Abstract
Hydrogen sulfide (H2S) is a colorless, water soluble, flammable gas that has the characteristic smell of rotten eggs. Like other members of the gasotransmitter family (nitric oxide and carbon monoxide), H2S has traditionally been considered to be a highly toxic gas and environmental hazard. However, much like for nitric oxide and carbon monoxide, the initial negative perception of H2S has evolved with the discovery that H2S is produced enzymatically in mammals under normal conditions. As a result of this discovery, there has been a great deal of work to elucidate the physiological role of H2S. H2S is now recognized to be cytoprotective in various models of cellular injury. Specifically, it has been demonstrated that the acute administration of H2S, either prior to ischemia or at reperfusion, significantly ameliorates in vitro or in vivo myocardial and hepatic ischemia-reperfusion injury. These studies have also demonstrated a cardioprotective role for endogenous H2S. This review article summarizes the current body of evidence demonstrating the cytoprotective effects of H2S with an emphasis on the cardioprotective effects. This review also provides a detailed description of the current signaling mechanisms shown to be responsible for these cardioprotective actions. Antioxid. Redox Signal. 12, 1203–1217.
Introduction
Gaseous signaling molecules (i.e., nitric oxide, carbon monoxide, and hydrogen sulfide) are labile biological mediators whose production and metabolism are enzymatically regulated (Fig. 1). These small gaseous molecules freely diffuse through cell membranes to invoke cellular signaling, thus alleviating the need for membrane receptors and second messengers systems (59). Nitric oxide (NO) was the first gasotransmitter identified and has been the most studied to date. NO was first discovered in the late 1700's, but its importance in the field of biology and medicine was not fully appreciated until the 1980's when, in a series of studies conducted by several independent groups, it was reported that NO is generated in mammals, including humans, by nitric oxide synthases (NOSs) (31). There are three isoforms of NOS that have been characterized, purified, and cloned: the endothelial isoform (eNOS); the neuronal isoform (nNOS); and the inducible isoform (iNOS). Moreover, NO possesses a number of physiological properties that make it a potent cardioprotective-signaling molecule (35). Carbon monoxide (CO), the second endogenous gasotransmitter to be identified, is derived from the breakdown of heme by the enzyme heme oxygenase (HO) (17). There are three known isoforms of HO: HO-1 is expressed ubiquitously and is inducible; HO-2 is constitutively active and expressed primarily in the brain and testes; and HO-3 is constitutively active as well, but less efficient than the other two oxygenases (51). Although long considered an insignificant and potentially toxic waste product of heme catabolism, CO is now recognized as a key-signaling molecule that regulates numerous cardiovascular functions (17).
FIG. 1.
Endogenously produced gases that modulate cardiovascular physiology. Nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) are three endogenously produced gases that are members of the gasotransmitter family of cellular signaling molecules. Each is produced enzymatically and all have been reported to possess cytoprotective effects. It should be noted that these gases are very labile and in general highly reactive. NO, CO, and H2S all activate multilple signal pathways and promote chemical modifications. CBS, cystathionine β-synthase, CGL or CSE, cystathionine γ-lyase, cGMP, cyclic guanosine monophosphate, eNOS, endothelial nitric oxide synthase, HO-1, heme oxygnase 1, iNOS, inducible nitric oxide synthase, KATP, ATP-sensitive K+ channel, 3MST, 3-mercaptopyruvate sulfur transferase, nNOS, neuronal nitric oxide synthase, sGC, soluble guanylyl cyclase. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Hydrogen sulfide (H2S) is the third endogenously produced gasotransmitter to be identified as an important cell signaling molecule. Like other members of the gasotransmitter family, H2S has classically been regarded as a toxic gas and environmental hazard. However, again much like NO and CO, the initial negative perception of H2S has evolved with the recent discovery that it is produced by a number of enzymes in mammals and modulates a number of physiological processes. The production of H2S in mammalian systems has been attributed to three endogenous enzymes (Fig. 2): cystathionine β-synthase (CBS), cystathionine γ-lyase (CGL or CSE), and 3-mercaptopyruvate sulfurtransferase (3MST). The endogenous production of H2S was initially described in the brain as a central component of long-term potentiation of neuronal circuitry (39) and was attributed to the activity of CBS (1). However, newer evidence suggests that in the brain 3MST produces greater quantities of neuronal H2S compared to CBS and contributes to ∼90% of total H2S production in the brain (54). In addition, H2S is produced in the vasculature (i.e., primarily smooth muscle cell) CGL where it mediates smooth muscle relaxation (28) and subsequent vasodilation independent of the guanylyl cyclase/cGMP pathway (77). The rate of H2S production in tissue homogenates has been reported to be in the range of 1–10 pmoles per second per mg protein, resulting in low micromolar extracellular concentrations (59). It is at these concentrations that H2S has been shown to be cytoprotective in various models of cellular injury (32), including the heart and liver (32).
FIG. 2.
Enzymatic synthesis of hydrogen sulfide. The physiological production of H2S in mammalian systems has been attributed to three enzymes that are part of the cysteine biosynthesis pathway. Cystathionine β-synthase (CBS) is predominantly found in the brain, nervous system, and liver. It produces H2S through a reaction involving the generation of cystathionine from homocysteine, serine, and cysteine. Cystathionine γ-lyase (CGL or CSE) is found in the vasculature and live. It produces H2S through a reaction involving the generation of L-cysteine, pyruvate, and ammonia from L-cystathionine and cysteine. 3-Mercaptopyruvate sulfur transferase (3MST) has been reported to be localized in the mitochondria and can be found in the brain and vasculature. It produces H2S through a reaction involving the generation of pyruvate from 3-mercaptopyruvate (3MP). The 3MP is provided through the metabolism of cysteine and α-ketoglutarate (α-KG) by cysteine aminotransferase (CAT). These enzymes regulate the physiological levels of H2S observed in the bloodstream and tissues and are critical for H2S homeostasis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
It is important to note that the previous reported low micromolar levels of H2S may not accurately reflect its actual physiological concentrations. For instance, the levels of H2S in the brain have previously been reported to be in the range of 30–100 μM. However, Furne et al. (22) recently reported the levels of H2S in the brain to be 15 nM. In this study, the authors measured H2S levels in the headspace of homogenized brain tissue. The discrepancy in the levels of H2S measured in Furne study versus those levels previously reported can be attributed to the assays used to measure H2S. Furne et al. (22) measured H2S in the gas space of tissue homogenates using a gas chromatograph equipped with a chemiluminescence sulfur detector, whereas previous studies measured H2S in tissue homogenates using polarographic probes (6) or colorimetric assays (47). Fundamentally, all of the techniques have limitations that may account for the different levels of H2S measured [see (61) for review of methods and their limitations], such as: the instability of sulfide, its high volatility, its great susceptibility to oxidation, its adherence to various materials (e.g., glass), and the erroneous release of sulfide out of some rubbers used or out of the often used reagent DTT (61). This might lead to artificially elevated or lowered levels and explains the large discrepancy among the various reports. Therefore, new reliable techniques need to be established to elucidate the levels of H2S required to elicit its physiological signaling.
Hydrogen Sulfide and Cardioprotection
The physiological actions of H2S make this gas ideally suited to protect the heart and blood vessels against injury during a number of cardiovascular disease states. The cardiovascular actions of hydrogen sulfide are summarized in Fig. 3. In recent years, the cardioprotective effects of endogenous and exogenous H2S have been investigated in models of in vitro (33, 66, 73, 76) and in vivo (18, 23, 55, 56, 58, 80) myocardial injury. The effects of endogenous H2S have primarily been studied by pharmacologically inhibiting CGL and by genetically targeting CGL in mice. The effects of exogenous H2S have been studied through the administration of H2S in the form of sodium hydrosulfide (NaHS) or sodium sulfide (Na2S). In these studies, H2S was administered before, during and after ischemia at concentrations ranging from 0.1 to 1000 μM (in vitro) or dosages ranging from 0.010 to 3 mg/kg (in vivo).
