Abstract
Reactive oxygen species (ROS), a heterogeneous population of biologically active intermediates, are generated as by-products of the aerobic metabolism and exhibit a dual role in biology. When produced in controlled conditions and in limited quantities, ROS may function as signaling intermediates, contributing to critical cellular functions such as proliferation, differentiation, and cell survival. However, ROS overgeneration and, particularly, the formation of specific reactive species, inflicts cell death and tissue damage by targeting vital cellular components such as DNA, lipids, and proteins, thus arising as key players in disease pathogenesis. Given the predominant role of hepatocytes in biotransformation and metabolism of xenobiotics, ROS production constitutes an important burden in liver physiology and pathophysiology and hence in the progression of liver diseases. Despite the recognized role of ROS in disease pathogenesis, the efficacy of antioxidants as therapeutics has been limited. A better understanding of the mechanisms, nature, and location of ROS generation, as well as the optimization of cellular defense strategies, may pave the way for a brighter future for antioxidants and ROS scavengers in the therapy of liver diseases. Antioxid. Redox Signal. 12, 1295—1331.
I. Introduction
Free radicals were first documented about 50 years ago in plant and animal tissues by using paramagnetic resonance absorption techniques (62). Despite this seminal finding, free radicals were considered mainly as mediators of the damaging effects of radiation, with little interest in biology, until McCord and Fridovich (210) described the existence of a specific enzyme dedicated to scavenge the superoxide anion (210). The landmark discovery of superoxide dismutase (SOD), ∼15 years ago, sparked a considerable interest in the chemistry and biology of free radicals, now being recognized as critical players in multiple cellular functions, diseases, and aging. Free radicals, including reactive oxygen/nitrogen species (ROS, RNS), are generated as by-products of biochemical reactions within cells and, hence, considered as inherent intermediates of many physiologic processes. However, when produced in large amounts or in an uncontrolled fashion, free radicals inflict tissue damage and are implicated in many pathologic processes. The understanding of the fine balance between the physiologic and pathologic effects of free radicals is an important driving force in this field of research that may have an impact on diverse disciplines, including physiology, cell biology, and clinical medicine.
One of the predominant foundations for the biologic actions of free radicals and reactive species lies in cellular redox signaling, which involves the posttranscriptional modification of proteins that use redox chemistry. A redox reaction involves the transfer of electrons between two molecules or atoms, resulting in their reduction (gain of electrons) and oxidation (loss of electrons). The paradigm of a redox reaction in cell signaling is illustrated by the reduction/oxidation state of cysteine residues of proteins, resulting in the breaking down or formation of a protein disulfide bond. Redox changes of target proteins are initiated by the generation of ROS and RNS, which can lead to the formation of disulfide bridges between two adjacent cysteine residues in a protein or the generation of S-nitrosothiols, resulting from the attack of nitric oxide or peroxynitrate on cysteine. In addition, methionine residues of proteins can be oxidized by ROS to methionine sulfoxide, which is specifically repaired by methionine sulfoxide reductases A and B (267). Interestingly, this particular modification of methionine residues is thought to serve as a free radical sink, thereby protecting other macromolecules from oxidation. Consistent with this view, the specific lack of methionine sulfoxide reductase A increases sensitivity to oxidative stress in mice (267). These thiol modifications can be regulated by factors that act on or modulate ROS/RNS generation.
Besides proteins, free radicals and ROS also target other cellular components, including DNA and lipids, whose oxidative modifications can alter signaling pathways and trigger cell death. Although ROS are produced in many cell types and recognized in the pathogenesis of different diseases (86, 326), given the bulk of the liver in the biotransformation of xenobiotics and metabolism, in the present review, we focus mainly on the role of ROS and redox control of liver function in health and disease. Although some aspects of the chemistry and impact of free radicals have been previously reviewed (272, 274), in the following sections, we briefly describe some basic notions about their nature, sources, and defense.
II. Free Radicals and Reactive Species: Sources and Defense
Free radicals are molecules or atoms with unpaired electrons, making them highly reactive and extremely likely to take part in a chemical reaction. Generally, radicals can be formed by homolytic bond cleavage, usually between two atoms of similar electronegativity (often O-O or O-N bonds), or by single-electron oxidation or reduction of an atom or molecule (e.g., superoxide anion). In addition, to this one-electron radical, two-electron oxidants, although not strictly radicals, arise from the metabolism or scavenging of the former, as best exemplified by the generation of hydrogen peroxide from superoxide anion. Within each class, some are much more reactive or strongly oxidizing than others, a property that can be estimated by the one-electron reduction potential, given that the activation energy for radical reactions is low. The reduction potential (in volts) is a measure of the affinity of a substance for electrons compared with that of hydrogen, which is set at 0 (e.g., reduction potentials for superoxide anion, hydrogen peroxide, and hydroxyl radical are 0.94, 0.32, and 2.31 V, respectively). Molecules that have lower reduction potentials are strongly electronegative and can oxidize. Among biologically relevant ROS, hydrogen peroxide has the lowest reactivity, the highest stability and intracellular concentration, and hence is highly regulated in cells to avoid its overgeneration (117).
A. Sources of ROS
1. Extramitochondrial
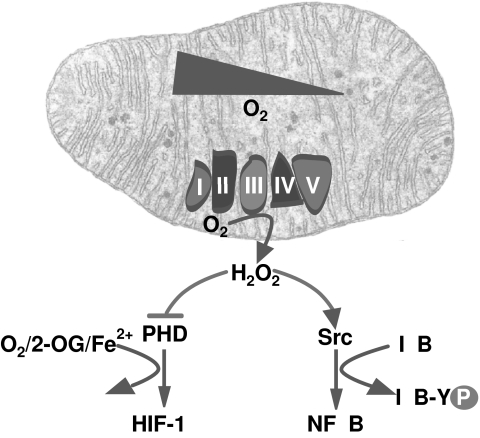
Biologic systems are constantly exposed to intrinsic and extrinsic sources of free radicals and reactive oxidants. Although in most cell types, ionizing and UV radiation are important sources of free radicals, in the liver, these species arise as by-products of the metabolism of a wide range of drugs and xenobiotics (e.g., phenols, aromatic amines) and, especially in the mitochondrial oxidative phosphorylation, as detailed later. The biotransformation of xenobiotics (e.g., quinones) through redox cycling generates superoxide anion. In this process, the parental compound is reduced by a flavoenzyme such as cytochrome P450 reductase to a radical that then reacts with oxygen to form superoxide anion (237, 238). This reaction has been commonly used to produce superoxide anion in in vitro conditions (63, 64). Free radicals and ROS can be generated by various enzymes and in different cellular locations. For instance, amino acids oxidases, cyclooxygenase, lipooxygenase, nitric oxide synthase, and xanthine oxidase, generate superoxide anions and other derived ROS in the cytosol. Whereas cyclooxygenase and lipooxygenase may link superoxide anion generation to arachidonic acid metabolism and inflammation, with important implications in pathogenesis and cancer, xanthine oxidase has been involved in ischemia/reperfusion injury and liver transplantation. Moreover, nitric oxide generated by nitric oxide synthase can interact with superoxide anion, resulting in the formation of the potent oxidant peroxynitrite, which in turn can target protein cysteine thiols (see later). Oxidants also are generated by sulfhydryl oxidase in the endoplasmic reticulum (ER) during protein folding and disulfide bond formation necessary for the assembly and secretory pathway for proteins (144), as well as in peroxisomes by peroxisomal oxidase. Of particular relevance in both liver physiology and pathophysiology is the burst of superoxide anion formed by NADPH oxidase. Although NADPH oxidase was first described in professional phagocytes of the innate immune system (e.g., neutrophils and macrophages), it is now known that its expression is ubiquitous. The burst of superoxide anion generated from NADPH as an electron donor and molecular oxygen is considered a first line of defense against ingested pathogens (254). However, NADPH oxidase has been involved in many pathologic processes including cardiovascular disorders and liver diseases (36, 79). A nonphagocytic form of NADPH oxidase has been demonstrated in vascular cell types (322). Like the phagocytic respiratory burst NADPH oxidase, nonphagocytic NADPH oxidase reduces molecular oxygen to superoxide, which is in turn converted to hydrogen peroxide. However, unlike the phagocytic type, the NADPH oxidase present in blood vessels is constitutively active, producing relatively low levels of ROS under basal conditions; however, in response to peptide hormones, such as angiotensin II (AngII), it can generate high levels of oxidants. Both the phagocytic and nonphagocytic oxidases are multimeric enzymes composed of plasma membrane–associated proteins as well as cytosolic factors (317). Recently, several smooth muscle cell homologues of gp91phox, termed Nox (nonphagocytic oxidase), have been identified (47). Studies in liver fibrogenesis demonstrated that NADPH oxidase mediates the actions of AngII on hepatic stellate cells and plays a critical role in liver fibrogenesis (16). Consistent with this function, recent data provided evidence that the disruption of the AngII-receptor type 1 extends longevity in mice, which is associated with reduced oxidative damage, preserved mitochondrial integrity, and upregulation of survival genes nicotinamide phosphoribosyltransferase (Nampt) and sirtuin 3 (Sirt3) (20). Hence, these findings point to a functional link between the renin-angiotensin system and NADPH oxidases in the modulation of oxidative stress and its associated comorbidities, including aging.
2. Mitochondrial
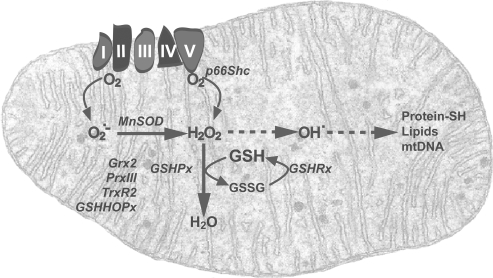
In addition to the preceding pathways, mitochondria are the largest source of ROS within cells (11). The partial reduction of oxygen during oxidative phosphorylation generates the superoxide anion, which acts on the matrix side of mitochondria where it is then transformed into other species, including hydrogen peroxide (88, 95, 130) (Fig. 1). The basal production of ROS from the mitochondrial electron-transport chain is low, with estimates of 2–4% of electrons leaking from the electron flow to molecular oxygen to form superoxide anion, although recent determinations have reduced this figure to ∼0.1–0.5% (17). Recent data described the onset of superoxide flashes originating from the mitochondrial permeability transition pore that occur randomly in space and time with all-or-none properties (320). Superoxide flashes are triggered by a functional coupling between the mitochondrial permeability transition pore activation and electron-transport chain–dependent superoxide production, which drive localized redox signaling in individual mitochondria under physiologic conditions. However, when produced in an uncontrolled fashion and with increased frequency, superoxide flashes contribute to global oxidative stress, playing a key role in hypoxia/reoxygenation injury (320). The precise site, however, within the mitochondrial electron-transport complexes responsible for the ROS formation is not well established. Recent data pointed to the flavin mononucleotide group of complex I as an important source of ROS through reverse electron transfer (185), as inferred from the ability of diphenyleneiodonium to inhibit succinate-supported ROS generation without affecting the flavin group of complex II. In addition, although ROS can activate caspases, resulting in cell death (see later), active caspases, such as caspase-3 have been described to disrupt electron-transport complexes I and II, which contribute to the loss of ΔΨm and ROS generation (257), establishing a vicious cycle. Moreover, complex III of respiration is also known to be an important source of ROS generation (26, 110). Respiratory-chain complex III has two ubiquinone-reactive sites: the Qo, where ubiquinol is oxidized by redox-reactive centers, cytochrome c1, and the Rieske [2Fe–2S] protein, and the Qi, where ubiquinone is reduced by the redox center cytochrome b. The Rieske cluster is a mobile structure, and this mobility may facilitate rapid electron transfer between cytochrome b and c1 (148). Besides this predominant mechanism, the redox activity of p66Shc within mitochondria has been shown to generate hydrogen peroxide in the absence of superoxide anion through oxidation of cytochrome c (116). Furthermore, purified apoptosis-inducing factor (AIF) exhibits a NADH oxidase activity, which generates ROS, including superoxide anion and hydrogen peroxide (77, 215). However, given the role of AIF in cell death, it is unclear whether this emerging function of AIF as a ROS-generating enzyme contributes to the mitochondria-dependent apoptosome activation.
FIG. 1.
Mitochondrial ROS generation and defense. Mitochondria are the major producers of ROS, in particular, superoxide anion, as a side effect of electron flow in the respiratory chain, principally from complex I and III. In addition, hydrogen peroxide can be generated by the adaptor protein p66Shc. The antioxidant defense includes a number of enzymatic systems as shown, as well as the GSH redox cycle, to prevent escalating effects through formation of hydroxyl radical that could target proteins, lipids, and DNA.
In analogy to the interaction between NO and superoxide anion in extramitochondrial compartments, mitochondrial nitric oxide synthase (mtNOS) has been shown to generate NO, hence constituting an important source of mitochondrial peroxynitrite, whose impact on redox-regulated processes, such as proliferation or mitochondrial dysfunction, depends on the extent of generation (33). However, although the existence of mtNOS has been described in the last decade in mitochondrial fractions isolated from different sources, recent evidence in ultrapurified rat liver mitochondria by using independent and complementary methods to detect nitric oxide synthase has questioned the existence of mtNOS (314). These carefully performed studies thus refute previous claims regarding the existence of mtNOS (at least in rat liver) and hence the in situ generation of NO within mitochondria, minimizing the possibility for the mitochondrial generation of peroxynitrite. In light of these new findings and considering that NO· is freely diffusible across membranes, it is conceivable that the mitochondrial production of peroxynitrite derives from the extramitochondrial NO diffusing into mitochondria to react with superoxide anion generated by the electron-transport chain.
B. Cellular defense
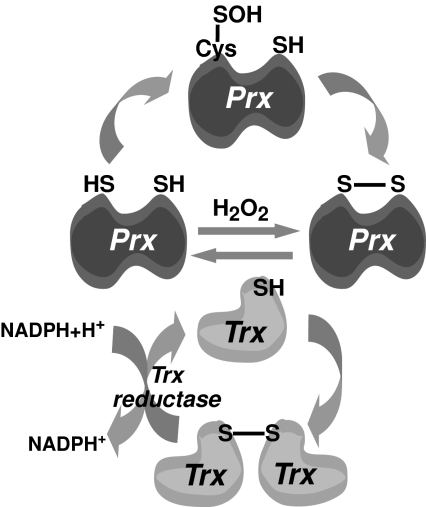
Despite the constant generation of free radicals and oxidant species, living organisms not only have adapted to an unfriendly coexistence with these potentially toxic species, but also have developed mechanisms for the advantageous use of them. The arsenal of cellular defenses to control the magnitude of ROS generation is extensive and includes enzymatic (superoxide anion dismutases, catalases, GSH peroxidases, peroxiredoxins, glutaredoxins, thioredoxins, sulfiredoxins) and nonenzymatic antioxidants (vitamins A, C, and E, GSH, urate, bilirrubin). The coordinated action of antioxidant enzymes ensures efficient ROS removal. For example, the superoxide dismutases (SODs) catalyze the dismutation of superoxide anion into hydrogen peroxide, which, in turn, is converted into water and oxygen by GSH peroxidases and catalase. Considering the reactivity and site localization where free radicals and ROS are generated within cells, enzymatic antioxidant defenses are compartmentalized to neutralize these species more efficiently. For instance, SOD is localized in the cytosol (Cu/Zn SOD) or in the mitochondria (Mn-SOD), thus handling different pools of superoxide anion generated extra- or intramitochondrially. In addition, extracellular SOD (ecSOD) is found predominantly in the extracellular matrix and is known to regulate endothelial cells by preventing NO from reacting with superoxide anion (102). Glutathione peroxidase (GSHPx), catalase, and peroxiredoxins control the fate of hydrogen peroxide produced from superoxide anion. Similar to ecSOD, GSHPx has also been found in plasma. GSHPx-1, a selenoprotein, is found in the cytosol and mitochondria of all cell types, whereas distinct peroxiredoxins can be located in the cytosol (Prx) or mitochondria (Prx-III) (42, 132). These couples are associated with thioredoxins (Trxs) and thioredoxin reductases (TRs) in the cytosol (Trx1/TR1/Prx) and mitochondria (Trx2/TR2/Prx-III) to regenerate oxidized Prx (Fig. 2) (134, 256). In addition to the disulfide-bond formation in Prx from cysteinyl thiols as a result of hydrogen peroxide reduction, Prx can be inactivated through the oxidation of the active cysteine into sulfinic acid (Cys-SO2H; see later), which is efficiently reduced specifically by sulfiredoxins (Srxs) but not by Trxs (327, 331). Srx regenerates inactive 2-Cys Prx, returning it to the catalytic cycle and preventing its permanent oxidative inactivation by oxidative stress. Regarding the role of Srxs in maintaining active Prx-III, it is interesting to note that they reside in different cellular sites, Srx in the cytosol and Prx-III in mitochondria, thus questioning the significance of Srxs in regenerating the hyperoxidized mitochondrial Prx-III in vivo (42, 236, 328). Recent findings, however, indicate that Srx translocates to mitochondria in response to oxidative stress to restore Prx-III and that cells overexpressing mitochondria-targeted Srx are resistant to mitochondrial ROS-mediated cell death through the restoration of the peroxidase activity of Prx-III (236). Comparative analyses in HeLa cells indicated that Prx-I is more susceptible than Prx-II to hyperoxidation of the cysteine thiol at their catalytic site to sulfonic acid, which is considered an irreversible step. The mechanism underlying this differential susceptibility involved the acetylation of Prx-II in the N-terminal domain, which prevents Prx-II from irreversible hyperoxidation without altering its affinity for hydrogen peroxide (280).
