dynamic microsystems, Inc., Silver Spring, MD). The data were initially analyzed by one-way analyses of variance (ANOVAs). If the main effects were statistically significant (p < 0.05), post hoc analyses of significant group comparisons were performed using Tukey’s protected t-tests (p < 0.05). Body weight and ethanol intake data were analyzed with appropriate ANOVA or t-tests.
RESULTS
Effects of Repeated Amygdala Injections of Flumazenil During Withdrawal on Anxiety-Like Behavior: Dose–Response Relationship
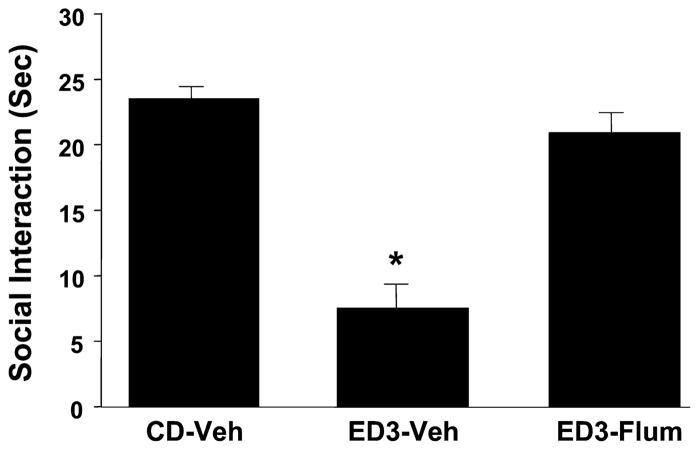
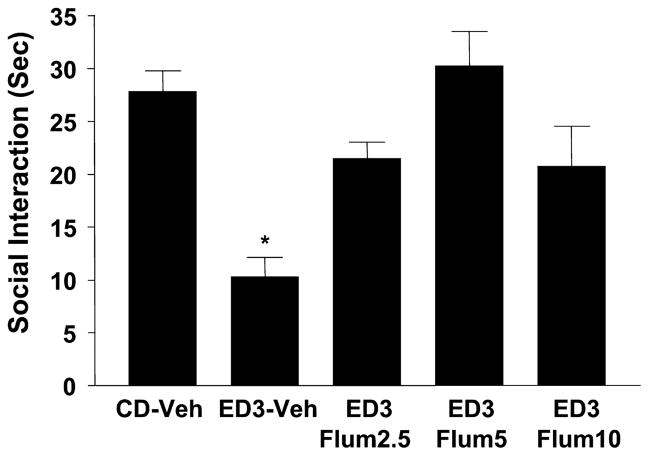
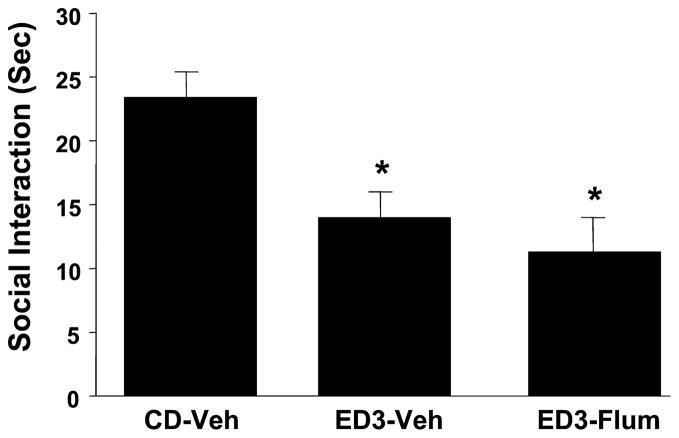
The results of bilateral injections of flumazenil into the amygdala are shown in Figs. 2 and 3. Figure 2 shows data from the initial study where there was a significant effect of treatments [F(2,34) = 38.4, p < 0.0001] and blockade of the withdrawal effect by intra-amygdala flumazenil injections (5 μgμl per side during the first 2 withdrawals significantly blocked the anxiety effect seen during a third withdrawal). Subsequently, doses of 2.5, 5, and 10 μg μl were examined to confirm this effect and to isolate the optimum dose for subsequent studies. Again, as shown in Fig. 3, there was a significant effect of treatments [F(4,22) = 7.23, p < 0.01] and significant blockade of the repeated withdrawal effect by flumazenil with the dose–response range appearing to lie between 0 and 5 μgμl. No additional benefit was seen with the higher dose of 10 μg μl. No treatment significantly altered locomotor behavior in these two experiments (data not shown).
Fig. 2.
Intra-amygdalar injection of flumazenil selectively counteracts ethanol withdrawal-induced anxiety-like behavior. Rats were implanted with cannulae above the amygdala and then subjected to 3 cycles of 4.5% ethanol diet (ED3). Injections were given 4 hours after ethanol from the first and second cycles of ED was withdrawn. Social-interaction behavior was assessed between 5 and 6 hours after the removal of the third cycle of ethanol. CD, control diet given to unoperated controls; ED3-Veh, ethanol diet with vehicle injections (artificial cerebrospinal fluid); ED3-Flum, Ethanol diet and flumazenil injections (5 μg/μl per side). *p < 0.05 versus CD-Veh.