FIG. 3.
Cardiovascular actions of hydrogen sulfide. H2S is produced in μM concentrations in the circulation and despite its very short half-life in blood and tissues exerts a number of critical effects on the cardiovascular system. Physiological actions include: vasodilation, inhibition of leukocyte-endothelial cell interactions, antioxidant effects, anti-apoptotic effects, increased angiogenesis, and modulation of mitochondrial respiration. Cystathionine gamma lyase (CGL or CSE) is localized primarily in the smooth muscle cell layer of blood vessels while the recently described enzyme, 3-mercaptopyruvate sulfur transferase (3MST) has been identified in vascular endothelial cells as well as in mitochondria. At present, it is not clear if 3MST is localized in cardiac mitochondria. These cytoprotective actions of H2S are observed at physiological levels of H2S (i.e., μM) while very high concentrations of H2S (mM) induce cellular injury and apoptosis. At present, very little is known regarding H2S levels in animals and humans in the setting of cardiovascular disease of the effects of risk factors (i.e., hypertension, diabetes mellitus, dyslipidemia, and obesity) on blood and tissue levels of H2S. Hydrogen sulfide therapy in cardiovascular disease is focused on the use of physiological levels of H2S. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Cardioprotective effects of exogenous hydrogen sulfide therapy
The vast majority of in vitro studies that have investigated the cardioprotective effects of exogenous H2S have employed a pharmacological preconditioning (PC) strategy. Using an in vitro Langendorf hanging heart model, Johansen et al. (34) investigated the cardioprotective effects exogenous H2S therapy by subjecting rat hearts to 30 min of left coronary artery (LCA) occlusion, followed by 120 min of reperfusion. In these experiments, NaHS was administered in the perfusate at doses from 0.1 to 10 μM starting 10 min prior to the onset of ischemia and continued for the first 10 min of reperfusion. NaHS was reported to provide a dose-dependent reduction in infarct size, with 1 μM providing the best reduction. Importantly, the cardioprotective effects of NaHS were found to be lost at a dose of 10 μM, demonstrating a very tight therapeutic dose range. In addition, it should be noted that all commercially available NaHS contains a significant number of impurities that can complicate the interpretation of results obtained using this H2S precursor. Bian et al. (7) also investigated the cardioprotective effects of exogenous H2S therapy in an in vitro Langendorf hanging heart model and in isolated adult rat myocytes. In the Langendorf hanging heart model, NaHS was administered at a dose of 100 μM in the perfusate in three cycles lasting 3 min each. Hearts were then subjected to 30 min of low-flow ischemia followed by 10 min of reperfusion. Treated hearts were found to have a significant decrease in ischemia-reperfusion-induced arrhythmias. In separate experiments, isolated adult cardiac myocytes were subjected to simulated ischemia through an exposure to a glucose-free Krebs buffer containing 10 mM 2-deoxy-D-glucose (2-DOG). A similar treatment strategy consisting of three cycles of superfusion with NaHS (1–1,000 μM) was employed and NaHS was found to dose dependently increase cell viability. Again, it is important to note that in terms of efficacy a U-shaped curve was observed for the doses of NaHS investigated. Hu et al. (29) recently investigated the efficacy of a single treatment of H2S. In these experiments, isolated adult cardiac myocytes were exposed to NaHS (100 μM) for 30 min. The cells were then subjected to simulated ischemia 20 h later. Following a 5-min expose to 2-DOG and 10 min of simulated reperfusion, NaHS significantly increased cell viability, increased the percentage of rod-shaped cells, and increased myocyte contractility, demonstrating the efficacy of a single expose of NaHS. This study demonstrated that a single expose of NaHS resulted in delayed cardioprotection in isolated cardiac myocytes.
While the in vitro investigations provided the first evidence that H2S is cardioprotective and has provided some mechanistic insights into its cardioprotective actions (discussed below), clinical translation of a therapeutic agent critically depends upon demonstration of efficacy in in vivo models of cardiovascular disease. Sivarajah et al. (57) provided the first report of the cardioprotective actions of H2S in an in vivo model of myocardial ischemia-reperfusion injury in 2006. In this study, rats were administered NaHS (3 mg/kg) 15 min prior to myocardial ischemia and then subjected to 25 min of coronary artery ligation and 2 h of reperfusion. The authors reported a 25% reduction in myocardial infarct size, which clearly provides strong evidence for the cardioprotective effects of H2S therapy. These results are, however, somewhat diminished by the fact that NaHS was administered prior to the onset of coronary artery ischemia and by the fact that this study only investigated a relatively short period of reperfusion (i.e., 2 h). This represents a significant limitation of the study since it is well appreciated that myocardial reperfusion injury evolves over a 24–72 h period following recannalization of a coronary artery. Furthermore, patients generally receive medical treatment for acute myocardial ischemia after the onset of symptoms. Therefore, a more clinically relevant approach would be to administer H2S at the time of reperfusion. Recently, Elrod et al. (18), therefore, investigated this approach using a well-established in vivo murine model of ischemia-reperfusion injury. In these studies, mice were subjected to 30 min of left coronary artery ischemia followed by 24 h reperfusion. Na2S, generated from authentic H2S gas was administered into the left ventricular lumen at the time of reperfusion at doses ranging from (10–500 μg/kg). Evaluation of infarct size at 24 h following reperfusion revealed dose-dependent reductions in myocardial infarct size, with 50 μg/kg resulting in a 72% reduction in infarct size. Additional studies revealed that H2S-treated mice also exhibited significantly less post-ischemic left ventricular dysfunction, as assessed by high-resolution, two-dimensional echocardiography. More evidence regarding the efficacy of H2S reperfusion therapy comes from Sodha and colleagues (58), who investigated the cardioprotective effects of H2S in a preclinical large animal model. In this study, Yorkshire pigs were subjected to regional left ventricular ischemia by left anterior descending (LAD) arterial occlusion distal to the second diagonal branch for 60 min. The treatment group received highly pure Na2S (100 μg/kg bolus + 1 mg/kg/h infusion) 10 min prior to the onset of reperfusion. At the end of the 120-min reperfusion period, global systolic left ventricular function, as determined from LV + dP/dt, was significantly better in the Na2S-treated group compared to the vehicle-treated group. Myocardial infarct size was also significantly reduced in Na2S-treated groups relative to vehicle-treated group.
