Abstract
Redox reactions are known to regulate many important cellular processes. In this review, we focus on the role of redox regulation in DNA repair both in direct regulation of specific DNA repair proteins as well as indirect transcriptional regulation. A key player in the redox regulation of DNA repair is the base excision repair enzyme apurinic/apyrimidinic endonuclease 1 (APE1) in its role as a redox factor. APE1 is reduced by the general redox factor thioredoxin, and in turn reduces several important transcription factors that regulate expression of DNA repair proteins. Finally, we consider the potential for chemotherapeutic development through the modulation of APE1's redox activity and its impact on DNA repair. Antioxid. Redox Signal. 12, 1247–1269.
I. Introduction
Although the importance of DNA-repair pathways in protecting the genome from damage caused by endogenous and exogenous DNA-damaging agents (40, 44, 60) has long been recognized, the role of redox regulation in these pathways is a relatively recent discovery. In writing this review, we attempted to guide the reader through general as well as specific aspects of DNA repair and redox regulation, focusing ultimately on the connection between the two. We begin with an overview of DNA-repair pathways leading to a more in-depth discussion of one specific DNA-repair pathway, the base excision repair (BER) pathway. We focus on the BER pathway, which is responsible for the repair of DNA damage caused by oxidation, alkylation, and ionizing radiation, and specifically on apurinic/apyrimidinic endonuclease 1 (APE1), the only DNA-repair protein currently known to serve a dual role as a repair enzyme and a redox factor. In its role as a redox factor, APE1 modifies downstream transcription factors such as AP-1, NF-κB, CREB, p53, and others, and thereby indirectly alters the activity of other DNA-repair pathways. To put the redox activity of APE1 in perspective, we provide an overview of general redox systems as well as an in-depth discussion of the redox activity of APE1. Finally, in considering the impact of redox regulation of DNA repair to human health, we discuss the modulation of the redox activity of APE1 by small molecules and the potential for chemotherapeutic development targeting redox regulation of DNA repair.
II. DNA-Repair Pathways
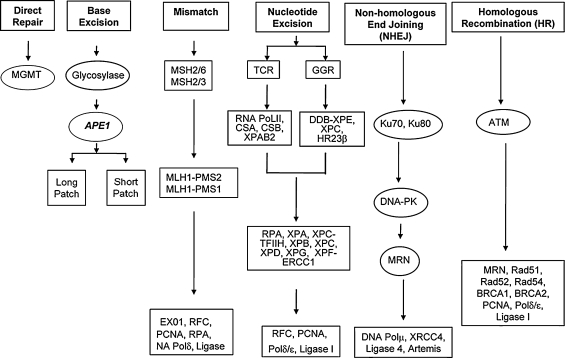
The genome of eukaryotic cells is constantly under attack from both endogenous and exogenous DNA-damaging agents. DNA damage resulting from endogenous agents includes oxidation by reactive oxygen species (ROS) generated from normal metabolic processes, alkylation by agents such as S-adenosylmethionine, adduct formation resulting from attack by reactive carbonyl species formed during lipid peroxidation, hydrolytic depurination leading to the formation of abasic sites, or deamination of bases, primarily cytidine, and to a lesser extent, adenine (44). Exogenous agents include environmental insults (chemicals, carcinogens, UV light), chemotherapeutic agents, and radiation damage (40, 60). Failure to repair DNA damage in both postmitotic and mitotic cells can result in apoptosis or accumulation of mutations and even cell-cycle arrest (58, 106). For example, the DNA-damage response in mitotic cells results in cell-cycle arrest involving the major cell-cycle machinery. In postmitotic cells, DNA damage may result in cell-cycle activation and subsequent arrest, leading to deleterious events in this cell population as well (110, 160). However, we have evolved a series of DNA-repair pathways to correct the damage, including direct repair (DR), base-excision repair (BER), nucleotide-excision repair (NER), mismatch repair (MMR), homologous recombination (HR), and nonhomologous end joining (NHEJ) (85, 86) (Fig. 1). The number of DNA-repair proteins and factors involved in the cellular response to DNA damage keeps growing as more and more information is obtained, not only on the DNA repair enzymes involved in each pathway, but also on the regulatory networks that are induced by persistence of DNA damage in the cell (182). Distinct DNA damage is repaired by the different pathways and mechanisms. Overlap and interaction between the various pathways and some overlap in mechanisms occur. For example, O6-methylguanine can be removed directly by O6-methylguanine-DNA methyltransferase (MGMT or AGT) in DR, but if this pathway is not successful, the O6mG mispairs and is recognized by the MMR pathway (59). Similarly, oxidative DNA damage is repaired mainly by BER, but some repair by NER also is possible (53). Single-strand DNA breaks (SSBs) unrepaired by BER lead to double-strand breaks (DSBs), which may be repaired by HR, and HR can also repair DNA DSBs that NHEJ pathways fail to process (49). Interaction of different DNA-repair pathways and mechanisms provides the most efficient defense for the cell genome, whereas reduced repair capacity can lead to genomic instability. A number of diseases are associated with defects in DNA repair, including xeroderma pigmentosum, Cockayne syndrome, tridothiodystrophy, Werner syndrome, and Bloom syndrome (118, 162). The reader is directed to recent comprehensive reviews for more specific information on each DNA-repair pathway (47, 111, 144, 145, 150, 157, 174). For updated information on the individual repair proteins, the following link may prove useful: http://www.cgal.icnet/DNA_Repair_Genes.html (182). What follows is an overview of DNA-repair pathways necessary to provide a context for understanding the role of redox regulation in DNA repair.
FIG. 1.
Schematic overview of DNA-repair pathway. Several DNA-repair pathways are involved in maintaining cell genomic stability; these include direct repair (DR), base-excision repair (BER), nucleotide-excision repair (NER), mismatch repair (MMR), homologous recombination (HR), and nonhomologous end joining (NHEJ). More than 150 proteins are involved. Only selected genes of each pathway are shown here. [Adapted from Fishel et al. (57).]
A. Mammalian direct repair: O6-alkylguanine-DNA methyltransferase or O6-methylguanine-DNA methyltransferase
This type of repair in mammals is termed direct reversal because the damaged base is repaired through removal of the alteration to the base instead of removal of the damaged base. It is unique in this sense and probably is the most efficient mechanism of repair (105). The protein that carries out this reaction, the AGT protein, removes alkyl groups through direct transfer from the O6 position of guanine and to a lesser extent from the O4 position from thymine to the protein, leaving a guanine or thymine in DNA and inactivated protein. This is a stoichiometric reaction, as one protein removes one alkyl group and is then degraded. It is essential to repair O6-meG adducts, as they cause errors by mispairing with thymine during replication, leading to G:C to A:T transitions or a strand break.
B. Base-excision repair
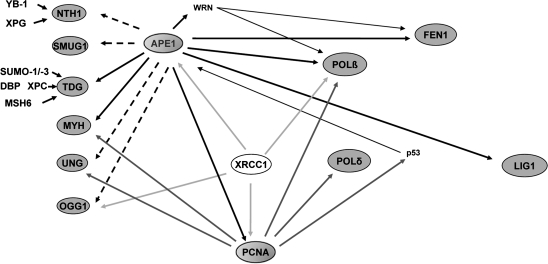
BER is responsible for the repair of DNA damage arising from alkylation, deamination, or oxidation of bases (8, 40, 50). Alkylation of bases arises from exposure to either endogenous agents such as S-adenosylmethionine or exogenous agents, including environmental and chemotherapeutic agents, whereas deamination of cytidines and adenines occurs spontaneously. Oxidative damage can result from ROS generated by normal cellular processes, in addition to environmental or chemotherapeutic agents. BER is initiated by the removal of the damaged base through enzymes called DNA glycosylases, which specifically recognize several different types of base damage. Glycosylases are of two types, monofunctional and bifunctional. Monofunctional glycosylases [e.g., N-methyl purine DNA glycosylase (MPG or AAG)] excise the damaged base to generate an apurinic/apyrimidinic (AP) or abasic site, which is acted on by the multifunctional AP endonuclease, APE1. Bifunctional glycosylases such as human 8-oxoguanine DNA glycosylase (hOGG1), human endonuclease VIII–like DNA glycosylase (NEIL1-3), and E. coli endonuclease III (NTH) glycosylase have an additional AP lyase function (36, 43) that excises the damaged base and nicks the phosphodiester backbone 3' to the AP site. The resulting AP site is processed by APE1, which hydrolyzes the phosphodiester backbone immediately 5' to the AP site, creating 3' OH and 5' deoxyribose phosphate (5' dRP) termini. At this stage, repair can proceed by two pathways: the short-patch BER (SP-BER) pathway and the long-patch BER (LP-BER) pathway. APE1 is responsible for 95% of the endonuclease activity in the cell and is a critical part of both the short-patch and the long-patch BER pathway (45, 46). SP-BER repairs normal AP sites. DNA polymerase β (pol β) removes the 5' dRP moiety by its dRPase activity and uses the 3' OH terminus to insert the correct base. The nick is ligated by DNA ligase III/XRCC1, and repair is completed. The LP-BER pathway preferentially repairs oxidized and reduced AP sites and is a minor branch of the BER pathway. A segment or flap of three to eight nucleotides surrounding the AP site is displaced, followed by insertion of the correct nucleotides by DNA polymerase δ, ɛ, or β, along with proliferating cell nuclear antigen (PCNA) and replication factor-C (RF-C). After resynthesis, flap endonuclease 1 (FEN1) removes the displaced strand, and DNA ligase I, or the DNA ligase III/XRCC1 complex ligates the nick. APE1 is the only AP endonuclease that performs these functions in the BER, and as such, is a key player in the BER process. APE1 also coordinates recruitment of other DNA-repair proteins involved in BER through a complex network of direct protein–protein interactions and indirect interactions, as shown in Fig. 2. No effective backup to APE1 activity exists in the cell, as is discussed in more detail later, including its other major redox-signaling function.
FIG. 2.
Network of protein–protein interactions involving APE1 (adapted from ref. 51). APE1 plays an essential role in BER through its enzymatic activity as well as its role in coordinating interactions, either directly (solid lines) or indirectly (dashed lines), with a large number of other proteins involved in BER. Proteins highlighted are central components with APE1, apurinic/apyrimidinic endonuclease, in the middle. Those proteins in the first column are DNA glycosylases; the remaining proteins include PCNA, an accessory protein; polymerases, pol β and pol δ; ligase 1, LIG1; and the flap endonuclease, FEN1.
C. Nucleotide-excision repair
The NER pathway is responsible for repairing large adducts such as ultraviolet-light–induced cyclobutane pyrimidine dimers, adducts induced by polycyclic aromatic hydrocarbons, and other bulky DNA lesions induced by cross-linking agents and base-damaging chemical carcinogens. Numerous proteins are required to complete NER. More than 25 proteins/complexes have been identified in eukaryotic cells; these can be further divided into two main subpathways; global genome repair (GGR) and transcription-coupled repair (TCR), depending on the complexes that initiate repair (8, 63). TCR is initiated when RNA polymerase II (RNA Pol II) stalls at sites of DNA damage. TCR-specific factors, including the Cockayne syndrome proteins, CSA and CSB, are recruited at the site of transcription arrest, followed by removal of the lesion by NER enzymes. In contrast, the heterodimer XPC/HR23B appears to be the major damage-recognition factor in human cells. The UV-DNA damage–binding protein UV-DDB is additionally required for NER of UV-induced cyclobutane pyrimidine dimers. After recognition, both TCR and GGR use the same proteins to repair the damaged DNA. The transcription-factor IIH (TFIIH) complex is recruited to the site of DNA damage, including its component helicases, XPB and XPD (xeroderma pigmentosum complementary group B and D proteins) that unwind the DNA strand on either side of the DNA damage. XPA and RPA (replication protein A) stabilize the exposed single-strand DNA followed by cleavage of the 27- to 30-nucleotide fragment 3' and 5' of the lesion by endonucleases XPG and ERCC1/XPF1/XPF. The resulting gap is filled in by the DNA polymerases δ or ɛ, along with PCNA, RPA, and replication factor C (RFC) by using the undamaged strand as a template.
D. Mismatch repair
In a broad definition, MMR is responsible for the recognition and repair of single mismatches or misaligned short nucleotide repeats. Mismatches can be endogenously caused by spontaneous deamination of 5-methylcytosine to thymine, resulting in a guanine-to-thymine mismatch, damage to the cellular nucleotide pool, cytosine deamination to uracil, resulting in a guanine-to-uracil mismatch, or incorrect incorporation by DNA polymerase. A complex of MSH2 and MSH6 recognizes the mismatch and initiates the pathway. Various combinations of MSH2 and either MSH3 or MSH6 are formed, which specify the type of mismatch recognized. For example, when MSH2 is paired with MSH6, it recognizes both insertion–deletion mispairs and single-base mismatches, whereas when it is paired with MSH3, the complex recognizes insertion–deletion mispairs. After recognition, MSH proteins recruit MLH1 and its binding partners, post–meiotic-segregation increased-1 protein (PMS1) and PMS2. An exonuclease removes the DNA lesion, a DNA polymerase synthesizes a new strand, and finally, a DNA ligase completes the repair. This has been previously reviewed (104, 126).
E. Nonhomologous DNA end-joining and homologous recombination
NHEJ is the main repair pathway for DSBs in mammalian cells. DNA DSBs may be caused by ionizing radiation (IR), chemotherapeutic drugs, cleavage during V (D) J-recombination, meiotic recombination, or the collapse of replication forks. DSBs are the most severe form of DNA damage and endanger genomic stability by coordinating deletion or translocation or both of chromosomal DNA. Proteins including, but not limited to, Ku 70, Ku 80, DNA ligase IV, and XRCC4 are part of the NHEJ-repair pathway. The Ku proteins bind to the ends of broken DNA and, as a complex with DNA-PKcs (DNA-dependent kinase catalytic subunit), interact with DNA ligase IV and XRCC4 to repair DNA through the NHEJ pathway. DNA ligase IV and XRCC4 function in a complex to ligate the nick and to complete repair (34). Discovery of new proteins involved in NHEJ includes Metnase or SETMAR (117), which has been shown to interact with DNA ligase IV and to enhance the efficiency and accuracy of NHEJ (92).
Homologous recombination (HR) also is involved in repairing DNA DSBs. HR is initiated through the DSB recognition by ATM (ataxia telangiectasia–mutated protein), which phosphorylates multiple downstream proteins. DSBs are processed by the MRN complex (Mre11/Rad50/Nbs1) nuclease activity to yield single-strand DNA (ssDNA). ATM-activated BRCA1 attracts BRCA2 and RAD51 to bind to the ssDNA ends, allowing the RAD52/RAD54 complex to join and form larger complexes with BLM and WRN proteins. These large protein complexes at the strand break direct pairing of the processed DNA with a homologous region on the sister chromatid and initiate strand exchange. This was previously reviewed in detail (167).