FIG. 2.
Peroxiredoxin/thioredoxin couple. Peroxiredoxins (Prxs) are known to detoxify hydrogen peroxide, thus protecting proteins, which results in Prx oxidation with the formation of disulfide bonds. The reduction of oxidized Prx occurs by thioredoxins (Trxs), which are subsequently reduced by Trx reductase by using NADPH.
A key mechanism to control the production of ROS is through the transcriptional regulation of these enzymatic strategies. For instance, a number of transcription factors, including Nrf1/2, which works in association with Keap1, along with PGC1-α, and FoxO have been described to modulate the expression of antioxidant enzymes such as MnSOD, Prx3, Prx5, Trx2, and TR2, thus protecting against ROS overgeneration and oxidative stress in a number of conditions and cell types (243, 290). Recently, a mutual dependence of FoxO3A and PGC-1α in the induction of oxidative-stress genes was described in endothelial cells (239). Expanding the role of PGC-1α in mitochondrial biogenesis and protection against oxidative stress, these data show a novel function of PGC-1α acting as a partner for FoxO3 in the regulation of antioxidant genes and hence ROS generation. Because FoxO3 also induces proapoptotic proteins, further work is required to identify additional coactivators that modulate FoxO target specificity.
In addition to these efficient enzymatic systems to scavenge ROS, critical nonenzymatic antioxidants exist, some of which collaborate with the enzymatic partners, such as GSH. This critical antioxidant is a tripeptide (l-γ-glutamyl-l-cysteinyl-glycine), which owes its antioxidant function to the sulfhydryl group of cysteine (78, 159, 193). It is synthesized in the cytosol of all cells from its constituents, amino acids, glutamate, cysteine, glycine, and is then compartmentalized in various suborganelles (see later), where it plays a critical function in the detoxification of hydrogen peroxide produced from superoxide anion (96, 159). In addition to its redox-modulating effects, GSH is a versatile antioxidant because of its function as a cofactor for GSHPx and reductase (GR) in the so-called GSH redox cycle. Given the relevance of GSH in the defense against oxidative stress and in the susceptibility to different stimuli, the regulation and role of GSH (in particular, nuclear and mitochondrial GSH) is further discussed later. Hydrophilic antioxidants such vitamin E play critical roles in protecting lipid membranes from peroxidation by reacting with lipid radicals produced in the lipid peroxidation reaction, which removes the free radical intermediates and prevents the oxidation reaction from continuing. The oxidized α-tocopheroxyl radicals produced in this process may be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol, or ubiquinol (301, 321). In most cases, the generation of ROS is controlled by the availability and function of antioxidants. However, when the former overwhelm the latter, oxidative stress arises, compromising several cell functions by targeting and modifying critical components, including proteins, lipids, and DNA.
III. Oxidative Stress: More Than an Imbalance Between Oxidants and Antioxidants
As originally defined in 1985, oxidative stress was an imbalance between oxidants and antioxidants, in favor of the former (284). This implies that either the overgeneration of free radicals and ROS and/or the limitation in the function of antioxidants results in the net accumulation of ROS, which exert deleterious effects in cell functions, ultimately contributing to aging and major disease processes, including cardiovascular disorders, pulmonary diseases, diabetes, neurodegeneration, and liver diseases (32, 117, 289). This view has fostered the idea that the downregulation of ROS by supplemented antioxidants would be useful in the treatment of diseases, which so far has not been proven effective. Large-scale interventional studies in humans with antioxidants, based on the concept that oxidative stress reflects an imbalance between prooxidants and antioxidants, have been inconsistent in demonstrating health benefits, particularly in cancer patients (182, 303). The idea that redox signaling may specifically involve discrete pathways within cells suggests the possibility that oxidative stress can actually occur without an overall imbalance of prooxidants and antioxidants, and that the disruption of redox-sensitive signaling pathways can lead to metabolic and organ specificity in oxidative stress. In this regard, the GSH/GSSG and cysteine/cystine redox potential have been considered a useful estimation of the balance between oxidative reactions and endogenous antioxidant defenses. However, studies in human plasma have shown that whereas GSH concentration correlated with that of cysteine, no correlations were found between GSSG and cystine (155). Moreover, the redox-potential values (calculated by using the Nernst equation) showed that the plasma GSH/GSSG redox potential (−137 ± 9 mV) was more oxidized than the values reported in cells (−185 to −285 mV), but less oxidized compared with the redox potential values of cysteine/cystine (−80 ± 9 mV). Thus, the lack of equilibration regarding GSH/GSSG and cysteine/cystine pools between plasma and tissues suggests that these plasma redox levels do not appropriately reflect the complexity of oxidative stress as an imbalance between prooxidants and antioxidant systems (155). Thus, given these considerations, the conception of oxidative stress has been reoriented to reflect the disruption of thiol redox states, which normally function in cell signaling and physiologic regulation, without necessarily a net increase in reactive species. Particularly in aging research, the concept of a beneficial role for ROS in longevity has been provided in the so-called hormesis theory. For instance, glucose restriction has been shown to extend Caenorhabditis elegans longevity by stimulating the mitochondrial ROS generation, which was prevented by antioxidant pretreatment (275). A similar outcome was reported in transgenic flies overexpressing Mn-SOD and catalase, ectopically targeted to the mitochondrial matrix, showing that the increased catabolism of superoxide anion and hydrogen peroxide significantly reduced the life span of flies (17). These findings indicate that the rate of mitochondrial ROS release is not simply and negatively related to the life span and suggest that the increased formation of mitochondrial ROS may lead to a secondary increase in stress defense, which ultimately results in reduced net stress levels and extended life span. Hence, although ROS may play a beneficial role by acting as signaling intermediates, their inappropriate generation linked to alterations in thiol redox status may participate in the pathogenesis of diseases.
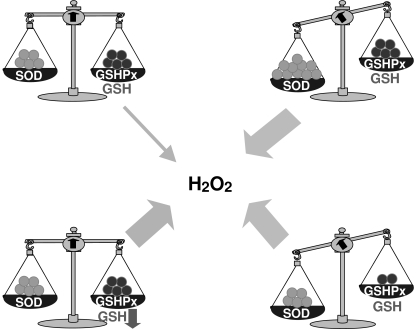
IV. Critical Balance Among Antioxidant Enzymes to Prevent Hydrogen Peroxide Accumulation
In addition to the control of ROS generation by antioxidants, a critical balance among antioxidant enzymes must operate to avoid the overgeneration of oxidants such as hydrogen peroxide. This has been well documented in the case of SOD and GSHPx, which modulate the conversion of superoxide anion to molecular oxygen and water in a two-step enzymatic process, involving first the dismutation of superoxide anion to hydrogen peroxide by SOD, followed by the conversion of hydrogen peroxide to molecular oxygen and water by GSHPx. Overabundance of SOD activity relative to that of GSHPx activity may lead to a net increase in hydrogen peroxide (Fig. 3), which can result in the harmful generation of the hydroxyl radical by the Haber-Weiss/Fenton reactions. Indeed, high levels of SOD have deleterious effects in cell function and life span. For instance, patients with Down syndrome carry three copies of Cu/ZnSOD gene in chromosome 21 and only two copies of GSHPx in chromosome 3 (75). Fibroblasts derived from these patients display increased activity of Cu/ZnSOD relative to GSHPx, resulting in increased basal levels of hydrogen peroxide. Bacteria that overexpress FeSOD or MnSOD show increased sensitivity to free-radical generators such as paraquat (23). In Drosophila melanogaster, overexpression of Cu/ZnSOD increases mortality during the development of the mature insect (240). Cultured mammalian cells overexpressing Cu/ZnSOD had increased lipid peroxidation and hypersensitivity to oxidative stress, which could be counterbalanced by increased catalase or GSHPx levels (2). The low-molecular-weight SOD mimetic mangafodipir (manganese dipyridoxyl diphosphate, MnDPDP) has been shown to kill colon and liver tumor cells by a poorly understood mechanism, which contrasts with the effect observed with two other mimetics, manganese(III)tetrakis(4-benzoic acid) porphyrin and copper(II)(3,5-diisopropylsalicylate)2 (177).
FIG. 3.
Critical balance among antioxidant enzymes to prevent hydrogen peroxide formation. Schematic representation indicating that the overabundance of SOD relative to that of GSHPx may lead to a net increase in hydrogen peroxide, as shown in the case of fibroblasts from patients with Down syndrome. In addition to this possibility and because GSHPx uses GSH as a cofactor, its limitation may also compromise the efficient elimination of hydrogen peroxide, resulting in its accumulation, which may lead to harmful effects.
Although these studies suggest that dismutation of superoxide anion is detrimental, the underlying mechanism remains speculative, with the most-accepted interpretation being that the increase in SOD levels leads to an increase in steady-state concentration of its dismutation product, hydrogen peroxide. In line with this interpretation, we specifically assessed the role of mitochondrial GSH in the efficacy of superoxide anion scavenging by an MnSOD mimetic in the protection of rat liver mitochondria and cultured primary hepatocytes against superoxide anion-induced dysfunction and cell death (319). Selective mitochondrial GSH depletion sensitized hepatocytes to antimycin A–mediated superoxide anion generation and subsequent death in the presence of MnTBAP compared with antimycin A alone. Together, these findings indicate that the dismutation of superoxide anion does not have a direct role in longevity and that the beneficial effect of its conversion to hydrogen peroxide depends on the fate of the resulting product, which is governed by the GSH redox cycle. A clear example of this is the elegant study of Giorgio et al. (116) in the characterization of cell and tissues from p66Shc-null mice. P66Shc is a redox enzyme that generates mitochondrial ROS, particularly hydrogen peroxide, as signaling molecules for apoptosis. For this function, p66Shc uses reducing equivalents of the mitochondrial electron-transfer chain through the oxidation of cytochrome c. Redox-defective mutants of p66Shc are unable to induce mitochondrial hydrogen peroxide and to mediate mitochondrial apoptosis in vivo, resulting in extended life span (116). Given the dual role of hydrogen peroxide in cell death and proliferation, p66Shc-mediated hydrogen peroxide production also is indispensable for cells to respond to the mitogenic effect of selected growth factors and the functional activation of the Ras-p53–dependent checkpoint (116). Overall, a better understanding of the regulation of redox signaling and the balance of pathways modulating the fate of hydrogen peroxide may lead to novel opportunities for the treatment of disease processes and aging.
Despite these findings regarding the deleterious effects of increased hydrogen peroxide generation in aging, some reports indicate that the overexpression of enzymes that downregulate hydrogen peroxide promotes disease pathogenesis. For instance, mice overexpressing GPx1 have been shown to develop insulin resistance and obesity (209), suggesting that increased GPx1 activity interferes with insulin function by overquenching the intracellular ROS required for insulin signaling. Moreover, as mentioned earlier, the SELECT (Selenium and Vitamin E Cancer Prevention Trial) reports showed inconsistent effects of antioxidants in cancer prophylaxis (182). In addition, the SELECT trial revealed a small increase in diabetes incidence (22, 291). These findings on longevity are consistent with the possibility that the extent of ROS release is directly proportional to the rate of accumulation of oxidatively damaged macromolecules, which in turn govern the rate of aging under normal conditions. However, the adverse effects of enhanced scavenging of ROS and hydrogen peroxide on cancer susceptibility and disease progression suggest that a critical physiological threshold is needed for proper cell function and signaling. At a more speculative level, it remains open whether, in addition to selenium and vitamin E, other antioxidants may have exerted beneficial effects in cancer prophylaxis. Overall, these studies imply that oxidative stress is far more complex than just an imbalance between oxidants and antioxidants, and that factors that regulate their source or compartmentalization or both may influence the final outcome of antioxidant strategies in disease pathogenesis.
V. Targets of Reactive Oxygen Species
A. Proteins
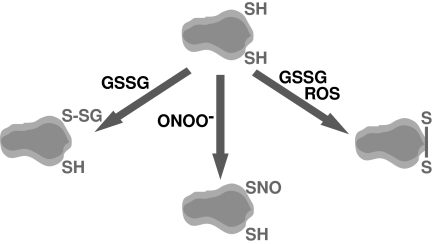
Cysteine is the most chemically reactive natural amino acid found in cells because of the thiol group. Thiols are found in proteins (PSH) and in low-molecular-mass metabolites such as GSH. Two thiols can be oxidized to form a disulfide bond, and disulfides are found mainly in proteins (PSSP), in GSSG, or as mixed disulfides between protein and glutathione (PSSG) (Fig. 4). In addition, thiols can be reversibly oxidized by ROS to nitrosothiols or sulfenic acids, which are regenerated by Trx/TR. Disulfides are important for protein structure and folding, and formation of disulfide bonds can regulate protein function (181). Furthermore, in addition to its putative role in the regulation of the targeted protein, glutathionylation (the formation of PSSG) has been suggested as a mechanism for protection against irreversible protein oxidation during oxidative stress (101).
FIG. 4.
Protein sulfhydryl fates. Cysteine is a chemically reactive amino acid present in proteins. It can be a target of ROS and oxidants. This scheme illustrates the major modifications of protein sulfhydryl moieties undergoing disulfide formation (which may then be restored by the Prx/Trx/TR system, shown in Fig. 2), glutathionylation, or nitrosylation, which may result in the alteration of protein function.
The primary function of Trx is to maintain reduced PSH pools in cells. The paradigm in this function is exemplified in the case of Prx, whose primary role is the reduction of hydrogen peroxide, which results in oxidized Prx. Six isoforms of mammalian Prx (Prx I to VI) are classified in three subfamilies: 2-Cys, atypical 2-Cys, and 1-Cys (256, 281). Prx I through IV belong to the 2-Cys Prx subfamily and exist as homodimers containing two conserved cysteine residues. In the catalytic cycle of 2-Cys Prxs, the conserved NH2-terminal Cys-SH is first oxidized by peroxides to cysteine sulfenic acid (Cys-SOH), which then reacts with the conserved COOH-terminal Cys-SH of the other subunit in the homodimer to form a disulfide bond, which is specifically reduced by Trx (Fig. 2). The resulting oxidized Trx is then regenerated by TR by using NADPH as reducing equivalents (38). Because of the slow conversion rate to a disulfide, the sulfenic intermediate can be further oxidized to cysteine sulfinic acid (Cys-SO2H), resulting in Prx inactivation, as this particular hyperoxidized state is not reduced by Trx (38, 236, 253). Besides Prx, ROS lead to the functional inactivation of Tyr and Ser/Thr phosphatases, including protein tyrosine phosphatase 1B, PTEN, low-molecular-weight phosphotyrosine-protein phosphatase, and Src homology phosphatase 2, through the formation of intramolecular disulfide bridges or sulfenyl-amide bonds (34, 49, 50, 52, 213, 285, 336). ROS also result in the activation of Tyr kinases by direct Cys oxidation (49). In this regard, recent findings indicate that hypoxia-mediated mitochondrial ROS stimulation results in c-Src activation through oxidation of the critical Cys 487 (188). Moreover, redox regulation of Src has been also involved in cell adhesion, spreading, anoikis resistance, and renal hypertrophy (48–50).
Another mechanism involved in the modification of thiols is through the addition of a NO moiety (S-nitrosylation) or a glutathione moiety (S-glutathionylation), both of which are produced by different reactions induced by nitric oxide–related species (71, 204, 336). S-nitrosylation induced by peroxynitrite formation shares some parallelism with sulfenic acid formation, as it is reversible (252). S-thiolation, the formation of a mixed disulfide between a protein thiol and a low-molecular-mass thiol, also is a reversible process. As glutathione is the most abundant intracellular low-molecular-mass thiol and a key regulator of the redox state, S-glutathionylation, the formation of a mixed disulfide with glutathione, is considered the most important and abundant thiolation mechanism in cells (39, 276). However, the formation of both modifications does not occur directly by addition of the respective species nitric oxide ( · NO) or glutathione (GSH) to the protein cysteine thiol (204). A critical line of defense against the glutathionylation of cysteine residues is the availability of glutaredoxins (Grx), which are compartmentalized in different subcellular sites, where they play a key role in restoring protein cysteine moieties to the sulfhydryl state (93).
B. Lipids
Phospholipids are predominant membrane components in eukaryotic cells, where they distribute asymmetrically across the bilayer. The most prominent phospholipids include phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, and cardiolipin, which consist of a hydrophilic head group with attached hydrophobic acyl chains. Membrane phospholipids are highly sensitive to oxidative modifications because oxygen and free radicals are more soluble in the fluid lipid bilayer than in aqueous solution, and tend to concentrate in organic regions. A paradigm of the significance of phospholipid oxidation in cell homeostasis is cardiolipin, a mitochondrial phospholipid, whose oxidation is a key event in the regulation of mitochondrial apoptosis, as discussed in detail in Section C.2.b. The phospholipid acyl chain normally varies from 14 to 22 carbons in length, with a ratio between saturated and unsaturated fatty acids of about 40:60, respectively (245). Thus, polyunsaturated fatty acids (PUFAs) are essential components of phospholipids and strongly affect their flexibility, selective permeability, and fluidity. PUFAs are extremely sensitive to oxidation, because the presence of a methylene group between two double bonds renders the fatty acid sensitive to ROS-induced damage, becoming more susceptible as the number of double bonds per fatty acid molecule increases. Reactive free radicals can pull off hydrogen atoms from PUFAs side chains, which are linked to a carbon in the fatty acid backbone by a covalent bond (Fig. 5). In this reaction, the carbon acquires an unpaired electron, becoming a free radical that combines with molecular oxygen dissolved in the membrane. The resulting peroxy-radical is highly reactive and more hydrophilic, contributing to its migration to the membrane surface, where it can alter membrane dynamics, react with membrane proteins and, more important, further oxidize adjacent PUFAs side chains. As a consequence, the process of lipid peroxidation becomes amplified through this free-radical chain reaction (35).