Protective effects of exogenous hydrogen sulfide therapy in other models of ischemia-reperfusion
The cytoprotective effects of exogenous H2S therapy are not limited to myocardial ischemia-reperfusion injury, as H2S has been shown to confer protection in other organ systems. Our laboratory has recently reported that cytoprotective effects of H2S in an in vivo model of hepatic ischemia-reperfusion injury (32). In this study, hepatic injury to the left lateral and median lobes of the liver was achieved by occluding the hepatic artery and portal vein to 60 min followed by 5 h of reperfusion. H2S in the form of Na2S was administered 5 min before reperfusion and was shown to attenuate the elevation in serum alanine aminotransferase by 68.6% and aspartate aminotransferase by 70.8% compared with vehicle group. Fu et al. (21) investigated the role of H2S in the pathogenesis of pulmonary ischemia-reperfusion injury using an isolated rat lung ischemia-reperfusion model. Lungs were subjected to 45 min ischemia, followed by 45 min of reperfusion, at which time lung injury was assessed by lung histological change, perfusion flow rate, ratio of lung wet weight to dry weight, and lung compliance. Lungs were pretreated with H2S to evaluate the effects of exogenous H2S or pretreated with D,L-propargylglycine (PAG) to evaluate the effects of endogenous H2S. Lungs pretreated with H2S exhibited less injury whereas lungs pretreated with PAG exhibited exacerbated injury. These findings suggest that endogenous H2S might be involved in protecting the lung from ischemia-reperfusion injury and that administration of exogenous H2S might be of clinical benefit in lung ischemia-reperfusion injury. Finally, Xu et al. (68) examined the effect of ischemia-reperfusion on CBS-mediated H2S production in the kidney and determined whether changes in the endogenous H2S generation had any impact on renal ischemia-reperfusion injury. In these experiments, the left kidney of rats was subjected to 45 min of ischemia, followed by a reperfusion period of 6 h. Renal ischemia-reperfusion led to a significant decrease in the CBS-mediated H2S production. Partial restoration of CBS activity by intraperitoneal injections of the NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide not only restored renal H2S levels but also alleviated ischemia-reperfusion-induced lipid peroxidation and reduced cell damage in the kidney tissue. Furthermore, administration of an exogenous H2S (NaHS, 100 μg/kg) improved renal function. H2S has also been reported to protect cultured neurons from of glutamate-induced oxidative cell death (40). However, there are contrasting reports regarding the in vivo effects of H2S on the brain. Qu et al. (47) reported that the administration of NaHS significantly increased infarct volume in rats following middle cerebral artery occlusion, whereas the inhibition of H2S synthesis with pharmacological inhibitors reduced infarct volume. In contrast, Florian and colleagues (20) reported that a 48-h exposure to 80 ppm H2S gas reduced infarct size by 50% in rats following focal ischemia by inducing hypothermia. The obvious difference between these two studies is the way H2S was administered (single injection vs. chronic exposure to H2S gas). The efficacy of the chronic exposure to H2S gas could be attributed to the hypothermia induced by H2S, as hypothermia is a known neuroprotective strategy (20). Given, the discrepancy in these studies, more work is certainly warranted to determine if H2S therapy will be an effective treatment strategy for cerebral ischemia-reperfusion injury.
Taken together, these results of these studies suggest that both maintenance of endogenous tissue levels of H2S and therapy with exogenous H2S may offer cytoprotection against ischemia-reperfusion injury in a number of organs, including the liver, lung, and kidney. However, there is still some work that needs to be done to address the effects H2S has on the brain during cerebral ischemia.
Cardioprotective effects of endogenous hydrogen sulfide
The growing interest in the use of exogenous H2S as a pharmacological agent for the treatment of myocardial ischemia-reperfusion injury has led investigators to evaluate the role that endogenous H2S plays in the development of myocardial injury following myocardial ischemia. Multiple studies have reported that when the production of endogenous H2S is reduced prior to myocardial ischemia by pharmacologically inhibiting CGL with PAG myocardial injury is exacerbated (8, 57). Bliksoen et al. (8) reported a 38% increase in myocardial infarct size following 40 min of low-flow ischemia when isolated hearts were exposed to PAG prior to the ischemic episode. This is supported by in vivo data, demonstrating that the administration PAG (50 mg/kg i.v.) 15 min before the onset of ischemia significantly augmented myocardial infarct size caused following ischemia and reperfusion (57). The use of PAG has certainly provided evidence that supports the idea that endogenous H2S is a cytoprotective-signaling molecule that can limit the extent of myocardial injury. It is important to note that many of the currently available pharmacological inhibitors of CGL and CBS are not highly specific for these enzymes, which complicates the interpretation of these findings. Development of specific and potent CGL and CBS inhibitors will prove to be extremely valuable for researchers investigating the physiological actions of H2S. More definitive evidence regarding the cardioprotective actions of endogenous H2S has emerged from studies using genetically altered mice. Mice with a cardiac-specific overexpression of CGL experience smaller infarct sizes in response to in vivo myocardial ischemia-reperfusion injury (18). Recently, our laboratory has investigated the effects of myocardial ischemia-reperfusion injury in a mouse model of systemic deletion of the CGL gene that was originally developed by Yang and colleagues (70). These mice were subjected to 45 min of left coronary artery occlusion and 24 h of reperfusion at which time myocardial infarct size was determined. These animals exhibited significantly larger areas of myocardial infarction compared to wild-type control animals (Fig. 4).
FIG. 4.
Deficiency of CGL exacerbates myocardial ischemia-reperfusion injury. In order to determine the role of endogenously produced H2S on myocardial reperfusion injury, mice deficient in CGL (CGL−/−) and wild-type littermates were subjected to 45 min of left coronary artery occlusion, followed by 24 h of reperfusion. At 24 h of reperfusion, myocardial area-at-risk and infarct size were evaluated with the TTC-Evans blue dual staining method. (A) Representative photomicrographs of midventricular heart sections from wild-type and CGL−/− mice at 24 h after reperfusion. Myocardial infarct size (white tissue) is significantly greater in CGL−/− mice as compared to wild-type control hearts. (B) Bar graph of the myocardial area-at-risk per left ventricle (AAR/LV), infarct size per AAR (Inf/AAR), and Inf/LV in wild-type and CGL−/− groups of mice. CGL−/− mice experienced a 48% increase in Inf/AAR (p = 0.004 vs. wild-type) and a 34% (p = 0.01) increase in Inf/LV when compared to wild-type mice. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Role of endogenous hydrogen sulfide in ischemic preconditioning
Of all the gaseous signaling molecules, NO has been the most extensively studied in the setting of myocardial ischemia-reperfusion injury. Previous studies clearly demonstrate that the deficiency of eNOS exacerbates myocardial ischemia-reperfusion injury, whereas the overexpression of eNOS, the administration of NO donors, and inhaled NO gas therapy all significantly protect the myocardium (12). Additionally, endogenously produced NO has been shown to play a prominent role in the cardioprotective signaling associated with IPC (42). Recent studies have evaluated if endogenous H2S plays a role in signaling initiated by IPC. Both in vitro (8) and in vivo (57) studies have clearly demonstrated that pharmacological inhibition of endogenous H2S production with PAG does not have an affect on IPC-induced cardioprotection, suggesting that endogenous H2S does not play a role in the setting of IPC signaling.
Summary of hydrogen sulfide and cytoprotection
In summary, extensive research performed in recent years has clearly demonstrated that the acute administration of H2S, either prior to ischemia (i.e., preconditioning) or at the time of reperfusion, significantly ameliorates in vitro or in vivo myocardial ischemia-reperfusion injury. Most importantly, these studies have provided important information regarding the doses of H2S that provide cardioprotection and suggest that the use of H2S at or near the levels considered to be produced under physiological conditions in vivo is optimal to protect the heart following coronary artery occlusion and reperfusion. Furthermore, H2S has been shown to attenuate the extent of cellular necrosis and apoptosis following ischemia-reperfusion in a number of organs including the heart, liver, lung, and kidney.