III. General Redox Systems
In living systems, two systems are primarily responsible for general reduction-oxidation (redox) regulation, the thioredoxin (TRX) and glutaredoxin/glutathione (GRX/GSH) systems. They maintain the redox cellular homeostasis as well as redox regulate several cellular processes through a thiol–redox mechanism (91, 142). Thiol-based redox mechanisms rely on the special properties of Cys residues, which can adopt 10 different sulfur oxidation states from +6 to −2 (the fully reduced state) (69, 100). Cys can exist in a number of different forms in vivo, including cysteinyl radical, sulfenic acid, sulfinic acid, sulfonic acid, cystine, and others [see Jacob et al. (100) for a recent review of sulfur chemistry associated with Cys]. Of the types of reactions involving Cys residues, the thiol/disulfide exchange reaction is of relevance to our discussion of general redox systems [reviewed in (69)]. In thiol/disulfide-exchange reactions, an oxidized protein, including a disulfide bond, is recognized by a reduced protein such as TRX or GRX/GSH and is then reduced through the formation of an intermediate mixed disulfide bond (Fig. 3). Cys acts as a nucleophile in this type of reaction (69, 100). Interestingly, TRX and GRX/GSH are reduced by thioredoxin reductase and glutathione reductase, respectively, in a reaction involving both thiol/disulfide exchange and electron-transfer reactions requiring cofactors such as FADH2 and NADPH (Fig. 4) (69, 100)
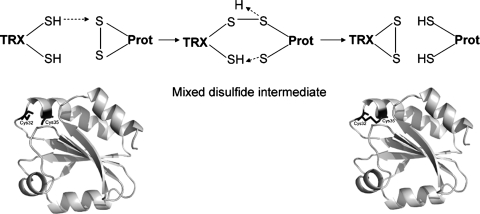
FIG. 3.
Reduction of oxidized proteins by TRX. Thioredoxin (TRX) reduces oxidized proteins containing a disulfide through the formation of a mixed disulfide intermediate involving nucleophilic attack by Cys 32 of the CXXC motif. The mixed disulfide is then resolved by Cys 35, resulting in the formation of a disulfide bond in thioredoxin. Ribbon renderings are shown for the reduced (PDB identifier, 1ERT) and oxidized thioredoxin (PDB identifier, 1ERU) along with the Cys residues of the CXXC motif in black-stick renderings.
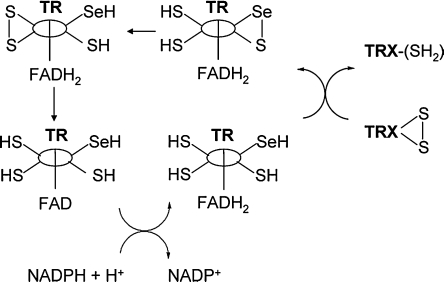
FIG. 4.
Thioredoxin reductase/thioredoxin (TR/TRX) redox cascade. Thioredoxin is reduced by thioredoxin reductase in a somewhat more-complex mechanism involving the formation of a selenylsulfide and subsequent reduction by a pair of Cys residues within another subunit of TR. Electron-transfer reactions involving the FADH2, a cofactor of TR, and NADPH are required to regenerate TR. [Adapted from Jacob et al. (100).]
A. The thioredoxin system
Components of the thioredoxin system include thioredoxin (TRX), NADPH, and thioredoxin reductase (TR) (90, 100). Thioredoxins (TRXs) comprise a large family of structurally conserved proteins that serve as general protein disulfide oxidoreductases and can reduce disulfide bonds in a variety of proteins through a thiol/disulfide exchange mechanism (143). Oxidized thioredoxin is then reduced by thioredoxin reductase, a flavoprotein containing a selenocysteine, in a reaction involving NADPH.
Thioredoxins (TRXs) share a similar active-site motif Cys-X-X-Cys and a common structural motif, known as the TRX fold (91, 120, 153), which consists of a four-stranded β-sheet surrounded by three α-helices (Fig. 4). The active-site motif is located on the loop connecting β-sheet 1 and α-helix 1. The N-terminal Cys residue in the active site is surface exposed and has a low pKa value; for example, Cys32 in human TRX has an estimated pKa of 6.3 (61), whereas the C- terminal Cys is buried in the molecule and has a much higher pKa value. It has been proposed that the low pKa value of the N-terminal Cys arises from the partial positive charge from the dipole moment associated with α-helix 2 (88), or alternatively, may be due to its hydrogen bond to the C-terminal Cys (181). The nucleophilicity of the thiolate group of the Cys is increased by the low pKa. The proposed reaction mechanism of disulfide reduction by thioredoxin is as follows: the N-terminal cysteine thiolate of TRX acts as a nucleophile and attacks the target disulfide, resulting in a transient mixed disulfide intermediate, which is, in turn, reduced by the C-terminal active-site Cys residue, generating a dithiol in the target protein and a disulfide in thioredoxin (91, 108, 120) (Fig. 3). The resulting disulfide in the active site of TRX can be reduced by TR with electrons from NADPH, completing the catalytic cycle.
The mechanism by which TR reduces TRX back to the dithiol involves the formation of a selenylsulfide in the active site of TR (100), as shown in Fig. 4. A second redox active site located in the other subunit of the dimeric TR contains two thiols that reduce the selenylsulfide back to a thiol and selenol, with the resultant formation of a disulfide bond. This disulfide is reduced by electron transfer from FADH2, and the resulting FAD is then reduced by electron transfer from NADPH (100).
B. The glutaredoxin/glutathione system
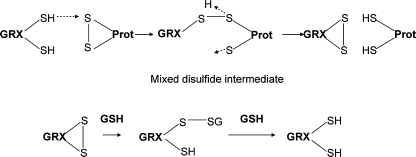
The glutaredoxin system is composed of NADPH, the flavoprotein glutathione reductase, glutathione, and glutaredoxin (54, 90, 121). This system also works through a cascade of disulfide oxidation and reduction. Glutaredoxins (GRXs) are small redox enzymes of ∼100 amino acid residues, which use glutathione as a cofactor. Structurally glutaredoxins are very similar to thioredoxins, retaining the same fold and active sites. However, the active site of GRXs includes Cys-X-X-Cys or Cys-X-X-Ser. By using a similar reactive mechanism, GRXs catalyze the reversible reduction of substrate protein disulfides, resulting in oxidation of the GRXs (Fig. 5). Oxidized GRXs are reduced nonenzymatically by glutathione (glutamyl-cysteinyl-glycine,GSH), and then the oxidized glutathionine disulfide (GSSG) is reduced by glutathione reductase at the expense of NADPH (121).
FIG. 5.
Reduction of oxidized proteins by glutaredoxin/glutathione (GRX/GSH). Through a mechanism similar to that used by TRX, GRX also forms a mixed-disulfide intermediate with an oxidized protein. This disulfide is resolved through involvement of a second Cys residue of the CXXC motif in GRX, resulting in reduction of the protein and formation of a disulfide bond in GRX. GSH directly reduces GRX again through a disulfide-exchange mechanism and is itself reduced by glutathione reductase. [Adapted from Lillig et al. (121).]
C. Roles of general redox systems
Through a thiol/disulfide exchange mechanism, thioredoxin and glutaredoxin systems maintain a reducing intracellular redox state, which is an important metabolic variable, influencing many aspects of cell function, like growth, apoptosis, and reductive biosynthesis. In addition, by redox signaling, they control the activation of a number of transcription factors and hence regulate a broad range of cellular functions (11, 153, 192). The two redox systems physiologically play many roles in different organisms and, conversely, are also pathophysiologic factors for a variety of human diseases, including cancer, viral disease, Alzheimer's disease, and others (7, 12, 33, 154), and hence serve as vital drug targets for cancer therapy and other disease treatments (20, 39, 122, 123, 141). Although the thioredoxin system and glutaredoxin system share a number of functions, they are not just simple duplicate systems; TRX and GRX act on different substrates (54, 121). TRX but not GRX, for example, has been implicated in the reduction of APE1 (6, 83, 94, 170).
The remainder of our discussion on redox factors focuses on APE1, which is the only DNA-repair protein known also to have a role in redox regulation affecting the expression of a number of other DNA-repair proteins.
IV. The Redox Activity of APE1
In a search to identify the nuclear factor responsible for reducing the transcription factor AP-1, a factor termed redox effector factor 1, Ref-1, was identified (184, 187). Since this initial discovery, APE1 (Ref-1) has been reported to reduce a number of other important transcription factors, including NF-κB, HIF-1α, p53, PAX, and others (35, 48, 83, 84, 112, 169, 175) (Fig. 6). And, as discussed below, the redox activity of APE1 plays an important role in regulating the expression of a large number of DNA-repair proteins.
FIG. 6.
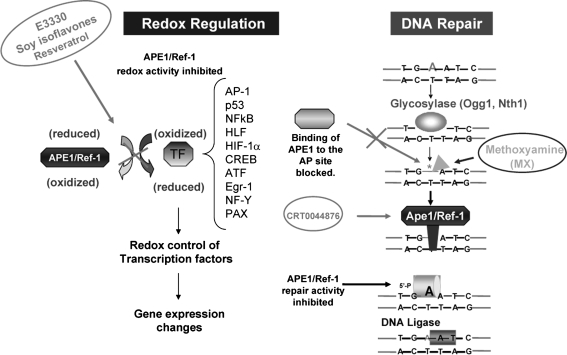
APE1 has dual roles in redox and DNA repair. APE1 possesses two major functions: redox regulatory/signaling and DNA repair. Through its redox function, APE1 regulates gene expression by modifying the redox status of some transcription factors involved in variety of cancer processes. Small molecules that block APE1 redox function are shown in ovals. In addition to its redox function, APE1 plays a critical role in the BER DNA-repair pathway as an AP endonuclease, which processes the AP sites. Blocking AP sites by using methoxyamine (MX) or APE1 or both directly by using APE1-specific inhibitors such as CRT0044876 may decrease DNA repair and lead to tumor-cell death. [Adapted from Luo et al. (128).]
A. Evolution of the redox function of APE1
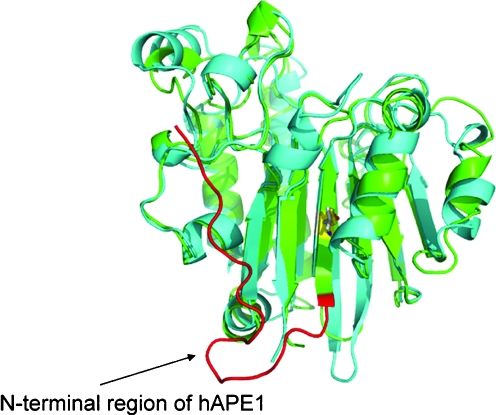
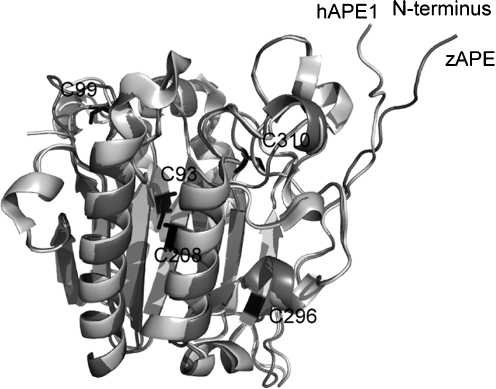
Although APE1 is reported to have distinct redox and repair domains (186) located within the N- and C-terminal regions of the protein, respectively, these functional domains do not correspond to independently folded domains within the protein (i.e., structural domains). Furthermore, the repair and redox activities do not appear to be coordinated within human APE1, and whereas the AP endonuclease activity of APE1 is conserved from bacteria to humans, the redox function is unique to mammals. Thus, as shown in Fig. 7, APE1 and E. coli exonuclease III, the major AP endonuclease found within E. coli, are closely related in terms of structure (r.m.s.d., 1.5 Å), retaining not only the same overall fold and topology but also very similar endonuclease active sites (Fig. 8). The sequence identity between APE1 and exonuclease III is 27.7%. The most obvious structural difference between human APE1 and exonuclease III is an additional 62 N-terminal residues found only in APE1 (Fig. 7). Within this N-terminal region of APE1 is a nuclear localization sequence. However, addition of N-terminal residues alone does not confer redox activity; zebrafish APE includes a similar N-terminal addition (Fig. 9) but lacks redox activity (68).
FIG. 7.
Comparison of human APE1 and exonuclease III from Escherichia coli. The structurally similar enzymes, human APE1 (PDB identifier, 1BIX, green ribbon rendering) and exonuclease III (PDB identifier, 1AKO, blue), share a similar fold; however, APE1 includes an additional 62 N-terminal residues, highlighted in red (residues 44-62). In exonuclease III, the residue equivalent to the APE1 Cys 65 (yellow stick rendering) is Val 4 (gray stick rendering). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 8.
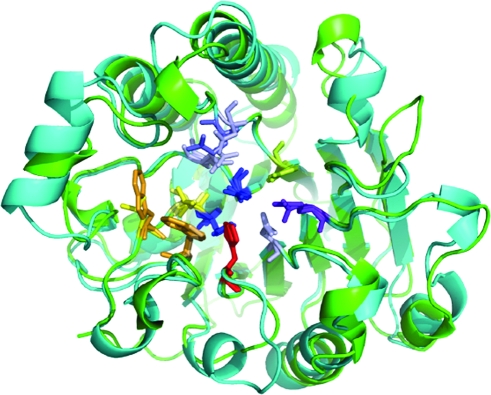
Comparison of active-site regions of human APE1 and exonuclease III. Stick renderings are shown for residues within the active sites of APE1 (PDB identifier, 1BIX, green ribbon rendering) and exonuclease III (PDB identifier, 1AKO, blue). Active-site residues are color coded as follows: light blue, Asn; slate blue, Gln; blue, Asp; purple blue, Glu; red, His; green, Tyr; light orange, Phe; bright orange, Trp; yellow, Leu; pale yellow, Ile. The active-site regions of APE1 and exonuclease III are highly conserved. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 9.
Comparison of zebrafish APE and human APE1. Although the zebrafish APE (PDB identifier, 203C, light gray) also includes an extended N-terminus similar to that found in the human APE1 (PDB identifier, 203H, dark gray), it does not have redox activity. As shown in these ribbon renderings, the zebrafish and human enzymes are structurally very similar, including the N-terminal residues. Conserved Cys residues, including 93, 99, 208, 296, and 310, are shown in black stick renderings for the human enzyme.