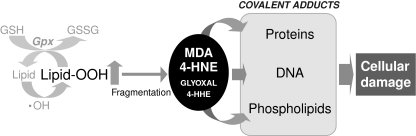
FIG. 5.
Lipid peroxidation and formation of reactive aldehydes. Free radicals, such as the hydroxyl radical, can react with lipids to generate hydroperoxides as well as endoperoxides. These species may undergo fragmentation to produce reactive intermediates, such as 4-hydroxynonenal (4-HNE), 4-hydroxyhexenal (4-HHE), malodialdehyde (MDA), and glyoxal, able to generate irreversibly covalent adducts with proteins, DNA, and phospholipids, leading to cell death.
Lipid peroxidation generates hydroperoxides as well as endoperoxides, which undergo fragmentation to produce a broad range of reactive intermediates called reactive carbonyl species (RCS), three to nine carbons in length, including highly reactive α,β-unsaturated hydroxyalkenals, such as 4-hydroxynonenal (4-HNE) and 4-hydroxyhexenal (4-HHE) (Fig. 5). Oxidation of n-6 PUFAs (mainly linoleic and arachidonic acid) leads to the formation of 4-HNE (89), whereas oxidation of n-3 PUFAs (docosahexaenoic acid, eicosapentaenoic acid, and linolenic acid) generates 4-HHE (308). 4-HNE-protein adducts are frequently formed by the Michael addition of 4-HNE to amino groups (Lys and His) or thiols (Cys or GSH), followed by cyclization and hemi-acetal or hemi-thioacetal formation, or by Schiff-base formation on primary amines (Lys). These reactive aldehydes have a much longer half-life (minutes to hours instead of microseconds to nanoseconds) than most ROS and RNS radicals. In addition, their noncharged structure allows them to migrate through hydrophobic membranes and hydrophilic media (cytosol), extending considerably the action area and increasing their damaging effects on target sites located within or outside membranes. In addition to the covalent modification of proteins, 4-HNE can react with DNA and activate cellular stress-response systems such as the transcription factors Nrf2 and Tfam (250, 305). Besides hydroxyalkenals, RCS include aldehydes and dicarbonyls, such as acrolein, malondialdehyde (MDA), glyoxal, and methylglyoxal (231). These aldehydes react with proteins to form advanced lipid peroxidation end products, altering protein functions and subsequent cellular responses. MDA and acrolein are formed during lipid peroxidation and can bind to nucleophiles (251). MDA is one of the most abundant aldehydes, resulting from peroxidation of arachidonic, eicosapentaenoic, and docosahexaenoic acid, and has been shown to alter protein functions (89). For instance, on reaction with Lys residues resulting in the formation of Schiff bases, MDA modulates low-density lipoproteins (287). Acrolein is a strong electrophile with high reactivity toward Cys, His, and Lys nucleophile residues (304). Another type of reactive lipid peroxidation end products is α-oxoaldehydes (methylglyoxal and glyoxal), which react with Lys and arginine residues (231).
Because of the reactivity of the by-products of lipid peroxidation, adducts or cross-linkings on proteins are formed, which progressively lead to impaired protein function, causing alterations in signal transduction that affect cell dysfunction, inflammatory response, and cell death (248). As a consequence, oxidative lipid damage is considered an important contributing factor in aging, atherosclerosis, alcoholic liver disease, and diabetes (31, 51, 245).
These deleterious effects are modulated by the presence of cellular metabolizing systems, in particular GSH, which plays a major role in the protection against lipid peroxidation. Although GSH can react spontaneously with 4-HNE through a Michael addition, the reaction catalyzed by GST is kinetically accelerated, resulting in the formation of a 4-HNE-GSH conjugate with the subsequent consumption of net GSH equivalents. GSTA4-4 is the main GST responsible for the metabolism of most long-chain α,β-unsaturated aldehydes, although other GSTs may be involved, in particular, GSTA3 (8). GSTA4 catalyzes the glutathionylation of α,β-unsaturated aldehydes to produce a poorly reactive conjugation product that is transported out of the cell. In addition, GSH reduces hydroperoxide formation during lipid peroxidation through GSHPx, thus limiting 4-HNE generation (147). This is a critical mechanism for cell protection, as 4-HNE has been described to impair mitochondrial function due to oxidative stress by limiting mitochondrial GSH, which could initiate a vicious circle between 4-HNE and mitochondrial dysfunction (179, 255). In addition, although it has been shown that mildly cross-linked HNE-modified proteins are preferentially degraded by the proteasome, extensive modifications by cross-linking aldehyde lead to the formation of protein aggregates that eventually inhibit the proteasome, contributing to cellular damage (122). In this regard, NF-κB activation has been shown to be inhibited by 4-HNE and acrolein, either through direct inhibition of the degradation of IκB by the proteasome or by specifically preventing the phosphorylation of IκB (242).
C. DNA
Cellular DNA is also subject to oxidative damage on exposure to a wide range of environmental genotoxins, anticancer drugs, or ROS, being particularly relevant in the case of mitochondrial DNA (10). Compared with the nuclear genome, the mitochondrial genome is highly vulnerable to oxidative stress, given its open circular structure, lack of histone protection, and proximity to the mitochondrial electron-transport chain, the main source of superoxide radical and hydrogen peroxide (225, 329). Oxidative mitochondrial DNA modifications include damage to purine and pyrimidine bases, sugar-phosphates, as well as single- or double-strand breaks. In this regard, the formation of 7,8-dihydro-8-oxoguanine (8oxodG) is commonly used as a measure of oxidative damage to DNA (128, 266). Consistent with this, mitochondrial GSSG has been shown to correlate with age-associated oxidative damage to mitochondrial DNA (76). Oxidative base damage does not involve direct superoxide anion or hydrogen peroxide attack on DNA but arises by the action of hydroxyl radical generated through reactions of hydrogen peroxide with metal ions (iron or copper) in close proximity to DNA. Moreover, superoxide anion and hydrogen peroxide (derived from quinone redox cycling) can induce DNA strand breaks reflective of endonuclease activation (128). Although lacking important nuclear nucleotide excision repair, mitochondria are endowed with an efficient base-excision repair system for the repair of oxidized bases (162, 258). Repair begins by the mitochondrial DNA glycosylase that recognizes and removes the damaged base, followed by the apurinic endonuclease that generates an apurinic/apyrimidinic site. Enhanced mitochondrial DNA repair induced by menadione has been associated with cell survival by targeting 8-oxodG glycosylase (OGG1) to the mitochondria (84). An adaptive response to increasing ROS concentrations has been linked to elevated apurinic endonuclease 1 levels, increased DNA repair rate, and attenuated oxidative mitochondrial DNA damage. In addition, a key feature of mitochondrial DNA is its organization as protein–DNA macrocomplexes, called nucleoids (113). Average nucleoids contain two to eight mitochondrial DNA molecules, which are organized by the histone-like mitochondrial transcription factor A. Recent evidence showed the presence of antioxidant enzymes, MnSOD and GSHPx1, as integral components of nucleoids to maintain mitochondrial DNA integrity (162). Thus, given the role of mitochondria as a source of ROS formation and in cell-death regulation, the preservation of intact mitochondrial DNA stability by mitochondrial antioxidants may be essential in the control of apoptosis and cell-death susceptibility during oxidative stress.
D. Signaling pathways
By targeting protein thiols, ROS generation can alter signaling pathways (e.g., kinases and phosphatases cascades, and transcription factors), which may have profound effects in multiple cell functions, including proliferation, differentiation, or cell death. ROS may ultimately commit hepatocytes for apoptotic/necrotic cell death by activating oxidant-induced hepatocyte apoptosis or by inhibiting cell-survival signaling cascades. The mitogen-activated protein kinases (MAPKs) constitute a family of serine/threonine kinases that includes extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK (69, 70). These enzymes are positively regulated by upstream kinases and inactivated by phosphatases. Activated MAPKs transduce a multitude of extracellular stimuli by phosphorylating and activating downstream transcription factors such as c-Jun and activating transcription factor-2 (ATF-2). Importantly, the role of this cascade in oxidant-dependent hepatocyte cell death depends on the intensity or duration or both of the stimuli. For instance, nontoxic concentrations of menadione induced low levels of transient ERK1/2 and JNK activation, whereas toxic concentrations of menadione led to markedly increased and prolonged activation of ERK1/2 and JNK. Although ERK1/2s are critical for hepatocyte resistance to superoxide anion generation by menadione, the sustained activation of JNK accounted for hepatocyte death, overcoming the effects of ERK1/2. In addition, ROS-mediated inactivation of MAPK phosphatase has been shown to contribute to hepatocyte death after TNF exposure (158). Thus, ROS can target different redox-sensitive pathways, whose interplay and final outcome in hepatocyte survival/death depends on the degree or duration or both of the activation/inactivation of these cascades, in particular, the relative level of activation of NF-κB, ERK1/2, or JNK. Because the underlying mechanism responsible for the modulation of cell-signaling cascades by ROS involves the redox status of protein cysteine thiols, antioxidant strategies also can control signaling pathways. In addition to Trx/TR/Prx, GSH and related enzymes have been shown to preserve PSH status and activity. For instance, GSH S-transferase (GST) Pi monomer has been reported to regulate the activation of the redox-sensitive kinase JNK (1). GSSG formation promotes the oligomerization of GSH S-transferase Pi, liberating active JNK, which regulates cell death. Similarly, ASK1, a MAPK kinase upstream of JNK and p38, is inhibited by reduced Trx. GSSG formation promotes oxidation of Trx, liberating active ASK1 (265). Moreover, GST mu1 (GST M1) protected primary hepatocytes against TGF-β1–induced apoptosis by blocking ASK1, a MAP kinase kinase kinase (MAPKKK) ubiquitously expressed, which mediates the activation of downstream targets, including JNK (c-Jun N-terminal kinase) and p38 MAP kinase by inflammatory cytokines (114).
VI. Hepatic GSH and Redox-Dependent Cell-Death Regulation
A. GSH synthesis and regulation
Among the armamentarium of antioxidants, the tripeptide GSH plays a central role, as it is the most abundant intracellular thiol, reaching millimolar concentrations in most cell types, especially in the liver. GSH is involved in many cellular functions, including antioxidant defense, by scavenging ROS in a chemical reaction driven by the potential of the redox environment toward equilibrium. In addition, GSH acts as a coenzyme of detoxification enzymes like GSH peroxidases and GSH-S-transferases (159, 193). GSH plays an essential role in maintaining the intracellular redox environment that is critical for the function of various cellular proteins. Although several reducing couples, such as GSH/GSSG, NADP+/NADPH, and Trx/TR, contribute to maintain the intracellular reducing environment, the GSH concentration is considerably greater than that of other couples and is therefore a determining factor of the redox potential, in which both GSH concentration and the molar ratio of GSH/GSSG contribute according to the Nernst equation
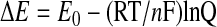 |
where Q denotes log([GSH]2)/[GSSG]. However, compared with GSH/GSSG ratio (about 100), which is maintained mainly by constant reduction of GSSG from NADPH reducing equivalents through catalysis of GR, the absolute GSH concentration may be a more-sensitive factor in redox potential, because of the square of the GSH concentration, according to the Nernst equation.
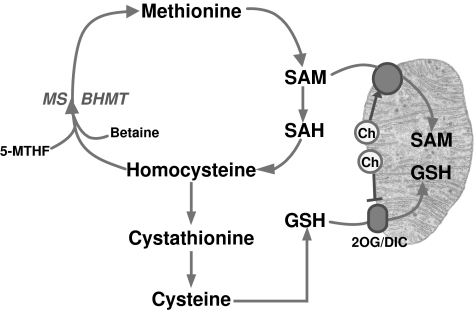
Hepatic GSH is maintained at a relatively constant concentration through GSH synthesis and turnover. De novo GSH synthesis occurs exclusively in the cytosolic compartment in two sequential ATP-dependent steps that are catalyzed by γ-glutamyl cysteine synthase (also called glutamate cysteine ligase, GCL) and GSH synthetase (212) (Fig. 6). GCL-catalyzed formation of γ-glutamylcysteine is the first and rate-limiting reaction in GSH synthesis, and it is feedback inhibited by GSH itself, a mechanism that is central in the regulation of cellular GSH concentrations (121). Cysteine is a rate-limiting substrate for de novo GSH synthesis and is derived from extracellular GSH breakdown by γ-glutamyl transferase (GGT) and/or from methionine-to-cysteine conversion in the transsulfuration pathway, in which the thiol of cysteine derives from methionine, with serine contributing to its carbon backbone. A key intermediate involved in this process is the synthesis of S-adenosyl-l-methionine (SAM) from methionine (193, 205). Thus, in addition to its role in methylation reactions in multiple cell types, the function of SAM as a GSH precursor is more restrictive, being particularly relevant in the maintenance of hepatic GSH homeostasis (95, 109, 205). Whereas the cystathionine pathway is characteristic in liver cells, the cleavage of circulating GSH takes place at the external plasma-membrane surface of various epithelial cells, such as kidney, pancreas, bile duct, and small intestine. In the GGT-catalyzed reaction, the γ-glutamyl moiety is transferred from GSH or GSH conjugates to acceptors like amino acids, dipeptides, or GSH itself, whereas cysteinylglycine is cleaved by membrane-bound dipeptidases. The resultant constituent amino acids and γ-glutamyl products are taken up into cells for de novo GSH synthesis. Thus, the GGT reaction is part of the γ-glutamyl cycle in intracellular GSH synthesis and GSH homeostasis (212).
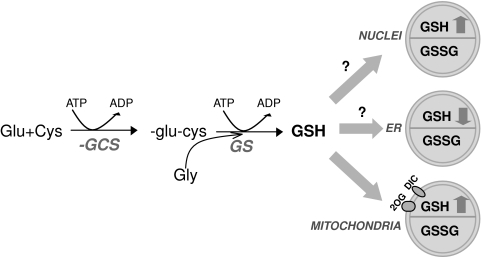
FIG. 6.
Glutathione synthesis and compartmentation. Glutathione (GSH) is synthesized in the cytosol by an ATP-dependent two-step process catalyzed by γ-glutamyl cysteine synthase (γ-GCS) and GSH synthetase (GS). Once synthesized, GSH is distributed to other organelles, such as the nucleus, the endoplasmic reticulum (ER), and the mitochondria, which constitute distinct redox pools in terms of GSH/GSSG distribution. To reach the mitochondria, GSH requires a carrier-mediated transport to cross the mitochondrial inner membrane. The dicarboxylate carrier (DIC) and the 2-oxoglutarate carrier (2OG) have been shown to function as GSH transporters.
In addition to the availability of cysteine, and because GCL catalyzes the rate-limiting step of GSH synthesis, the regulation of GCL constitutes a major step in the homeostasis of GSH. In general, GCL gene expression is upregulated under conditions in which increased cellular defense is necessary, and many agents have been described to elevate its expression, including xenobiotics (e.g., 5,10-dihydroindeno-[1,2-b]indole, tert-butyl hydroquinone), metals (e.g., zinc), Michael reaction acceptors (e.g., diethyl maleate), and lipid peroxidation products, such as HNE and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (25, 127, 333). Selenium deficiency also has been shown to upregulate the expression of the catalytic GCL subunit, resulting in the downregulation of plasma homocysteine and in the increase of plasma GSH (307). Consensus NF-κB, Sp-1, AP-1, activator protein-2 (AP-2), metal response, and antioxidant response (ARE)/electrophile-responsive elements have been identified in the human GCLC promoter. Previous studies have identified a proximal AP-1 element (−263 to −269) to be critical in mediating the effect of oxidative stress in the transcriptional induction of human GCLC (223). A critical distal ARE element (ARE4), located 3.1 kb upstream of the transcriptional start site, has been described, which mediated constitutive and xenobiotic inducible expression in HepG2 cells (226). In this response, the transcription factor Nrf2 plays an essential role by forming complexes with other Jun or Maf proteins, which bind to ARE4 in response to β-naphthoflavone, tert-butyl hydroquinone, or hydrogen peroxide (82, 324). Nrf1 and Nrf2 are members of the basic leucine-zipper proteins that can trans-activate ARE (151, 175), and these transcription factors have been shown to regulate rat GCLC promoter by modulating the expression of key AP-1 and NF-κB family members (193).