Cardioprotective Mechanisms of Hydrogen Sulfide
H2S possesses a diverse physiological profile that contributes to its cardioprotective actions (Fig. 3). Of the reported physiological effects of H2S there are several that are likely responsible for protection against cardiovascular events such as acute myocardial ischemia-reperfusion. H2S has been reported to regulate blood pressure and previous experimental work focused on exogenous H2S demonstrated that intravenous injections of H2S transiently decreased blood pressure in rats (78) through endothelium dependent vasodilatation (14). Recently, it has been reported that mice deficient in CGL (CGL-/-) have markedly reduced serum H2S levels, which results in pronounced hypertension and diminished endothelium-dependent vasodilation (70). These findings provide direct evidence that H2S is a physiologic vasodilator that can influence blood pressure. This could be of importance in the treatment of myocardial I/R injury as hypertension is known to exacerbate the severity of acute myocardial infarction by increasing myocardial oxygen demand and cellular injury. It has also become evident that H2S acutely serves as a potent antioxidant and under more chronic conditions upregulates antioxidant defenses (40). The reported antioxidant effects of H2S may be of critical importance for the treatment of cardiovascular disease, as oxidative stress plays a prominent role in the development of injury following acute myocardial I/R and LV dysfunction associated with heart failure (16). It has also been demonstrated that H2S effectively promotes cytoprotection in a number of cell types through the inhibition of apoptotic cell death (49), which could also limit the development of LV remodeling and dysfunction associated with myocardial I/R and heart failure. Another physiological characteristic of H2S that might provide cardioprotection relates to the evidence that H2S can alter the metabolic state of organisms by modulating mitochondrial function. Although the physiological and cardioprotective effects of H2S have previously been documented, the cellular and molecular signaling mechanisms that mediate these effects have just begun to be elucidated. The rest of this review article will highlight some of the key signaling targets and pathways that have been associated with H2S.
Hydrogen Sulfide and ATP-Sensitive K+ Channels
Perhaps the most widely characterized cellular target associated with H2S is the ATP-sensitive K+ (KATP) channel, since H2S-induced vasorelaxation has been shown to be mediated mainly by the opening of KATP channels in vascular SMCs (78). KATP channels are found on the surface of cell membranes and mitochondria of many different cell types, including pancreatic β-cells, neurons, cardiac myocytes, liver, and skeletal and smooth muscle cells (5). These channels are weakly inwardly rectifying K+ (Kir) channels that stabilize the membrane potential close to the equilibrium potential for K+. At the molecular level, functional KATP channels are understood to be multi-subunit protein complexes. Transmembrane Kir6 pore forming subunits allow K+ ions to permeate the channel complex, whereas sulfonylurea (SUR) accessory subunits serve to act as receptors for a variety of pharmacological compounds that either activate or inhibit KATP channel opening. Classically, cardiac sarcolemmal KATP channels are thought to be composed of Kir6.2 and SUR2A subunits; however, the evidence is strong that SUR1 and Kir6.1 subunits are also expressed in the heart and that they may have a functional role (63). KATP channels are known to play an important role in the cardioprotective signaling of IPC. In addition, pharmacological agents that selectively open the KATP channel have been shown to have infarct-limiting effects as potent as IPC. There is still debate, however, as to whether the sarcolemmal KATP channel or mitochondrial KATP channel plays a more prominent role in this cardioprotection (24), in part since the mitochondrial KATP channel has yet to be identified.
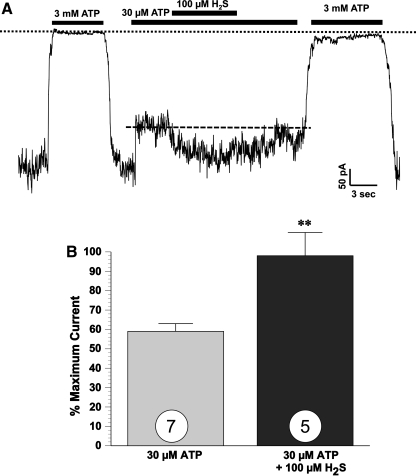
Emerging evidence indicates that KATP channels are critical targets of endogenous and exogenous H2S (7). Patch-clamp studies have demonstrated that H2S in a concentration dependent and reversible manner increases whole cell KATP channel currents in smooth muscle cells isolated from both aortic and mesenteric arteries (14, 78). Additionally, it has been suggested that the endogenous production of H2S in the heart protects the myocardium during ischemia-reperfusion injury through a KATP channel-mediated pathway (23). Early work suggested that H2S mediates cardioprotection through sarcolemmal KATP channels and not through mitochondrial channels (7). In this study it was found that specific blockade of mitochondrial KATP channels with 5-hydroxydeconoate (5-HD) had no effect on the protective effect of H2S on cell viability and cell function, whereas glibenclamide (nonselective) and HMR-1098 (a putative sarcolemmal KATP channel blocker) both significantly attenuated this effect. Recent data from our lab provided direct evidence suggesting that H2S activates sarcolemmal KATP channels (Fig. 5). For these experiments, ventricular myocytes were isolated from 8–10-week-old male mice (C57BL/6J) using a collagenase perfusion method as described previously (63). Isolated myocytes were allowed to stabilize for 1 h before being used for electrophysiological recording experiments. Recordings of single-KATP channel activity were made by standard inside-out patch-clamp techniques. For each patch, activity was first recorded in the presence of 0 mM ATP (maximum current) and 3 mM ATP (zero current; indicated by the dotted line) to determine the presence of ATP sensitive K+ channels. Channel activity was then recorded in the presence of 0.03 mM ATP, which resulted in a 41% decrease in channel activity (59% of maximum current). When 100 μM H2S was applied in the presence of 0.03 mM ATP, we observed a 66% increase in channel activity (98% vs. 59%, p < 0.01, n = 5). The effect of H2S was reversible when returning to the 0.03 mM ATP solution. Interestingly, subsequent application of 3 mM ATP failed to completely block KATP channel activity, suggestive of a change in ATP sensitivity. The current amplitude was unchanged when washing off the ATP. These data demonstrate that H2S may directly act on sarcolemmal KATP channels in excised membrane patches from cardiac myocytes to increase channel activity. In contrast, a recent study by Sivarajah and colleagues (56) has reported that the cardioprotective effects of NaHS were abolished by 5-HD, suggesting a role for mitochondrial KATP channels. Given these diverse findings, more work is needed to determine which channel (if not both) plays a role is H2S-mediated cardioprotection. Additionally, the precise molecular mechanisms underlying the effect of H2S on KATP channels are still largely unknown. Since H2S is a strong reductant, it is possible that H2S directly interacts with KATP channel proteins (60), inducing the reduction of disulfide bonds of the channel protein (67). Signaling through protein kinase C (PKC) has also been reported as a possible mechanism of action (7). Bian et al. (7) demonstrated that PKC inhibition attenuated the protective effects of H2S, but the authors did not investigate if PKC was upstream or downstream of H2S. Studies have also indicated that H2S does not induce second messengers to open KATP channels, but rather directly opens the channels (60). Therefore, it is possible that PKC is downstream of KATP channel opening.
FIG. 5.
Hydrogen sulfide activates myocardial KATP channels. (A) Representative recording of ATP-sensitive potassium (KATP) channels measured in the inside-out configuration at −100 mV membrane potential. Note that inward currents are depicted as downward deflections of the current trace. ATP and H2S were applied as shown in the top bars. (B) Mean patch current. On average, the mean patch current recorded with 30 μM ATP was 41% of that recorded in the absence of ATP (n = 7). H2S increased mean patch current by 66%. Values are means ± S.E.M. **p < 0.01 compared to 0.03 mM ATP.
Hydrogen sulfide and protein kinase C
PKC is a family of serine/threonine kinases that regulates a diverse set of cellular processes, including proliferation, migration, and cell survival. There are several isoforms of PKC, which have distinct roles in these processes. Additionally, the isoforms of PKC have different roles in different tissue. PKC epsilon (PKCɛ) and PKC alpha (PKCα) play a role in cardioprotective signaling in response to various stimuli following ischemia-reperfusion injury (46), whereas PKC delta (PKCδ) plays a key role in the injury associated with ischemia-reperfusion injury (15). When activated, PKC translocates from the cytosol to the sarcolemmal membrane, sarcoplasmic reticulum, and mitochondria. At the sarcolemmal membrane, PKCɛ can activate Ras, which is an upstream mediator of Erk activation. At the sarcoplasmic reticulum, PKC can activate sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) and accelerate the clearance of Ca2+ from the cytoplasm (64). The translocation of PKCδ to the mitochondria is associated with diminished mitochondrial respiration, increased reactive oxygen species (ROS) generation, and induction of apoptosis (15). H2S has previously been reported to induce PKC activation (29, 72). Pan et al. (45) reported that H2S activates PKCα, PKCɛ and PKCδ in isolated cardiomyocytes. Interestingly the activation of PKCɛ was the only isoform found to be secondary to KATP channel opening and the activation of PKCδ did not seem to cause any detrimental effects. They also demonstrated that PKC activation intervenes in the development of Ca2+ overload and myocyte hypercontracture induced by ischemia-reperfusion insults by facilitating cytosolic Ca2+ clearing through SERCA and the sodium calcium exchanger (NCX).