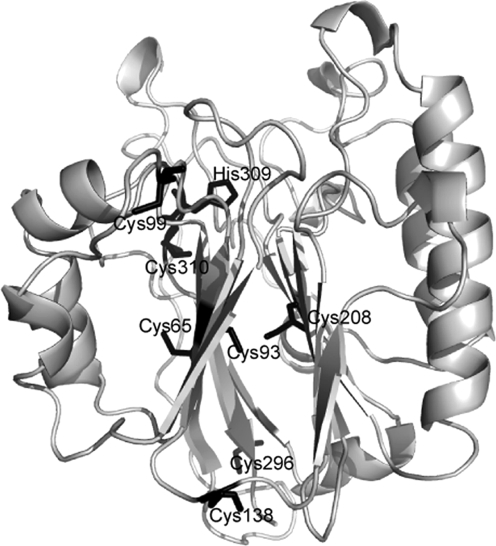
So the question then becomes, what is required for the redox activity of human APE1 (hAPE1)? This continues to be a source of controversy in the literature. Of the seven Cys residues present in hAPE1, Cys 65 was identified as the critical residue required for redox activity through analysis of single Cys-to-Ala substitutions within APE1 (see Fig. 10) (178). Investigation of the role of Cys residues within APE1 was based on the finding that a Cys residue within the DNA-binding domain of the transcription factor c-Jun was subject to oxidation, leading to loss of DNA bindings and was reduced by APE1 (2, 184, 187). Subsequently, the crystal structure of human APE1 was reported (70), revealing that Cys 65, a residue unique to mammalian sequences, is a buried residue located on the first β strand in the fold, which is part of a β sheet in the core of the protein (Fig. 10). The residue equivalent to the hAPE1 Cys 65 in exonuclease III, based on structural alignment, is Val 4, whereas that in zebrafish APE1 (zApe) is Thr 58 (68). Conservation of Cys residues between the hAPE1 and the E. coli enzyme is limited to Cys 208 and Cys 310, but within vertebrate APEs, all Cys residues except Cys 65 and Cys138 are conserved (68). The structure of APE in vertebrates also is conserved, based on a comparison of the zAPE and human APE1 structures. Within the vicinity of Thr 58, the structure and chemical environment is highly conserved (68). In contrast, residues in the vicinity of Val 4 in the E. coli enzyme exonuclease III are not similar to those found in the vertebrate APEs.
FIG. 10.
Positions of Cys residues within APE1. The human APE1 (PDB identifier, 1BIX, gray ribbon rendering) includes seven Cys residues (black sticks), whose positions relative to the active-site His 309 are shown. None of the Cys residues is appropriately positioned to form a disulfide bond, and the redox-critical Cys residue, Cys 65, is a buried residue located in the first β strand of the APE1 fold.
The report of a viable C64A knockin mouse and data within challenged the validity of a role for Cys 65 in the redox activity of APE1 (148). However, we note that the mouse knockin study does not directly address the role of Cys 65 (64 in mouse) in the redox activity, but rather suggests that Cys 65 is not essential for the development of the mouse, potentially confirming that redox systems are redundant. In an effort to clarify this issue, we substituted Thr 58 with Cys in zAPE, thereby conferring redox activity to the enzyme, as measured both in EMSA redox and transactivation redox assays (68). To date, the preponderance of evidence supports a role for Cys 65 in the redox activity of hAPE1. Thus, we conclude that evolution of redox activity in hAPE1 is coincident with the appearance of Cys 65 in mammalian sequences.
B. Comparison of APE1 with other redox factors
In contrast to molecules such as TRX and GRX, which maintain the general redox status of the cell, APE1 does not contain two Cys residues within a C-X-X-C motif. Thus, the mechanism by which APE1 reduces transcription factors is likely to differ from that of thioredoxin or glutaredoxins. In the crystal structures reported to date of APE1, no disulfide bonds are present, and the only Cys residue reported to be absolutely required for redox activity is Cys 65, which is a buried residue. The Cys residues positioned closest to one another are 93 and 208, but their respective S atoms are ∼3.5 Å apart, too far apart to form a disulfide bond, which is typically ∼2.2 Å in length. Further, these residues are buried in the core of the protein and are not accessible. The solvent-accessible Cys residues include 99 and 138; however, these residues are not in close proximity to one another and would not be expected to interact to form a disulfide bond. Furthermore, substitution of Ala for either Cys 99 or 138 has no effect on redox activity (178). Before the determination of the crystal structure of APE1, it was proposed that Cys 65 and 93 form a disulfide bond (178). Given the respective locations of Cys 65 and Cys 93 in the protein, >8 Å apart and positioned on opposite sides of the β sheet (Fig. 10), a substantial conformational change in the structure of the protein would be required for a disulfide bond to form between these residues.
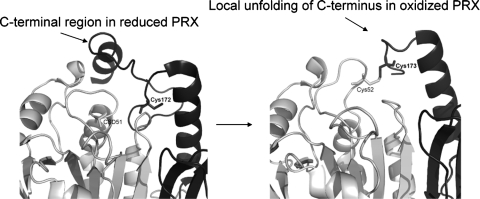
Another group of redox proteins, peroxiredoxins, are responsible for sensing hydrogen peroxide in the cell and serve as catalysts to detoxify this extremely reactive molecule (87). These enzymes are dissimilar to thioredoxin- or glutaredoxin-type molecules in that they lack a C-X-X-C motif, but they do include two Cys residues that are required for activity (183). These Cys residues are located ∼9 Å from one another in the fully folded dimeric structure (Fig. 11); one Cys from each monomer forms the active site (183). The nucleophilic thiolate is sequestered before a local unfolding event near the dimer interface. The resolving thiolate is located near the C-terminus of the molecule and, on local unfolding, is placed in close proximity to the nucleophilic thiolate (183) (Fig. 11). Peroxiredoxins reduce hydrogen peroxide and not other proteins (87), but on overoxidation to the sulfenic or sulfinic acid state, are themselves reduced by sulfiredoxin (29, 177). The requirement for local unfolding for peroxiredoxin to complete its catalytic cycle in the detoxification of hydrogen peroxide is very interesting and may be a general mechanism used by other redox factors. Although APE1 does not include two Cys residues positioned similarly to those found in peroxiredoxin, the possibility remains that a conformational change or local unfolding event may result in more favorable positioning of Cys residues. An interesting similarity between APE1 and peroxiredoxin is the proximity of a Cys residue to a terminus; in the case of hAPE1, Cys 65 is located relatively close to the N-terminus of the protein; it is located within the first secondary structural element (a β strand) in the fold (Fig. 7). In summary, we conclude that hAPE1 is unique as a redox factor, having evolved this additional function while maintaining its essential base-excision repair activity.
FIG. 11.
Redox mechanism of peroxiredoxin (PRX) involves local unfolding. Peroxiredoxin is the enzyme responsible for detoxification of hydrogen peroxide. In a mechanism dissimilar to that used by TRX or GRX, PRX has two Cys residues, one located in each subunit of the dimeric structure, which participate in the reduction of hydrogen peroxide, leading to the formation of disulfide bond in PRX. Reduced PRXII (PDB identifier, 1QMV) is shown in the left panel as a ribbon rendering with one subunit in light gray, the second in dark gray, and Cys residues in black. In the right panel, the oxidized PRXI (PDB identifier, 1QQ2) is rendered similarly. Unfolding of the C-terminus of the dark-gray subunit allows the formation of a disulfide bond between the Cys residues that are located ∼9 Å apart in the reduced form of the protein.
C. Mechanism of redox regulation by APE1
Although a role for Cys 65 in the redox activity of hAPE1 has been established (68, 178), a detailed mechanism has yet to be elucidated. It is possible that another as-yet-unidentified Cys residue in APE1 is involved in the redox activity. In this case, after the formation of a mixed disulfide intermediate between APE1 and a transcription factor, a resolving Cys would serve to restore the thiolates in the transcription factor. As noted earlier, this interaction would likely involve a different conformation of hAPE1 than has been reported in the crystal structures to date (17, 68, 70, 139). Alternatively, a residue other than Cys might be involved in the redox activity, perhaps a Ser residue, as found in some glutaredoxins. A similar mechanism would be proposed in this case, although involvement of a Ser residue would require a significant reduction in its pKa. As the stoichiometry of the relevant redox complex has yet to be established, it is possible that more than one APE1 molecule is involved in the reduction of transcription factors. In this case, a Cys or Ser from a second molecule of APE1 may serve as the resolving thiolate, again, in a mechanism similar to that proposed for thioredoxin. As this problem is of considerable interest, we are actively investigating the mechanism of redox regulation by hAPE1.
V. Transcription Factors Regulated by the Redox Activity of APE1
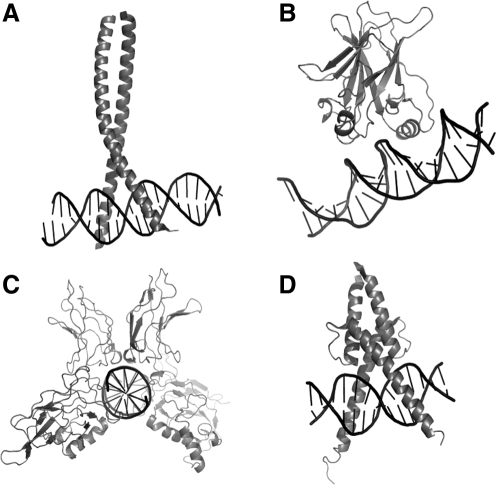
Because APE1 is a multifunctional protein involved in both the repair of DNA damaged by oxidative or alkylating compounds and in the redox regulation of a number of stress-inducible transcription factors, such as AP-1, NF-κB, HIF-1α, and p53, it clearly plays a pivotal role between redox signaling that alters the cellular response to DNA damage and DNA-repair functions. These transcription factors are multidomain proteins that are structurally unrelated, suggesting that no common structural motif is recognized by APE1. Further, the DNA-binding domains of these factors, which are redox regulated, are structurally distinct (Fig. 12); AP-1 is a heterodimeric bZip family protein; p53, a single immunoglobulin-like domain; NF-κB, a dimer of two-domain immunoglobulin-like subunits; and HIF-1α, a basic helix-loop-helix domain. Redox regulation of transcription factors serves as one of several mechanisms that control sequence-specific DNA-binding and thereby gene expression. Of the transcription factors regulated by APE1 that play an important role in the DNA-damage response or DNA-repair pathways including DR, BER, HR, MMR and GGR, p53, AP-1, and HIF-1α are discussed here.
FIG. 12.
Structural comparison of DNA-binding domains from transcription factors that are reported to be redox regulated by APE1/Ref-1 bound to DNA. Proteins are shown in a gray ribbon rendering, and the DNA in a spline/stick representation in black. The DNA-binding domains of the leucine zipper–motif protein AP-1 (c-Jun/c-Fos) bound to DNA (PDB identifier 1FOS) is shown in (A), the immunoglobulin-like fold of p53 (PDB identifier 1TSR) with an α helix bound in the major groove (B), and the heterodimeric two-domain immunoglobulin-like folds of p65/p50 NF-κB (PDB identifier 1VKX) in (C). A representative basic leucine-zipper/basic helix-loop-helix–containing protein (MyoD) bound to DNA (PDB identifier 1MDY) is shown in (D) to illustrate the type of interaction expected for HIF-1α bound to DNA.
A. p53
The tumor-suppressor p53 is a sequence-specific transcription factor that serves as a potent tumor suppressor and functions in part by preserving genomic integrity (113). Not surprisingly, it has been demonstrated that p53 promotes genomic integrity by regulating some of the DNA-repair pathways (80, 162). Regulation of DNA repair by p53 is complex, involving both transactivation-dependent and -independent mechanisms, reviewed by Sengupta and Harris (162), and p53 is itself regulated by both redox-dependent and -independent mechanisms involving Ref-1 (APE1).
Investigation of redox regulation of p53 was initiated based on the finding that oxidized p53 bound DNA very poorly (75) and led to the discovery that Ref-1 was the factor responsible for enhancing the DNA-binding activity of wild-type p53 (101). Although Ref-1 is believed to interact transiently with p53, and this interaction has been detected only by far Western analysis (67) and not by coimmunoprecipitation (101), Ref-1 stimulates DNA-binding activity in vitro and transactivation in vivo by wild-type p53 (67, 101). The mechanism by which Ref-1 enhances the DNA-binding activity of p53 has not yet been fully elucidated but has been proposed to include redox-dependent activation of the DNA-binding domain, potentially through direct reduction of a disulfide bond, as well as a redox-independent interaction with the C-terminal regulatory domain of p53 (101). Most recently, Ref-1 was shown to interact with the tetramerization domain, promoting formation of tetramers from dimers and thereby enhancing sequence-specific DNA-binding activity of p53 through a redox-independent mechanism (77). Redox signaling involving p53 has also been shown to depend on a general redox factor as well as Ref-1. TRX has been shown to enhance stimulation of p53-dependent expression of p21 by Ref-1 suggesting a link between TRX and Ref-1 in cellular response to oxidative stress (175). A summary of p53 regulation of DR, BER, NER, MMR, HR, and NHEJ repair pathways follows.
BER is initiated by highly specialized DNA glycosylases that cleave the DNA base, creating an AP site, as discussed earlier. Regulation of the BER pathway by p53 involves both activation and negative regulation of BER enzymes under different conditions and in different cell lines. In response to γ-ray treatment, p53 was demonstrated to promote activation of AAG. However, this initial step in BER was also shown to be under negative transcriptional regulation by p53 after exposure to nitric oxide (NO) (202). Recent studies demonstrated that p53 downregulated APE1 expression through binding to the promoter region of APE1 that includes an SP1 site. APE1 mRNA and protein levels decreased in a time-dependent manner in the human colorectal cancer line HCT116 p53(+/+), but not in the isogenic p53-null mutant after treatment with camptothecin. Overexpression of wild-type p53 in the p53-null cells significantly reduced both endogenous APE1 and APE1 promoter–dependent luciferase expression in a dose-dependent fashion (196). Thus, Ref-1 (APE1) regulates DNA-binding of p53, and p53 in turn regulates both expression and protein levels of APE1 in this example.
Another BER enzyme, DNA polymerase β (pol β), involved in SP-BER, has been shown to be regulated by p53. Pol β has associated dRP lyase activity that is important and often rate limiting in BER and acts after APE1 activity. Pol β also plays a role in single nucleotide gap filling in LP-BER. In p53-deficient cells, it has been shown that pol β protein expression is altered (35). Additionally, pol β gene expression in CHO cells and HeLa cells can be upregulated by CREB in response to DNA alkylating agent exposure (79). As discussed, CREB is under redox control for its binding to DNA and activating transcription. Therefore, a tight link and interaction occurs in the DNA BER process between a number of transcription factors that are under redox control and the DNA-repair response (Table 1).
Table 1.
Role of APE1 in Regulating Expression of DNA Repair Genes
|
Ape1/Ref-1 Transcription Factor | ||||
|---|---|---|---|---|
| NFkB | AP-1 | CREB | p53 | HIF-1α |
| Direct Repair | Direct Repair | |||
| AGT | AGT | |||
| Homologous Recombination | Homologous Recombination | Homologous Recombination | ||
| ATM | RAD51 | NBS1 | ||
| RAD50 | WRN | |||
| Global Genome Repair | Global Genome Repair | |||
| ERCC1 | XPC | |||
| XPA | DDB2 | |||
| RAD23B | ||||
| ERCC3 | ||||
| Mismatch Repair | Mismatch Repair | Mismatch Repair | ||
| MLH1 | MLH1 | MSH2 | ||
| MSH2 | MSH2 | MSH6 | ||
| PMS2 | PCNA | |||
| MSH6 | PMS2 | |||
| Base Excision Repair | Base Excision Repair | Base Excision Repair | ||
| UNG2 | Polβ | Polβ | ||
| APE1 | ||||
| AAG | ||||
NER, which is divided into TCR and GGR, as discussed earlier, is affected differentially by p53. For example, several studies found that p53 selectively affected GGR, but not TCR. Two main proteins in GGR, DDB2 and XPC, which are involved in DNA-damage recognition, are transcriptionally regulated by p53 (3, 96, 168). Loss of p53 and subsequent deficiencies in the GGR proteins DDB2 and XPC appear to lead to genome instability. This has been effectively demonstrated in knockout mouse studies. In this study, 100% of XPC-/- mice develop lung cancer, and DDB2-/- mice develop skin tumors (89). Again, if p53 is not fully functional through redox modification, it cannot turn on DDB2 and XPC, which would result in defective GGR (Table 1).
