Abstract
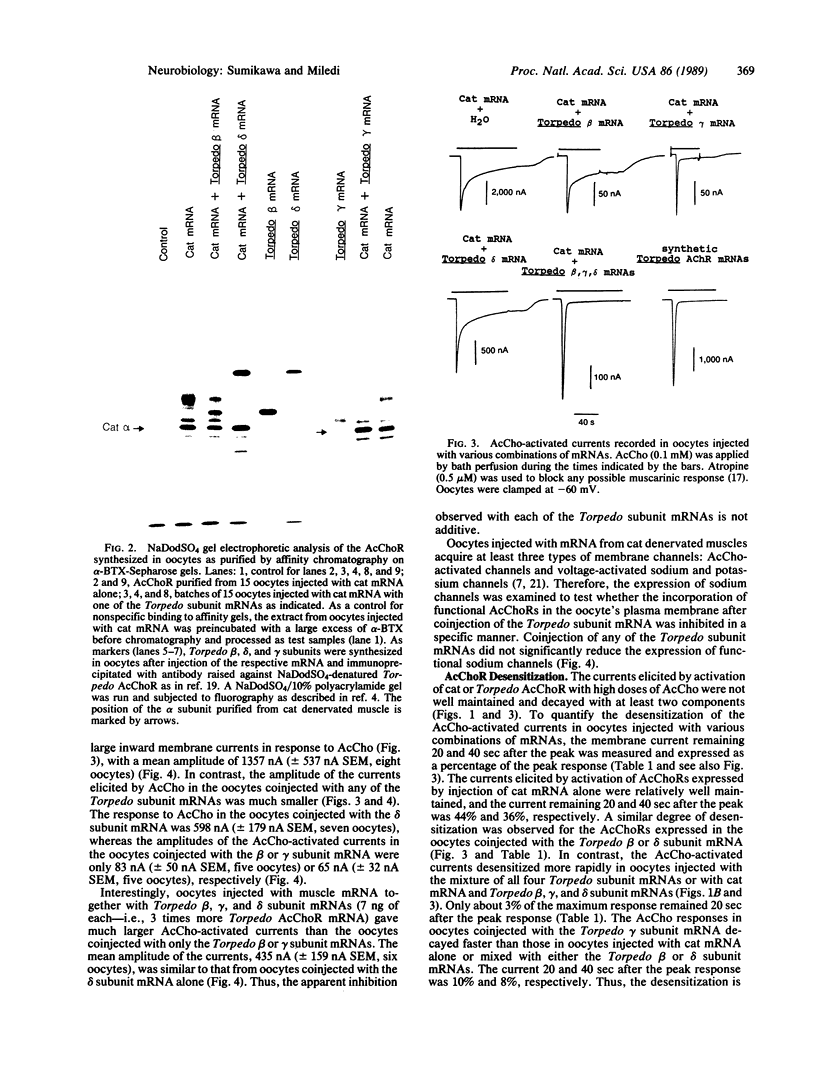
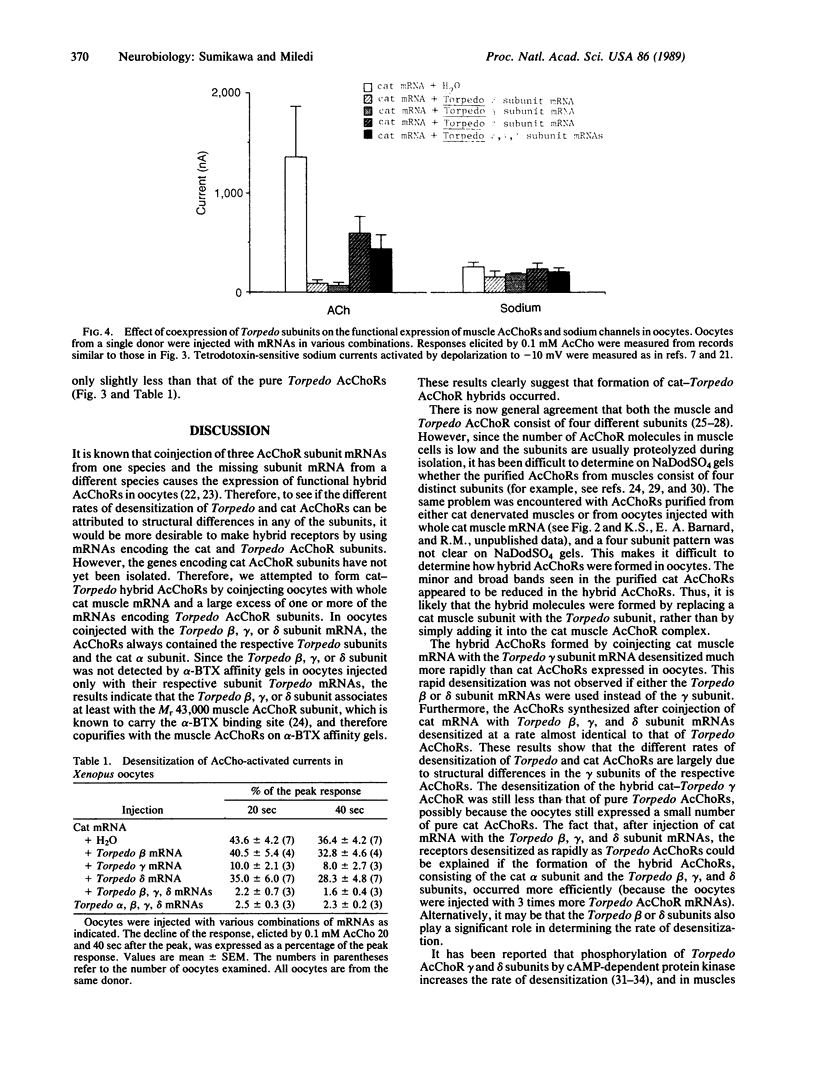
Cat muscle acetylcholine receptors (AcChoR) expressed in Xenopus oocytes desensitized more slowly than Torpedo electric organ AcChoRs, also expressed in oocytes. To examine the bases for the different degrees of desensitization, cat-Torpedo AcChoR hybrids were formed by injecting oocytes with cat denervated muscle mRNA mixed with a large excess of cloned Torpedo AcChoR subunit mRNAs. Hybrid AcChoRs formed by coinjection of cat muscle mRNA with the Torpedo beta or delta subunit mRNAs desensitized as slowly as cat AcChoR. In contrast, the hybrid AcChoRs expressed by coinjection with the Torpedo gamma subunit mRNA desensitized much more rapidly than cat AcChoR. The AcChoRs expressed in oocytes injected with cat muscle mRNA together with the Torpedo beta, gamma, and delta subunit mRNAs desensitized as rapidly as Torpedo AcChoR, indicating that the cat alpha subunit does not play an important role in determining the slow rate of desensitization. It is concluded that the difference in the rates of desensitization of cat and Torpedo AcChoRs is determined mainly by differences in their respective gamma subunits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claudio T., Ballivet M., Patrick J., Heinemann S. Nucleotide and deduced amino acid sequences of Torpedo californica acetylcholine receptor gamma subunit. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1111–1115. doi: 10.1073/pnas.80.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio T., Green W. N., Hartman D. S., Hayden D., Paulson H. L., Sigworth F. J., Sine S. M., Swedlund A. Genetic reconstitution of functional acetylcholine receptor channels in mouse fibroblasts. Science. 1987 Dec 18;238(4834):1688–1694. doi: 10.1126/science.3686008. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Gotti C. M., Hunkapiller M. W., Raftery M. A. Mammalian muscle acetylcholine receptor: a supramolecular structure formed by four related proteins. Science. 1982 Dec 17;218(4578):1227–1229. doi: 10.1126/science.7146904. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Einarson B., Gullick W., Conti-Tronconi B., Ellisman M., Lindstrom J. Subunit composition of bovine muscle acetylcholine receptor. Biochemistry. 1982 Oct 12;21(21):5295–5302. doi: 10.1021/bi00264a027. [DOI] [PubMed] [Google Scholar]