Anti-apoptotic properties of hydrogen sulfide
Previous studies have reported the anti-apoptotic effects of H2S (18, 56, 58). Apoptotic cell death during reperfusion has been implicated as an important contributor to reperfusion-induced injury. Therefore, targeting anti-apoptotic mechanisms may offer a potential strategy to attenuate reperfusion-induced cell death (25). In this regard, the activation of pro-survival kinases, such as PI3K-Akt, PKC, and Erk1/2 (termed reperfusion injury salvage kinase (RISK) pathway) have been demonstrated to confer cardioprotection against myocardial ischemia-reperfusion through an inhibition of apoptosis (71). The downstream anti-apoptotic effectors of the RISK pathway have not been fully elucidated, but the Erk1/2 component of the RISK pathway has been shown to signal through signal transducers and activators of transcription-3 (STAT-3), p90RSK, Bcl-2, Bcl-xL, and heat shock proteins (HSPs). The STAT pathway has recently been shown to be an integral part of the response of the myocardium to various cardiac insults, including myocardial infarction (4). In particular, the overexpression of STAT-3 provides protection (41), whereas cardiac-specific deficiency of STAT-3 exacerbates cardiac injury (26). HSPs have also been demonstrated to provide cardioprotection in the setting of ischemia-reperfusion. In particular, HSP70 suppresses apoptosis in a caspase-dependent (52) and caspase-independent manner (48). Past studies have reported that exogenous H2S activates several of these pro-survival kinases, including PKC/Erk1/2 and PI3K/Akt (8, 30, 72). A more recent study provides in vivo evidence for the activation of a PKCɛ-STAT-3 signaling cascade by H2S (11). In this study, H2S increased the phosphorylation of PKCɛ and STAT-3, increased the expression of HSP90, HSP70, Bcl-2, and Bcl-xL, and also inactivated the pro-apoptogen Bad. The activation of this signaling pathway resulted in a reduction in apoptotic cell death following myocardial ischemia-reperfusion, as evidenced by a reduction in TUNEL staining. Another pathway by which H2S may reduce apoptotic cell death relates to its cross talk with the NO pathway. Yong et al. (72) have shown that H2S confers protection against ischemia-reperfusion injury through the activation of eNOS. NO is known to inhibit apoptosis (43) either directly or indirectly by inhibiting caspase-3-like activation via a cGMP-dependent mechanism and by direct inhibition of caspase-3-like activity through protein S-nitrosylation (38). This evidence suggests that H2S does not simply reduce apoptotic cell death following myocardial ischemia-reperfusion through a simple reduction in cellular injury, but actually has the ability to promote direct anti-apoptotic signaling.
Antioxidant properties of hydrogen sulfide
H2S has also been reported to protect neurons (40), vascular smooth muscle cells (69), and myocytes (23) from oxidative stress. Under physiological conditions, small amounts of ROS produced as a consequence of electron transfer reactions in mitochondria, peroxisomes, and cytosol are quenched by cellular antioxidant defense systems. Antioxidants act by scavenging oxidative species and their precursors, inhibiting their formation and enhancing endogenous antioxidant defenses (36). There is considerable evidence that implicates the production of ROS as an initial cause of injury to the myocardium following ischemia-reperfusion. ROS formed during oxidative stress can initiate lipid peroxidation, oxidize proteins to inactive states, and cause DNA strand breaks, all potentially damaging to normal cellular function and outcome (65). Therefore, the capacity of cardiac myocytes to maintain homeostasis during periods of oxidative stress resides in the ability to activate or induce protective enzymes (37). Geng and colleagues (23) reported that H2S decreases lipid peroxidation in the heart following isoproterenol–induced myocardial ischemic injury by scavenging hydrogen peroxide and superoxide. Kimura and colleagues (40) demonstrated using a model of glutamate-induced oxidative stress that H2S protects neurons from cell death by increasing the levels of the antioxidant, glutathione. They found that H2S increased glutathione levels by enhancing the activity of γ-glutamylcysteine synthetase and upregulating cystine transport. This suggests that H2S reduces oxidative stress through two distinct mechanisms; it can act as a direct scavenger of ROS and also upregulate antioxidant defenses. A recent study indicates that H2S may upregulate endogenous antioxidants through a nuclear-factor-E2-related factor-2 (Nrf2) dependent signaling pathway (11). Nrf2, a member of the NF-E2 family of nuclear basic leucine zipper transcription factors, regulates the gene expression of a number of enzymes that serve to detoxify pro-oxidative stressors (19). This regulation is mediated by Nrf2 binding to the antioxidant responsive element (ARE), a cis-acting regulatory element or enhancer sequence, found in the promoter region of certain genes (62). Genes that contain an ARE include: heme oxygenase–1 (HO-1), thioredoxin (Trx), thrioredoxin reductase (TrxR), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione S-transferase (GST), and catalase (79). Calvert et al. (11) found that as early as 30 min following the administration of H2S, Nrf2 accumulated in the nucleus of cardiac tissue and remained at an elevated level for at least 2 h. Subsequently, the protein expression of Trx1 and HO-1 were found to be elevated 24 h following the administration H2S. A role for Nrf2 in mediating the antioxidant effects of H2S is further supported by the finding that garlic oil, a reported H2S donor (6), can promote Nrf2 activation (19).
Anti-inflammatory effects of hydrogen sulfide
H2S also inhibits leukocyte-endothelial cell interactions in vivo (75). Zanardo et al. (75) utilized intravital microscopy to demonstrate that H2S inhibits leukocyte adherence in the endothelium in the rat mesenteric microcirculation during vascular inflammation. Inhibition of leukocyte–endothelial cell adhesion suppressed edema formation following carrageenan administration to the paw of rats and attenuated leukocyte infiltration into an air pouch model. In contrast, inhibition of endogenous H2S biosynthesis with inhibitors of H2S generating enzymes elicited leukocyte adherence to the microvascular endothelium (75). In support of this finding, Yusof et al. (74) also demonstrated that acute NaHS treatment in mice promotes a significant transformation to an anti-inflammatory phenotype in small intestine postcapillary venules such that these vessels fail to support leukocyte rolling and leukocyte adhesion in response to ischemia-reperfusion injury. These elegant studies strongly suggest that H2S is a potent anti-inflammatory molecule. However, further studies are required to more fully elucidate these potent anti-leukocyte effects in animal model systems of inflammatory and cardiovascular disease states.