MMR, which is in charge of DNA repair after DNA polymerase errors, removes mismatches in DNA. MSH2 in complex with MSH6 or MSH3 is active in the recognition of single-base mismatches and short insertion/deletion mispairs or larger loops of unpaired nucleotides, respectively. MSH2, MLH1, and PMS2 have all been shown to be under p53 regulation, similar to DDB2 and XPC in NER (38, 159). PCNA, another member of the NER pathway, also is under p53 regulation (Table 1) (189).
DSBs threaten severely genomic stability by facilitating deletion or translocation or both of chromosomal DNA. Because either a deficit or an excess in HR may lead to genomic instability, it is not surprising that HR is highly regulated. Once again, p53 plays an important role in the repair of DSBs through the regulation of both DSB repair pathways, HR and NHEJ. Increased levels of HR have been observed in mice that are deficient in wild-type p53 (26, 127). Arias-Lopez et al. (10) demonstrated that p53 inhibits HR through repression of RAD51 expression. Additionally, p53 has been demonstrated to repress the transcription of the RecQ4 helicases, WRN and RecQ4 (163, 191).
Finally, some studies in murine fibroblast cell lines have demonstrated that AGT also is under p53 regulation (72, 155). AGT also appears to be regulated by NF-κB, another transcription factor that is under redox control by APE1. This was demonstrated by overexpression of the p65 subunit of NF-κB in HEK293 cells, resulting in an increase in AGT expression (116).
All of these studies point to the possible relevance of redox controlling DNA-repair responses through p53. Therefore, if reduced p53 is required to bind DNA and either activate or repress the transcription of DNA-repair genes, as discussed earlier, then it follows that Ref-1 (APE1) plays an important role not only in the redox modulation of p53 but also in the regulation of DNA repair.
B. AP-1
Activator protein-1 (AP-1) refers to a family of structurally and functionally related basic leucine zipper proteins (bZIPs) that intermix to form heterodimeric sequence-specific DNA-binding proteins, including primarily Jun proteins, c-Jun, JunB, and JunD, Fos proteins, c-Fos, FosB, Fra-1 and Fra-2, and some ATF-family members, ATFa, ATF-2, and ATF-3 (82). These proteins recognize AP-1 sites that are also referred to as tetradecanoylphorbol-13-acetate (TPA)-responsive elements. AP-1 transcription factors are inducible factors that respond to environmental changes, including stress and radiation, or to growth-factors signals. Proliferation, differentiation, apoptosis, and transformation are some of the processes that are mediated by AP-1 (82).
The first report of redox activity associated with APE1 and its identification as Ref-1 resulted from efforts to identify the nuclear factor responsible for reducing AP-1 (c-Jun/c-Fos) and thereby enhancing its DNA-binding activity (1, 184). Redox regulation of AP-1 was found to result from oxidation/reduction of conserved Cys residues within the basic DNA-binding domains of c-Jun and c-Fos (AP-1) (2). Oxidized AP-1 shows little affinity for DNA as compared with reduced AP-1. c-Fos and c-Jun dimerize through a coiled-coil interaction involving the leucine-zipper motifs and bind DNA as a heterodimer; c-Jun also forms a homodimeric species with lower DNA-binding affinity than the heterodimeric c-Jun/c-Fos, whereas c-Fos does not exhibit any DNA-binding activity on its own (2). The conserved Cys residue within c-Jun is mutated to a Ser residue in the oncogenic v-Jun, which is constitutively active and exhibits enhanced DNA-binding relative to c-Jun (2). Thus, Ref-1 regulates DNA binding of AP-1 in a redox-dependent manner involving direct reduction of the conserved Cys residues within the DNA-binding domains of c-Jun and c-Fos. Ref-1 has been shown to copurify with AP-1 (184) and thus interacts more stably with this transcription factor than with p53. TRX has also been identified as a factor modulating transcriptional activation of AP-1 through its reduction of Ref-1 (83). As summarized later, AP-1, which is rapidly induced in response to a number of cellular stimuli, regulates the expression of several proteins involved primarily in NER and MMR DNA-repair pathways.
In one example of regulation of DNA-repair proteins by AP-1 composed of c-Jun and ATF2, Hayakawa et al. (78) identified 23 DNA-repair or repair-associated genes whose promoters are bound on phosphorylation of ATF2 and c-Jun after cisplatin treatment. These genes were identified by using chromatin immunoprecipitation (ChIP) with antibodies against ATF2 and c-Jun, followed by hybridization to promoter arrays (78). These include ERCC1, ERCC3, XPA, MSH2, MSH6, RAD50, RAD23B, MLH1, PMS2, UNG2, and ATM. Confirmatory studies directly established expression of some genes, including ERCC1, ERCC3, XPA, RAD23B, and MSH2, and some genes that have been specifically implicated in the repair of DNA-cisplatin adducts, such as RAD23B, XPA, ERCC3, XPF-ERCC1, MSH2, and PMS2. DNA adducts induced by cisplatin are repaired mainly by the NER pathway. Several important proteins including XPA, RAD23B, ERCC1, and ERCC3 in NER were observed on the promoter array. The members of the MMR complex, including MSH2, MSH6, MLH1, and PMS2, which are included in a large complex involved in the recognition of DNA-cisplatin adducts, are bound strongly by activated c-Jun or ATF2 or both. AP-1 also was discovered to upregulate MSH2 expression in the myeloid leukemia U937 cell line treated with a phorbol ester (TPA) (95). Undeniably, ERCC1 has been recognized to be regulated by AP-1. Li et al. (119) demonstrated that AP-1 is transcriptionally up-regulated ERCC-1 in response to TPA in human ovarian cancer cells (119).
All of these studies support the role of the involvement of AP-1 in regulating a significant number of DNA-repair proteins that are involved mainly in the NER and MMR pathways (Table 1). Because AP-1 must be converted from an oxidized to a reduced state to bind to its target sequence, redox control of this protein would have significant implications. As for p53, APE1 is implicated as a potential point of control for regulating the DNA-binding activity of AP-1 and thereby modulating the expression of DNA-repair proteins.
C. HIF-1α and hypoxia
Hypoxia-inducible factor −1 (HIF-1) is a heterodimeric transcription factor comprising HIF-1α and HIF-1β (also known as aryl hydrocarbon–receptor nuclear translocator) subunits (93) that plays an important role under hypoxic conditions in the cell. Of the two subunits, HIF-1α has been shown to be crucial for regulating cellular response to hypoxia and is frequently overexpressed in human cancers. Under normal oxygen conditions, HIF-1α is targeted for ubiquitin-mediated proteasomal degradation by von Hippel-Landau protein (pVHL) (97, 98, 132). Under hypoxic conditions, HIF-1α translocates to the nucleus and dimerizes with HIF-1β, forming HIF-1. HIF-1, along with coactivators, binds hypoxia-response elements (HREs) within promoters and regulates the expression of its downstream genes, including vascular endothelial growth factor (VEGF) (62, 102). The DNA-binding activity of HIF-1 has been shown to be regulated by redox signaling, and the redox-dependent stabilization of the HIF-1α protein is required for activation of HIF-1 (93). Overexpression of TRX and Ref-1 enhanced the DNA-binding activity of HIF-1, as detected in a reporter assay. The results, taken together, suggest a role for Ref-1 as a redox regulator of HIF-1α. Ref-1 (APE-1) also has been shown to be required for the binding of transcriptional proteins to the HIF-1 DNA-recognition element within the rat pulmonary artery endothelial cell VEGF gene (198). Although this study did not specifically address the role of redox regulation, the authors found that Ref-1 was required for the formation of the hypoxia-inducible transcriptional complex, including HIF-1 and transcriptional coactivators, p300 and cyclic AMP response element–binding protein (CREB). In a second example of Ref-1 as a coactivator, a transcriptional complex including HIF-1α, signal and transducer of transcription 3 (STAT3), CREB-binding protein (CBP)/p300, and Ref-1 (APE1) was reported to regulate the protein tyrosine kinase Src-dependent expression of VEGF in response to hypoxia in pancreatic and prostate carcinomas (71). More recently, HIF-1α was shown to play a role in downregulating mRNA and protein levels of Ref-1 under hypoxic conditions in human microvascular endothelial cells (125). Thus, HIF-1α regulates expression levels of Ref-1 and is itself regulated by Ref-1.
Increasing evidence reveals that hypoxic stress in the tumor microenvironment can cause genetic instability in cancer cells. Hypoxia induces changes in the expression of several genes involved in DNA-repair pathways. These studies suggest that hypoxia downregulates the expression of key genes within the MMR pathway, including MLH1 and MSH2, and several critical mediators of HR, BRCA1, BRCA2, and RAD51, resulting in significant genetic instability (21, 22, 24, 25, 32, 109, 135, 138) [reviewed in Bindra et al., 2007(23), and Bristow and Hill, 2008 (32)]. The MMR genes appear to be repressed through a mechanism involving c-Myc (25).
In most studies, repression of MMR and HR has been shown to be independent of HIF-1α (21, 22, 24, 25, 32, 135, 138). However, this has been contradicted by Koshiji et al. (109), who demonstrated that HIF-1α is responsible for genetic instability during hypoxia at the nucleotide level by inhibiting MSH2 and MSH6, which recognize DNA base mismatches. These investigators demonstrated that HIF-1α displaces the transcriptional activator Myc from Sp1 binding to repress MSH2-MSH6 in a p53-dependent manner (109). HIF-1α also has been shown to be associated with the loss of MSH2 expression in human sporadic colon cancers (172), linking hypoxia to DNA repair in the induction of these cancers. The decrease of the expression of NBS1, a member of the MRe11–RAD50–NBS1 (MRN) complex that initially recognizes DNA DSBs, has also been shown to be HIF-1α dependent (173). These studies demonstrate that the regulation of DNA repair is an integral part of the hypoxic response and that hypoxia affects DNA repair partially through HIF-1α, a critical mediator in the hypoxic response (Table 1). As Ref-1 has been implicated in the redox regulation of HIF-1α, it then also plays a role in the regulation of DNA-repair genes controlled by HIF-1α.
VI. The Multifunctional APE1 and Redox Control
APE1 is a vital multifunctional protein that acts as an essential master regulator contributing to the maintenance of the genome stability (Fig. 6). Functional activities associated with APE1 include apurinic/apyrimidinic endonuclease activity essential for BER, redox activity, transcriptional regulatory activity (19), and most recently, RNA- cleavage activity (15, 176). In this review, we have limited our discussion to the APE1 BER repair and redox activities, the functions relevant to redox regulation of DNA repair. APE1 also is subject to a number of interesting posttranslational modifications, including acetylation, phosphorylation, and nitrosylation. The implications of these modifications were reviewed recently (19, 171) and are not further discussed here.
APE1 has a pleiotropic role in controlling cellular response to oxidative stress. In addition to its repair function in BER as an AP endonuclease, APE1 controls the redox status of either ubiquitous (i.e., AP-1, Egr-1, NF-κB, p53, CREB, HIF-1α) or tissue-specific transcription factors (i.e., PEBP-2, Pax-5 and −8, and TTF-1) (Fig. 6) (6, 35, 48, 84, 94, 112, 169, 175, 184, 185).
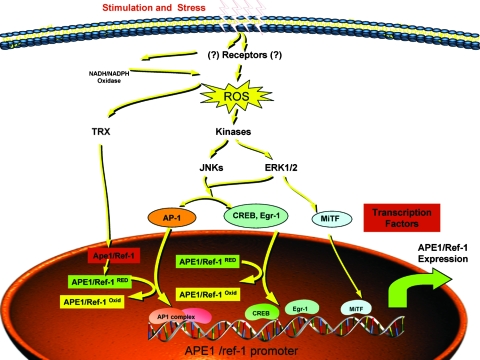
ROS induced by different oxidative or toxic agents have been shown to increase APE1 expression transiently (Fig. 13). A number of transcription factors, including Egr-1, CREB, and Jun/ATF4, are involved in the inducible expression of APE1 (66, 73, 152). In a recent study, the level of APE1 was shown to be increased transcriptionally in response to ROS in melanoma cells by microphthalmia-associated transcription factor (MiTF), a key transcription factor for melanocyte lineage survival that plays an important role in development and carcinogenesis (122). In this study, melanoma cells were classified into MiTF-positive and -negative groups to explore the function of MiTF in regulating cellular responses to ROS. A high level of APE1/Ref-1 was discovered in the MiTF-positive melanoma cell lines. Knocking down MiTF reduced the APE1 protein level and abolished induction of APE1 by ROS. MiTF-negative melanoma cells survived more poorly under ROS stress than did the MiTF-positive cells. Overexpression of APE1 partially rescued ROS-induced cell death when MiTF was depleted. The MiTF regulation of APE1 is direct through E-boxes on the APE1 promoter. It also was found that exposure of HeLa cells to H2O2 and to histone deacetylase inhibitors increases acetylation of APE1 at residues Lys6/Lys7, leading to Egr-1–mediated induction of the tumor-suppressor PTEN gene expression (52). In addition, increasing evidence demonstrates that functional triggering of membrane-bound receptors (such as TSH, CD40L, ATP, IL-2) can lead to APE1 functional activation through intracellular generation of sublethal doses of ROS (170). APE1 also is directly responsible for the control of the intracellular ROS levels through inhibiting the ubiquitous small GTPase Rac1, the regulatory subunit of NADPH oxidase system (9, 149). Recently, Park et al. (151) found that overexpressing APE1/Ref-1 increased inhibition of angiotensin II (Ang II) to the whole-cell conductance Ca2+-activated K+ (BKca) currents in human umbilical vein endothelial cells (HUVECs) through blocking NADPH oxidase–dependent ROS production. The inhibitory effect of Ang II on BKca channel function is NADPH oxidase dependent (151). It also was demonstrated that NADPH-mediated ROS production induced by the P2Y purinergic receptor triggering was able to promote APE1 functional activation (152) and results in the proposition of an autoregulatory loop between these two systems.
FIG. 13.
Overview of ROS signaling. Reactive oxygen species (ROS) are generated through external stimuli or stress, as well as metabolic processes, and act as a signal for JNK and ERK1/2 kinases, resulting in the activation of a number of transcription factors, including Egr-1, CREB, and Jun/ATF4, which are involved in the inducible expression of APE1. In melanoma cells, MiTF also was shown to upregulate transcription of APE1. In its role as a redox factor, APE1 reduces a number of transcription factors, including AP-1, Egr-1, NF-κB, p53, CREB, and HIF-1α. Thus, APE1 controls the redox status of several transcription factors that in turn regulate expression of APE1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
We demonstrated that reducing expression of APE1 in neuronal cultures by using small interfering RNA (siRNA) enhances cisplatin-induced ROS generation, cell killing, and apoptosis (103, 136). Another recent study showed that Ape1 can antagonize the generation of ROS. Overexpression of APE1 inhibits, whereas silencing APE1 expression potentiates ROS accumulation under treatment with oxidative reagents or loading with granzyme K in cytotoxic T lymphocyte (CTL)/natural killer (NK) cells. APE1 is a physiological substrate of granzyme K, and cleavage by granzyme K facilitates intracellular ROS accumulation and enhances granzyme K-induced cell death (74). Merluzzi et al. (136) also found that repression of APE1 by antisense overexpression determines an additional increase in CD40-mediated B-cell proliferation, and the increase is abolished by pretreatment of cells with the antioxidant N-acetyl-l-cysteine (NAC). They proposed that APE1, through control of the intracellular redox state, may also affect the cell cycle by inducing nucleus-cytoplasm redistribution of p21 (136).