B. GSH compartmentation
1. Endoplasmic reticulum
Once synthesized in the cytosol, GSH is compartmentalized in discrete organelles such as ER, nuclei, and mitochondria, where it constitutes distinct redox pools in terms of the GSH/GSSG distribution, redox potential, and control of cellular activities (Fig. 6). The ER pool typically exhibits a total GSH (GSH+GSSG) concentration similar to that found in the cytosolic compartment (2– 10 mM). Based on previous findings (144), a feature of the ER redox environment is that GSH is in a predominant oxidized state, which is thought to favor disulfide bond formation and proper folding of nascent proteins in a process catalyzed by protein disulfide isomerase, in which oxidation of catalytic thiols is essential for proper enzyme activity. Despite its relevance to ER physiology, only a few studies have reported the status of GSH in the ER. An initial study using a tetrapeptide probe in a hybridoma cell line reported a GSH/GSSG ratio that varied from 1.5:1 to 3.3:1 (144), thus fostering the notion that a significant proportion of GSH is in the oxidized form. Another approach with monobromobimane estimated an ER GSH/GSSG status of 3:1, confirming the relative oxidizing GSH redox in the ER compared with the cytosol. Because of this oxidation state, the accurate quantification of the GSH redox status in ER is difficult and is subject to artifacts. In this regard, by using iodoacetic acid to preserve the GSH redox state and to avoid ex vivo oxidation during sample preparation, GSH concentration in rat liver microsomes was reported to be ∼4– 6 mM, which is in the range of previous estimations (15). Intriguingly, although the use of iodoacetic acid to minimize the extensive oxidation during microsomal isolation estimated a GSH/GSSG ratio somewhat higher (5:1) than previously reported (15, 144), this approach did not affect the extent of glutathionylation of ER-resident proteins, which represented a minor fraction of the total GSH found in the ER (83), as opposed to previous reports (15). Although further work in understanding the homeostasis of GSH in the ER is needed, these new insights suggest that mixed disulfides between GSH and ER proteins is a rate-limiting step in enzyme-catalyzed protein folding. Furthermore, it could be speculated that glutathionylation in the ER is a highly regulated process kept to a minimum to limit protein transit time through the secretory pathway to regulate protein maturation and degradation in the ER. Moreover, the reductase activity of disulfide isomerase has been shown to be dependent on a reduced GSH status (83). These features imply that the role of GSH in the ER is to maintain oxidoreductase catalytic function and the appropriate redox environment to control ER-generated ROS and redox state, which otherwise may disrupt ER function, resulting in the activation of the unfolding protein response and subsequent cell death (40, 153).
2. Nuclei
The nuclei exhibit an independent GSH pool, which plays an important role in the protection against oxidant- and ionizing radiation–induced DNA damage, as well as in the maintenance of nuclear proteins in an adequate redox status required for gene transcription (65, 225, 244). In addition, GSH functions as a hydrogen donor in ribonucleotide reductase–catalyzed reduction of ribonucleotides to deoxyribonucleotides, thus playing a critical role in DNA synthesis (142). Recent studies showed that the nuclear GSH pool is not in equilibrium with cytosolic GSH, with higher nuclear-to-cytosolic GSH levels reported in different cell types, including rat hepatocytes and developing neurons (18, 296). The trafficking of nuclear GSH is a dynamic process, which correlates with cell-cycle progression. A higher nuclear-to cytosolic GSH gradient has been associated with proliferative states, whereas an even distribution between the two compartments predominates in confluent cells (202, 244). However, because the nuclear membrane breaks down during cell division, it is conceivable that the homogeneous distribution of GSH between nuclei and cytosol after cell proliferation may reflect the loss of compartmentation. Nevertheless, the mechanisms underlying the trafficking and dynamics of nuclear GSH distribution are poorly understood, although a passive diffusion of GSH from the cytosol to the nuclei via nuclear pores has been suggested as the responsible mechanism (Fig. 6) (139). Intriguingly, a novel role for Bcl-2 in the maintenance of high nuclear GSH levels has been described (318). Although the mechanism underlying this observation remains to be fully established, another related observation indicated the ability of GSH to bind to the Bcl-2 homology-3 domain groove in mitochondria (342). Whether this binding described in mitochondria occurs in nuclei remains to be further investigated.
3. Mitochondria
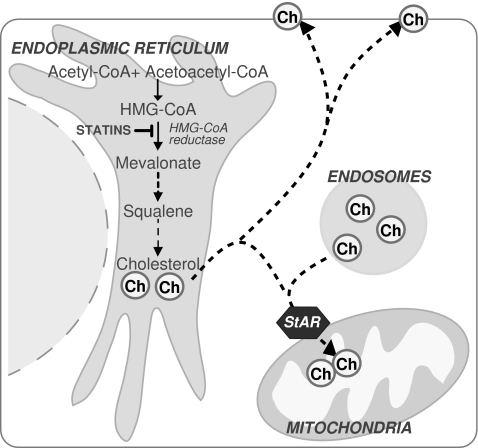
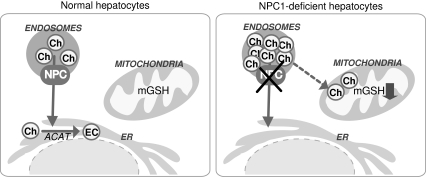
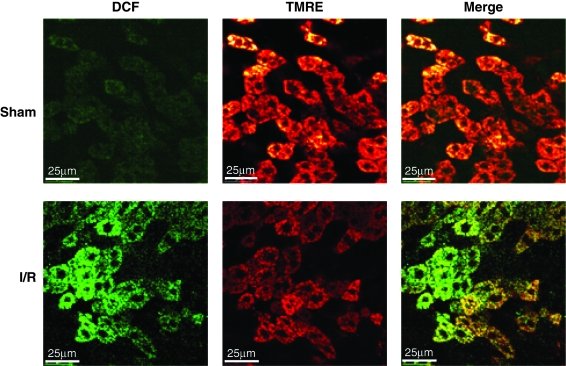
Mitochondria are the main consumers of molecular oxygen in the cell, which is used as a transducing device to provide the energy required for ATP synthesis in the oxidative phosphorylation. One of the collateral effects of this constant oxygen consumption is the leakiness of electrons to molecular oxygen, resulting in the formation of ROS (Fig. 1). As indicated earlier, mitochondria are endowed with a wide range of antioxidant strategies, most notably GSH, which represents a metabolically separated pool with respect to the cytosol in terms of synthesis rate and turnover, first described in the 1970s (154). As mitochondria do not contain catalase, the GSH redox cycle is the main defense to avoid hydrogen peroxide accumulation. Mitochondrial GSH (mGSH) is predominantly in the reduced form and represents a minor fraction of the total GSH pool (10–15%) (Fig. 6). Considering the volume of the mitochondrial matrix, the concentration of mGSH may be similar to that of cytosol (∼10 mM) (111, 120). Because the mGSH concentration is high, moderate depletion of mGSH would not be expected to affect negatively the disposal of hydrogen peroxide by GSHPx or the mitochondrial function. However, the depletion of mGSH below a critical level can compromise the adequate reduction of hydrogen peroxide, particularly in conditions of stimulated ROS generation from the mitochondrial electron-transport chain. Thus, under complex III inhibition by antimycin A, increased hydrogen peroxide generation was observed only when GSH was depleted to 2–3 nmol/mg protein (110), which corresponds to the range of the Km of GSHPx for GSH (183). Depletion of mGSH below 40% leads to stimulated hydrogen peroxide generation from complex I (130). Furthermore, GSH contributes to the reduction of organic hydroperoxides, including products of lipid peroxidation, through GST and GSHPx (21, 44). Despite the high GSH concentration existing in mitochondrial matrix, de novo GSH synthesis does not occur in this organelle, and mitochondrial GSH arises by the activity of specific carriers, the 2-oxoglutarate carrier (2-OG) and the dicarboxylate carrier (DIC) that have been partially characterized in kidney and liver (45, 46, 61). These antiport carriers have been shown to import cytosolic GSH into mitochondria against an electrochemical gradient in the exchange of matrix dicarboxylates (201). Whether these carriers also participate in the efflux of mGSH in exchange for cytosolic GSH is unknown. Remarkably, a recent study with an immortalized human colonic epithelial cell line reported that butylmalonate and phenylsuccinate, inhibitors of DIC and 2-OG, respectively, increased rather than decreased mGSH levels without changing the cytosolic GSH pool, suggesting that these carriers exchanged mGSH for cytosolic GSH (54). However, whether these observations are specific for the particular cell line used remains to be established. Furthermore, recent evidence in cultured neuronal cells implied a role for the antiapoptotic Bcl-2 member in the modulation of mGSH pool at mitochondrial membrane sites (342). As with the nuclear trafficking of GSH, the role of Bcl-2 in the trafficking of GSH into mitochondria remains to be fully understood. Moreover, a role for UCP2 in the transport of mitochondrial GSH has been described in neurons, suggesting that the transport of protons back into the matrix by UCP2 may favor the movement of GSH (73). Unlike 2-OG and DIC carriers, the evidence for these alternative carriers in the transport of mitochondrial GSH in hepatic liver mitochondria remains to be established. A key feature of the transport of GSH through 2-OG is its strict dependence on appropriate membrane dynamics (96), which is determined by fatty acid composition and the cholesterol/phospholipid molar ratio (61, 189). Reduction of appropriate membrane fluidity impairs this transport system, resulting in mGSH depletion. This selective pool of GSH has been shown to play a key role in controlling cell susceptibility to oxidative stress, Ca2+ overload, TNF/Fas, and hypoxia (7, 188, 190, 192, 197, 198), and its depletion has been shown to contribute to a number of pathologies (96, 104, 169). Interestingly, the hepatocellular susceptibility to TNF induced by mGSH depletion was observed despite unimpaired GSHPx, Trx2, and PrxIII, thus highlighting the relevance of this antioxidant in cell protection under oxidative stress. By regulating the mitochondrial ROS generation, mGSH was recently shown to play a key role in the preservation of the reduced cardiolipin status (198), which regulates cell-death pathways, as described later.
C. Role of GSH in cell death
1. Mechanisms of cell death
Among the various recognized forms of cell death that include necrosis and autophagy, apoptosis is evolutionarily conserved, highly organized, and characterized by distinctive nuclear changes, chromatin shrinkage, DNA fragmentation, membrane blebbing, and the formation of apoptotic bodies that contain components of the dying cell. Since its rediscovery in the early 1970s (161), it has become an intense field of research because of its involvement in the development of pathologic states characterized by either the decreased (e.g, cancer) or increased (e.g., steatohepatitis) incidence of apoptosis (98, 119, 196). The morphologic features of apoptosis are generated during the activation of specific cellular cysteine proteases (caspases) (268) that can occur through two main pathways: death receptor– (also called extrinsic) or mitochondria- (also called intrinsic) mediated pathways.
The extrinsic death-receptor–mediated pathway is triggered by extrinsic signals, such as components of the tumor necrosis factor family (TNF), like TNF, Fas/CD95 ligand, or TRAIL, which bind to death receptors on the plasma membrane. At the level of the activated receptor, proapoptotic proteins interact through their death domains or death-effector domains, resulting in the formation of the death-inducing signaling complex (DISC) (214, 227). Initiator caspases, such as caspase-8 or caspase-10, are recruited to the DISC, and on activation, they trigger a caspase cascade that determines the downstream activation of executioner caspases-3 and −7, followed by apoptosis-induced cellular demise. In certain cell types, type II cells, such as hepatocytes, the activation of caspase-8 by TNF is weak and, therefore, insufficient to complete cell apoptosis (270). In type II cells, the cleavage of the proapoptotic protein Bid by active caspase-8 engages the mitochondrial apoptotic cascade, establishing a cross-talk between the death-receptor and mitochondrial pathways in apoptotic signaling. In addition to the TNF receptor 1 internalization, another critical step in TNF signaling involves endosomal trafficking and activation of acidic signaling components, including sphingomyelinases (224, 273) (see later).
The intrinsic mitochondrial apoptotic pathway involves the release of proapoptotic proteins from mitochondria to the cytosol. Different apoptotic stimuli, such as ROS/RNS and mitochondrial DNA damage, can mediate mitochondrial outer membrane (MOM) permeabilization and the release of mitochondrial proapoptotic proteins, like cytochrome c, AIF, or second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI (Smac/Diablo) (98, 119, 316). Within the cytosol, these apoptogenic proteins trigger caspase-dependent or caspase-independent signaling events. For example, cytochrome c binds to the apoptotic protease-activating factor-1 (Apaf-1) and forms the apoptosome to which procaspase-9 is recruited. The ATP-dependent cleavage of procaspase-9 signals downstream activation of effector caspases, such as caspase-3 and −6/7. Additionally, Smac/Diablo, which antagonizes inhibitors of caspases, enhances caspase activation. Interestingly, through mitochondria-to-nuclear translocation, AIF participates in caspase-independent mitochondria-mediated apoptosis, in which it induces chromatin condensation and DNA fragmentation (297). Because MOM constitutes a physical barrier that prevents the release of these apoptotic proteins, the breakage of this membrane controls cell death. At least two mechanisms underlying MOM permeabilization have been suggested, which differentiate depending on whether the mitochondrial inner membrane (MIM) permeabilization occurs, involving either a primary role of the mitochondrial permeability transition (MPT) or the Bcl-2 network (13, 171). In the former, the usually impermeable MIM prevents unrestrained influx of low-molecular-weight solutes into the mitochondria. The MPT features mitochondrial swelling, uncoupling, and MIM permeabilization to small solutes, which results in a colloidal osmotic pressure that leads primarily to massive swelling of the mitochondrial matrix (171). As a consequence of MPT, the MOM ruptures, and cytochrome c and other mitochondrial intermembrane space proteins are released into the cytosol. MTP is most likely assembled at the contact sites formed by the physical interaction of MOM and MIM. Although the actual components of the MTP remain to be fully described, evidence indicates a role for VDAC, ANT, and cyclophylin D in this process. However, the role of VDAC in apoptosis is uncertain (9), whereas cyclophilin D has been shown to be a key factor in necrosis induced by ischemia/reperfusion (229). The other model proposes that Bcl-2 family proteins, particularly BH1-3 members such as Bax/Bak, control MOM permeabilization. In this regard, Bax oligomerization is considered a critical regulatory point in cell death by the formation of pores in MOM (119, 171). However, attempts to visualize these oligomers have shown that large clusters of Bax are localized near, but not on, the MOM. An alternative model suggests that the insertion of activated, oligomerized Bax or Bak or both into the MOM creates a positive curvature stress on the membrane, leading to supramolecular pores that include lipids (lipidic pores) in the MOM (14, 174). Clearly, understanding the mechanisms underlying MOM permeabilization may provide novel strategies to regulate cytochrome c release and hence apoptosis.
2. Regulation by GSH
a. Regulation of the extrinsic pathway
Critical signal-transduction and transcriptional pathways are activated by redox stress, which contributes to many forms of liver injury and diseases. Because GSH modulates these redox pathways, the role of GSH in hepatocellular death has been critically examined in response to TNF and Fas, two ligands of the TNF-receptor family with relevance in liver diseases (100, 196). An important aspect in the signaling of TNF on binding to its receptor is the stimulation of both survival and death signals, which are exemplified by the formation of two sequential signaling complexes, the balance of which determines the ultimate fate of cells (214, 227). In brief, the rapidly forming complex I is assembled on the receptor's cytoplasmic tail and consists of the adaptor TRADD, the protein kinase RIP1, and the signal transducer TRAF2. This complex signals inflammation and survival through IκB kinase (IKK)-dependent activation of transcription factor NF-κB. Subsequently, complex I dissociates from the receptor, and TRADD together with RIP1 associate with the adaptor protein FADD and procaspase-8 to form complex II. Activation of caspase-8 can lead to the proteolytic activation of executioner caspase-3, which contributes to the apoptotic demise of the target cell. As mentioned earlier, in hepatocytes, the activation of caspase-3 requires the participation of mitochondria and the apoptosome assembly through the cleavage of Bid by the active caspase-8, resulting in the formation of a truncated fragment that elicits the mitochondrial membrane permeabilization requiring the participation of Bax/Bak (270).