Fig. 3.
Dose–response for intra-amygdala flumazenil pretreatments that selectively counteract ethanol withdrawal-induced anxiety-like behavior. Rats were implanted with cannulae above the amygdala, exposed to 3 cycles of chronic ethanol diet with or without flumazenil pretreatments (2.5, 5, or 10 μg/μl per side) during the first 2 withdrawals. Other groups and abbreviations are as per Fig. 2. *p < 0.05 versus CD-Veh.
Regional Specificity of Flumazenil Action Against Repeated Ethanol Withdrawal-Induced Anxiety-Like Behavior
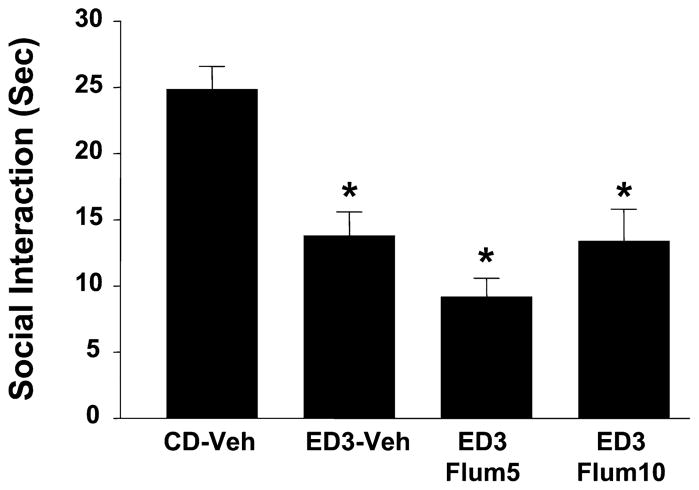
The responses to flumazenil found after injections into other brain regions are shown in Figs. 4–7. First, significant effects were found across the groups in each of the respective experiments involving the raphe [F(2,25) = 14.53, p <0.001] (experiment 3, Fig. 4), paraventricular nucleus of the hypothalamus (PVN) [F(2,21) = 6.31, p < 0.01] (experiment 4, Fig. 5), nucleus accumbens [F(2,18) = 11.74, p < 0.001] (experiment 5, Fig. 6), or inferior colliculus [F(3,35) = 13.12, p < 0.001] (experiment 6, Fig. 7). However, while the prophylactic flumazenil injected into the amygdala strongly inhibited the social-interaction deficits caused by repeated exposure and withdrawal from ethanol diet, injections into these other brain regions were ineffective. None of the groups of animals in these experiments exhibited an effect of treatment on locomotor behavior (data not shown).
Fig. 4.
Intra-raphe injection of flumazenil fails to counteract ethanol withdrawal- induced anxiety-like behavior. Rats were implanted with single cannulae above the dorsal raphe nucleus and then subjected to 3 cycles of 4.5% ethanol diet (ED3). Flumazenil treatment was 2.5 μg (0.5 μg/μl). Groups and abbreviations are as per Fig. 2. *p < 0.05 versusCD-Veh.
Fig. 7.
Failure of injections of flumazenil in the inferior colliculus to counteract ethanol withdrawal-induced anxiety-like behavior. Rats were implanted with cannulae above the inferior colliculus and then subjected to 3 cycles of 4.5% ethanol diet (ED3). Flum5 and Flum10 injections (5 or 10 μg/μl per side, respectively) were given 4 hours after ethanol from the first and second cycles of ED was withdrawn. Other groups and abbreviations are as per Fig. 2. *p < 0.05 versus CD-Veh.
Fig. 5.
Intra-paraventricular nucleus of the hypothalamus injections of flumazenil fails to counteract ethanol withdrawal-induced anxiety-like behavior. Rats were implanted with cannulae above the paraventricular nucleus and then subjected to 3 cycles of 4.5% ethanol diet (ED3). Flumazenil treatment was 2.5 μg (0.5 μg/μl). Groups and abbreviations are as per Fig. 2. *p < 0.05 versus CD-Veh.
Fig. 6.
Intra-accumbens injections of flumazenil into the accumbens fail to counteract ethanol withdrawal-induced anxiety-like behavior. Rats were implanted with cannulae above the nucleus accumbens and then subjected to 3 cycles of 4.5% ethanol diet (ED3). Flumazenil treatments, groups, and abbreviations are as per Fig. 2. *p < 0.05 versus CD-Veh.