A genetic study has demonstrated that APE1 is essential for cell survival and organism development. Knockout of APE1 in mice leads to postimplantation embryonic lethality on days E5 to E9 (188). Conditional knockout and knockdown strategies also confirmed the crucial role of this protein in cells (58, 65, 99). In addition, studies demonstrated that altering APE1 levels leads to a change of cell growth, survival, and sensitivity (28, 37, 56, 58, 81, 115, 134, 147, 179, 180). However, these studies used either overexpression of APE1, APE1 antisense oligonucleotides, or APE1 siRNA to change the total amount of cellular APE1 and thereby all functions of APE1, including its repair and redox activities. Thus, the function of APE1 involved in each case of altered cellular function cannot be identified. Because APE1 plays complex and critical roles in cell survival, proliferation, differentiation, and apoptosis in physiologic and pathologic conditions, as well as in the growth and development of the organism, it is important to distinguish and characterize which function of APE1 is involved in different biologic events, especially those that may differ in normal cells and various pathologic cells, such as cancer cells. The use of specific small-molecule inhibitors blocking either repair or redox, but not both functions of APE1, will give a clearer picture and will be helpful for modifying its function in treatment of the different diseases. Our recent data demonstrated that E3330, 2E-3-[5-(2, 3 dimethoxy-6-methyl-1,4-benzoquinolyl)]-2-nonyl-2-propenoic acid]), a novel quinone derivative, specifically blocks the redox function of APE1 and has no effect on APE1-repair activity and other members of the BER pathway (128). By using E3330, we demonstrated that APE1 is required in normal embryonic hematopoiesis and that the redox function, but not repair activity, of APE1 is critical in normal embryonic hematopoietic development (199).
VII. Modulating APE1 Activities as a Cancer Therapeutic Approach
Elevated APE1 levels have been demonstrated in a variety of cancers and are typically associated with aggressive proliferation, increased resistance to therapeutic agents, and poor prognosis (50, 140, 180, 190). Previous studies demonstrated that decreasing APE1/Ref-1 levels leads to the blockage of cell growth and the increase of cellular sensitivity to DNA-damage agents by using anti-sense oligonucleotides and siRNA of APE1/Ref-1 (56, 58, 115, 147, 180). Not yet known is the relative importance of APE1 redox versus the repair function in cancer. Efforts to determine the effects of inhibiting either the redox or repair function of APE1 are ongoing. Targeting of a specific protein, particularly one that plays an important role in cellular response to stress, by chemical knockout (i.e., through use of a small-molecule inhibitor) may have unintended consequences. However, exploration of novel targets is clearly an avenue that merits pursuit, particularly in the case of cancers for which current treatments are ineffective. There has been considerable debate in the literature regarding the wisdom of inhibiting essential DNA-repair enzymes (14, 42, 57, 106, 129). However, small-molecule inhibitors have been identified for several DNA-repair enzymes, including MGMT, poly ADP-ribose polymerase (PARP1), ataxia–telangiectasia mutated kinase (ATM kinase), APE1, and DNA PKcs (42, 57, 129). Targeting of the redox function of APE1 represents a novel approach in the development of a cancer therapeutic agent. Blocking of the redox function of APE1 would be expected to affect the activity of a number of downstream transcription factors and the gene products that they regulate. In this section, we focus primarily on redox inhibitors, those known specifically to inhibit APE1 and those that may affect APE1 either directly or indirectly. We briefly discuss APE1-repair inhibitors as they have been reviewed recently (106).
A. APE1 redox inhibitors
1. E3330
One molecule previously discussed was demonstrated to bind specifically to APE1 with a binding constant estimated by surface plasmon resonance analysis (SPR) of 1.6 nM, which suggests a specific interaction between APE1 and E3330 (166). We recently demonstrated that E3330 blocks the redox function of APE1 with AP-1 as the downstream target in vitro, as well as after the treatment of ovarian cancer cells with E3330 (128). Additionally, we found that E3330 blocks APE1 redox activity with HIF-1α and other downstream transcription factors (Table 1). This demonstrates that the redox inhibition is not specific for the downstream target. Although E3330 blocked the redox function of APE1, it had no effect on APE1-repair endonuclease activity. We also found that E3330 does have single-agent cancer cell–killing abilities in a variety of cancer cell lines representing ovarian, colon, lung, breast, brain, pancreatic, prostate, and multiple myeloma cancers, but does not have significant cell killing in our studies with normal cells, such as embryonic hematopoiesis cells, retinal vascular endothelial cells (RVECs), and human CD34+ progenitor cells. These data implicate the redox role of APE in cancer, but not in “normal” cell survival. Inhibition of the APE1 redox function significantly attenuates RVEC proliferation and capillary formation in vitro but does not cause cell death. Furthermore, the capillary formation of RVECs appears much more sensitive to redox inhibition of APE1 than to the proliferation. This is the first time that this role of APE1 has been clearly demonstrated. Additionally, our data demonstrate a new role of APE1 in angiogenesis, and inhibition of APE1 redox function by E3330 abrogates this role (128). E3330 also has been shown to inhibit the growth of pancreatic cancer cell lines, an effect that is enhanced under hypoxic conditions (200), as well as pancreatic cancer–associated endothelial and endothelial progenitor cells (201). Consistent with redox regulation by Ref-1, the DNA-binding activity of HIF-1 is inhibited by E3330 in the aforementioned pancreatic cancer studies. Thus, collectively our data and those of others suggest that APE1 redox function will be a promising target of cancer treatment and will open a new avenue for cancer treatment.
2. Other redox inhibitors
A number of natural products reported to affect either directly or indirectly the redox function of APE1 in cells were recently reviewed (57, 107, 128, 133) and are therefore discussed only briefly here. Soy isoflavones, a component in soybeans, are thought to have potential as chemopreventive agents in prostate cancer (137). Treatment of PC-3 prostate cells and xenograft mice with soy isoflavones after radiation treatment resulted in increased cell killing, reduced NF-κB binding to DNA, and reduced APE1 levels. The authors concluded that the soy isoflavones reduced APE1 levels and subsequently resulted in a reduction of the ability of APE1 to reduce NF-κB, resulting in the inability of the cells to respond to the stress (156). However, at this point, the data are merely correlative.
Another natural product, resveratrol, a component of red wine and grapes, was reported to affect the redox activity of APE1 (193). Resveratrol was shown to inhibit both the endonuclease activity of APE1 and the DNA-binding activities of AP-1 in cellular extracts, presumably through inhibition of APE1 redox function. However, this has not been corroborated by others, nor has it been shown to be effective at levels that are physiologically relevant.
B. APE1 repair inhibitors
To date, two different classes of small molecules have been reported to inhibit the AP endonuclease activity of APE1, methoxyamine (18, 124) and negatively charged molecules including CRT0044876 (130), an aryl stibonic acid 13755 (161), and a number of pharmacophore-based compounds (197). Methoxyamine (MX) blocks repair by reacting with apurinic/apyrimidinic sites in the DNA and forming stable adducts that prevent endonucleolytic cleavage by APE1 (18, 64, 124). As MX acts at the level of the DNA, it would also be expected to block the activity of other DNA-repair enzymes such as DNA polymerase β activity. However, none of the aforementioned compounds has been reported to inhibit the redox activity of APE1; this is not discussed further here. A number of recent reviews discuss the status of these types of agents (14, 57, 106).
VIII. Chemoprevention, Redox Modulation, and DNA Repair
As was clearly documented, DNA damage leading to genome instability is a key step for the initiation and progression of cancer (16). Both endogenous and exogenous DNA-damaging agents and particularly those that induce oxidative stress are some of the main factors that cause DNA damage in cells. Therefore, agents that reduce the oxidative stress and subsequent DNA damage, as well as those that increase the repair of DNA damage, are considered to be pertinent in cancer prevention. It is estimated that nearly one third of all cancer deaths in the United States could be prevented. Accumulating research evidence has shown that some dietary antioxidants are able to reduce the incidence of cancer by increasing DNA repair and reducing oxidative stress.
A. Dietary antioxidants
1. Ellagic acid
Ellagic acid, a component in berries (blueberry, strawberry, and red raspberry), was reported to reduce oxidative DNA damage both in vitro and in vivo (4, 5). In vitro, ellagic acid has demonstrated a >95% inhibition of 8-oxodeoxyguosine (8-oxodG) production. It also was shown to reduce other oxidative DNA adducts caused by 4-hydroxy-17β-estradiol and CuCl2. In an in vivo study, female CD-1 mice were fed pure ellagic acid, and formation of DNA adducts was related to ellagic acid in a dose-dependent manner. Further study found both ellagic acid and its natural source resulted in overexpression of genes involved in DNA-repair pathways such as XPA, ERCC5, and DNA ligase III, mainly those involved in NER. These results demonstrated that ellagic acid is effective in preventing oxidative DNA damage both in vitro and in vivo by increasing DNA repair.
2. Selenium
Selenium is found in plentiful amounts in dairy, eggs, fish, meat, grains, and Brazil nuts. Selenium, in the form of selenocysteine, is a major constituent of many antioxidant enzymes known as selenoproteins. Not surprisingly, Se was reported to be preventive for cancer initiation from oxidative DNA damage through reducing oxidative stress and increasing DNA repair. The active species of Se include hydrogen selenide (H2Se) and its methylated metabolite, methylselenol (MeSeH), selenomethionine (SeMet), and selenoproteins. Se, in the form of selenomethionine, was reported to promote BER activity by p53 activation in normal human fibroblasts in vitro (164). This study demonstrated that Se-induced p53 activation promotes BER activity by reducing specific cysteine residues in p53. A dominant-negative APE1 redox mutant blocks reductive activation of p53 by Se. Se also was shown to stimulate the activity of a selenoprotein, thioredoxin reductase (TR) (165). These data suggest that Se reduces p53 through interactions involving TR, which reduces TRX and APE1, as well as redox interactions between APE1 and p53. Se-induced activation of p53 is also dependent on the BRCA1 protein in recombinational repair, which is frequently mutated in familial breast cancer (55). It also was reported that Se inhibited DNA-binding activity of transcription factors such as AP-1, NF-κB, SP-1, and SP-3, as well as the DNA-repair proteins XPA in the NER pathway and formamidopyrimidine DNA glycosylase (FPG) in the BER pathway (27, 76, 131, 195). These data imply that Se may reduce cancer incidence through modulating DNA repair, redox status of cells, and the cellular transcriptional response to oxidative stress. It also suggests that the redox function of APE1 is a major component of this interaction. Additional clinical studies have found an inverse relation between the levels of Se and the prevalence of several types of cancer.
3. Oltipraz
Oltipraz, a synthetic dithiolethione, is similar to the thiolethione, an antioxidant component in cruciferous vegetables. Oltipraz has been shown to inhibit the development and progression of multiple organ tumors, including breast, bladder, colon, stomach, liver, lymph nodes, lung, pancreas, and skin, induced by a variety of structurally diverse carcinogens in preclinical studies (41). Clinical studies demonstrated that oltipraz has minimal toxicity in humans and significant chemopreventive activity (41). Oltipraz increased NER activity by decreasing platinum-DNA adducts, but not BER activity, as measured by determining the levels of AP sites in HT29 colon adenocarcinoma cells (146). Further study found that oltipraz also increases APE1 protein level and AP-1 DNA-binding, which is partially dependent on APE1 in HT29 cells (194). Oltipraz also was shown to inhibit microvessel formation in both human and rodent bioassays in a dose-dependent manner (158). These data suggest that the redox, not the repair, function of APE1 may be involved in chemopreventive effect of oltipraz.
All of these compounds are natural agents that have been implicated in protecting against cancer.
B. Direct regulation of DNA repair by altered redox status of the cell
The evolutionarily conserved enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a key redox-sensitive protein with an active-site cysteine sulfhydryl. GAPDH was shown to interact directly with APE1 (13). By using recombinant proteins, Azam et al. (13) found that GAPDH interacts with APE1 through the active-site cysteine 152 to convert the “oxidized” form of APE1 to its reduced form and reestablishes the ability to cleave AP sites. This reduction process also enhanced the detection of APE1 by anti-APE1 antibodies, suggesting a structural change. siRNA knockdown of GAPDH in HCT116 cells enhanced sensitivity to the alkylating DNA-damaging agent methyl methane sulfonate (MMS), which produces AP site, and increased the level of spontaneous AP sites in the genomic DNA. These data imply that GAPDH plays an important role in promoting BER activity by maintaining APE1, a key AP endonuclease, in an active reduced state.
OGG1, an 8-oxoG DNA glycosylase in the BER pathway, is responsible for recognizing and repairing 8-oxoguanine (8-oxoG), a common and mutagenic form of oxidized guanine in DNA. A recent study with human lymphoblastoid cells treated with cadmium resulted in an almost complete reduction in the 8-oxoG DNA glycosylase activity of OGG1, presumably because of an alteration in the redox status of the cells. OGG1 activity returned to normal once the redox cellular status was returned to normal. The reversible inactivation of OGG1 activity by cadmium was strictly associated with reversible oxidation of the protein, as demonstrated by the use of cysteine-modifying agents, such as diamide, with the pure protein and crude extracts (30). A frequently found polymorphism of OGG1, S326C OGG1, associated with cancer development, has been shown to have lower 8-oxoG DNA glycosylase activity, and the lower activity of OGG1-Cys326 is associated with the easy oxidation of Cys326 to form a disulfide bond (31). In this study, the 8-oxoG repair activity was analyzed in the cells and cell extracts of lymphoblastoid cell lines established from individuals carrying either Ser/Ser or Cys/Cys genotypes. The cells homozygous for the Cys variant display increased genetic instability, reduced 8-oxoG repair rates, and almost twofold lower basal 8-oxoG DNA glycosylase activity in their cell extracts. Reducing agents increase the repair capacity to the level of the Ser variant, but do not affect the activity of the latter. The 8-oxoG DNA glycosylase activity in cells carrying the Cys/Cys alleles is more sensitive to oxidizing agents (31).
NER, which repairs mainly bulky DNA adducts and helix-distorting lesions, has some crossover activity on oxidative DNA damage. On exposure of human pulmonary epithelial cells (A549) to nontoxic doses of H2O2, increased expression of XPA, XPC, ERCC4, and ERCC5 was observed, whereas ERCC1 expression decreased (114). Functional studies also demonstrated a decrease in NER activity.