Through maintenance of protein sulfhydryls in the appropriate redox state, GSH can regulate the activity of caspases and NF-κB, thus modulating the death/survival balance and the susceptibility to TNF (55, 85, 97). The effect of GSH depletion on DISC and caspase-8 activation by death ligands is controversial, with the outcome dependent on the specific cell type used (138). Thus, mouse hepatocytes are rendered susceptible to TNF-induced cell death by GSH depletion in the cytosol by impairing TNF-mediated NF-κB transactivation and the subsequent activation of survival pathways by IκB kinase–dependent and –independent mechanisms (191, 206, 228). Moreover, severe GSH depletion induced necrosis of primary mouse hepatocytes, whereas the addition of TNF sensitized to apoptosis, involving increased caspase activity and cytochrome c release. Thus, the extent and site of GSH depletion (cytosol or mitochondria or both) can determine the fate of hepatocytes in response to TNF and the mode of death (apoptosis versus necrosis). Moreover, redox regulation of NF-κB constitutes a primary mechanism determining the susceptibility of hepatocytes to TNF, primarily by modulating the expression of survival genes, which control caspase activation. However, in addition to this function, NF-κB also is known to modulate the generation of ROS from mitochondria, resulting in the subsequent sustained activation of stress kinases such as JNK (158, 315). In this regard, NF-κB contributes to the transcriptional upregulation of Mn-SOD as well as to the induction of the ferritin heavy chain, which modulate TNF-mediated ROS generation (29, 249). Interestingly, a novel mechanism of noncanonic NF-κB activation through a multistep pathway was described recently, involving a Bcl-3/p50 complex in U937 cells after GSH depletion by BSO (68). This process requires two steps: an early phase accompanied by substantial GSH depletion produces a cytosolic preparative complex consisting of p50 and its interactor Bcl-3 linked by interprotein disulfide bridges. In a late phase that coincides with ROS production, the complex is targeted to the nucleus, resulting in the transactivation of Bcl-2 and resistance to BSO-mediated apoptosis. Overall, these data suggest that the outcome of GSH depletion on NF-κB may be dependent on the type of cell/stimuli used, as well as the pathway of NF-κB activation (canonic versus noncanonic). Another key aspect in this differential outcome may relate to the compartmentation of GSH depletion (cytosol versus nuclei) and the consequent redox regulation of Trx, particularly in the nuclei, which promotes the reduced state of NF-κB (208). Cross-talk between caspase activation and redox regulation of Grx-1 in response to Fas was recently described in type I cells (murine alveolar epithelial cells, fibroblasts, and CD4+ T lymphocytes) (3). Stimulation of Fas ligand induced S-glutathionylation of Fas at cysteine 294 due to caspase-dependent Grx1 degradation, which amplified subsequent caspase activation and apoptosis. Although this process defines a novel redox-based mechanism to propagate Fas-dependent apoptosis, it remains to be established whether this mechanism occurs in hepatocytes. Consistent with this possibility, it is known that Fas stimulates ROS generation in hepatocytes, which favors the formation of its reduced form GSSG and hence the S-glutathionylation of target proteins, such as Fas.
b. Regulation of the intrinsic pathway
In addition to the critical role of the MOM permeabilization in cytochrome c release and subsequent apoptosome activation and cell death, cytochrome c mobilization from the mitochondrial intermembrane space constitutes a central mechanism in cell-death regulation. It has been proposed that during apoptosis, cytochrome c detaches from the MIM by dissociating from the membrane phospholipid cardiolipin (117). A significant proportion of the cytochrome c in the mitochondria seems to be associated with cardiolipin, involving two major mechanisms. At physiologic pH, cytochrome c has +8 net charges, establishing an electrostatic bond with the anionic cardiolipin. In addition, cytochrome c has a hydrophobic channel through which one of the four acyl chains of cardiolipin inserts. The other chains of cardiolipin remain in the membrane, anchoring cytochrome c to the MIM (157). One of the mechanisms involved in cytochrome c detachment from MIM includes cardiolipin oxidation, because oxidized cardiolipin has a much lower affinity for cytochrome c than the unoxidized form (170, 198). Cardiolipin oxidation occurs by different mechanisms, including the formation of a cardiolipin–cytochrome c complex with a stimulated peroxidase activity (117, 156). Furthermore, mGSH has been shown to control the formation of peroxidized cardiolipin during TNF-mediated hepatocellular cell death by regulating ROS generation (198). In addition, by using liposomes mimicking the composition of MOM entrapping fluorescent dextrans, it was observed that the incorporation of peroxidized cardiolipin in the bilayer enhanced the pore-forming activity of active Bax by restructuring the lipid bilayer through a mechanism promoting the lamellar-to-inverted hexagonal lipid-phase transition (198). Despite these data indicating a role of cardiolipin in the regulation of cytochrome c mobilization and MOM permeabilization, events that are associated with cristae remodeling, other evidence neglects this function. It has been suggested that the remodeling of cristae is a required step in the release of the inner pool of cytochrome c (278), and recent studies correlated the disassembly of Opa1 oligomers, a structural determinant of cristae morphology, with the remodelling of cristae (99). Hence to account for the rapid and extensive release of cytochrome c during apoptosis, it has been shown that cristae are remodeled such that cytochrome c is redistributed in the mitochondria before translocation through the MOM. However, by using fluorescence microscopy followed by three-dimensional electron-microscope tomography, it was found that cristae remodeling was not required for efficient release of cytochrome c (294). Moreover, besides its putative role in cytochrome c mobilization, recent studies provided evidence for a novel role for cardiolipin acting as an essential activating platform for caspase-8 on mitochondria (118). In type II cells, TNF or Fas caused the translocation of caspase-8 to mitochondria, where it binds to cardiolipin, resulting in the oligomerization and activation of caspase-8. Because in type I cells, DISC assembly and caspase-8 activation by death ligands occur in specific domains of the plasma membrane, lipid rafts, it is conceivable that the new role of mitochondria in activating caspase-8 may also take place in raftlike domains, which requires further investigation. Whereas these findings are of significance in disorders in which tafazzin, a mitochondrial transacylase enzyme required for cardiolipin maturation, is mutated, such as in the Barth syndrome, the relevance of this novel function of cardiolipin to anchor caspase-8 to mitochondria in liver diseases remains to be established.
Another interesting role of GSH in cell-death regulation was described recently in neurons and cancer cells through the redox inactivation of cytochrome c (311). The proapoptotic activity of cytochrome c is influenced by its redox state, so that increased ROS following an apoptotic insult leads to the oxidation and hence activation of cytochrome c. Thus, this novel finding suggests that even a modest increase in ROS can prime cells to undergo apoptosis in response to otherwise potentially nonlethal events of mitochondrial damage and cytochrome c release. Hence, these emerging mechanisms related to cardiolipin and cytochrome c redox regulation may be dependent on mGSH, pointing to this antioxidant as a critical therapeutic target for the control of cell death and oxidative stress–related diseases (201).
D. Redox control of caspases
In addition to the redox modulation of cardiolipin and cytochrome c, another level of apoptosis regulation is through the redox control of caspases. Caspases are a growing family of cysteine proteases that play a critical role during apoptosis. Their name refers to the active cysteine group and the characteristic cleavage of their targets at aspartate residues. The presence of a cysteine moiety in the catalytic site is essential for their function, as its replacement by other amino acids results in loss of function (325). As with many other proteins, the enzymatic activity of caspases is regulated by the redox state of the cysteine residue (129, 306). As described in Jurkat cells after Fas challenge, caspase-3 activity exhibited a dual regulation, depending on the extent of ROS generation by hydrogen peroxide. In an oxidizing state induced by a high dose of hydrogen peroxide, caspase-3 became inhibited because of the oxidation of the critical cysteine residue (129). Another mechanism of redox caspase regulation is by nitric oxide and the subsequent S-nitrosylation of the active-site thiol (219). In addition to directly modulating caspase-3 activity, ROS have been described to regulate the processing of the caspase-3 proenzyme, by regulating the activity of caspase-9 (235, 306). Furthermore, recent findings have shown the regulation of caspase-1 by superoxide dismutase 1 (211). SOD1-deficient macrophages exhibited higher superoxide anion generation, decreased redox potential, and inhibited caspase-1 by reversible oxidation and glutathionylation of the redox-sensitive residues Cys397 and Cys362. As a consequence of the redox regulation of caspases, the apoptotic phenotype is compatible with low to moderate oxidative stress, whereas high doses of ROS shift cell death toward necrosis. As many forms of liver diseases are accompanied with a burst of ROS and subsequent oxidative stress, therapeutic strategies using caspase inhibitors may be of limited value, as they may prevent the acquisition of an apoptotic phenotype without efficiently protecting against ROS-mediated necrosis.
VII. Lipid-Mediated Reactive Oxygen Species Generation and Liver Diseases
Lipids such as sphingolipids (SLs), cholesterol, and free fatty acids are integral components of biologic membranes that play a major structural role. However, recent evidence has shown that these lipids are more than just structural membranes components. Thus, lipids actively participate in and regulate signaling pathways and ROS generation, potentially contributing to cell-death susceptibility. In particular, cholesterol and SLs are not randomly distributed within membranes, but are concentrated in specific domains called lipid rafts, where specific signaling pathways occur (309). In this section, we briefly describe their mechanism of action linked to oxidative stress, ROS generation, and the role in hepatocellular injury.
A. Sphingolipids
SLs constitute a class of lipids that function as second messengers in different cellular processes such as cell differentiation, growth, and cell death (166, 224). Ceramide is the prototypic SL that has been most intensively studied in relation to cell death and stress response. Ceramide levels increase before the onset of cell death, arguing in favor of ceramide upregulation as a cause rather than a consequence of cell death (133). Among the different known cellular targets, ceramide has been shown to disrupt mitochondrial electron flow at complex III, resulting in enhanced ROS generation, which facilitates cytochrome c release and caspase activation (108, 123). Cellular ceramide levels can increase by several mechanisms. In addition to the de novo synthesis through activation of serine-palmitoyl transferase, the rate-limiting enzyme in ceramide synthesis, or ceramide synthetase, ceramide can arise from hydrolysis of sphingomyelin-engaging sphingomyelinases (SMases) (166, 224) (Fig. 7). This pathway may be of significance in promoting specific macrodomain formation in the plasma membrane, allowing oligomerization of certain cell-surface proteins such as TNF and Fas (66). Several SMases have been characterized, two of which are of particular relevance in cell signaling. The membrane-bound neutral SMase (NSMase), with an optimum pH of ∼7.5, and an acidic SMase (ASMase), with an optimum pH of ∼4.8, are further subclassified as an endosomal/lysosomal ASMase and a secretory Zn2+-dependent SMase (200, 224). Apoptotic stimuli, such as death ligands (e.g., Fas and TNF), chemotherapeutic agents or ionizing radiation activate these SMases, which account for the ability of the inducing stimuli to generate ceramide with different kinetics and possibly at distinct intracellular locations. Although the precise intracellular site of ceramide generation by individual SMases remains to be clearly established, the domains within the intracytoplasmic region of the death-ligand receptor responsible for the activation of NSMase and ASMase are different (166). Conflicting data on the role of ceramide and SMases in cell-death signaling have been published (277). For instance, NSMase is activated through FAN (factor associated with NSMase) and leads to the accumulation of ceramide at the plasma membrane; however, its role in TNF-mediated cell death remains unclear (105, 278). Activation of ASMase is dependent on TNFR1 internalization and is mediated through the death domain of TNFR1 by the recruitment of the adaptor proteins TRADD and FADD (277). A role for ASMase in transmitting apoptotic signals of death receptors has been reported for TNF (105, 136, 137), Fas ligand (27, 53), and TRAIL (87). Inhibition of the formation of clathrin-coated pits blocks the activation of the endolysosomal ASMase and JNK, as well as TNF-induced cell death. In contrast, inhibition of TNFR1 internalization does not affect the interaction of the adaptor molecules FAN and TRADD with TNFR1 at the cell surface, the activation of plasma-membrane–associated NSMase, or the stimulation of proline-directed protein kinases. The role of ASMase in cell death has been shown to involve two distinct mechanisms, including the recruitment of mitochondria through ganglioside GD3 generation (106), and the downregulation of liver-specific methionine adenosyltransferase-1A (MAT1A), the rate-limiting enzyme responsible for the synthesis of S-adenosyl-l-methionine (SAM) (Fig. 8) (199). In addition to recruiting mitochondria to stimulate ROS generation, MOM permeabilization, and cytochrome c release (107, 260), ganglioside GD3 has been shown to prevent the nuclear translocation of DNA binding competent members of NF-κB, thus disabling survival factors and sensitizing hepatocytes to TNF-mediated cell death (60). Interestingly, Garofalo et al. (112) suggested the existence of lipid microdomains composed of GD3/Bax in mitochondria from T cells. Whether these microdomains exist also in liver mitochondria has not been reported yet, and it deserves further research. As a key intermediate of TNF/Fas, ASMase contributes to hepatocyte apoptosis, thus emerging as a novel therapeutic target for liver diseases (see later).
FIG. 7.
Ceramide metabolism. Scheme depicting the different pathways that lead to the generation (e.g., de novo synthesis in the endoplasmic reticulum and hydrolysis of sphingomyelin by sphingomyelinases), or catabolism (e.g., synthesis of gangliosides in the Golgi, and generation of ceramide-1-phosphate and sphingosine-1-phosphate) of ceramide.
FIG. 8.
Dual role of GD3 in cell death. Ganglioside GD3, generated after activation of acid sphingomyelinase, has been shown to promote cell death by two different mechanisms: GD3 is able to migrate to the mitochondria, where it generates ROS, cytochrome c release, and cell death; conversely, GD3 downregulates the liver-specific methionine adenosyltransferase-1A (MAT1A), the rate-limiting enzyme responsible for the conversion of methionine (Met) into S-adenosyl-l-methionine (SAM), a GSH precursor, leading to the depletion of GSH, and therefore to an increased susceptibility to cell death.
B. Cholesterol
Cholesterol is a critical component of biologic membranes, which plays an essential role in determining membrane physical properties and in the regulation of multiple signaling pathways (103, 146). Cholesterol is distributed to different membranes, most prominently to plasma membrane, where it participates in the physical organization of specific domains. Mitochondria are considered cholesterol-poor organelles and obtain their cholesterol load by the action of specialized proteins involved in cholesterol delivery from extramitochondrial sources followed by regulated trafficking within mitochondrial membranes (Fig. 9) (103). Because of its structural role in membrane dynamics, the homeostasis of cholesterol is highly regulated, as its accumulation in cells is toxic and causes fatal diseases. For instance, Niemann-Pick type C (NPC) disease is a fatal neurodegenerative disease characterized by lysosomal storage of cholesterol and glycosphingolipids. Although most patients with NPC exhibit neurologic symptoms, cholesterol accumulates in the liver and pancreas, and some patients die early of liver failure before manifestations of the neurologic symptoms. Moreover, NPC disease is the second most common cause of neonatal cholestasis. Ten percent of NPC infants with neonatal cholestasis die of liver failure before they reach 6 months of age, and patients who survive often live with persistent liver disease accompanied by fibrosis, and, in some rare cases, cirrhosis. Recent studies in NPC-1–deficient mice showed that hepatocyte apoptosis is a primary cause of liver dysfunction and liver failure (19). In addition, recent data demonstrated a key role for TNF in NPC-1 deficiency–mediated hepatocyte apoptosis (259). These data suggest that cholesterol accumulation in NPC sensitizes to TNF-induced hepatocellular cell death. Although the molecular mechanisms underlying these observations are not completely understood, recent findings have shown that free cholesterol in NPC-1–deficient hepatocytes accumulates in mitochondria but not in endoplasmic reticulum, resulting in a selective mGSH depletion and subsequent sensitization to TNF-mediated cell death by overgeneration of mitochondrial ROS (197) (Fig. 10). Although hepatic mitochondrial cholesterol fulfills an important physiological function, such as bile acid synthesis, its overaccumulation in mitochondria impairs vital membrane functions, including transport of GSH from the cytosol into mitochondria, leading to mGSH depletion (96, 189). Consistent with the selective accumulation of mitochondrial cholesterol in NPC disease, this particular pool of cholesterol has emerged as an important factor in steatohepatitis through mGSH depletion (Fig. 11). However, the mechanisms underlying the mitochondrial cholesterol loading in hepatic steatosis are currently unknown. In hepatoma cells, however, an overexpression of StAR, a polypeptide involved in the intramitochondrial trafficking of cholesterol, has been observed (288). StAR silencing resulted in the net decrease of mitochondrial cholesterol loading (222), suggesting its involvement in the delivery of mitochondrial cholesterol, besides its role in intramitochondrial cholesterol trafficking. Thus, given the impact of cholesterol-mediated changes on membrane dynamics with subsequent regulation of mGSH and ROS generation, a better understanding about the cell biology and trafficking of this particular cholesterol pool may be of relevance in the modulation of redox signaling and cell death.
FIG. 9.
Cellular cholesterol synthesis and transport. Cholesterol is synthesized in the endoplasmic reticulum (ER) and distributed to different membranes, principally to the plasma membrane, bypassing the Golgi. In addition, cholesterol from the ER or coming from endosomes (through low-density lipoprotein receptor–mediated cholesterol uptake) also can be targeted to the mitochondria through the action of a cholesterol-transfer protein, steroidogenic acute regulatory protein (StAR).
FIG. 10.
Role of free cholesterol in NPC-1–deficient hepatocytes. In normal hepatocytes, LDL-derived free cholesterol (Ch) can be transported, in a process that involves NPC proteins, from the endosomes to the endoplasmic reticulum (ER), where it is esterified to cholesteryl esters (ECs). However, in NPC-1–deficient hepatocytes, free cholesterol is not able to move to the ER, and it accumulates in mitochondria, resulting in selective mGSH depletion.
FIG. 11.
Mitochondrial cho-lesterol in different pathologic settings. In normal cells, overaccumulation of cholesterol in mitochondria impairs GSH transport from cytosol to mitochondria, leading to mitochondrial GSH depletion, and consequently, to increased sensitivity to cytokines such as TNF and Fas, as observed in hepatocytes from alcoholic- (ASH) and nonalcoholic (NASH) steatohepatitis experimental models, in which mGSH depletion was accompanied by increased mitochondrial ROS generation and cardiolipin peroxidation. However, in cancer cells, cholesterol accumulation in mitochondria contributes to the mitochondrial dysfunction of cancer cells, favoring enhanced dependence on aerobic glucolysis for energy production. Remarkably, under these conditions, and in contrast to the effect observed in normal cells, hepatocellular carcinoma cells were able to maintain optimal mGSH levels. This enhanced mitochondrial cholesterol was accompanied by increased resistance to chemotherapy, Bax, and hypoxia, selectively acting through mitochondria.