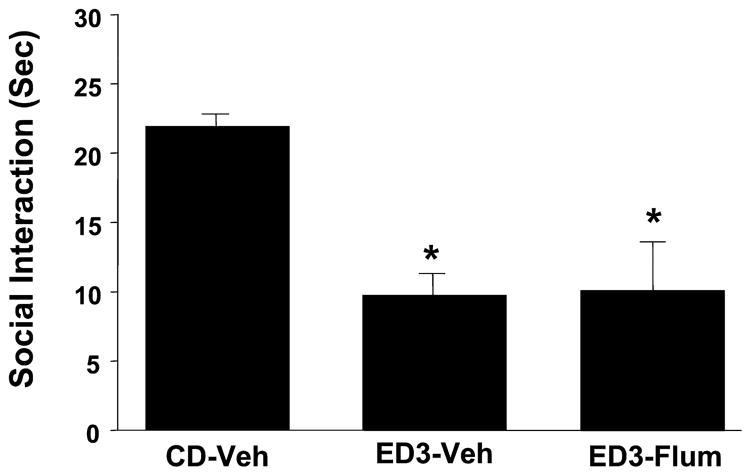
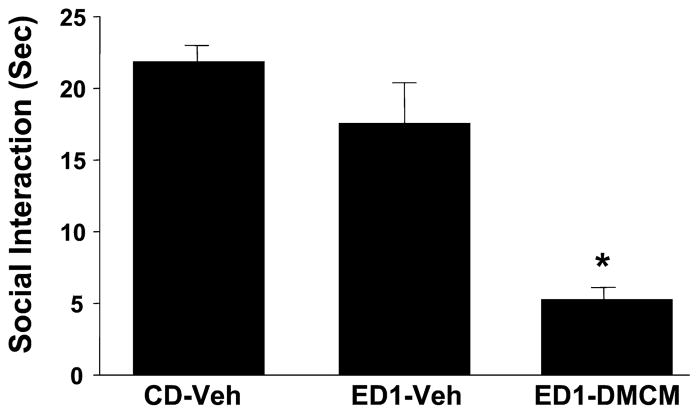
Repeated Amygdala DMCM Actions on Repeated Ethanol Withdrawal-Induced Anxiety-Like Behavior
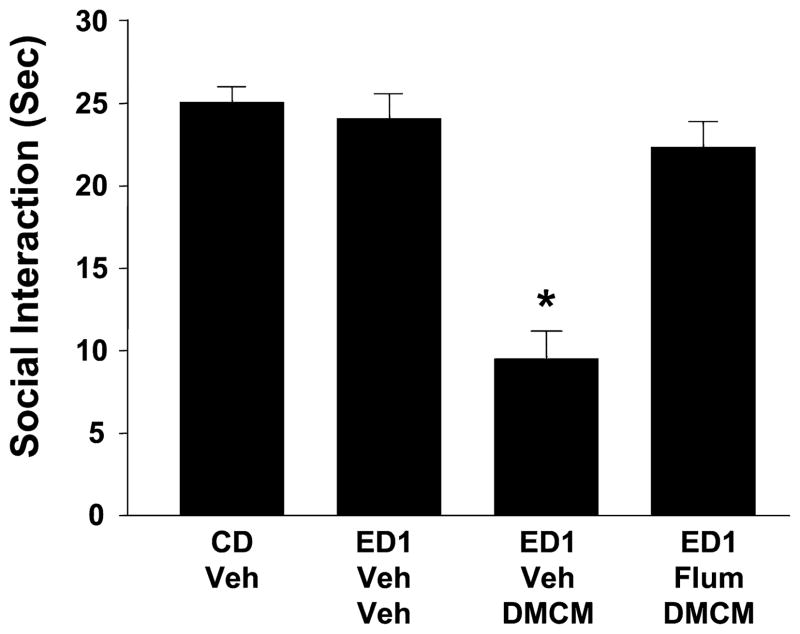
Across treatment groups in experiment 7, there were significant effects [F(2,31) = 11.52, p < 0.001] (Fig. 8) with DMCM injections into the amygdala in lieu of the first 2 cycles of ethanol treatment and withdrawal mimicking the effect of repeated withdrawal on anxiety-like behavior. Compared with control-diet treated animals or animals exposed to a single 5-day cycle of ethanol diet and withdrawal, these DMCM-treated and withdrawn animals fared worse. While there was no effect of withdrawal alone on social interaction, there was a moderate effect of the single cycle of ethanol exposure on locomotor behavior (p < 0.01) that was not seen in DMCM-treated animals. Significant effects on social-interaction behavior also arose in experiment 8, where peripheral flumazenil treatments preceded the amygdala DMCM injections [F(3,35) = 32.52, p < 0.001] (Fig. 9). Here, flumazenil DMCM-treated and withdrawn animals showed no social-interaction deficit relative to controls or animals exposed only to the single cycle of ethanol diet. Locomotor effects were not significantly different among the groups in this study (data not shown).
Fig. 8.
Repeated intra-amygdalar injection of methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM) sensitizes ethanol withdrawal-induced anxiety-like behavior. Rats were implanted with cannulae above the amygdala, treated with DMCM twice, and then subjected to 1 cycle of 4.5% ethanol diet (ED1). Other groups and abbreviations are as per Fig. 2. *p < 0.05 versus CD-Veh.
Fig. 9.
Peripheral pretreatment with flumazenil prevents sensitization of ethanol withdrawal-induced anxiety-like behavior by intra-amygdala injection of methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM). Rats were implanted with cannulae above the amygdala, treated with DMCM twice with or without flumazenil pretreatment (5 μg/μl per side), and then subjected to 1 cycle of 4.5% ethanol diet (ED1). Other groups and abbreviations are as per Fig. 2. *p < 0.05 versusCD-Veh.