Hydrogen sulfide and mitochondria
As noted above, another physiological characteristic of H2S that could provide cardioprotection relates to the evidence that H2S can alter the metabolic state of organisms by modulating mitochondrial function. H2S is known to be a potent and reversible inhibitor of cytochrome c oxidase (complex IV of the mitochondrial electron transport chain) (27) and the profound nature of H2S to influence whole organism metabolism was recently demonstrated by its induction of a suspended animation-like state in mice (50). Another possible mechanism for H2S's protective action on mitochondrial function may lie in the capacity of H2S to modulate cellular respiration, as the inhibition of mitochondrial respiration has been shown (13) to protect against myocardial ischemia-reperfusion injury by limiting the generation of ROS and diminishing the degree of mitochondrial uncoupling. H2S may also affect the mitochondria indirectly via off-target effects. For instance, a common target of the anti-apoptotic signaling activated by the RISK pathway is the mitochondria. Specifically, activation of the RISK pathway has been shown to inhibit the opening of mitochondria permeability transition pores (MPTP) (15). MPTP occupy a fundamental role in determining cellular survival in the setting of myocardial ischemia-reperfusion injury because MPTP opening causes mitochondrial membrane potential (ΔΨm) depolarization (2). As a result, the loss of ΔΨm causes a rapid impairment of mitochondrial function, which can lead to apoptotic cell death through the release of pro-apoptotic proteins. Additionally, by reducing overall cellular oxidative stress, H2S protects mitochondria from becoming damaged and activating a pro-apoptotic signaling cascade.
Summary of the cardioprotective mechanisms of hydrogen sulfide
A common characteristic among gasotransmitters is that they can freely diffuse across cellular membranes and very rapidly activate multiple intracellular targets. This is unique in that gasotransmitters can subvert the need for receptor-mediated signaling. Although the cellular targets of H2S have not been fully defined, work over the past several years has provided some important insights into its signaling capability. Figure 6 summarizes some of the primary signaling cascades that have been described for H2S. As discussed above, the most defined cellular target of H2S is the KATP channel. Activation of the KATP channel during myocardial ischemia-reperfusion injury gives H2S the ability to regulate intracellular calcium. Through this regulation, H2S prevents mitochondrial damage and prevent the initiation of a pro-apoptotic cascade. Additionally, H2S-mediated antioxidant actions alleviate cellular oxidative stress and indirectly reduce mitochondrial dysfunction and apoptotic signaling. Furthermore, the initiation of a direct anti-apoptotic signaling cascade can also prevent cell death. Individually, these pathways are distinct enough to stand-alone and provide cardioprotection, but together, they are able to feed off of one another and present a formidable barrier for cardiac injury.
FIG. 6.
Summary of the key H2S signaling targets and pathways. Although the physiological and cytoprotective effects of H2S have previously been documented, the signaling mechanisms that mediate these effects have just begun to be elucidated. The activation of multiple signaling cascades allows H2S to target pathways that can provide cytoprotection independently of each other, but most importantly allows for the merging of multiple pathways into a single cytoprotective signaling cascade. Some of the known protective signal pathways are illustrated. Given the highly reactive property of hydrogen sulfide and the fact that H2S induces S-sulfhydration, it is likely that additional signaling pathways not shown are activated by H2S. Additionally, signaling pathways involved in pharmacological preconditioning may differ from the cytoprotective effects of H2S administered during the pathogenesis of ischemia-reperfusion injury. GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione S-transferase; HO-1, hemeoxygenase-1; HSP, heat shock protein; MPTP, mitochondrial permeability transition pore; Nrf-2, nuclear-factor-E2-related factor; PKC, protein kinase C; ROS, reactive oxygen species; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase; Trx, thioredoxin; TrxR, thioredoxin reductase; STAT-3, signal transducers and activators of transcription-3. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Garlic and Garlic Derivatives as a Source of Hydrogen Sulfide
It has long been appreciated that diet exerts important long-term effects on vital body functions and impacts overall cardiovascular health and disease. There has been a well-known connection between increased risk for cardiovascular disease and poor diet, often involving high intake of saturated fat and limited fruit and vegetable intake, characteristic of a contemporary Western diet (10). In contrast, epidemiological studies have shown that diets rich in fruits, herbs, and spices are associated with a low risk of cardiovascular disease. In particular, garlic has gained a formidable reputation as a prophylactic and therapeutic medicinal agent (3). Garlic has attracted attention of modern medicine because of its widespread health use around the world and its ability to enhance antioxidants, to inhibit platelet aggregation, and to reduce hypertension (3, 9, 53). The mechanisms by which garlic and its derivatives elicit these physiological effects have remained unknown for quite some time. However, a recent study by Benavides et al. (6) suggests that H2S may underlie the beneficial physiological effects garlic exerts on the cardiovascular system. In this study, the authors demonstrated that garlic and garlic-derived organic polysulfides, such as diallyl trisulfide (DATS) and diallyl disulfide (DADS), induce H2S production in a thiol-dependent manner and that this signal molecule mediates the vasoactivity of garlic.
Toxic Effects of Hydrogen Sulfide
Despite the more recently described cytoprotective actions, H2S has long been viewed as an environmental hazard. In fact, there is an extensive body of literature describing the toxicological profile of H2S, most of which is focused on the toxic effects occurring after the inhalation of gaseous H2S (See (59)). A significant body of human data has accumulated with inhaled H2S in conjunction with toxicological studies and environmental surveys. The toxic effects of H2S are usually observed at relatively high levels, nonphysiological levels (i.e., high μM to mM) and not observed at the physiological levels (i.e., low μM) reported in the circulation and tissue. Toxic effects of inhaled H2S at high acute exposure levels or following very chronic lower-level exposure include olfactory epithelial cell toxicity and transient loss of olfaction, pulmonary inflammation, and toxicity, and effects on blood pressure and cardiovascular responsiveness. There is also a clear neurological toxicity of inhaled H2S at very high doses involving inhibition of glutamate reuptake, as well as inhibition of monoamine oxidase. Very little is currently known regarding the toxicity of H2S administered directly into the circulation (i.e., intravenous injection) and the majority of studies have reported robust cytoprotection in various models of cardiovascular diseases after administration of physiological or low levels of H2S. Clearly, the development of H2S or H2S-releasing agents as therapeutic agents for the treatment for cardiovascular and other diseases requires a more detailed understanding of the toxicological profile, as well as the pharmacokinetics, distribution, metabolism, and mechanisms of action.
Summary and Future Research Directions
The field of H2S studies has exploded during the past decade. Consider that not too long ago, H2S was only thought of as a noxious toxic gas produced in dangerous quantities primarily under industrial and agricultural conditions. In fact, the majority of previous H2S research was focused on the toxic effects associated with exposures to excessive amounts of H2S. With the relatively recent discovery that H2S is continuously generated by a number of enzymes in mammals and modulates a vast array of physiological processes, H2S is now considered a highly important gaseous signaling molecule. A direct result of this improved understanding of the physiological properties of H2S is a growing interest in the use of H2S to treat various disease states. As such, extensive work has found that H2S is cytoprotective in various models of cellular injury (7, 34, 44). The fields of H2S physiology and pharmacology have been very rapidly growing in recent years, but a number of critical issues must be addressed to significantly advance our understanding of the biology and clinical potential of H2S in the future. At present there is a paucity of information on the regulation of CBS, CGL, and 3MST under normal physiological conditions and during various disease states. It is unclear if these enzymes can be upregulated via post-translational modifications to increase systemic levels of H2S. It is also important to determine if the H2S-generating enzymes are downregulated during the progression of cardiovascular diseases and does the diminution of H2S promote hypertension, atherogenesis, coronary artery disease, and heart failure. Future studies should define the role of various cardiovascular risk factors and disease states on enzymatic synthesis of H2S and the effects of these alterations on the severity of cardiovascular disease states. Some important information can also be gleaned from transgenic or iRNA animal models in which H2S enzymes have been deleted or knocked down.
At present, there is also a dire need for highly potent and specific inhibitors of CBS, CGL, and 3MST. All of the agents currently used to inhibit these enzymes lack specificity, are not potent, and do not easily enter into cells. The development of specific and highly active pharmacological inhibitors of these enzyme pathways are clearly required to further define the role(s) of physiological production of H2S on a number of organ systems. Gene targeted animals do provide some insights, but these models do have significant limitations. The combination of knockout animals and pharmacological inhibitors represents a very powerful approach to more fully understand the biology of H2S.