C. Free fatty acids and lipotoxicity
Since the first description in pancreatic β cells associated with the impairment of insulin secretion, free fatty acids (FFAs) are considered the main cause of lipotoxicity, with saturated FA (i.e., palmitate) being one of the most potent triggers of lipotoxicity (180). Lipotoxic effects of FFA are of relevance not only in type 2 diabetes, but also in the development and progression of hepatic steatosis (4, 195). The mechanisms and alterations in the cellular metabolism caused by FFA are multifactorial, including activation of JNK, induction of proinflammatory cytokines, inhibition of mitochondrial β-oxidation, increased ROS production, as well as the generation of toxic lipid intermediates and lipid derivatives (24, 81, 195). In particular, the generation of oxidative stress is an important factor in lipotoxicity because of its contribution to cellular stress signaling and interference with mitochondrial functions (4). For instance, in a recent study, palmitate was shown to limit GSH synthesis by inhibition of the cysteine transporter and the subsequent limited substrate supply (286). This outcome, coupled with the targeting and impairment of mitochondrial function and subsequent generation of ROS, suggests that mitochondrial oxidative stress may be a primary mechanism of saturated FFAs, with a negative impact on the intracellular redox state in different subcellular organelles, including the ER, mitochondria, and lysosomes (24, 92, 286). Palmitate-mediated induction of ROS in hepatocytes can be prevented by inhibition of carnitine palmitoyltransferase or the blocking of the mitochondrial electron-transport chain (230). Another potential mechanism involved in palmitate-induced lipotoxicity may relate to enhanced ceramide generation, as this sphingolipid is synthesized de novo from palmitoyl CoA and serine in the ER (see earlier; Fig. 7). A recent study showed that the lipotoxicity induced by palmitate involved Bim upregulation through transcription factor FoxO3A, which was not altered by fumonisin B1, an inhibitor of de novo ceramide synthesis (12). However, the effect of this inhibitor on the homeostasis of ceramide in hepatocytes after palmitate exposure was not examined (12). Protein phosphatase 2A (PP2A) is a known target of ceramide (264), and it has been shown that inhibition of ceramide synthase by fumonisin B1 in vivo results in the activation of other sphingolipid-metabolizing systems (e.g., SMase and SPT), contributing to an imbalance of the sphingolipid metabolism (135). It is, therefore, possible that increased activity of SMase could compensate for reduced cellular ceramide levels by production of ceramide through sphingomyelin hydrolysis. Thus, these findings indicate that the involvement of ceramide in FoxO3-dependent Bim expression and hepatic lipoapoptosis cannot be excluded and deserves further investigation.
Finally, another factor contributing to palmitate-induced lipotoxicity involves lysophophatidylcholine generation by phospholipase A2 (131). Interestingly, palmitic acid–induced hepatocyte death was accompanied by decreased cardiolipin content, mitochondrial depolarization, and cytochrome c release, events that were prevented by phospholipase A2 inhibition and reproduced by lysophosphotidylcholine administration. Moreover, oleic acid abrogated the toxic effects of palmitic acid due to the diversion of palmitic acid to triglyceride storage, resulting in decreased lysophosphotidylcholine content. These findings suggest that FFAs lead to steatosis or lipoapoptosis, depending on the abundance of saturated/unsaturated FFAs by several mechanisms, including the mitochondrial stimulation of ROS production.
VIII. Impact of ROS in Liver Diseases
Given the regulation of signaling pathways through redox mechanisms, in this section, we briefly describe the impact of oxidative stress and redox signaling in liver diseases.
A. Hepatitis and liver failure
Death receptors, in particular, TNF and Fas, are known to mediate many forms of liver injury, including fulminant hepatitis (196). The signal transduction of these ligands with their binding to the corresponding plasma membrane receptors is complex and includes protein–protein interactions and the generation of second messengers, such as sphingolipids that control the balance between the survival and prodeath signals generated by either ligand. As described earlier, an important player is the overgeneration of ROS, which can subsequently regulate JNK activation (198, 158, 315). Normally, JNK activation in response to TNF is transient because NF-κB diminishes its activation (29, 158). Thus, NF-κB suppression sensitizes hepatocytes to TNF by allowing sustained JNK activation through ROS generation. NF-κB controls the magnitude of ROS generation after TNF by inducing the expression of MnSOD and the ferrritin heavy chain (158, 198, 249). An important source of TNF-mediated ROS production is the mitochondrial complex III, which is targeted by ceramide derived from ASMase (105, 108, 197, 198). In acute liver inflammation and liver failure, JNK activation induces apoptosis by targeting distinct factors upstream of the mitochondrial death pathway, among which Itch and Bid play central roles (41). JNK phosphorylates and activates Itch, an E3 ubiquitin ligase. The active Itch catalyzes the ubiquitination of c-FLIPL that subsequently leads to its proteosomal degradation. Moreover, JNK also translocates to mitochondria, where it might directly trigger the release of cytochrome c (6). Fulminant hepatitis in mice can be induced with treatment with the lectin concanavalin A by a TNF-dependent mechanism, as shown in mice deficient in both TNFR1 and TNFR2, that requires JNK (41, 158). Two JNK isoforms, JNK1 and JNK2, are expressed in the liver. However, their relative contribution to TNF-mediated hepatitis is currently unsettled. For instance, recent findings indicated that jnk2-/- hepatocytes are resistant to TNF-induced apoptosis, as compared with wild-type or jnk1-/- hepatocytes, by a mechanism involving jnk1-mediated expression of the antiapoptotic gene Mcl-1 (164, 165). Deletion of Mcl-1 in jnk2-/- hepatocytes increases TNF and galactasomine/LPS-induced hepatocellular apoptosis and liver injury in vivo. Whereas these findings indicated that jnk1 plays a critical role in determining TNF-mediated hepatocellular apoptosis, Das et al. (72) showed that deletion of both jnk1 and jnk2 in hepatocytes did not prevent TNF-mediated hepatocellular apoptosis and liver failure in vivo. In contrast, mice with loss of jnk1 and 2 in hematopoietic cells exhibited a profound defect in hepatitis that was associated with reduced expression of TNF (72). Thus, in addition to the individual role of jnk1 and jnk2 in TNF-mediated hepatitis, their site of generation appears to be critical in determining the final outcome in the susceptibility of hepatocytes to TNF. Nevertheless, these findings confirm a role for JNK in the development of hepatitis and also provide compelling evidence pointing to hematopoietic cells as the sites of the essential function of JNK. In addition to JNK, another critical mediator of TNF/Fas-mediated hepatocellular death and fulminant liver failure is ceramide generation, particularly through ASMase activation (105, 199). In these studies, it was shown that mice deficient in ASMase but not in NSMase are resistant to galactosamine plus TNF or LPS-induced hepatocellular injury, characterized by the mitochondrial generation of ROS and MAT1A downregulation and consequent SAM depletion. As mentioned earlier, decreased SAM levels by TNF caused GSH depletion, contributing to TNF-induced ROS overgeneration. Importantly, SAM therapy was able to rescue mice from galactosamine plus TNF-mediated liver failure (199), and most of this effect was mediated by the ability of SAM to replenish the mitochondrial pool of GSH.
B. NASH and alcohol-induced liver injury
Steatohepatitis (SH) represents an advanced stage in the spectrum of fatty liver diseases that encompasses alcoholic (ASH) and nonalcoholic steatohepatitis (NASH), two of the most common forms of liver disease worldwide. Although the primary etiology of ASH and NASH is different, these two diseases show almost identical histologic features characterized by steatosis, inflammation (predominantly characterized by leukocytes and mononuclear cells infiltration), and hepatocellular cell death due to sensitivity to oxidative stress (5). Despite significant progress in recent years, the pathogenesis of SH is still incompletely understood and, in particular, the mechanisms that determine the transition from simple steatosis to SH and hence disease progression. Several interrelated factors contribute to disease progression, including insulin resistance, hepatic fat accumulation, inflammation, and oxidative stress (203). The accumulation of lipids in the cytoplasm of hepatocytes, mostly in the form of FFAs and triglycerides, is considered the first step in the development of SH. However, SH progression beyond hepatic steatosis usually does not occur in the absence of a second hit that promotes oxidative stress, inflammation, cell death, and fibrosis. In this regard, cytokine overexpression and the membrane receptors have been shown to contribute to hepatocellular apoptosis and SH (5, 67, 91). TNF is overexpressed in the livers of obese mice and mediates insulin resistance in both diet-induced and genetic models of obesity (143). Recent findings by using mice deficient in both TNF receptors 1 and 2 showed a critical role for TNF signaling in diet-induced NASH (300). Thus, understanding the factors that determine the susceptibility of the fatty liver to TNF is of potential significance. Although the two-hit hypothesis is the most prevalent view to explain disease progression, recent findings provided evidence that the type rather than the amount of fat is critical in the sensitization of the fatty liver to TNF/Fas-mediated steatohepatitis (197). By using nutritional and genetic models of hepatic steatosis, it was described that cholesterol accumulation in hepatocytes, as opposed to triglycerides or FFA, played a critical role in NASH (197, 207). Although cholesterol loading occurred in different cell sites, cholesterol accumulation in the ER or the plasma membrane did not cause ER stress or alter TNF signaling. Rather, the trafficking of cholesterol to mitochondria accounted for the hepatocellular susceptibility to TNF due to mGSH depletion (Fig. 11). These findings were not only observed in mice fed a hypercholesterolemic diet but also were observed in NPC-1–deficient mice as well as in obese ob/ob mice. Boosting the pool of mGSH or preventing its depletion by blocking cholesterol synthesis with atorvastatin attenuated the susceptibility of obese ob/ob mice to LPS-mediated liver injury, highlighting the relevance of mitochondria-mediated oxidative stress in the susceptibility to TNF-induced liver injury and NASH. Furthermore, a correlation between cholesterol accumulation and disease progression was described recently in morbidly obese patients (30). Interestingly, in this patient population, they found a significant overexpression of StAR, a polypeptide involved in the mitochondrial delivery of cholesterol (222, 288), and SREBP-2, a transcription factor that controls the de novo cholesterol synthesis, compared with healthy controls or patients with simple steatosis.
In addition to cholesterol, SLs also may play a role in NASH. Blocking glucosylceramide synthase, the first step in glycosphingolipid synthesis from ceramide, ameliorated hepatic steatosis and increased insulin sensitivity in obese mice (340), indicating a role for glycosphingolipids in NASH. Because GD3 is known to target mitochondria (see earlier) causing ROS generation, further research on the potential role of GD3 in NASH is needed.
Similar to these findings with NASH, TNF plays a key role in alcohol-induced liver injury, which is characterized by the susceptibility to TNF-mediated cell death (58, 145, 246, 335). Although the mechanisms for the transition from resistance to susceptibility to TNF with alcohol intake may be multifactorial, including elevated PTEN levels (283), it has been reported that alcohol feeding causes mGSH depletion because of alcohol-stimulated cholesterol synthesis (59, 61, 96, 189). Cholesterol accumulation in mitochondrial membranes impairs the hepatic mitochondrial transport of GSH from the cytosol, resulting in its depletion (59, 61, 323, 341), with similar findings observed in alveolar type-II cells (312). mGSH has been associated with increased susceptibility to ethanol-induced liver injury and lethality in mice deficient in Nrf2, a transcription factor that regulates GSH biosynthesis and homeostasis (176). The mechanism involved in the mGSH-dependent hepatocellular susceptibility to TNF/Fas after mGSH depletion involved mitochondrial generation of ROS through ASMase-induced ceramide production, causing the peroxidation of cardiolipin, which facilitated the permeabilizing activity of oligomerized Bax in the MOM (198). Remarkably, the potentiating effect of peroxidized cardiolipin did not involve modifications in the oligomerization or penetration of active Bax in the bilayer; instead, the presence of peroxidized cardiolipin restructured the lipid bilayer, indicating that this lipid facilitates the formation or expansion or both of the proteolipidic pore itself. Another interesting finding was that alcohol feeding induced the depletion of the mitochondrial pool of SAM (94). As mentioned earlier, the transulfuration pathway provides cysteine from methionine for the synthesis of GSH through generation of SAM (Fig. 12). Both SAM and GSH share metabolic similarities, as both are exclusively synthesized in the cytosol but are found in the mitochondria as well, where they play pivotal roles in the maintenance of mitochondrial functions (205). The time-dependent analysis indicated that the mitochondrial SAM depletion by alcohol feeding preceded that of mGSH. Moreover, unlike alcohol-induced cholesterol loading in mitochondria, which impaired the transport of GSH from the cytosol, the mitochondrial transport of SAM was insensitive to alcohol-mediated changes in membrane dynamics (94). Rather, the mechanism of mitochondrial SAM depletion by alcohol feeding involved cytosolic SAM limitation and S-adenosylhomocysteine accumulation, which competed for SAM to be transported to the mitochondria. In addition to the critical role of SAM in methylation reactions, another prevalent mechanism of action is the modulation of membrane fluidity through phosphatidylcholine synthesis from phosphatidylethanolamine (109). Hence, the earlier depletion of mitochondrial SAM by alcohol intake can contribute to that of GSH, suggesting that strategies to preserve this pool of SAM may be of potential relevance in alcohol-induced liver injury by protecting hepatocytes from TNF-induced liver injury due to the maintenance of mGSH stores. In contrast to this potential therapeutic effect of SAM, the use of N-acetylcysteine in the face of alcohol consumption has been shown to be inefficient in boosting mGSH levels, despite significantly increasing those of cytosolic GSH (109), underlying the impairment in the transport of GSH into mitochondria imposed by cholesterol accumulation in mitochondria.
FIG. 12.
The transsulfuration pathway. Dietary methionine is converted to the methyl donor S-adenosylmethionine (SAM) and is demethylated to S-adenosylhomocysteine (SAH) and homocysteine. In the transsulfuration pathway, homocysteine is converted to cystathionine that can be transformed into cysteine. Cysteine, in turn, can be used in glutathione (GSH) synthesis. Homocysteine also can be remethylated to methionine, either through the folate cycle by using 5,10-methylenetetrahydrofolate (5-MTHF) involving the enzyme methionine synthase (MS), or by using betaine and the enzyme betaine homocysteine methyltransferase (BHMT). Both GSH and SAM can be transported to the mitochondria; however, whereas cholesterol loading in the mitochondria inhibits GSH transport, mitochondrial SAM transport is insensitive to membrane dynamics.
In addition to these mechanisms, another key player in NASH and alcohol-induced liver injury is ER stress, which contributes to lipogenesis, fatty liver, and hepatocellular injury (160). Among the several branches that constitute this pathway, XBP-1, a transcription factor that belongs to the bZip family and is activated by accumulating unfolded proteins in the ER, plays an essential role in the control of ER stress (241). With ER stress, the spliced XBP-1 regulates a subset of ER-resident chaperone genes to protect cells from ER stress. Interestingly, in addition to this established role, a novel function of XBP-1 in the regulation of hepatic lipogenesis has been described (178). XBP-1 protein expression in mice was elevated after a carbohydrate-enriched diet feeding that corresponded with the induction of critical genes involved in fatty acid synthesis. However, inducible and selective depletion of XBP-1 in the liver resulted in marked hypocholesterolemia and hypotriglyceridemia, secondary to a decreased production of lipids from the liver, demonstrating a novel function for XBP-1 in the regulation of lipogenesis and fatty liver. Remarkably, this phenotype was not accompanied by hepatic steatosis or impairment in protein secretory function. Moreover, a protective role for XBP-1 against oxidative stress was recently described in XBP-1–deficient embryonic fibroblasts (184). In XBP-1–deficient cells, hydrogen peroxide induced more-extensive ROS generation and prolonged p38 phosphorylation than in wild-type cells. Interestingly, the expression of different antioxidant enzymes, including catalase, was lower in XBP-1–deficient cells, suggesting a novel role for this transcription factor in oxidative-stress regulation. Thus, the role of XBP-1 in fat accumulation and oxidative stress in NASH and alcohol-induced liver injury should be further investigated. Nevertheless, these findings defining XBP1 as a regulator of lipogenesis may have important implications, suggesting that the targeting of this ER protein may be a promising approach for the treatment of fatty liver disease.
Finally, another aspect that may regulate fatty liver disease is the activation or recruitment or both of hepatic stem cells to the site of injury. Hepatic stem cells have attracted attention as potential candidates for liver-directed gene therapy and regenerative medicine. Although hepatic stem cells are known to contribute to liver regeneration when proliferation of hepatocytes is impaired or in the face of significant tissue damage, the mechanisms controlling lineage commitment and response of hepatic stem cells to environmental signals remain poorly defined. In alcohol-fed and obese ob/ob mice, it was shown that the expansion of hepatic oval cells helps to compensate for the increased turnover of damaged mature hepatocytes, thus limiting the progression of fatty liver disease (332). Given the relevance of TNF in NASH and alcohol-mediated liver injury through ROS overgeneration, the susceptibility of hepatic progenitor cells to TNF may be a critical factor involved in disease progression. In this regard, it was shown that differentiated hepatic progenitor cells (rat liver epithelial cells) are more susceptible to TNF-mediated apoptosis accompanied by ROS overproduction and GSH depletion (269). Furthermore, a correlation between the degree of oval cell activation and extent of oxidative damage in mice and humans with fatty livers was described (262), suggesting a survival advantage against oxidative stress. In addition, the upregulation of stress-inducible genes, such as acute-phase reactants (APRs) may limit tissue damage in response to stress, inflammation, or oxidative stress. The acute-phase reaction is characterized by altered transcription (positive or negative) of numerous target genes, many of which are expressed exclusively or predominantly by the liver to regulate diverse processes such as blood coagulation, innate immunity, metal sequestration, or antiproteolytic activities. Taken together, it appears that oxidative stress plays a dual role in regulating hepatic progenitor cells, stimulating recruitment and apoptosis resistance at early stages of activation that culminate in apoptosis susceptibility in differentiated cells.