Glutathione (GSH) also is directly implicated in the regulation of NER. GSH-depletion in cells preincubated with BSO, l-buthionine-sulfoximine, completely abolished the downregulation of ERCC1 expression and the decrease in NER capacity by H2O2 and increased significantly the upregulation of ERCC4 expression. These data suggest that NER capacity as well as the expression of NER-related genes can be modulated by oxidative stress (114).
IX. Concluding Remarks
In conclusion, redox regulation clearly plays an important role in DNA repair, with implications for human health and cancer therapeutic development. We have highlighted the role of APE1, an important DNA-repair protein and redox factor, in this review as a protein directly linking redox regulation and DNA repair. It is our expectation that future research in this area will bring additional insights into the importance of redox regulation in DNA repair and will likely result in the identification of other proteins that also play important roles in redox regulation and DNA repair.
Abbreviations Used
- 8-oxoG
8-oxoguanine
- AAG
alkyladenine DNA glycosylase
- AGT
O-6-alkylguanine–DNA methyltransferase
- Ang II
angiotensin II
- AP
apurinic/apyrimidinic
- AP-1
activator protein 1
- APE1
apurinic/apyrimidinic endonuclease 1
- ATF4
activating transcription factor 4
- ATM
ataxia-telangiectasia–mutated protein
- BER
base-excision repair
- BKca
large-conductance Ca2+-activated K+ channels
- BLM
Bloom syndrome gene product
- BRCA1
breast cancer 1, early onset
- BRCA2
breast cancer 2, early onset
- CD40L
CD40 ligand
- CREB
cyclic AMP response element–binding protein
- CSA
Cockayne syndrome protein A
- CSB
Cockayne syndrome protein B
- CTL
cytotoxic T lymphocyte
- DDB1
DNA damage binding protein 1
- DDB2
DNA damage binding protein 2
- DNA-PKcs
DNA-dependent kinase catalytic subunit
- DR
direct repair
- dRP
deoxyribophosphate
- dRPase
deoxyribophosphodiesterase
- DSBs
double-strand breaks
- E3330
2E-3-[5-(2, 3 dimethoxy-6-methyl-1,4-benzoquinolyl)]-2-nonyl-2-propenoic acid
- ERCC1
excision-repair cross-complementation group 1
- ERCC3
excision-repair cross-complementation group 3
- ERCC5
excision-repair cross-complementation group 5
- FEN1
flap endonuclease 1
- FPG
formamidopyrimidine DNA glycosylase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GGR
global genome repair
- GRX
glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide
- HIF-1α
hypoxia-inducible factor-1
- HR
homologous recombination
- HR32B
human homologue of the yeast RAD23 protein
- HUVECs
human umbilical vein endothelial cells
- IL-2
interleukin 2
- LP-BER
long-patch base-excision repair
- MeSeH
methylselenol
- MGMT
O6-methylguanine-DNA methyltransferase
- MiTF
microphthalmia-associated transcription factor
- MLH1
MutL homologue 1
- MMR
mismatch repair
- MMS
methyl methane sulfonate
- MPG
N-methylpurine DNA glycosylase
- MRN
Mre11/Rad50/Nbs1
- MSH2
MutS homologue 2
- MSH3
MutS homologue 3
- MSH6
MutS homologue 6
- MYH
MutY homologue
- NAC
N-acetyl-l-cysteine
- NEIL
fgp/nei family DNA glycosylase
- NER
nucleotide-excision repair
- NF-κB
nuclear factor kappa B
- NHEJ
nonhomologous end joining
- NK
natural killer
- NTH
homologue of Escherichia coli endonuclease III (nth)
- O6-meG
O6 methyl guanine
- P2Y
purinergic receptor
- PCNA
proliferating cell nuclear antigen
- PMS1
postmeiotic-segregation increased-1 protein
- PMS2
postmeiotic-segregation increased-2 protein
- Pol β
DNA polymerase β
- PRX
peroxiredoxin
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- Ref-1
redox effector factor 1
- RFC
replication factor C
- RNA Pol II
RNA polymerase II
- ROS
reactive oxygen species
- RPA
replication protein A
- SeMet
selenomethionine
- siRNA
small interfering RNA
- SMUG1
mammalian 5-formyluracil DNA glycosylase
- SP-BER
short-patch base-excision repair
- ssDNA
single-strand DNA
- TCR
transcription-coupled repair
- TDG
thymine-DNA glycosylase
- TFIIH
transcription factor IIH
- TR
thioredoxin reductase
- TRX
thioredoxin
- TSH
thyroid-stimulating hormone
- UNG
uracil-DNA glycosylase
- WRN
Werner protein, deficient in Werner syndrome
- XPB
xeroderma pigmentosum complementary group B protein
- XPC
xeroderma pigmentosum complementary group C protein
- XPD
xeroderma pigmentosum complementary group D protein
- XRCC1
x-ray repair complementing defective repair in Chinese hamster cells 1
- XRCC4
x-ray repair complementing defective repair in Chinese hamster cells 4
Footnotes
Reviewing Editors: Margherita Bignami, Diego Bonatto, Dindial Ramotar, Young R. Seo, and Silvia Tornaletti
Acknowledgments
Financial support for this work was provided by the National Institutes of Health, National Cancer Institute CA114571 to M.M.G., CA94025, CA106298, CA114571 and CA121168 to M.R.K., IU Simon Cancer Center Translational initiative pilot funding (ITRAC) to M.M.G. and M.R.K., and the Riley Children's Foundation (M.R.K.).
References
- 1.Abate C. Luk D. Gentz R. Rauscher FJ ., 3rd Curran T. Expression and purification of the leucine zipper and DNA-binding domains of Fos and Jun: both Fos and Jun contact DNA directly. Proc Natl Acad Sci U S A. 1990;87:1032–1036. doi: 10.1073/pnas.87.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 3.Adimoolam S. Ford JM. p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc Natl Acad Sci U S A. 2002;99:12985–12990. doi: 10.1073/pnas.202485699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aiyer HS. Kichambare S. Gupta RC. Prevention of oxidative DNA damage by bioactive berry components. Nutr Cancer. 2008;60(suppl 1):36–42. doi: 10.1080/01635580802398448. [DOI] [PubMed] [Google Scholar]
- 5.Aiyer HS. Srinivasan C. Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60:227–234. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 6.Akamatsu Y. Ohno T. Hirota K. Kagoshima H. Yodoi J. Shigesada K. Redox regulation of the DNA binding activity in transcription factor PEBP2: the roles of two conserved cysteine residues. J Biol Chem. 1997;272:14497–14500. doi: 10.1074/jbc.272.23.14497. [DOI] [PubMed] [Google Scholar]
- 7.Akterin S. Cowburn RF. Miranda-Vizuete A. Jimenez A. Bogdanovic N. Winblad B. Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 8.Altieri F. Grillo C. Maceroni M. Chichiarelli S. DNA damage and repair: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:891–937. doi: 10.1089/ars.2007.1830. [DOI] [PubMed] [Google Scholar]
- 9.Angkeow P. Deshpande SS. Qi B. Liu YX. Park YC. Jeon BH. Ozaki M. Irani K. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–725. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- 10.Arias-Lopez C. Lazaro-Trueba I. Kerr P. Lord CJ. Dexter T. Iravani M. Ashworth A. Silva A. p53 modulates homologous recombination by transcriptional regulation of the RAD51 gene. EMBO Rep. 2006;7:219–224. doi: 10.1038/sj.embor.7400587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arner ES. Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 12.Arner ES. Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Azam S. Jouvet N. Jilani A. Vongsamphanh R. Yang X. Yang S. Ramotar D. Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J Biol Chem. 2008;283:30632–30641. doi: 10.1074/jbc.M801401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bapat A. Fishel ML. Kelley MR. Going ape as an approach to cancer therapeutics. Antioxid Redox Signal. 2009;11:651–669. doi: 10.1089/ars.2008.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes T. Kim WC. Mantha AK. Kim SE. Izumi T. Mitra S. Lee CH. Identification of apurinic/apyrimidinic endonuclease 1 (APE1) as the endoribonuclease that cleaves c-myc mRNA. Nucleic Acids Res. 2009;37:3946–3958. doi: 10.1093/nar/gkp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckman RA. Loeb LA. Efficiency of carcinogenesis with and without a mutator mutation. Proc Natl Acad Sci U S A. 2006;103:14140–14145. doi: 10.1073/pnas.0606271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beernink PT. Segelke BW. Hadi MZ. Erzberger JP. Wilson DMI. Rupp B. Two divalent metal ions in the active site of a new crystal form of human apurinic/apyrimidinic endonuclease, Ape1: implication for the catalytic mechanism. J Mol Biol. 2001;307:1023–1034. doi: 10.1006/jmbi.2001.4529. [DOI] [PubMed] [Google Scholar]
- 18.Bennett SE. Kitner J. Characterization of the aldehyde reactive probe reaction with AP-sites in DNA: influence of AP-lyase on adduct stability. Nucleosides Nucleotides Nucleic Acids. 2006;25:823–842. doi: 10.1080/15257770600726133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhakat KK. Mantha AK. Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid Redox Signal. 2009;11:621–638. doi: 10.1089/ars.2008.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biaglow JE. Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther. 2005;4:6–13. doi: 10.4161/cbt.4.1.1434. [DOI] [PubMed] [Google Scholar]
- 21.Bindra RS. Schaffer PJ. Meng A. Woo J. Maseide K. Roth ME. Lizardi P. Hedley DW. Bristow RG. Glazer PM. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindra RS. Schaffer PJ. Meng A. Woo J. Maseide K. Roth ME. Lizardi P. Hedley DW. Bristow RG. Glazer PM. Alterations in DNA repair gene expression under hypoxia: elucidating the mechanisms of hypoxia-induced genetic instability. Ann N Y Acad Sci. 2005;1059:184–195. doi: 10.1196/annals.1339.049. [DOI] [PubMed] [Google Scholar]
- 23.Bindra RS. Crosby ME. Glazer PM. Regulation of DNA repair in hypoxic cancer cells. Cancer Metastasis Rev. 2007;26:249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 24.Bindra RS. Glazer PM. Repression of RAD51 gene expression by E2F4/p130 complexes in hypoxia. Oncogene. 2007;26:2048–2057. doi: 10.1038/sj.onc.1210001. [DOI] [PubMed] [Google Scholar]
- 25.Bindra RS. Glazer PM. Co-repression of mismatch repair gene expression by hypoxia in cancer cells: role of the Myc/Max network. Cancer Lett. 2007;252:93–103. doi: 10.1016/j.canlet.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Bishop AJ. Hollander MC. Kosaras B. Sidman RL. Fornace AJ., Jr Schiestl RH. Atm-, p53-, and Gadd45a-deficient mice show an increased frequency of homologous recombination at different stages during development. Cancer Res. 2003;63:5335–5343. [PubMed] [Google Scholar]
- 27.Blessing H. Kraus S. Heindl P. Bal W. Hartwig A. Interaction of selenium compounds with zinc finger proteins involved in DNA repair. Eur J Biochem. 2004;271:3190–3299. doi: 10.1111/j.1432-1033.2004.04251.x. [DOI] [PubMed] [Google Scholar]
- 28.Bobola MS. Finn LS. Ellenbogen RG. Geyer JR. Berger MS. Braga JM. Meade EH. Gross ME. Silber JR. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 2005;11:7405–7414. doi: 10.1158/1078-0432.CCR-05-1068. [DOI] [PubMed] [Google Scholar]
- 29.Bozonet SM. Findlay VJ. Day AM. Cameron J. Veal EA. Morgan BA. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J Biol Chem. 2005;280:23319–23327. doi: 10.1074/jbc.M502757200. [DOI] [PubMed] [Google Scholar]
- 30.Bravard A. Vacher M. Gouget B. Coutant A. de Boisferon FH. Marsin S. Chevillard S. Radicella JP. Redox regulation of human OGG1 activity in response to cellular oxidative stress. Mol Cell Biol. 2006;26:7430–7436. doi: 10.1128/MCB.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravard A. Vacher M. Moritz E. Vaslin L. Hall J. Epe B. Radicella JP. Oxidation status of human OGG1-S326C polymorphic variant determines cellular DNA repair capacity. Cancer Res. 2009;69:3642–3649. doi: 10.1158/0008-5472.CAN-08-3943. [DOI] [PubMed] [Google Scholar]
- 32.Bristow RG. Hill RP. Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 33.Burke-Gaffney A. Callister ME. Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26:398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Burma S. Chen BP. Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5:1042–1048. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Cao X. Kambe F. Ohmori S. Seo H. Oxidoreductive modification of two cysteine residues in paired domain by Ref-1 regulates DNA-binding activity of Pax-8. Biochem Biophys Res Commun. 2002;297:288–293. doi: 10.1016/s0006-291x(02)02196-4. [DOI] [PubMed] [Google Scholar]
- 36.Chen DS. Herman T. Demple B. Two distinct human DNA diesterases that hydrolyze 3'-blocking deoxyribose fragments from oxidized DNA. Nucleic Acids Res. 1991;19:5907–5914. doi: 10.1093/nar/19.21.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen DS. Olkowski ZL. Biological responses of human apurinic endonuclease to radiation-induced DNA damage. Ann N Y Acad Sci. 1994;726:306–308. doi: 10.1111/j.1749-6632.1994.tb52834.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen J. Sadowski I. Identification of the mismatch repair genes PMS2 and MLH1 as p53 target genes by using serial analysis of binding elements. Proc Natl Acad Sci U S A. 2005;102:4813–4818. doi: 10.1073/pnas.0407069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chew EH. Lu J. Bradshaw TD. Holmgren A. Thioredoxin reductase inhibition by antitumor quinols: a quinol pharmacophore effect correlating to antiproliferative activity. FASEB J. 2008;22:2072–2083. doi: 10.1096/fj.07-101477. [DOI] [PubMed] [Google Scholar]
- 40.Christmann M. Tomicic MT. Roos WP. Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. doi: 10.1016/s0300-483x(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 41.Clapper ML. Chemopreventive activity of oltipraz. Pharmacol Ther. 1998;78:17–27. doi: 10.1016/s0163-7258(97)00164-2. [DOI] [PubMed] [Google Scholar]
- 42.Damia G. D'Incalci M. Targeting DNA repair as a promising approach in cancer therapy. Eur J Cancer. 2007;43:1791–1801. doi: 10.1016/j.ejca.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 43.David SS. Williams SD. Chemistry of glycosylases and endonucleases involved in base-excision repair. Chem Rev. 1998;98:1221–1262. doi: 10.1021/cr980321h. [DOI] [PubMed] [Google Scholar]
- 44.De Bont R. van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 45.Demple B. Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 46.Doetsch PW. Cunningham RP. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990;236:173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- 47.Eker AP. Quayle C. Chaves I. van der Horst GT. DNA repair in mammalian cells: direct DNA damage reversal: elegant solutions for nasty problems. Cell Mol Life Sci. 2009;66:968–980. doi: 10.1007/s00018-009-8735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ema M. Hirota K. Mimura J. Abe H. Yodoi J. Sogawa K. Poellinger L. Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Essers J. van Steeg H. de Wit J. Swagemakers SM. Vermeij M. Hoeijmakers JH. Kanaar R. Homologous and non-homologous recombination differentially affect DNA damage repair in mice. EMBO J. 2000;19:1703–1710. doi: 10.1093/emboj/19.7.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans AR. Limp-Foster M. Kelley MR. Going APE over Ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 51.Fan J. Wilson DM., 3rd Protein-protein interactions and posttranslational modifications in mammalian base excision repair. Free Radic Biol Med. 2005;38:1121–1138. doi: 10.1016/j.freeradbiomed.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Fantini D. Vascotto C. Deganuto M. Bivi N. Gustincich S. Marcon G. Quadrifoglio F. Damante G. Bhakat KK. Mitra S. Tell G. APE1/Ref-1 regulates PTEN expression mediated by Egr-1. Free Radic Res. 2008;42:20–29. doi: 10.1080/10715760701765616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farmer H. McCabe N. Lord CJ. Tutt AN. Johnson DA. Richardson TB. Santarosa M. Dillon KJ. Hickson I. Knights C. Martin NM. Jackson SP. Smith GC. Ashwort A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes AP. Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 55.Fischer JL. Lancia JK. Mathur A. Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006;26:899–904. [PubMed] [Google Scholar]