The vast majority of agents including authentic H2S gas that are currently utilized to augment endogenous H2S levels are very short-acting agents that do not allow for sustained H2S release in vivo; this represents a significant limitation for the use of H2S therapy in chronic cardiovascular and other disease states. The development of long-lasting H2S therapies as well as agents that promote prolonged activation of the H2S generating enzyme systems may prove highly beneficial for treatment of patients with chronic cardiovascular diseases.
Given the very robust preclinical data regarding the cytoprotective effects of H2S in various animal models of ischemia-reperfusion injury, it is very logical to consider the clinical translation of H2S-based therapies for acute myocardial infarction, stroke, and as an adjunctive therapy for transplantation of various organs (i.e., liver and lung).
Clinical Translation of Hydrogen Sulfide Therapy
At present there are two clinical studies underway (ClinicalTrials.gov) to investigate the potential safety and effectiveness of H2S (administered in the form of sodium sulfide). One trial will evaluate the safety of H2S therapy in coronary artery bypass graft (CABG) patients to potentially reduce the damage done to the heart during cardiac surgery. A second clinical study will assess the pharmacokinetics of sodium sulfide in healthy volunteers and in subjects with varying degrees of impaired renal function. The study subjects with receive a single intravenous infusion of Na2S for 3 h and then followed for a period of 7 days. The results of these ongoing clinical trials and others will provide very important insights into the potential clinical benefits of H2S therapy and will certainly set the stage for the design of additional clinical trials aimed at evaluating the cardioprotective effects of H2S.
It is also very important to consider the potential toxic effects of H2S when considering the translation of the preclinical animal studies to patients that suffer from cardiovascular disease. It is well appreciated that high concentrations of H2S are very toxic and this may potentially limit the clinical usefulness of H2S in patients. At present, it is not clear how broad the H2S therapeutic window is in most animal models systems or in patients and this must be established for scientists and clinicians to move forward to the clinic. Furthermore, it is also possible that patients suffering from various chronic cardiovascular diseases with a number of risk factors and ailments may not tolerate the same levels of H2S that have been reported to be highly therapeutic in normal healthy animals that are subsequently subjected to ischemia-reperfusion injury or other insults.
Undoubtedly, the H2S field will continue to expand in the coming years with the identification of new cellular signaling targets, the development of novel agents to augment endogenous H2S levels (i.e., H2S donors), agents that modulate CGL and CBS activity, a better understanding of the regulation of H2S producing enzymes, novel transgenic mouse models with altered H2S levels, as well as the identification of new disease states to treat.
In summary, much like NO and CO, H2S has proven to be an important cellular signaling molecule that possesses profound cytoprotective effects. In fact, the efficacy of H2S in providing these cytoprotective effects, especially in the heart, has proven to be similar to that of NO. The exogenous administration of both gasotransmitters has been shown to protect against myocardial ischemia-reperfusion injury at concentrations thought to be in the physiological range, and studies have clearly shown that endogenous NO and H2S are cardioprotective signaling molecules. Furthermore, the mechanisms by which H2S and NO protect the heart are very similar, with some differences. Clearly, the field of H2S is an exciting area of research that will continue to grow in the coming years and will challenge the way we view the role that this gaseous signaling molecule plays in physiology and in pathophysiology.
Abbreviations Used
- 2-DOG
2-deoxy-D-glucose
- 3MST
3-mercaptopyruvate sulfurtransferase
- CBS
cystathionine β-synthase
- CGL or CSE
cystathionine γ-lyase
- CO
carbon monoxide
- DADS
diallyl disulfide
- DATS
diallyl trisulfide
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GST
gluatathione s-transferase
- HO
heme oxygenase
- H2S
hydrogen sulfide
- IPC
ischemic preconditioning
- KATP
ATP sensitive K+ channel
- MPTP
mitochondrial permeability transition pore
- NaHS
sodium hydrosulfide
- Na2S
sodium sulfide
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
nuclear factor-E2-related factor-2
- PAG
D,L-propargylglycine
- RISK
reperfusion injury salvage cascade
- STAT-3
signal transducer and activators of transcription 3
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Acknowledgments
Supported by grants from the American Diabetes Association (7-09-BS-26) to JWC, and the National Institutes of Health (NIH) National Heart Lung and Blood Institute (NHLBI) 2RO1 HL-060849-09 and 5R01 HL-092141-01 to DJL, and by R01 HL-085820 to WAC. This work was also supported by funding from the Carlyle Fraser Heart Center (CFHC) of Emory University Hospital Midtown.
References
- 1.Abe K. Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aon MA. Cortassa S. Akar FG. O'Rourke B. Mitochondrial criticality: A new concept at the turning point of life or death. Biochim Biophys Acta. 2006;1762:232–240. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee SK. Maulik SK. Effect of garlic on cardiovascular disorders: A review. Nutr J. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry SP. Townsend PA. Latchman DS. Stephanou A. Role of the JAK-STAT pathway in myocardial injury. Trends Mol Med. 2007;13:82–89. doi: 10.1016/j.molmed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Baukrowitz T. Fakler B. KATP channels gated by intracellular nucleotides and phospholipids. Eur J Biochem. 2000;267:5842–5848. doi: 10.1046/j.1432-1327.2000.01672.x. [DOI] [PubMed] [Google Scholar]
- 6.Benavides GA. Squadrito GL. Mills RW. Patel HD. Isbell TS. Patel RP. Darley-Usmar VM. Doeller JE. Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian JS. Yong QC. Pan TT. Feng ZN. Ali MY. Zhou S. Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 8.Bliksoen M. Kaljusto ML. Vaage J. Stenslokken KO. Effects of hydrogen sulphide on ischaemia-reperfusion injury and ischaemic preconditioning in the isolated, perfused rat heart. Eur J Cardiothorac Surg. 2008;34:344–349. doi: 10.1016/j.ejcts.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Bordia A. Verma SK. Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1998;58:257–263. doi: 10.1016/s0952-3278(98)90034-5. [DOI] [PubMed] [Google Scholar]
- 10.Bryan NS. Calvert JW. Elrod JW. Gundewar S. Ji SY. Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvert JW. Jha S. Gundewar S. Elrod JW. Ramachandran A. Pattillo CB. Kevil CG. Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvert JW. Lefer DJ. Myocardial protection by nitrite. Cardiovasc Res. 2009;83:195–203. doi: 10.1093/cvr/cvp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q. Moghaddas S. Hoppel CL. Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y. Ndisang JF. Tang G. Cao K. Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 15.Churchill EN. Mochly-Rosen D. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35:1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