C. Ischemia/reperfusion liver injury and liver transplantation
Hepatic ischemia/reperfusion (I/R) damage can occur in diverse clinical settings, including liver transplantation, trauma, hemorrhagic shock, or liver surgery, and is a serious clinical complication that may compromise liver function because of extensive hepatocellular loss. Despite intensive research in this area, the cellular and molecular mechanisms responsible for hepatic I/R injury are not well understood. Molecular events include NF-κB activation, JNK activation, mitochondrial dysfunction followed by ROS generation, and the activation of Toll-like receptors (TLR). Although this family of receptors is known to play a fundamental role in innate immunity and inflammation, recent evidence showed that TLR, particularly TLR4, is an important mediator of hepatic I/R injury through an interferon regulatory factor-3–dependent pathway (337). Moreover, deficiency of TLR4 signaling in the donor organ has been shown to reduce I/R injury in a murine liver-transplantation model (282). Early protein targets of I/R during liver transplantation have been described by using differential proteomic analysis (271). Besides a cluster of proteins functionally involved in lipid, energy, and metabolic pathways, the active-site thiol of PrxI was found to be overoxidized into sulfonic acid, indicating that the redox state of Prx may be an early and sensitive marker of oxidative stress in hepatic I/R and liver transplantation (37, 310). Although these factors may allow the design of biochemical and genetic strategies to modulate hepatic I/R injury, ischemic preconditioning is a surgical strategy of therapeutic value during I/R, involving a number of mechanisms, including the redox-sensitive mitochondrial ATP-sensitive K+ channels that control ROS production (90, 221).
TNF has been identified as a key player in hepatic I/R damage (263). Consistent with these findings, ischemic preconditioning with TNF/Fas was shown to reduce ischemic injury in the liver through the activation of oxidative stress that induces a cytoprotective response (152). In line with its role in TNF/Fas signaling, it was shown that ASMase plays a role in hepatic I/R injury (186). Indeed, ASMase-induced ceramide generation targeted mitochondria through JNK and BimL activation. Thus, in addition to the previously described mechanisms and consistent with the role of ceramide in recruiting the mitochondrial pathway of cell death, ASMase emerges as a potential target for intervention against hepatic I/R.
The role of NF-κB and ROS in hepatic I/R injury is of interest, with the former playing a controversial role because of its dual action in the induction of survival/inflammatory genes. Whereas the generation of ROS during I/R derives from various sources, mitochondrial ROS constitute a predominant mechanism in this condition, as examined in vivo with two-photon confocal microscopy (Fig. 13). Hepatic NF-κB activation has been shown to diminish hepatic I/R injury and improve orthotopic liver transplantation, whereas NF-κB inactivation has been shown to protect against hepatic I/R (172, 293). These apparently conflicting results have been explained on the basis of different degree of residual NF-κB activation. Moreover, ROS and oxidative stress exert a dual effect on NF-κB activation, which involves its release from the inhibitory moiety in the cytosol, its nuclear translocation, and the subsequent transactivation of target genes. Recent data showed that NF-κB transactivation is diminished in hepatocytes after GSH depletion, involving IKK-dependent and -independent mechanisms (191). Moreover, GSSG formation subsequent to the overgeneration of ROS inactivates NF-κB (85). In addition to this dual behavior, the activation of NF-κB in different population of cells in the liver also may contribute to the dichotomy of NF-κB in I/R-induced liver injury. Recent findings showed that ROS mediate liver injury through selective parenchymal inactivation of NF-κB in I/R (187). Thus, whereas in hepatocytes, the activation of NF-κB after I/R occurred through a noncanonic pathway without IκB degradation through Src activation, in Kupffer cells, this process involves a canonic pathway likely mediated by TLR. These findings have strong implications, as they suggest that strategies that increase GSH in hepatocytes would maintain NF-κB activation and hence the expression of survival genes without changing the induction of proinflammatory cytokines, which may thus contribute to the protection against I/R damage. In contrast, marked GSH depletion resulted in the downregulation of survival genes and in the enhanced generation of TNF/IL-1β, which translated into exaggerated liver damage after I/R.
FIG. 13.
Mitochondrial ROS generation during hepatic ischemia/reperfusion (I/R) injury. In vivo two-photon confocal microscopy during partial (70% of the liver) hepatic I/R (90 min of ischemia followed by 60 min of reperfusion) in mice. The generation of ROS, by using 2',7'-dichlorofluorescin (DCF) fluorescence, occurs largely in mitochondria and is accompanied by loss of mitochondrial membrane potential, as detected by using tetramethylrhodamine ethyl ester (TMRE). The imaging of the liver of living mice was performed by the using spectral confocal inverted microscope Leica TCS SP5. DCF (200 μM, incubated for 45 min) and TMRE (5 μM, incubated for 30 min) labelings were imaged by using one-photon excitation at 485 nm and emission at 520 nm for the DCF and excitation at 516 nm and emission at 582–677 nm for the TMRE. The emissions were acquired with internal spectral detector (PMT) at high speed to avoid the movement from animal breathing and heart beating during acquisition. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
D. Cholestasis and bile acid–induced liver injury
Cholestatic liver disease is a prominent cause of morbidity and mortality because of the retention of toxic bile acids, which are known to play a key role in cell toxicity (233, 334). Chronic retention of toxic bile acids can cause oxidative stress, apoptosis, and fibrosis, leading to cirrhosis. The role of GSH in cholestatic liver diseases is controversial, although most studies have shown that experimental cholestasis in rats and hepatocytes subjected to toxic bile acids results in a decrease in GSH levels (233, 261). Moreover, other mechanisms involved in bile acids–induced cytotoxicity include ER stress (298) and mitochondrial targeting (334), which can further limit antioxidant defense and contribute to liver injury. Bile duct ligation in rats was reported to cause mitochondrial dysfunction and ROS generation, events associated with increased cholesterol/phospholipids in mitochondria (168). Interestingly, elevated mitochondrial volume per hepatocyte was described in perfused liver from bile-duct–ligated rats, which constitutes an adaptive mechanism to offset the impairment in mitochondrial metabolism during secondary biliary cirrhosis (169).
Another mechanism that can contribute to ROS overgeneration relates to decreased glutamate cysteine ligase (GCL) expression and activity by bile acids (233). Remarkably, ursodeoxycholic acid, which is currently in use for the treatment of primary biliary cirrhosis, was shown to prevent the decrease in GCL expression during chronic cholestasis and to increase GCL expression in cultured rat hepatocytes (217). Furthermore, its taurine derivative may have far-reaching effects in GSH regulation, other than modulating GCL expression. For instance, tauroursodeoxycholic acid has been shown to preserve membrane dynamics, preventing the depletion of mGSH after alcohol feeding (56) and to alleviate ER stress in type-2 diabetes (241). In this regard, and consistent with cholesterol enrichment in mitochondria, secondary biliary cirrhosis has been reported to deplete mGSH levels (167). However, the therapeutic effect of tauroursodeoxycholic acid in secondary biliary cirrhosis in relation to its ability to prevent mGSH depletion by bile acids remains to be fully established. Thus, the therapeutic effect of ursodeoxycholic acid and its taurine derivative may be multifactorial. The balance between hydrophobic and hydrophilic bile acids determines the onset of liver disease through mechanisms involving mitochondria/ER targeting, oxidative stress, and the limitation in antioxidant defense, including mitochondrial GSH. Consequently, this disorder is potentially susceptible for the beneficial effects of antioxidants, although this remains to be fully established.
E. Endotoxemia
The liver plays a key role in the clearing of gut-derived lipopolysaccharide (LPS), a major component of the outer membrane of all gram-negative bacteria that can trigger the synthesis and release of proinflammatory cytokines such as TNF-α, interleukin 1β (IL-1β), and inducible nitric oxide synthase (iNOS) (292, 338). A major mechanism of LPS-mediated effects is the activation of NF-κB through TLR (339), although NADPH oxidase–induced ROS also has been reported to contribute to endotoxin-mediated liver injury (124). Endotoxemia frequently occurs in patients with liver cirrhosis, with the extent of endotoxemia correlating with the degree of liver failure. Moreover, endotoxemia also is believed to participate in the pathogenesis of many liver diseases, including alcoholic liver disease and NASH, in which TNF plays a central role (67, 145). Consistent with the involvement of oxidative stress after LPS exposure, GSH has been shown to play an important role in the susceptibility to LPS-induced liver injury (247). For instance, in experimental models, LPS has been reported to deplete GSH stores in the liver, and mice deficient in GSHPx exhibited enhanced susceptibility to LPS-mediated liver damage (149). Consistent with these findings, the exogenous administration of GSH has been shown to decrease LPS-induced systemic inflammatory response and mortality (295). The molecular mechanism underlying the protective effect of GSH relates to the modulation of TLR4 signaling and the potentiation of LPS-induced TNF secretion under low-GSH status. Consequently, Nrf2-deficient mouse embryonic fibroblasts exhibited greater activation of NF-κB and interferon regulatory factor 3 in response to LPS and polyinosinic-polycytidylic acid [poly(I:C)] stimuli (299), indicating a critical role for Nrf2 in the regulation of GSH to modulate optimal NF-κB activation in response to LPS and TNF. The downregulation of GSH levels by LPS involves several mechanisms, including its depletion as a consequence of oxidative stress (338), an event that may be due to the reduction of GCL activity during endotoxemia because of its decreased expression (247). Moreover, inhibition of nitric oxide generation ameliorated the decrease in GSH, suggesting that the hepatic expression of GCL is inhibited by increased availability of NO stores (247).
Despite these findings, the regulation of GCL by NO is not well established and appears to depend on the cell type analyzed. For instance, NO did not influence the basal GSH levels or GCL expression in rat hepatocytes, although IL-1 increased the expression of GCL (173). These findings contrast with the outcome observed in rat aortic vascular smooth muscle cells, in which NO increased GSH levels and the expression of both subunits of GCL (218). Thus, although the regulation of GSH during endotoxemia remains to be fully understood, the evidence available suggests that prodrugs that boost GSH levels may be of therapeutic relevance. Further research is needed to establish whether this beneficial effect of GSH is through control of oxidative stress or modulation of proinflammatory genes through NF-κB activation or both.
F. Hepatocellular cancer
Liver cancer constitutes the final stage in the progression of chronic liver diseases; it is the fifth-greatest cause of cancer-related death worldwide. Because current effective therapy is lacking, an urgent need exists for a better understanding of the molecular pathways regarding hepatocarcinogenesis and the mechanisms involved in therapy resistance. One of the prominent characteristics of solid tumors is the onset of hypoxia along the edge of tumor growth. This anatomic feature arises because of the disorganized structure and architecture of tumor vasculature, resulting in irregular and inefficient oxygen delivery, which is considered a negative prognostic factor for response to treatment and survival of cancer patients (80). In addition to the prominent role of prolylhydroxylases as oxygen sensors, another critical player is the mitochondrial generation of ROS after oxygen deprivation (28, 126). Hypoxia-induced mitochondrial ROS regulate HIF-1α stabilization and NF-κB activation (126, 188). In particular, the NF-κB activation by hypoxia-induced mitochondrial ROS generation occurs by a noncanonic pathway involving the phosphorylation of IκB in tyrosine residues through activation of the c-Src tyrosine kinase (Fig. 14) (188). With Src mutants, the cysteine 487 was identified as a critical determinant in the activation of Src by ROS. Although this pathway involving Src–NF-κB activation in response to hypoxia is essential for hepatocellular carcinoma cell survival, the overproduction of mitochondrial ROS can be exploited to destroy cancer cells by an oxidant-dependent mechanism (188). In this regard, the depletion of mGSH has been shown to potentiate the mitochondrial ROS generation by hypoxia, resulting in necrotic cell death. Thus, whereas ROS promote carcinogenesis through activation of both HIF1α and NF-κB, its enhanced production by mGSH depletion emerges as an attractive approach to killing cancer cells. Consistent with this notion, it was observed that oncogenically transformed cells are sensitive to the generation of ROS by β-phenylethyl isothiocyanate, which depletes GSH in both cytosol and mitochondrial fractions and inhibits NF-κB activation (302). Hence, mitochondrial ROS generation by hypoxia exhibits a dual role in cancer biology: it fosters carcinogenesis by activating NF-κB and HIF1α, which, in turn, promote cancer cell survival, angiogenesis, and tumor expansion; conversely, its overproduction after mitochondrial GSH depletion switches hypoxia from a cancer-promoting to a cancer-killing environment. Finally, because GD3 has been characterized as a proapoptotic lipid effector by a dual role involving mitochondria targeting and the inactivation of the NF-κB survival pathway (see earlier), its effect on the sensitization of hepatocellular carcinoma to hypoxia and chemotherapy deserves further investigation (Fig. 15). Regarding this, we have observed that the transfection of human hepatocarcinoma cells with GD3 synthase, the enzyme that synthesizes GD3 from GM3, inactivates the Src–NF-κB pathway translating into the sensitization to hypoxia (Lluis et al., unpublished data). Thus, GD3, in addition to its ability to prevent the activation of NF-κB by the classic pathway through IκB degradation (60), also is efficient in disabling its activation through the noncanonic pathway involving phosphorylation of IκB at tyrosine residues.
FIG. 14.
Mitochondrial reactive oxygen species in hypoxic signaling. Mitochondrial ROS generation contributes to cancer cell survival during hypoxia by a dual mechanism: first, by stabilizing HIF-1α through the inhibition of the oxygen-sensing enzymes prolylhydroxylase (PHD), which requires oxygen, 2-oxoglutarate (2-OG), and Fe2+, and second, by inducing NF-κB activation by using a noncanonic pathway involving the phosphorylation of IκB in tyrosine residues through activation of the c-Src tyrosine kinase.
FIG. 15.
Ganglioside GD3 enhances apoptosis by suppressing the NF-κB–dependent survival pathway. Scheme showing NF-κB activation through the classic (A) and the c-Src–dependent pathways (B). GD3 is able to inhibit the translocation of the p50/p65–NF-κB subunits in both settings, resulting in suppression of NF-κB–dependent gene transcription and, thus, enhancing cell death. The classic pathway (A) involves the phosphorylation of IκB at serine residues by the IκB kinase complex (formed by NEMO, IKK1, and IKK2), followed by IκB-ubiquitination and proteasomal degradation, allowing translocation of the p50/p65–NF-κB subunits to the nuclei. In the noncanonic pathway (B), c-Src phosphorylates IκB at tyrosine residues, resulting in its release and in translocation of the p50/p65–NF-κB subunits to the nuclei.
Another key feature of tumorigenesis is the deregulation of cholesterol metabolism, which is reflected by the desensitization of hydroxymethylglutaryl-CoA reductase (HMG-CoAR) to inhibition by sterols, and by the continued cholesterol synthesis in growing solid tumors. Interestingly, cholesterol synthesis requires molecular oxygen for the transformation of squalene to cholesterol, and hypoxia has been shown to mediate the degradation of HMG-CoAR (234). Although mitochondria are cholesterol-poor organelles, another level of deregulation of carcinogenesis is the recognized enrichment of mitochondrial membranes in free-cholesterol content (Fig. 11). Because cholesterol enrichment can adversely affect mitochondrial functions (57, 189, 197), it is conceivable that the accumulation of cholesterol within mitochondrial membranes may account for or contribute to the known mitochondrial dysfunction of cancer cells, underlying the Warburg effect and dependence on aerobic glucolysis for energy production (80). By using human and rat hepatocellular carcinoma cell lines or the mitochondrial fraction isolated from patients with hepatocellular carcinoma, a dramatic increase in the levels of mitochondrial cholesterol compared with untransformed cells was observed, which translated into an increased membrane order parameter (222). This outcome was accompanied by enhanced mitochondrial-chemotherapy resistance and was reversed by either cholesterol extraction or fluidization of mitochondrial membranes. Moreover, given the role of StAR in regulation of mitochondrial cholesterol homeostasis (288), the downregulation of StAR in hepatocellular carcinoma cell lines by siRNA reduced the net level of mitochondrial cholesterol, increasing the susceptibility to mitochondria-targeted chemotherapy. In line with these data, Lucken-Ardjomande (194) recently showed that treatment of HeLa cells with U18666A, a cholesterol transport–inhibiting agent that is used widely to mimic Niemann-Pick type C disease, caused mitochondrial cholesterol upregulation, resulting in a delay in the release of Smac/Diablo and cytochrome c, as well as in Bax oligomerization and partial protection against stress-induced apoptosis. Moreover, the inhibitory effect of cholesterol on mitochondrial Bax activation was demonstrated in liposomes, and this effect was exerted by a dual mechanism involving changes in membrane order parameter and the decrease of Bax penetration into the membrane bilayer (222). Thus, by inhibiting Bax-driven MOM permeabilization, cholesterol modulates cell-death susceptibility. Furthermore, the potentiation of hepatocellular carcinoma chemotherapy by squalene synthase inhibition, which reduces cholesterol levels without perturbing isoprenoid metabolism, validates the specificity of cholesterol in chemotherapy resistance and revitalizes the potential benefit of cholesterol downregulation in cancer therapy.
An interesting contrast emerges in comparing the effect of mitochondrial cholesterol loading occurring in SH and hepatocarcinoma. Whereas in the former, mitochondrial cholesterol accumulation plays a proapoptotic role, in the latter, it acts in an antiapoptotic fashion, thus establishing a paradox in cell-death regulation (Fig. 11). Intriguingly, the mitochondrial cholesterol loading in SH results in mGSH depletion, whereas hepatocarcinoma cells are able to maintain optimal mGSH levels despite mitochondrial cholesterol accumulation by a mechanism that is currently under investigation (Montero et al., unpublished data).