- 56.Fishel ML. He Y. Smith ML. Kelley MR. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin Cancer Res. 2007;13:260–267. doi: 10.1158/1078-0432.CCR-06-1920. [DOI] [PubMed] [Google Scholar]
- 57.Fishel ML. Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28:375–395. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Fishel ML. He Y. Reed AM. Chin-Sinex H. Hutchins GD. Mendonca MS. Kelley MR. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst) 2008;7:177–186. doi: 10.1016/j.dnarep.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fishel R. Wilson T. MutS homologs in mammalian cells. Curr Opin Genet Dev. 1997;7:105–113. doi: 10.1016/s0959-437x(97)80117-7. [DOI] [PubMed] [Google Scholar]
- 60.Fleck O. Nielsen O. DNA repair. J Cell Sci. 2004;117:515–517. doi: 10.1242/jcs.00952. [DOI] [PubMed] [Google Scholar]
- 61.Forman-Kay JD. Clore GM. Gronenborn AM. Relationship between electrostatics and redox function in human thioredoxin: characterization of pH titration shifts using two-dimensional homo- and heteronuclear NMR. Biochemistry. 1992;31:3442–3452. doi: 10.1021/bi00128a019. [DOI] [PubMed] [Google Scholar]
- 62.Forsythe JA. Jiang BH. Iyer NV. Agani F. Leung SW. Koos RD. Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer. 2001;1:22–33. doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- 64.Frosina G. Fortini P. Rossi O. Carrozzino F. Abbondandolo A. Dogliotti E. Repair of abasic sites by mammalian cell extracts. Biochem J. 1994;304:699–705. doi: 10.1042/bj3040699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fung H. Demple B. A vital role for Ape1/Ref1 protein in repairing spontaneous DNA damage in human cells. Mol Cell. 2005;17:463–470. doi: 10.1016/j.molcel.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 66.Fung H. Liu P. Demple B. ATF4-dependent oxidative induction of the DNA repair enzyme Ape1 counteracts arsenite cytotoxicity and suppresses arsenite-mediated mutagenesis. Mol Cell Biol. 2007;27:8834–8847. doi: 10.1128/MCB.00974-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaiddon C. Moorthy NC. Prives C. Ref-1 regulates the transactivation and pro-apoptotic functions of p53 in vivo. EMBO J. 1999;18:5609–5621. doi: 10.1093/emboj/18.20.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Georgiadis M. Luo M. Gaur R. Delaplane S. Li X. Kelley M. Evolution of the redox function in mammalian apurinic/apyrimidinic endonuclease. Mutat Res. 2008;643:54–63. doi: 10.1016/j.mrfmmm.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giles NM. Giles GI. Jacob C. Multiple roles of cysteine in biocatalysis. Biochem Biophys Res Commun. 2003;300:1–4. doi: 10.1016/s0006-291x(02)02770-5. [DOI] [PubMed] [Google Scholar]
- 70.Gorman MA. Morera S. Rothwell DG. La Fortelle E. Mol CD. Tainer JA. Hickson ID. Freemont PS. The crystal structure of the human DNA repair endonuclease HAP1 suggests the recognition of extra-helical deoxyribose at DNA abasic sites. EMBO J. 1997;16:6548–6558. doi: 10.1093/emboj/16.21.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray MJ. Zhang J. Ellis LM. Semenza GL. Evans DB. Watowich SS. Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 72.Grombacher T. Eichhorn U. Kaina B. p53 is involved in regulation of the DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) by DNA damaging agents. Oncogene. 1998;17:845–851. doi: 10.1038/sj.onc.1202000. [DOI] [PubMed] [Google Scholar]
- 73.Grosch S. Kaina B. Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress requires CREB. Biochem Biophys Res Commun. 1999;261:859–863. doi: 10.1006/bbrc.1999.1125. [DOI] [PubMed] [Google Scholar]
- 74.Guo Y. Chen J. Zhao T. Fan Z. Granzyme K degrades the redox/DNA repair enzyme Ape1 to trigger oxidative stress of target cells leading to cytotoxicity. Mol Immunol. 2008;45:2225–2235. doi: 10.1016/j.molimm.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Hainaut P. Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993;53:469–44. [PubMed] [Google Scholar]
- 76.Handel ML. Watts CK. deFazio A. Day RO. Herland RL. Inhibition of AP-1 binding and transcription by gold and selenium involving conserved cysteine residues in Jun and Fos. Proc Natl Acad Sci U S A. 1995;92(10):4497–4491. doi: 10.1073/pnas.92.10.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanson S. Kim E. Deppert W. Redox factor 1 (Ref-1) enhances specific DNA binding of p53 by promoting p53 tetramerization. Oncogene. 2005;24:1641–1647. doi: 10.1038/sj.onc.1208351. [DOI] [PubMed] [Google Scholar]
- 78.Hayakawa J. Mittal S. Wang Y. Korkmaz KS. Adamson E. English C. Ohmichi M. McClelland M. Mercola D. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol Cell. 2004;16:521–535. doi: 10.1016/j.molcel.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 79.He F. Yang XP. Srivastava DK. Wilson SH. DNA polymerase beta gene expression: the promoter activator CREB-1 is upregulated in Chinese hamster ovary cells by DNA alkylating agent-induced stress. Biol Chem. 2003;384:19–23. doi: 10.1515/BC.2003.003. [DOI] [PubMed] [Google Scholar]
- 80.Helton ES. Chen X. p53 modulation of the DNA damage response. J Cell Biochem. 2007;100:883–8896. doi: 10.1002/jcb.21091. [DOI] [PubMed] [Google Scholar]
- 81.Herring CJ. West CM. Wilks DP. Davidson SE. Hunter RD. Berry P. Forster G. MacKinnon J. Rafferty JA. Elder RH. Hendry JH. Margison GP. Levels of the DNA repair enzyme human apurinic/apyrimidinic endonuclease (APE1, APEX, Ref-1) are associated with the intrinsic radiosensitivity of cervical cancers. Br J Cancer. 1998;78:1128–1133. doi: 10.1038/bjc.1998.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hess J. Angel P. Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 83.Hirota K. Matsui M. Iwata S. Nishiyama A. Mori K. Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci U S A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirota K. Murata M. Sachi Y. Nakamura H. Takeuchi J. Mori K. Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 85.Hoeijmakers JH. DNA repair mechanisms. Maturitas. 2001;38:17–22. doi: 10.1016/s0378-5122(00)00188-2. discussion 22–23. [DOI] [PubMed] [Google Scholar]
- 86.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 87.Hofmann B. Hecht HJ. Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 88.Hol WG. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45:149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- 89.Hollander MC. Philburn RT. Patterson AD. Velasco-Miguel S. Friedberg EC. Linnoila RI. Fornace AJ., Jr Deletion of XPC leads to lung tumors in mice and is associated with early events in human lung carcinogenesis. Proc Natl Acad Sci U S A. 2005;102:13200–12305. doi: 10.1073/pnas.0503133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 91.Holmgren A. Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure. 1995;3:239–243. doi: 10.1016/s0969-2126(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 92.Hromas R. Wray J. Lee SH. Martinez L. Farrington J. Corwin LK. Ramsey H. Nickoloff JA. Williamson EA. The human set and transposase domain protein Metnase interacts with DNA Ligase IV and enhances the efficiency and accuracy of non-homologous end-joining. DNA Repair (Amst) 2008;7:1927–1937. doi: 10.1016/j.dnarep.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang LE. Arany Z. Livingston DM. Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 94.Huang RP. Adamson ED. Characterization of the DNA-binding properties of the early growth response-1 (Egr-1) transcription factor: evidence for modulation by a redox mechanism. DNA Cell Biol. 1993;12:265–273. doi: 10.1089/dna.1993.12.265. [DOI] [PubMed] [Google Scholar]
- 95.Humbert O. Achour I. Lautier D. Laurent G. Salles B. hMSH2 expression is driven by AP1-dependent regulation through phorbol-ester exposure. Nucleic Acids Res. 2003;31:5627–5634. doi: 10.1093/nar/gkg781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hwang BJ. Ford JM. Hanawalt PC. Chu G. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci U S A. 1999;96:424–428. doi: 10.1073/pnas.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iliopoulos O. Levy AP. Jiang C. Kaelin WG., Jr Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ivan M. Kondo K. Yang H. Kim W. Valiando J. Ohh M. Salic A. Asara JM. Lane WS. Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 99.Izumi T. Brown DB. Naidu CV. Bhakat KK. Macinnes MA. Saito H. Chen DJ. Mitra S. Two essential but distinct functions of the mammalian abasic endonuclease. Proc Natl Acad Sci U S A. 2005;102:5739–5743. doi: 10.1073/pnas.0500986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacob C. Giles GI. Giles NM. Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed Engl. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 101.Jayaraman L. Murthy KG. Zhu C. Curran T. Xanthoudakis S. Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 102.Jiang BH. Rue E. Wang GL. Roe R. Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Y. Guo C. Vasko MR. Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008;68:6425–6434. doi: 10.1158/0008-5472.CAN-08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 105.Kaina B. Christmann M. Naumann S. Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Kelley MR. Fishel ML. DNA repair proteins as molecular targets for cancer therapeutics. Anticancer Agents Med Chem. 2008;8:417–425. doi: 10.2174/187152008784220294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khan N. Afaq F. Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 108.Kondo N. Nakamura H. Masutani H. Yodoi J. Redox regulation of human thioredoxin network. Antioxid Redox Signal. 2006;8:1881–1890. doi: 10.1089/ars.2006.8.1881. [DOI] [PubMed] [Google Scholar]
- 109.Koshiji M. To KK. Hammer S. Kumamoto K. Harris AL. Modrich P. Huang LE. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 110.Kruman II. Wersto RP. Cardozo-Pelaez F. Smilenov L. Chan SL. Chrest FJ. Emokpae R., Jr Gorospe M. Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 111.Kunz C. Saito Y. Schar P. DNA Repair in mammalian cells: mismatched repair: variations on a theme. Cell Mol Life Sci. 2009;66:1021–1038. doi: 10.1007/s00018-009-8739-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lando D. Pongratz I. Poellinger L. Whitelaw ML. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1alpha and the HIF-like factor. J Biol Chem. 2000;275:4618–4627. doi: 10.1074/jbc.275.7.4618. [DOI] [PubMed] [Google Scholar]
- 113.Lane DP. Cancer: p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 114.Langie SA. Knaapen AM. Houben JM. van Kempen FC. de Hoon JP. Gottschalk RW. Godschalk RW. van Schooten FJ. The role of glutathione in the regulation of nucleotide excision repair during oxidative stress. Toxicol Lett. 2007;168:302–309. doi: 10.1016/j.toxlet.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 115.Lau JP. Weatherdon KL. Skalski V. Hedley DW. Effects of gemcitabine on APE/ref-1 endonuclease activity in pancreatic cancer cells, and the therapeutic potential of antisense oligonucleotides. Br J Cancer. 2004;91:1166–1173. doi: 10.1038/sj.bjc.6602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavon I. Fuchs D. Zrihan D. Efroni G. Zelikovitch B. Fellig Y. Siegal T. Novel mechanism whereby nuclear factor kappaB mediates DNA damage repair through regulation of O(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007;67:8952–8959. doi: 10.1158/0008-5472.CAN-06-3820. [DOI] [PubMed] [Google Scholar]
- 117.Lee SH. Oshige M. Durant ST. Rasila KK. Williamson EA. Ramsey H. Kwan L. Nickoloff JA. Hromas R. The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc Natl Acad Sci U S A. 2005;102:18075–18080. doi: 10.1073/pnas.0503676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 119.Li Q. Zhang L. Tsang B. Gardner K. Bostick-Bruton F. Reed E. Phorbol ester exposure activates an AP-1-mediated increase in ERCC-1 messenger RNA expression in human ovarian tumor cells. Cell Mol Life Sci. 1999;55:456–466. doi: 10.1007/s000180050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lillig CH. Holmgren A. Thioredoxin and related molecules: from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 121.Lillig CH. Berndt C. Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 122.Liu F. Fu Y. Meyskens FL., Jr MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/ref-1. J Invest Dermatol. 2009;129:422–431. doi: 10.1038/jid.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z. Huang SL. Li MM. Huang ZS. Lee KS. Gu LQ. Inhibition of thioredoxin reductase by mansonone F analogues: implications for anticancer activity. Chem Biol Interact. 2009;177:48–57. doi: 10.1016/j.cbi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Liuzzi M. Talpaert-Borle M. A new approach to the study of the base-excision repair pathway using methoxyamine. J Biol Chem. 1985;260:5252–5258. [PubMed] [Google Scholar]
- 125.Loboda A. Stachurska A. Dorosz J. Zurawski M. Wegrzyn J. Kozakowska M. Jozkowicz A. Dulak J. HIF-1 attenuates Ref-1 expression in endothelial cells: reversal by siRNA and inhibition of geranylgeranylation. Vasc Pharmacol. 2009;51:133–139. doi: 10.1016/j.vph.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Lopez de Saro FJ. Marinus MG. Modrich P. O'Donnell M. The beta sliding clamp binds to multiple sites within MutL and MutS. J Biol Chem. 2006;281:14340–14349. doi: 10.1074/jbc.M601264200. [DOI] [PubMed] [Google Scholar]
- 127.Lu X. Lozano G. Donehower LA. Activities of wildtype and mutant p53 in suppression of homologous recombination as measured by a retroviral vector system. Mutat Res. 2003;522:69–83. doi: 10.1016/s0027-5107(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 128.Luo M. Delaplane S. Jiang A. Reed A. He Y. Fishel M. Nyland II RL. Borch RF. Qiao X. Georgiadis MM. Kelley MR. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small molecule inhibition of Ape1's redox function. Antioxid Redox Signal. 2008;10:1853–1867. doi: 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Madhusudan S. Hickson ID. DNA repair inhibition: a selective tumour targeting strategy. Trends Mol Med. 2005;11:503–511. doi: 10.1016/j.molmed.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 130.Madhusudan S. Smart F. Shrimpton P. Parsons JL. Gardiner L. Houlbrook S. Talbot DC. Hammonds T. Freemont PA. Sternberg MJ. Dianov EL. Hickson ID. Isolation of a small molecule inhibitor of DNA base excision repair. Nucleic Acids Res. 2005;33:4711–4724. doi: 10.1093/nar/gki781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mathers JC. Coxhead JM. Tyson J. Nutrition and DNA repair: potential molecular mechanisms of action. Curr Cancer Drug Targets. 2007;7:425–431. doi: 10.2174/156800907781386588. [DOI] [PubMed] [Google Scholar]