- Evans S., Goldman D., Heinemann S., Patrick J. Muscle acetylcholine receptor biosynthesis. Regulation by transcript availability. J Biol Chem. 1987 Apr 5;262(10):4911–4916. [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Monaco L., Eusebi F. Acetylcholine receptor channel properties in rat myotubes exposed to forskolin. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1000–1007. doi: 10.1016/s0006-291x(87)80169-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Voltage-operated channels induced by foreign messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):131–140. doi: 10.1098/rspb.1983.0092. [DOI] [PubMed] [Google Scholar]
- Harland R., Weintraub H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. J Cell Biol. 1985 Sep;101(3):1094–1099. doi: 10.1083/jcb.101.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Fukuda K., Konno T., Mori Y., Tanaka K., Mishina M., Numa S. Functional properties of nicotinic acetylcholine receptor subunits expressed in various combinations. FEBS Lett. 1987 Apr 20;214(2):253–258. doi: 10.1016/0014-5793(87)80065-0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Gullick W., Conti-Tronconi B., Ellisman M. Proteolytic nicking of the acetylcholine receptor. Biochemistry. 1980 Oct 14;19(21):4791–4795. doi: 10.1021/bi00562a012. [DOI] [PubMed] [Google Scholar]
- Lyddiatt A., Sumikawa K., Wolosin J. M., Dolly J. O., Barnard E. A. Affinity labelling by bromoacetylcholine of a characteristic subunit in the acetylcholine receptor from muscle and Torpedo electric organ. FEBS Lett. 1979 Dec 1;108(1):20–24. doi: 10.1016/0014-5793(79)81169-2. [DOI] [PubMed] [Google Scholar]
- Mayne K. M., Yoshii K., Yu L., Lester H. A., Davidson N. Expression of mouse-Torpedo acetylcholine receptor subunit chimeras and hybrids in Xenopus oocytes. Brain Res. 1987 Sep;388(3):191–197. doi: 10.1016/0169-328x(87)90026-x. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Lindstrom J. Assembly in vivo of mouse muscle acetylcholine receptor: identification of an alpha subunit species that may be an assembly intermediate. Cell. 1983 Oct;34(3):747–757. doi: 10.1016/0092-8674(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Properties of acetylcholine receptors translated by cat muscle mRNA in Xenopus oocytes. EMBO J. 1982;1(11):1307–1312. doi: 10.1002/j.1460-2075.1982.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Gundersen C. B., Miledi R. Expression of ACh-activated channels and sodium channels by messenger RNAs from innervated and denervated muscle. Proc R Soc Lond B Biol Sci. 1988 Apr 22;233(1272):235–246. doi: 10.1098/rspb.1988.0021. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Methfessel C., Mishina M., Takahashi T., Takai T., Kurasaki M., Fukuda K., Numa S. Role of acetylcholine receptor subunits in gating of the channel. Nature. 1985 Dec 12;318(6046):538–543. doi: 10.1038/318538a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Barnard E. A., Dolly J. O. Similarity of acetylcholine receptors of denervated, innervated and embryonic chicken muscles. 2. Subunit compositions. Eur J Biochem. 1982 Sep 1;126(3):473–479. doi: 10.1111/j.1432-1033.1982.tb06804.x. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Repression of nicotinic acetylcholine receptor expression by antisense RNAs and an oligonucleotide. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1302–1306. doi: 10.1073/pnas.85.4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- White M. M., Mayne K. M., Lester H. A., Davidson N. Mouse-Torpedo hybrid acetylcholine receptors: functional homology does not equal sequence homology. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4852–4856. doi: 10.1073/pnas.82.14.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray D. Prolonged exposure to acetylcholine: noise analysis and channel inactivation in cat tenuissimus muscle. J Physiol. 1981 Jan;310:37–56. doi: 10.1113/jphysiol.1981.sp013536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee G. H., Huganir R. L. Determination of the sites of cAMP-dependent phosphorylation on the nicotinic acetylcholine receptor. J Biol Chem. 1987 Dec 5;262(34):16748–16753. [PubMed] [Google Scholar]