- 16.Dieterich S. Bieligk U. Beulich K. Hasenfuss G. Prestle J. Gene expression of antioxidative enzymes in the human heart: increased expression of catalase in the end-stage failing heart. Circulation. 2000;101:33–39. doi: 10.1161/01.cir.101.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Durante W. Johnson FK. Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elrod JW. Calvert JW. Morrison J. Doeller JE. Kraus DW. Tao L. Jiao X. Scalia R. Kiss L. Szabo C. Kimura H. Chow CW. Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher CD. Augustine LM. Maher JM. Nelson DM. Slitt AL. Klaassen CD. Lehman-McKeeman LD. Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 20.Florian B. Vintilescu R. Balseanu AT. Buga AM. Grisk O. Walker LC. Kessler C. Popa-Wagner A. Long-term hypothermia reduces infarct volume in aged rats after focal ischemia. Neurosci Lett. 2008;438:180–185. doi: 10.1016/j.neulet.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Fu Z. Liu X. Geng B. Fang L. Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Furne J. Saeed A. Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 23.Geng B. Chang L. Pan C. Qi Y. Zhao J. Pang Y. Du J. Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 24.Gross GJ. Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 25.Hausenloy DJ. Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Hilfiker-Kleiner D. Hilfiker A. Fuchs M. Kaminski K. Schaefer A. Schieffer B. Hillmer A. Schmiedl A. Ding Z. Podewski E. Poli V. Schneider MD. Schulz R. Park JK. Wollert KC. Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 27.Hill BC. Woon TC. Nicholls P. Peterson J. Greenwood C. Thomson AJ. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem J. 1984;224:591–600. doi: 10.1042/bj2240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosoki R. Matsuki N. Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 29.Hu LF. Pan TT. Neo KL. Yong QC. Bian JS. Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflugers Arch. 2008;455:971–978. doi: 10.1007/s00424-007-0346-8. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y. Chen X. Pan TT. Neo KL. Lee SW. Khin ES. Moore PK. Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 31.Ignarro LJ. Nitric oxide: A unique endogenous signaling molecule in vascular biology. Biosci Rep. 1999;19:51–71. doi: 10.1023/a:1020150124721. [DOI] [PubMed] [Google Scholar]
- 32.Jha S. Calvert JW. Duranski MR. Ramachandran A. Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y. Pang QF. Xu G. Wang L. Wang JK. Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 34.Johansen D. Ytrehus K. Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury. Evidence for a role of K ATP channels. Basic Res Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 35.Jones SP. Greer JJ. Kakkar AK. Ware PD. Turnage RH. Hicks M. van Haperen R. de Crom R. Kawashima S. Yokoyama M. Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–H282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 36.Kaminski KA. Bonda TA. Korecki J. Musial WJ. Oxidative stress and neutrophil activation–the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 37.Kang KW. Lee SJ. Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 38.Kim YM. Talanian RV. Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 39.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun. 2000;267:129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- 40.Kimura Y. Kimura H. Hydrogen sulfide protects neurons from oxidative stress. Faseb J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 41.Kunisada K. Negoro S. Tone E. Funamoto M. Osugi T. Yamada S. Okabe M. Kishimoto T. Yamauchi–Takihara K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2000;97:315–319. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebuffe G. Schumacker PT. Shao ZH. Anderson T. Iwase H. Vanden Hoek TL. ROS and NO trigger early preconditioning: Relationship to mitochondrial KATP channel. Am J Physiol Heart Circ Physiol. 2003;284:H299–H308. doi: 10.1152/ajpheart.00706.2002. [DOI] [PubMed] [Google Scholar]
- 43.Li J. Bombeck CA. Yang S. Kim YM. Billiar TR. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem. 1999;274:17325–17333. doi: 10.1074/jbc.274.24.17325. [DOI] [PubMed] [Google Scholar]
- 44.Pan TT. Feng ZN. Lee SW. Moore PK. Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Pan TT. Neo KL. Hu LF. Yong QC. Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol. 2008;294:C169–C177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- 46.Ping P. Zhang J. Pierce WM., Jr. and Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 47.Qu K. Chen CP. Halliwell B. Moore PK. Wong PT. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke. 2006;37:889–893. doi: 10.1161/01.STR.0000204184.34946.41. [DOI] [PubMed] [Google Scholar]
- 48.Ravagnan L. Gurbuxani S. Susin SA. Maisse C. Daugas E. Zamzami N. Mak T. Jaattela M. Penninger JM. Garrido C. Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 49.Rose P. Moore PK. Ming SH. Nam OC. Armstrong JS. Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J Gastroenterol. 2005;11:3990–3997. doi: 10.3748/wjg.v11.i26.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roth MB. Nystul T. Buying time in suspended animation. Sci Am. 2005;292:48–55. doi: 10.1038/scientificamerican0605-48. [DOI] [PubMed] [Google Scholar]
- 51.Ryter SW. Otterbein LE. Morse D. Choi AM. Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saleh A. Srinivasula SM. Balkir L. Robbins PD. Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 53.Saravanan G. Prakash J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol. 2004;94:155–158. doi: 10.1016/j.jep.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 54.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfur transferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 55.Simon F. Giudici R. Duy CN. Schelzig H. Oter S. Groger M. Wachter U. Vogt J. Speit G. Szabo C. Radermacher P. Calzia E. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock. 2008;30:359–364. doi: 10.1097/SHK.0b013e3181674185. [DOI] [PubMed] [Google Scholar]
- 56.Sivarajah A. Collino M. Yasin M. Benetti E. Gallicchio M. Mazzon E. Cuzzocrea S. Fantozzi R. Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 57.Sivarajah A. McDonald MC. Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 58.Sodha NR. Clements RT. Feng J. Liu Y. Bianchi C. Horvath EM. Szabo C. Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 60.Tang G. Wu L. Liang W. Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 61.Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 62.Tanito M. Agbaga MP. Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Tong X. Porter LM. Liu G. Dhar-Chowdhury P. Srivastava S. Pountney DJ. Yoshida H. Artman M. Fishman GI. Yu C. Iyer R. Morley GE. Gutstein DE. Coetzee WA. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol. 2006;291:H543–551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Usachev YM. Marsh AJ. Johanns TM. Lemke MM. Thayer SA. Activation of protein kinase C in sensory neurons accelerates Ca2+ uptake into the endoplasmic reticulum. J Neurosci. 2006;26:311–318. doi: 10.1523/JNEUROSCI.2920-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venardos KM. Perkins A. Headrick J. Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: A review. Curr Med Chem. 2007;14:1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 66.Wang Q. Liu HR. Mu Q. Rose P. Zhu YZ. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: The contribution of the hydrogen sulfide-mediated pathway. J Cardiovasc Pharmacol. 2009;54:139–146. doi: 10.1097/FJC.0b013e3181ac8e12. [DOI] [PubMed] [Google Scholar]
- 67.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 68.Xu Z. Prathapasinghe G. Wu N. Hwang SY. Siow YL O K. Ischemia-reperfusion reduces cystathionine-{beta}-synthase-mediated hydrogen sulfide generation in the kidney. Am J Physiol Renal Physiol. 2009;297:F27–35. doi: 10.1152/ajprenal.00096.2009. [DOI] [PubMed] [Google Scholar]
- 69.Yan SK. Chang T. Wang H. Wu L. Wang R. Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351:485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 70.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S. Snyder SH. Wang R. H2S as a physiologic vasorelaxant: Hhypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yellon DM. Baxter GF. Reperfusion injury revisited: Is there a role for growth factor signaling in limiting lethal reperfusion injury? Trends Cardiovasc Med. 1999;9:245–249. doi: 10.1016/s1050-1738(00)00029-3. [DOI] [PubMed] [Google Scholar]
- 72.Yong QC. Lee SW. Foo CS. Neo KL. Chen X. Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330–H1340. doi: 10.1152/ajpheart.00244.2008. [DOI] [PubMed] [Google Scholar]
- 73.Yong QC. Pan TT. Hu LF. Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J Mol Cell Cardiol. 2008;44:701–710. doi: 10.1016/j.yjmcc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Yusof M. Kamada K. Kalogeris T. Gaskin FS. Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zanardo RC. Brancaleone V. Distrutti E. Fiorucci S. Cirino G. Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z. Huang H. Liu P. Tang C. Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol. 2007;85:1248–1253. doi: 10.1139/Y07-120. [DOI] [PubMed] [Google Scholar]
- 77.Zhao W. Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 78.Zhao W. Zhang J. Lu Y. Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu H. Itoh K. Yamamoto M. Zweier JL. Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 80.Zhu XY. Yan XH. Chen SJ. H2S protects myocardium against ischemia/reperfusion injury and its effect on c-Fos protein expression in rats. Sheng Li Xue Bao. 2008;60:221–227. [PubMed] [Google Scholar]