- 132.Maxwell PH. Wiesener MS. Chang GW. Clifford SC. Vaux EC. Cockman ME. Wykoff CC. Pugh CW. Maher ER. Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 133.McEligot AJ. Yang S. Meyskens FL., Jr Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Annu Rev Nutr. 2005;25:261–295. doi: 10.1146/annurev.nutr.25.050304.092633. [DOI] [PubMed] [Google Scholar]
- 134.McNeill DR. Wilson DM., 3rd A dominant-negative form of the major human abasic endonuclease enhances cellular sensitivity to laboratory and clinical DNA-damaging agents. Mol Cancer Res. 2007;5:61–70. doi: 10.1158/1541-7786.MCR-06-0329. [DOI] [PubMed] [Google Scholar]
- 135.Meng AX. Jalali F. Cuddihy A. Chan N. Bindra RS. Glazer PM. Bristow RG. Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother Oncol. 2005;76:168–176. doi: 10.1016/j.radonc.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 136.Merluzzi S. Gri G. Gattei V. Pagano M. Pucillo C. APE/Ref-1 makes fine-tuning of CD40-induced B cell proliferation. Mol Immunol. 2008;45:3731–3739. doi: 10.1016/j.molimm.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Messina M. Kucuk O. Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006;89:1121–1134. [PubMed] [Google Scholar]
- 138.Mihaylova VT. Bindra RS. Yuan J. Campisi D. Narayanan L. Jensen R. Giordano F. Johnson RS. Rockwell S. Glazer PM. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–3273. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mol CD. Izumi T. Mitra S. Tainer JA. DNA-bound structure and mutants reveal abasic DNA binding by APE1 DNA repair and coordination. Nature. 2000;403:451–455. doi: 10.1038/35000249. [DOI] [PubMed] [Google Scholar]
- 140.Moore DH. Michael H. Tritt R. Parsons SH. Kelley MR. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers. Clin Cancer Res. 2000;6:602–609. [PubMed] [Google Scholar]
- 141.Mukherjee A. Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol. 2008;81:S57–S68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura H. Nakamura K. Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 143.Nakamura H. Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal. 2005;7:823–828. doi: 10.1089/ars.2005.7.823. [DOI] [PubMed] [Google Scholar]
- 144.Nouspikel T. DNA repair in mammalian cells: nucleotide excision repair: variations on versatility. Cell Mol Life Sci. 2009;66:994–1009. doi: 10.1007/s00018-009-8737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nouspikel T. DNA repair in mammalian cells: So DNA repair really is that important? Cell Mol Life Sci. 2009;66:965–967. doi: 10.1007/s00018-009-8734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O'Dwyer PJ. Johnson SW. Khater C. Krueger A. Matsumoto Y. Hamilton TC. Yao KS. The chemopreventive agent oltipraz stimulates repair of damaged DNA. Cancer Res. 1997;57:1050–1053. [PubMed] [Google Scholar]
- 147.Ono Y. Furuta T. Ohmoto T. Akiyama K. Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat Res. 1994;315:55–63. doi: 10.1016/0921-8777(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 148.Ordway JM. Eberhart D. Curran T. Cysteine 64 of Ref-1 is not essential for redox regulation of AP-1 DNA binding. Mol Cell Biol. 2003;23:4257–4266. doi: 10.1128/MCB.23.12.4257-4266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ozaki M. Suzuki S. Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- 150.Pardo B. Gomez-Gonzalez B. Aguilera A. DNA repair in mammalian cells: DNA double-strand break repair: how to fix a broken relationship. Cell Mol Life Sci. 2009;66:1039–1056. doi: 10.1007/s00018-009-8740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Park WS. Ko EA. Jung ID. Son YK. Kim HK. Kim N. Park SY. Hong KW. Park YM. Choi TH. Han J. APE1/Ref-1 promotes the effect of angiotensin II on Ca2+ -activated K+ channel in human endothelial cells via suppression of NADPH oxidase. Arch Pharm Res. 2008;31:1291–1301. doi: 10.1007/s12272-001-2109-y. [DOI] [PubMed] [Google Scholar]
- 152.Pines A. Perrone L. Bivi N. Romanello M. Damante G. Gulisano M. Kelley MR. Quadrifoglio F. Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Powis G. Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Pharmacol Toxicol. 2001;41:261–195. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- 154.Raffel J. Bhattacharyya AK. Gallegos A. Cui H. Einspahr JG. Alberts DS. Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 155.Rafferty JA. Clarke AR. Sellappan D. Koref MS. Frayling IM. Margison GP. Induction of murine O6-alkylguanine-DNA-alkyltransferase in response to ionising radiation is p53 gene dose dependent. Oncogene. 1996;12:693–697. [PubMed] [Google Scholar]
- 156.Raffoul JJ. Banerjee S. Singh-Gupta V. Knoll ZE. Fite A. Zhang H. Abrams J. Sarkar FH. Hillman GG. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–2149. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 157.Robertson AB. Klungland A. Rognes T. Leiros I. DNA repair in mammalian cells: base excision repair: the long and short of it. Cell Mol Life Sci. 2009;66:981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ruggeri BA. Robinson C. Angeles T. Wilkinson JT. Clapper ML. The chemopreventive agent oltipraz possesses potent antiangiogenic activity in vitro, ex vivo, and in vivo and inhibits tumor xenograft growth. Clin Cancer Res. 2002;8:267–274. [PubMed] [Google Scholar]
- 159.Scherer SJ. Maier SM. Seifert M. Hanselmann RG. Zang KD. Muller-Hermelink HK. Angel P. Welter C. Schartl M. p53 and c-Jun functionally synergize in the regulation of the DNA repair gene hMSH2 in response to UV. J Biol Chem. 2000;275:37469–37473. doi: 10.1074/jbc.M006990200. [DOI] [PubMed] [Google Scholar]
- 160.Schwartz EI. Smilenov LB. Price MA. Osredkar T. Baker RA. Ghosh S. Shi FD. Vollmer TL. Lencinas A. Stearns DM. Goropse M. Kruman II. Cell cycle activation in postmitotic neurons is essential for DNA repair. Cell Cycle. 2007;6:318–329. doi: 10.4161/cc.6.3.3752. [DOI] [PubMed] [Google Scholar]
- 161.Seiple LA. Cardellina JH., 2nd Akee R. Stivers JT. Potent inhibition of human apurinic/apyrimidinic endonuclease 1 by arylstibonic acids. Mol Pharmacol. 2008;73:669–677. doi: 10.1124/mol.107.042622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sengupta S. Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 163.Sengupta S. Shimamoto A. Koshiji M. Pedeux R. Rusin M. Spillare EA. Shen JC. Huang LE. Lindor NM. Furuichi Y. Harris CC. Tumor suppressor p53 represses transcription of RECQ4 helicase. Oncogene. 2005;24:1738–1748. doi: 10.1038/sj.onc.1208380. [DOI] [PubMed] [Google Scholar]
- 164.Seo YR. Kelley MR. Smith ML. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc Natl Acad Sci U S A. 2002;99:14548–14553. doi: 10.1073/pnas.212319799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seo YR. Sweeney C. Smith ML. Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene. 2002;21:3663–3669. doi: 10.1038/sj.onc.1205468. [DOI] [PubMed] [Google Scholar]
- 166.Shimizu N. Sugimoto K. Tang J. Nishi T. Sato I. Hiramoto M. Aizawa S. Hatakeyama M. Ohba R. Hatori H. Yoshikawa T. Suzuki F. Oomori A. Tanaka H. Kawaguchi H. Watanabe H. Handa H. High-performance affinity beads for identifying drug receptors. Nat Biotechnol. 2000;18:877–881. doi: 10.1038/78496. [DOI] [PubMed] [Google Scholar]
- 167.Sung P. Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol. 2006;7:739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- 168.Tan T. Chu G. p53 Binds and activates the xeroderma pigmentosum DDB2 gene in humans but not mice. Mol Cell Biol. 2002;22:3247–3254. doi: 10.1128/MCB.22.10.3247-3254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tell G. Zecca A. Pellizzari L. Spessotto P. Colombatti A. Kelley MR. Damante G. Pucillo C. An “environment to nucleus” signaling system operates in B lymphocytes: redox status modulates BSAP/Pax-5 activation through Ref-1 nuclear translocation. Nucleic Acids Res. 2002;28:1099–1105. doi: 10.1093/nar/28.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tell G. Damante G. Caldwell D. Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 171.Tell G. Quadrifoglio F. Tiribelli C. Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.To KK. Koshiji M. Hammer S. Huang LE. Genetic instability: the dark side of the hypoxic response. Cell Cycle. 2005;4:881–882. doi: 10.4161/cc.4.7.1839. [DOI] [PubMed] [Google Scholar]
- 173.To KK. Sedelnikova OA. Samons M. Bonner WM. Huang LE. The phosphorylation status of PAS-B distinguishes HIF-1alpha from HIF-2alpha in NBS1 repression. EMBO J. 2006;25:4784–4794. doi: 10.1038/sj.emboj.7601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tornaletti S. DNA repair in mammalian cells: transcription-coupled DNA repair: directing your effort where it's most needed. Cell Mol Life Sci. 2009;66:1010–1020. doi: 10.1007/s00018-009-8738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ueno M. Masutani H. Arai RJ. Yamauchi A. Hirota K. Sakai T. Inamoto T. Yamaoka Y. Yodoi J. Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- 176.Vascotto C. Fantini D. Romanello M. Cesaratto L. Deganuto M. Leonardi A. Radicella JP. Kelley MR. D'Ambrosio C. Scaloni A. Quadrifoglio F. Tell G. APE1/Ref-1 interacts with NPM1 within nucleoli and plays a role in the rRNA quality control process. Mol Cell Biol. 2009;29:1834–1854. doi: 10.1128/MCB.01337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vivancos AP. Castillo EA. Biteau B. Nicot C. Ayte J. Toledano MB. Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci U S A. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Walker LJ. Robson CN. Black E. Gillespie D. Hickson ID. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993;13:5370–5376. doi: 10.1128/mcb.13.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Walker LJ. Craig RB. Harris AL. Hickson ID. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA damaging agents and hypoxic stress. Nucleic Acids Res. 1994;22:4884–4889. doi: 10.1093/nar/22.23.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang D. Luo M. Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 2004;3:679–686. [PubMed] [Google Scholar]
- 181.Weichsel A. Gasdaska JR. Powis G. Montfort WR. Crystal structures of reduced, oxidized, and mutated human thioredoxins: evidence for a regulatory homodimer. Structure. 2996;4:735–751. doi: 10.1016/s0969-2126(96)00079-2. [DOI] [PubMed] [Google Scholar]
- 182.Wood RD. Mitchell M. Lindahl T. Human DNA repair genes. Mutat Res. 2005;577:275–283. doi: 10.1016/j.mrfmmm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 183.Wood ZA. Poole LB. Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 184.Xanthoudakis S. Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xanthoudakis S. Miao G. Wang F. Pan YC. Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xanthoudakis S. Miao GG. Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A. 1994;91:23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xanthoudakis S. Curran T. Redox regulation of AP-1: a link between transcription factor signaling and DNA repair. Adv Exp Med Biol. 1996;387:69–75. [PubMed] [Google Scholar]
- 188.Xanthoudakis S. Smeyne RJ. Wallace JD. Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci U S A. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu J. Morris GF. p53-mediated regulation of proliferating cell nuclear antigen expression in cells exposed to ionizing radiation. Mol Cell Biol. 1999;19:12–20. doi: 10.1128/mcb.19.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu Y. Moore DH. Broshears J. Liu L. Wilson TM. Kelley MR. The apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme is elevated in premalignant and malignant cervical cancer. Anticancer Res. 1997;17:3713–3719. [PubMed] [Google Scholar]
- 191.Yamabe Y. Shimamoto A. Goto M. Yokota J. Sugawara M. Furuichi Y. Sp1-mediated transcription of the Werner helicase gene is modulated by Rb and p53. Mol Cell Biol. 1998;18:6191–6200. doi: 10.1128/mcb.18.11.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamawaki H. Berk BC. Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens. 2005;14:149–153. doi: 10.1097/00041552-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 193.Yang S. Irani K. Heffron SE. Jurnak F. Meyskens FL., Jr Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–1935. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]
- 194.Yao KS. Hageboutros A. Ford P. O'Dwyer PJ. Involvement of activator protein-1 and nuclear factor-kappaB transcription factors in the control of the DT-diaphorase expression induced by mitomycin C treatment. Mol Pharmacol. 1997;51:422–430. [PubMed] [Google Scholar]
- 195.Youn BW. Fiala ES. Sohn OS. Mechanisms of organoselenium compounds in chemoprevention: effects on transcription factor-DNA binding. Nutr Cancer. 2001;40:28–33. doi: 10.1207/S15327914NC401_7. [DOI] [PubMed] [Google Scholar]
- 196.Zaky A. Busso C. Izumi T. Chattopadhyay R. Bassiouny A. Mitra S. Bhakat KK. Regulation of the human AP-endonuclease (APE1/Ref-1) expression by the tumor suppressor p53 in response to DNA damage. Nucleic Acids Res. 2008;36:1555–1566. doi: 10.1093/nar/gkm1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zawahir Z. Dayam R. Deng J. Pereira C. Neamati N. Pharmacophore guided discovery of small-molecule human apurinic/apyrimidinic endonuclease 1 inhibitors. J Med Chem. 2009;52:20–32. doi: 10.1021/jm800739m. [DOI] [PubMed] [Google Scholar]
- 198.Ziel KA. Campbell CC. Wilson GL. Gillespie MN. Ref-1/Ape is critical for formation of the hypoxia-inducible transcriptional complex on the hypoxic response element of the rat pulmonary artery endothelial cell VEGF. FASEB J Epub. 2004. [DOI] [PubMed]
- 199.Zou GM. Luo MH. Reed A. Kelley MR. Yoder MC. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood. 2007;109:1917–1922. doi: 10.1182/blood-2006-08-044172. [DOI] [PubMed] [Google Scholar]
- 200.Zou GM. Maitra A. Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration. Mol Cancer Ther. 2008;7:2012–2021. doi: 10.1158/1535-7163.MCT-08-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zou GM. Karikari C. Kabe Y. Handa H. Anders RA. Maitra A. The Ape-1/Ref-1 redox antagonist E3330 inhibits the growth of tumor endothelium and endothelial progenitor cells: therapeutic implications in tumor angiogenesis. J Cell Physiol. 2009;219:209–218. doi: 10.1002/jcp.21666. [DOI] [PubMed] [Google Scholar]
- 202.Zurer I. Hofseth LJ. Cohen Y. Xu-Welliver M. Hussain SP. Harris CC. Rotter V. The role of p53 in base excision repair following genotoxic stress. Carcinogenesis. 2004;25:11–19. doi: 10.1093/carcin/bgg186. [DOI] [PubMed] [Google Scholar]