Effects of Intra-amygdalar Flumazenil on Stressor Sensitized Ethanol Withdrawal-Induced Anxiety
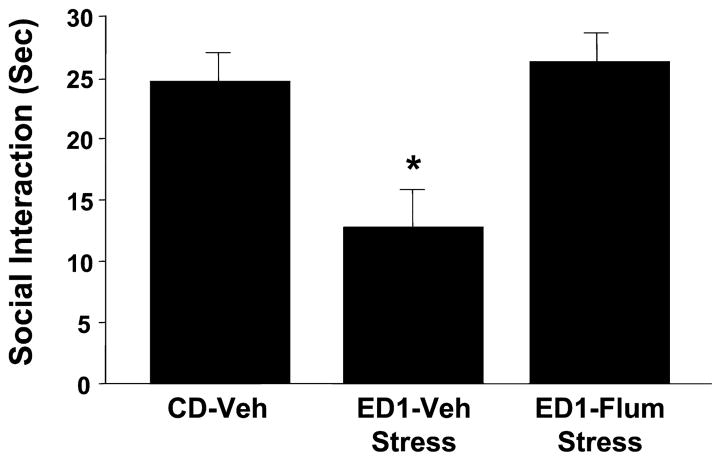
To assess the similarities among withdrawal, stress, and DMCM treatment effects, a subgroup of animals was pretreated with flumazenil, (15 minutes) prior to 60 minutes of restraint stress on 2 occasions 6 days apart. Rats were then exposed to the ethanol diet for 1 cycle of 5 days, prior to behavioral testing during withdrawal. Significant effects were seen across treatment groups [F(2,26) = 8.28, p < 0.001] (Fig. 10). Rats that usually show no significant anxiety-like response to such a short ethanol exposure period became responsive, if they had a history of exposure to a stressor. This effect was prevented by pretreating the animals with intra-amygdalar flumazenil prior to each stressor session. Locomotor effects were not significantly different among the groups (data not shown).
Fig. 10.
Intra-amygdala pretreatment with flumazenil prevents sensitization of ethanol withdrawal-induced anxiety-like behavior by restraint stress. Rats were implanted with cannulae above the amygdala, restraint stressed twice with or without intra-amygdala flumazenil injection (5 μg/μl per side), and then subjected to 1 cycle of 4.5% ethanol diet (ED1). Other groups and abbreviations are as per Fig. 2. *p < 0.05 versus CD-Veh.
Body Weights and Alcohol Intake
All animals readily gained weight throughout the course of dietary manipulation, and reasonable control was obtained over body weights across the various groups within and across experiments (Table 1). Rats generally consumed between 7.5 and 9 gkgd of ethanol.
Table 1.
Body Weights and Ethanol Intakesa
| Treatment group | Ethanol intake (g/kg/d) | Body weight (g) |
|---|---|---|
| Experiment 1: flumazenil/amygdala | ||
| CD-Veh | NA | 344 ± 4 |
| ED3-Veh | 8.2 ± 0.2 | 331 ± 8 |
| ED3-flumazenil | 8.1 ± 0.4 | 332 ± 12 |
| t(17) = 0.33; NS | F(2,34) = 1.57; NS | |
| Experiment 2: flumazenil/amygdala, dose–response | ||
| CD-Veh | NA | 343 ± 5 |
| ED3-Veh | 8.3 ± 0.5 | 335 ± 12 |
| ED3-flumazenil (2.5 μg per side) | 7.8 ± 0.3 | 340 ± 7 |
| ED3-flumazenil (5 μg per side) | 7.4 ± 0.4 | 338 ± 10 |
| ED3-flumazenil (10 μg per side) | 7.9 ± 0.2 | 343 ± 5 |
| F(3,16) = 1.01; NS | F(4,22) = 0.13; NS | |
| Experiment 3: flumazenil/raphe | ||
| CD-Veh | NA | 300 ± 4 |
| ED3-Veh | 8.4 ± 0.3 | 322 ± 8 |
| ED3-flumazenil | 8.7 ± 0.3 | 334 ± 6 |
| t(13) = 0.56; NS | F(2,25) = 5.44, p < 0.01 | |
| Experiment 4: flumazenil/PVN | ||
| CD-Veh | NA | 364 ± 5 |
| ED3-Veh | 8.6 ± 0.5 | 335 ± 6 |
| ED3-flumazenil | 8.7 ± 0.2 | 341 ± 6 |
| t(14) = 0.02; NS | F(2,21) = 7.07; p < 0.01 | |
| Experiment 5: flumazenil/accumbens | ||
| CD-Veh | NA | 343 ± 7 |
| ED3-Veh | 8.9 ± 0.7 | 327 ± 9 |
| ED3-flumazenil | 8.7 ± 0.2 | 310 ± 12 |
| t(11) = 0.15; NS | F(2,18) = 3.27; NS | |
| Experiment 6: flumazenil/colliculus | ||
| CD-Veh | NA | 356 ± 4 |
| ED3-Veh | 10 ± 0.6 | 358 ± 10 |
| ED3-flumazenil (5 μg per side) | 9.5 ± 0.3 | 353 ± 8 |
| ED3-flumazenil (10 μg per side) | 9.0 ± 0.5 | 335 ± 15 |
| F(2,35) = 1.04; NS | F(3,35) = 1.06; NS | |
| Experiment 7: DMCM/amygdala | ||
| CD-Veh | NA | 353 ± 4 |
| ED1-Veh | 8.5 ± 0.2 | 347 ± 7 |
| ED1-DMCM | 7.9 ± 0.3 | 348 ± 6 |
| t(14) = 1.51; NS | F(2,31) = 0.36; NS | |
| Experiment 8: flumazenil DMCM | ||
| CD-Veh | NA | 324 ± 6 |
| ED1-Veh | 8.6 ± 0.6 | 326 ± 7 |
| ED1-DMCM | 9.0 ± 0.4 | 314 ± 5 |
| ED1-flumazenil-DMCM | 8.7 ± 0.4 | 324 ± 6 |
| F(2,21) = 0.23; NS | F(3,35) = 0.49; NS | |
| Experiment 9: flumazenil/amygdala stress | ||
| CD-Veh | NA | 338 ± 7 |
| ED1-Veh | 8.1 ± 0.2 | 335 ± 6 |
| ED1-flumazenil | 7.7 ± 0.3 | 318 ± 0 |
| t(22) = 1.17; NS | F(2,26) = 4.15, p < 0.05 | |
Rats consumed ethanol or control diets for one or three 5-day cycles with flumazenil treatments during periods of withdrawal at the end of the first 2 of 3 cycles (experiments 1 to 6), or prior to repeated DMCM treatment (experiments 7 to 8) or repeated stress (experiment 9).
NA, not applicable; NS, not significant; CD, control diet; ED, ethanol diet; PVN, paraventricular nucleus of the hypothalamus; ED1, ED3, 1 or 3 cycles of ethanol diet, respectively; DMCM, methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate.
DISCUSSION
The results of these experiments indicate that flumazenil can selectively affect an index of anxiety-like behavior while exerting no major effects on locomotor behavior in a model of repeated ethanol withdrawal-induced anxiety. Importantly, this effect appears to be brain site specific as well. One site of relevance appears to be the amygdala, at least so far as ethanol withdrawal-induced anxiety-like behavior is concerned. The results also suggest that flumazenil’s action may be specifically mediated within the amygdala rather than the paraventricular nucleus of the hypothalamus, nucleus accumbens, inferior colliculus, and dorsal raphe. These results complement and extend findings that peripherally administered DMCM and flumazenil in ethanol withdrawal-related models regulate anxiety-like behavior (e.g., File et al., 1989; Knapp et al., 2005; Moy et al., 1997, 2000) and that the amygdala is involved more generally in withdrawal-related emotional responding (e.g., Lack et al., 2005; Overstreet et al., 2006; Rassnick et al., 1993).
While limited in scope, the stressor experiment reiterates that exposure to a stressor likely mimics to some extent the actions of withdrawal to sensitize neuroadaptations that render animals more likely to express a negative emotional response during withdrawal (see also Breese et al., 2004, 2005a; Weiss et al., 2001). More specifically, repeated restraint stress applied prior to chronic ethanol exposure or a single stress during forced abstinence from repeated ethanol exposure rendered animals more likely to express elevated anxiety-like behavior during withdrawal or during abstinence (Breese et al., 2004, 2005b). The antagonistic effect of intra-amygdalar flumazenil administration on sensitized anxiety-like response during withdrawal suggests that both stress and chronic ethanol/withdrawal-associated mechanisms within the amygdala contribute to this maladaptation. In this regard, it is notable that flumazenil may also exert anxiolytic effects under conditions that would appear to induce relatively high levels of stress or anxiety, such as in the shuttle-box avoidance procedure (Criswell and Breese, 1993). Overall, these interactions may exacerbate the course of development and maintenance of alcoholism through effects on craving and relapse (e.g., see Breese et al., 2005a; Le et al., 2000; Sinha, 2001). Stress experienced prior to, during, or after chronic ethanol exposure may exert relevant effects on these processes.
While the effect of flumazenil and its site of action appears clear enough, several caveats should be borne in mind in interpreting the data. First, the clinical data on the utility of flumazenil in treating alcoholics are mixed. For example, in a small trial of flumazenil in ethanol-withdrawn subjects, Potokar et al. (1997) found that flumazenil was neither anxiolytic nor anxiogenic, although total withdrawal scores were reduced. In contrast, Gerra et al. (1991) found anxiolytic effects of flumazenil in withdrawing alcoholics. It is possible that more established alcoholics or animals with more intensive exposure to ethanol are more resistant to flumazenil’s beneficial actions, although the consistent ameliorative actions of flumazenil across a host of studies and laboratories argue against this point (Breese et al., 2004; Buck et al., 1991; Criswell and Breese, 1993; File et al., 1989, 1992; Knapp et al., 2004, 2005, 2007; Little, 1991; Moy et al., 1997, 2000). Better isolation of the relevant mechanism should assist in focusing pharmaceutical development on new drugs that might find more consistent utility in humans. It should also be noted that the action of flumazenil appears to apply broadly across various chronic ethanol treatment regimens; therefore, the uniqueness of the effect to a cycled chronic ethanol paradigm should not be overemphasized. Further, the potential importance of brain regions other than the amygdala and doses other than those used herein cannot be ruled out, particularly when considering potential spread of the drug away from the injector site. In the amygdala, for example, drug may well have reached the central, basolateral, and/or medial nuclei, and determining whether one or more of these contributed to the effects found will require more focused study to ascertain their potential impact on the stress-, DMCM-, or ethanol withdrawal-sensitized response. Further considerations of the exact flumazenil concentration that reaches the relevant receptors become all the more interesting, in that the action of flumazenil may shift toward an agonist profile at higher doses (e.g., Barnard et al., 1998; Criswell and Breese, 1993; Knoflach et al., 1996; Skerritt and Macdonald, 1984), a possibility that complicates a full interpretation of data from the current studies. These caveats notwithstanding, the sensitivity of the amygdala to these manipulations (in which flumazenil was administered at doses as low as 2.5 μgμl per side) was conclusively demonstrated in the current studies.
The mechanism through which flumazenil exerts these effects is unknown, but interesting and not necessarily opposing ideas have emerged. The first possibility is that flumazenil may block an endogenous benzodiazepine receptor inverse agonist that was more active and/or present in greater amounts during withdrawal (e.g., the anxiogenic peptide diazepam-binding inhibitor) (Katsura et al., 1995a,b, 1998; Mohri et al., 2003, but see Adinoff et al., 1996; Roy et al., 1990). A second idea relates to the involvement of GABA-modulatory neurosteroids in chronic ethanol effects (Cagetti et al., 2004; Devaud et al., 1995; Korpi et al., 2001). For example, Devaud et al. (1995) found that lowered seizure thresholds induced by ethanol withdrawal were ameliorated by the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one (3α, 5α-THP, allopregnanolone) and that sensitivity to this steroid increases with chronic ethanol exposure (Devaud et al., 1996). This profile is reminiscent of flumazenil’s actions in control rats versus chronic ethanol-treated rats. One interpretation consistent with each of these potential mechanisms is that changes in GABA receptor subunit compositions after chronic ethanol exposure endow these agents with different intrinsic activity. Flumazenil appears to be particularly relevant to α4 subunit-containing GABA receptors (Barnard et al., 1998; Wallner et al., 2006). Given that this subunit may increase in some brain regions after chronic ethanol exposure (e.g., Devaud et al., 1995; Follesa et al., 2003; Sanna et al., 2003), it is possible that functional effects of flumazenil that are unique to withdrawn animals may hinge at least in part on this mechanism. Moreover, a “switch” from a predominantly antagonist activity to one that includes agonist activity (at least so far as these specific α4-containing receptors are concerned) may be responsible for the switch from a relatively innocuous antagonist in many models to an active agonist in models incorporating chronic ethanol-treated tissue (Follesa et al., 2006; see also Barnard et al., 1998; Wafford et al., 1996; Whittemore et al., 1996, but see Papadeas et al., 2001). Finally, another potential mechanism that may be engaged by flumazenil involves an interaction with adenosine. Adenosine effects during acute and chronic ethanol exposure are well described (Diamond et al., 1991; Mailliard and Diamond, 2004), and flumazenil reportedly antagonizes adenosine uptake in various brain regions (e.g., Phillis and Stair, 1987; Stone, 1999), an effect that would decrease neurotransmitter release. Systematic assessments of these respective mechanisms within the relevant brain regions (e.g., subnuclei of the amygdala and cells therein) should provide new insight into the neural regulation of emotional phenotypes aroused by cycles of chronic ethanol exposure.
CONCLUSION
Repeated ethanol withdrawal or repeated stresses prior to ethanol withdrawal sensitizes anxiety-like behavior. These effects are attenuated by pretreating these manipulations with flumazenil in the amygdala but not in a number of other brain regions. In contrast, pretreating the amygdala with the benzodiazepine receptor inverse agonist DMCM prior to ethanol exposure sensitizes anxiety-like behavior during withdrawal. Although other interpretations are possible, the results are consistent with the idea that benzodiazepine inverse receptor agonist action in the amygdala during withdrawal or stress/withdrawal sensitizes emotional responding.
Acknowledgments
We wish to thank Lara Marr for technical assistance and grants AA-11605 and AA-14284 from the NIAAA.
References
- Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996;15:288–295. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol Alcohol. 1993;28:593–598. [PubMed] [Google Scholar]
- Borlikova GG, Le Merrer J, Stephens DN. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology (Berl) 2006;185:188–200. doi: 10.1007/s00213-005-0301-3. [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brady KT, Lydiard RB. The association of alcoholism and anxiety. Psychiatr Q. 1993;64:135–149. doi: 10.1007/BF01065866. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005a;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA. Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology. 2007 Jun 6; doi: 10.1038/sj.npp.1301468. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KT, Lee G, Koob GF. Corticotropin releasing factor and amphetamine exaggerate partial agonist properties of benzodiazepine antagonist Ro 15-1788 in the conflict test. Psychopharmacology (Berl) 1988;94:306–311. doi: 10.1007/BF00174680. [DOI] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC. Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry. 1988;23:507–514. doi: 10.1016/0006-3223(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Heim H, Harris RA. Reversal of alcohol dependence and tolerance by a single administration of flumazenil. J Pharmacol Exp Ther. 1991;257:984–989. [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. Similar effects of ethanol and flumazenil on acquisition of a shuttle-box avoidance response during withdrawal from chronic ethanol treatment. Br J Pharmacol. 1993;110:753–760. doi: 10.1111/j.1476-5381.1993.tb13876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha C, Roozendaal B, Vazdarjanova A, McGaugh JL. Microinfusions of flumazenil into the basolateral but not the central nucleus of the amygdala enhance memory consolidation in rats. Neurobiol Learn Mem. 1999;72:1–7. doi: 10.1006/nlme.1999.3912. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of gamma-aminobutyric acidA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative reverse transcriptase-polymerase chain reaction analysis. Mol Pharmacol. 1995;48:861–868. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Diamond I, Nagy L, Mochly-Rosen D, Gordon A. The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence. Possible biologic and genetic markers of alcoholism. Ann N Y Acad Sci. 1991;625:473–487. doi: 10.1111/j.1749-6632.1991.tb33878.x. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Kindling of withdrawal: a study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2002;26:785–795. [PubMed] [Google Scholar]
- Engel J, Liljequist S. The effect of long-term ethanol treatment on the sensitivity of the dopamine receptors in the nucleus accumbens. Psychopharmacology (Berl) 1976;49:253–257. doi: 10.1007/BF00426825. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcott PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psychopharmacology (Berl) 1989;98:262–264. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Zharkovsky A, Hitchcot PK. Effects of nitrendipine, chlordiazepoxide, flumazenil and baclofen on the increased anxiety resulting from alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:87–93. doi: 10.1016/0278-5846(92)90011-3. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, Biggio G. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology. 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Follesa P, Mancuso L, Biggio F, Mostallino MC, Manca A, Mascia MP, Busonero F, Talani G, Sanna E, Biggio G. Gamma-hydroxybutyric acid and diazepam antagonize a rapid increase in GABA(A) receptors alpha(4) subunit mRNA abundance induced by ethanol withdrawal in cerebellar granule cells. Mol Pharmacol. 2003;63:896–907. doi: 10.1124/mol.63.4.896. [DOI] [PubMed] [Google Scholar]
- Frye GD, Chapin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, Breese GR. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: a comparison with ethanol. J Pharmacol Exp Ther. 1981;216:306–314. [PubMed] [Google Scholar]
- Gerra G, Caccavari R, Volpi R, Maninetti L, Delsignore R, Coiro V. Effectiveness of flumazenil in the treatment of alcohol withdrawal. Curr Ther Res. 1991;50:62–66. [Google Scholar]
- Gonsalves SF, Gallager DW. Persistent reversal of tolerance to anti-convulsant effects and GABAergic subsensitivity by a single exposure to benzodiazepine antagonist during chronic benzodiazepine administration. J Pharmacol Exp Ther. 1988;244:79–83. [PubMed] [Google Scholar]
- Grobin AC, Fritschy JM, Morrow AL. Chronic ethanol administration alters immunoreactivity for GABA(A) receptor subunits in rat cortex in a region-specific manner. Alcohol Clin Exp Res. 2000;24:1137–1144. [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1988;9:41–48. [PubMed] [Google Scholar]
- Katsura M, Ohkuma S, Tsujimura A, Kuriyama K. Increase of diazepam binding inhibitor mRNA levels in the brains of chronically ethanol-treated and -withdrawn mice. J Pharmacol Exp Ther. 1995a;273:1529–1533. [PubMed] [Google Scholar]
- Katsura M, Ohkuma S, Tsujimura A, Kuriyama K. Increase of diazepam binding inhibitor mRNA levels in the brains of chronically ethanol-treated and -withdrawn mice. J Pharmacol Exp Ther. 1995b;273:1529–1533. [PubMed] [Google Scholar]
- Katsura M, Ohkuma S, Tsujimura A, Xu J, Hibino Y, Ishikawa E, Kuriyama K. Functional involvement of benzodiazepine receptors in ethanol-induced increases of diazepam binding inhibitor (DBI) and its mRNA in the mouse brain. Brain Res Mol Brain Res. 1998;54:124–132. doi: 10.1016/s0169-328x(97)00330-6. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal- induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine GABA ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam- insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Korpi ER, Makela R, Romeo E, Guidotti A, Uusi-Oukari M, Furnari C, di Michele F, Sarviharju M, Xu M, Rosenberg PH. Increased behavioral neurosteroid sensitivity in a rat line selectively bred for high alcohol sensitivity. Eur J Pharmacol. 2001;421:31–38. doi: 10.1016/s0014-2999(01)01035-4. [DOI] [PubMed] [Google Scholar]
- Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348:1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- Lack AK, Floyd DW, McCool BA. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 2005;36:83–90. doi: 10.1016/j.alcohol.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, Osman S, Bloomfield PM, Jones T, Patsalos PN, O’Connell MT, Duncan JS, Andersen JV. Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab. 1995;15:152–165. doi: 10.1038/jcbfm.1995.17. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lechtenberg R, Worner TM. Relative kindling effect of detoxification and non-detoxification admissions in alcoholics. Alcohol Alcohol. 1991;26:221–225. doi: 10.1093/oxfordjournals.alcalc.a045104. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic–pituitary–adrenal axis. J Neurosci. 1997;17:8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG, Greenblatt DJ, Abernethy DR, File SE. Pharmacokinetic studies on Ro 15-1788, a benzodiazepine receptor ligand, in the brain of the rat. Brain Res. 1984;290:183–186. doi: 10.1016/0006-8993(84)90752-2. [DOI] [PubMed] [Google Scholar]
- Little HJ. The benzodiazepines: anxiolytic and withdrawal effects. Neuropeptides. 1991;19(Suppl):11–14. doi: 10.1016/0143-4179(91)90077-v. [DOI] [PubMed] [Google Scholar]
- Little HJ, Taylor SC, Nutt DJ, Cowen PJ. The benzodiazepine antagonist, Ro 15-1788 does not decrease ethanol withdrawal convulsions in rats. Eur J Pharmacol. 1985;107:375–377. doi: 10.1016/0014-2999(85)90265-1. [DOI] [PubMed] [Google Scholar]
- Mailliard WS, Diamond I. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol Ther. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts J-S, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–164. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Wasterlain CG. Anticonvulsant effects of four neuropeptides in the rat hippocampus during self-sustaining status epilepticus. Neurosci Lett. 2002;331:123–127. doi: 10.1016/s0304-3940(02)00847-9. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Bivens BS, Breese GR. Amino acid influences on seizures elicited within the inferior colliculus. J Pharmacol Exp Ther. 1987;243:603–608. [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Moak DH, Anton RF. Alcohol-related seizures and the kindling effect of repeated detoxifications: the influence of cocaine. Alcohol Alcohol. 1996;31:135–143. doi: 10.1093/oxfordjournals.alcalc.a008124. [DOI] [PubMed] [Google Scholar]
- Mohri Y, Katsura M, Shuto K, Tsujimura A, Ishii R, Ohkuma S. L-type high voltage-gated calcium channels cause an increase in diazepam binding inhibitor mRNA expression after sustained exposure to ethanol in mouse cerebral cortical neurons. Brain Res Mol Brain Res. 2003;113:52–56. doi: 10.1016/s0169-328x(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology (Berl) 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology (Berl) 2000;152:208–215. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1269. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology (Berl) 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(−) uptake in mesocorti-colimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Elsevier Academic Press; San Diego: 2005. [Google Scholar]
- Pesold C, Treit D. The septum and amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1994;638:295–301. doi: 10.1016/0006-8993(94)90662-9. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effets of benzodiazepines. Brain Res. 1995;671:213–221. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Stair RE. Ro 15-1788 both antagonizes and potentiates adenosine-evoked depression of cerebral cortical neurons. Eur J Pharmacol. 1987;136:151–156. doi: 10.1016/0014-2999(87)90706-0. [DOI] [PubMed] [Google Scholar]
- Potokar J, Coupland N, Glue P, Groves S, Malizia A, Bailey J, Wilson S, Nutt D. Flumazenil in alcohol withdrawal: a double-blind placebo-controlled study. Alcohol Alcohol. 1997;32:605–611. doi: 10.1093/oxfordjournals.alcalc.a008302. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Roy A, DeJong J, Adinoff B, Barbaccia M, Costa E, Guidotti A, Linnoila M. CSF diazepam-binding inhibitor in alcoholics and normal controls. Psychiatry Res. 1990;31:261–266. doi: 10.1016/0165-1781(90)90095-m. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Skerritt JH, Macdonald RL. Benzodiazepine receptor ligand actions on GABA responses. Benzodiazepines, CL 218872, zopiclone. Eur J Pharmacol. 1984;101:127–134. doi: 10.1016/0014-2999(84)90038-4. [DOI] [PubMed] [Google Scholar]
- Stone TW. Actions of benzodiazepines and the benzodiazepine antagonist flumazenil may involve adenosine. J Neurol Sci. 1999;163:199–201. doi: 10.1016/s0022-510x(98)00273-1. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminbutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci U S A. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factor. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressing Xenopus laevis oocytes. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- Woodward DJ, Chang JY, Janak P, Azarov A, Anstrom K. Meso-limbic neuronal activity across behavioral states. Ann N Y Acad Sci. 1999;877:91–112. doi: 10.1111/j.1749-6632.1999.tb09263.x. [DOI] [PubMed] [Google Scholar]