G. Hepatic insulin resistance
One of the characteristic metabolic features of hepatic steatosis is the resistance to the peptide hormone insulin, which stimulates the uptake and storage of glucose and other nutrients in skeletal muscle and adipose tissue while simultaneously repressing glucose efflux from the liver. Insulin resistance occurs when a normal dose of the hormone is incapable of eliciting these anabolic responses, and the condition is a component of or a risk factor for many metabolic diseases, including diabetes, hypertension, atherosclerosis, and cancer. Obesity predisposes to the development of insulin resistance, and several mechanisms have been proposed to explain how increased adiposity antagonizes the insulin stimulation of nutrient uptake and storage. First, increased adipose tissue may trigger the synthesis or secretion or both of glucocorticoids or inflammatory cytokines such as TNF that inhibit insulin action in peripheral tissues (143). Moreover, excess lipids may be delivered to nonadipose tissues that are not suited for fat storage (i.e., skeletal muscle and the liver), leading to the formation of specific metabolites that directly antagonize the insulin action (140). Hence, emerging evidence suggests that nutrient excess is associated with an increase in specific lipid-derived metabolites that inactivate signaling intermediates and cause insulin resistance. However, the mechanism by which specific lipid metabolites cause insulin resistance is incompletely resolved. Although hepatic steatosis is accompanied by hepatic insulin resistance, recent studies provided evidence dissociating the degree of fat accumulation in the liver with the onset of hepatic steatosis (220). Mice engineered to overexpress acyl-CoA:diacylglycerol acyltransferase 2 (DGAT2), which catalyzes the final step of triacylglycerol biosynthesis in the liver, exhibit massive hepatic steatosis with 20- to 30-fold higher triglyceride levels, and yet they become insulin sensitive (220). These findings clearly indicate that DGAT-mediated lipid accumulation in the liver is insufficient to cause insulin resistance and show that hepatic steatosis can occur independent of insulin resistance.
Unlike the accumulation of triglycerides, recent data provided clear-cut evidence in favor of SLs accumulation, in particular ceramide, in hepatic insulin resistance (140). Excessive levels of FFAs (saturated but not unsaturated), TNF-α, and glucocorticoids, through different mechanisms, stimulate the accumulation of the sphingolipid ceramide and various ceramide metabolites (140). Increased ceramide synthesis in response to excessive TNF, saturated FFAs, or glucocorticoids is associated with a reduction of insulin signal transduction by inhibiting PKB/Akt phosphorylation and activation. Ceramide promotes the dephosphorylation of PKB/Akt by protein phosphatase 2A (PP2A) and blocks the translocation of PKB/Akt from the cytoplasm to the plasma membrane (264). By using pharmacologic inhibitors and genetic mice deficient in dehydroceramide desaturase, it was shown that the de novo ceramide synthesis from saturated FFAs and glucocorticoids plays a key role in hepatic insulin resistance (140). In addition to this pathway of ceramide generation, recent evidence showed a key role for ASMase in hepatic steatosis and insulin resistance induced by high-fat feeding (74), thus pointing to this pathway of ceramide stimulation as another contributing factor in ceramide upregulation and hence in hepatic insulin resistance. These data, coupled with the preceding findings showing a role for ASMase in NASH, suggest that the pharmacologic inhibition of this enzyme may be of relevance in various forms of liver diseases, including the hepatic manifestation of the metabolic syndrome and associated comorbidities. Moreover, further studies are needed to investigate the role of complex glycosphingolipids in hepatic insulin resistance, as these lipids arise from ceramide in the Golgi and have been shown to modulate the insulin response (330, 340). Moreover, because ceramidases regulate ceramide metabolism and turnover by generating sphingosine, which can undergo different metabolic fates (Fig. 7), their role in insulin resistance and hepatic steatosis also merits further research.
Finally, protein tyrosine phosphatases (PTPs) are redox regulated by ROS generation and are known to modulate multiple signaling pathways (48, 213). Because tyrosine phosphorylation of insulin receptor is essential for signaling, its regulation at this step by PTPs modulates insulin signaling. Insulin stimulation in hepatic cell lines resulted in the rapid and transient oxidation and subsequent inhibition of PTP1B and TC45, enhancing phosphorylation of Tyr-972 in the insulin-receptor β-subunit. Tyr-972 is an important residue for the recruitment of insulin-receptor signaling-1 and the activation of PKB/Akt. Thus, the role of PTPs in obesity and insulin resistance deserves further investigation.
H. Drug-induced liver injury
Because of its fundamental role in metabolism, the liver is a primary target of xenobiotic biotransformation. The hepatotoxic potential of common therapeutic drugs, herbal remedies, or dietary supplements is widely recognized as an increasing health problem. Drug-induced liver injury reflects hepatocyte stress and death, which can arise directly by parent drugs or toxic metabolites, and it is modulated by a number of factors, including nutritional status and genetic determinants. One of the best and most relevant examples of drug-induced liver injury is acetaminophen-induced hepatotoxicity. Acetaminophen (N-acetyl-p-aminophenol, APAP) is a widely used analgesic, and it is considered to be safe when taken at therapeutic doses. However, at higher doses or under conditions that determine enhanced susceptibility to APAP (e.g. alcohol feeding), hepatocellular death and subsequent liver injury ensues. APAP is metabolized in vivo by cytochrome P450, particularly cytochrome P450 2E1 (CYP2E1) to a toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which can attach and covalently modify proteins (216, 232). A primary line of defense against NAPQI is through GSH conjugation that results in its depletion, especially if GSH consumption by NAPQI is not matched by synthesis. Although APAP can decrease GSH in both cytosol and mitochondria, the kinetics of GSH depletion in hepatocytes exposed to APAP showed that the depletion of mGSH preceded that of cytosol GSH (313), indicating the relevance of this pool of GSH in the hepatotoxicity of APAP. Interestingly, N-acetyl-m-aminophenol (AMAP), a nonhepatotoxic regioisomer of APAP, depletes cytosol GSH but not mGSH (141). Like that of many other drugs, APAP cytotoxicity in hepatocytes is mediated through mitochondrial membrane permeabilization through MPT, as described earlier. Thus, agents that block MPT, such as cyclosporin A, protect hepatocytes against APAP-induced cell death. However, despite MPT and cytochrome c release, necrosis rather than apoptosis is the predominant mode of cell death induced by NAPQI. This outcome likely reflects insufficient ATP levels due to impaired mitochondrial oxidative phosphorylation, together with caspase inhibition by the redox change and ROS overgeneration, which prevent the apoptotic phenotype.
A novel mechanism of APAP hepatotoxicity involves JNK activation. APAP induced sustained JNK activation and necrosis that was prevented by a synthetic JNK inhibitor (SP600125) both in isolated hepatocytes and in intact mice (125). Although jnk2 seems to play a more important role in mediating APAP toxicity than jnk1, full protection was achieved by silencing of both jnk1 and jnk2 (125). However, it remains unknown whether the protective strategy of jnk1/2 silencing in APAP hepatoxicity is due to their downregulation in hepatocyte versus hematopoietic cells. In contrast to JNK activation, the role of innate immunity, Kupffer cells, neutrophils, and natural killer or natural killer T cells in APAP-mediated hepatotoxicity remains controversial (150). However, the fact that isolated mouse hepatocytes can be killed by APAP with a mechanism similar to the one observed in vivo supports a limited role of innate immunity in APAP hepatotoxicity. Nevertheless, although the participation of innate immunity may account for the individual susceptibility to APAP-induced liver injury, the primary antidote for APAP-induced hepatocellular death is still N-acetylcysteine to boost hepatic GSH stores and mitochondrial function to keep up with NAPQI detoxification.
IX. Conclusions
The cumulative evidence provided over the years clearly supported a role for ROS and oxidants as important factors in many different pathologic processes, including liver diseases. The foundation for this role in pathophysiology derives from the reactivity of these reactive species with different cellular components such as lipids or DNA, and, more especially, with proteins because of the presence of cysteine residues. In most cases, as shown in cell-free or in in vitro cell systems, ROS and oxidant generation can perturb the functions of these vital cellular constituents, resulting in cell dysfunction or death. As a consequence, treatment with antioxidants has been shown to be beneficial under these controlled conditions. Unfortunately, the expectation of this translational application to human disease has not been fulfilled, as most clinical trials with antioxidants have been disappointing, with no effective antioxidant drug being available for widespread use today. Although this could be viewed as an indictment of the free radical theory, it may well reflect our limitation in the understanding the complex interactions/mechanisms of oxidant and antioxidant actions in designing effective therapies. Moreover, some antioxidants, such as ascorbate, can exhibit a dual nature, acting as an antioxidant or oxidant, depending on the redox environment. In addition to our continued efforts in basic research, to better understand the chemistry and biology of free radicals and their counterparts, we need larger, well-designed, randomized, double-blind, controlled trials to test the role of antioxidants in disease outcome. Moreover, ROS and oxidants may exhibit a dual life, acting as friends or foes, depending on factors that control their site, specificity, or magnitude of generation. A sound example of the unwanted effects of using antioxidants centers on aging research with the hormetic control of eukaryotic life span, in which an increase in ROS leads to a secondary development in stress defense, resulting in reduced net stress levels. Thus, as our understanding about the mechanisms, nature, and sites of ROS generation and their impact on biologic targets increases, it will likely translate into better management of the use of antioxidants and ROS scavengers in the therapy for liver diseases.
Abbreviations Used
- 2-OG
2-oxoglutarate carrier
- 4-HNE
4-hydroxynonenal
- AIF
apoptosis-inducing factor
- AngII
angiotensin II
- ARE
antioxidant responsive element
- ASH
alcoholic steatohepatitis
- ASMase
acidic sphingomyelinase
- DCF
dichlorofluorescein
- DGAT2
acyl-CoA:diacylglycerol acyltransferase 2
- DIC
dicarboxylate carrier
- ER
endoplasmic reticulum
- GCL
glutamate cysteine ligase
- GGT
γ-glutamyl transferase
- GR
GSH reductase
- Grx
glutaredoxin
- GSH
reduced GSH
- GSHPx
GSH peroxidase
- GSSG
oxidized GSH
- GST
GSH-S transferase
- mGSH
mitochondrial GSH
- MIM
mitochondrial inner membrane
- MOM
mitochondrial outer membrane
- MPT
mitochondrial permeability transition
- NASH
nonalcoholic steatohepatitis
- NSMase
neutral sphingomyelinase
- PP2A
protein phosphatase 2A
- Prx
peroxiredoxin
- PSH
reduced proteic SH
- PSSG
mixed GSH and proteic SH disulfide
- PSSP
proteic disulfide
- PUFA
polyunsaturated fatty acid
- RCS
reactive carbonyl species
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAM
S-adenosyl-l-methionine
- SH
steatohepatitis
- SH
thiol
- SLs
sphingolipids
- SMase
sphingomyelinase
- SOD
superoxide dismutase
- SPT
serinepalmitoyl transferase
- Srx
sulfiredoxin
- StAR
steroidogenic acute regulatory polypeptide
- TLR
Toll-like receptor
- TMRE
tetramethylrhodamine methyl ester
- TR
thioredoxin reductase
- Trx
thioredoxin
Footnotes
Reviewing Editors: Maria A. Aller, Paola Chiarugi, Jennifer S. Laurence, Andreas Mueller, Karl Oetti, Juan J. Poderoso, Gianluca Tell, and Jian Wu
Acknowledgments
The work described in this review was supported in part by the grant P50-AA-11999 from the Research Center for Liver and Pancreatic Diseases, U.S. National Institute of Alcohol Abuse and Alcoholism (NIAAA); the CIBEREHD and grants PI070193 and PI0900056 from the Instituto de Salud Carlos III; and by grants SAF2006-06780, SAF2008-2199, SAF2008-04974 and SAF2009-11417 from Plan Nacional de I+D and the Mutua Madrileña, Spain. We thank Dr. Laura Conde de la Rosa and Amanda Wellington for critically reading the article.
References
- 1.Adler V. Yin Z. Fuchs S. Regulation of JNK by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amstad P. Moret R. Cerutti P. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J Biol Chem. 1994;269:1606–1609. [PubMed] [Google Scholar]
- 3.Anathy V. Aesif SW. Guala AS. Havermans M. Reynaert NL. Ho YS. Budd RC. Janssen-Heininger YMW. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson N. Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Aoki H. Kang PM. Hampe J. Yoshimura K. Noma T. Matsuzaki M. Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong JS. Jones DJ. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi YC. Ansari GA. Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods Enzymol. 2005;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- 9.Baines CP. Kaiser RA. Sheiko T. Craigen WJ. Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakkenist CJ. Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Balaban RS. Nemoto S. Finkel T. Mitochondria, oxidants and ageing. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Barreyro FJ. Kobayashi S. Bronk SF. Werneburg NW. Malhi H. Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282:27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 13.Basañez G. Hardwick JM. Unravelling the bcl-2 apoptosis code with a simple model system. PlosBiol. 2008;6:e154. doi: 10.1371/journal.pbio.0060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basañez G. Sharpe JC. Galanis J. Brandt TB. Hardwick JM. Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- 15.Bass R. Ruddock LW. Klappa P. Freedman RB. A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J Biol Chem. 2004;279:5257–5262. doi: 10.1074/jbc.M304951200. [DOI] [PubMed] [Google Scholar]
- 16.Bataller R. Schwabe RF. Choi YH. Yang L. Paik YH. Lindquist J. Qian T. Schoonhoven R. Hagedorn CH. Lemasters JJ. Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayne AC. Mockett RJ. Orr WC. Sohal RS. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and ageing in transgenic Drosophila. Biochem J. 2005;391:277–284. doi: 10.1042/BJ20041872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellomo G. Palladini G. Vairetti M. Intranuclear distribution, function and fate of glutathione and glutathione-S-conjugate in living rat hepatocytes studied by fluorescence microscopy. Microsc Res Tech. 1997;36:243–252. doi: 10.1002/(SICI)1097-0029(19970215)36:4<243::AID-JEMT3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Beltroy EP. Richardson JA. Horton JD. Turley SD. Dietschy JM. Cholesterol accumulation and liver cell death in mice with Niemann Pick type C disease. Hepatology. 2005;42:886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- 20.Benigni A. Corna D. Zoja C. Sonzogni A. Latini R. Salio M. Conti S. Rottoli D. Longaretti L. Cassis P. Morigi M. Coffman TM. Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhagwat SV. Vijayasarathy C. Raza H. Mullick J. Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–839. doi: 10.1016/s0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 22.Bleys J. Navas-Acien A. Guallar E. Serum selenium levels and all-cause, cancer and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 23.Block CA. Ausubel FM. Paraquat-mediated selection for mutations in the manganeso-superoxide dismutase gene sodA. J Bacteriol. 1986;168:795–798. doi: 10.1128/jb.168.2.795-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borradaile NM. Han X. Harp JD. Gale SE. Ory DS. Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Borroz KI. Buetler TM. Eaton DL. Modulation of γ-glutamylcysteine synthetase large subunit mRNA expression by butylated hydroxianisole. Toxicol Appl Pharmacol. 1994;126:150–155. doi: 10.1006/taap.1994.1101. [DOI] [PubMed] [Google Scholar]
- 26.Boveris A. Cadenas E. Stoppani A. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner B. Ferlinz K. Grassmé H. Weller M. Koppenhoefer U. Dichgans J. Sandhoff K. Lang F. Gulbins E. Fas/CD95/Apo-I activates the acidic sphingomyelinase via caspases. Cell Death Differ. 1998;5:29–37. doi: 10.1038/sj.cdd.4400307. [DOI] [PubMed] [Google Scholar]
- 28.Brunelle JK. Bell EL. Quesada NM. Vercauteren K. Tiranti V. Zeviani M. Scarpulla RC. Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Bubici C. Papa S. Pham CG. Zazzeroni F. Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol. 2006;21:69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- 30.Caballero F. Fernandez A. De Lacy AM. Fernandez-Checa JC. Caballeria J. Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol. 2009;50:789–796. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Cantero AV. Portero-Otin M. Ayala V. Auge N. Sanson M. Elbaz M. Thiers JC. Pamplona R. Salvayre R. Negre-Salvayre A. Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-{beta}: implications for diabetic atherosclerosis. FASEB J. 2007;21:1–11. doi: 10.1096/fj.06-7536com. [DOI] [PubMed] [Google Scholar]
- 32.Cantin AM. Potential for antioxidant therapy of cystic fibrosis. Curr Opin Pulm Med. 2004;10:531–536. doi: 10.1097/01.mcp.0000138997.29276.a1. [DOI] [PubMed] [Google Scholar]
- 33.Carreras MC. Converso DP. Lorenti AS. Barbich M. Levisman DM. Jaitovich A. Antico Arciuch VG. Galli S. Poderoso JJ. Mitochondrial nitric oxide synthase drives redox signals for proliferation and quiescence in rat liver development. Hepatology. 2004;40:157–166. doi: 10.1002/hep.20255. [DOI] [PubMed] [Google Scholar]
- 34.Caselli A. Marzocchini R. Camici G. Manao G. Moneti G. Pieraccini G. Ramponi G The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 35.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Cave AC. Brewer AC. Narayanapanicker A. Ray R. Grieve DJ. Walker S. Shah AM. NADPH oxidases in cardiovascular health and disease. Antiox Redox Signal. 2006;8:692–714. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 37.Cesaratto L. Vascotto C. D'Ambrosio C. Scaloni A. Baccarani U. Paron I. Damante G. Calligaris S. Quadrifoglio F. Tiribelli C. Tell G. Overoxidations of peroxiredoxins as an immediate and sensitive marker of oxidative stress in HepG2 cells and its application to the redox effects induced by ischemia/reperfusion in human liver. Free Radic Res. 2005;39:255–268. doi: 10.1080/10715760400029603. [DOI] [PubMed] [Google Scholar]
- 38.Chae HZ. Chung SJ. Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 39.Chai YC. Hendrich S. Thomas JA. Protein S-thiolation in hepatocytes stimulated by t-butyl hydroperoxide, menadione, and neutrophils. Arch Biochem Biophys. 1994;310:264–272. doi: 10.1006/abbi.1994.1166. [DOI] [PubMed] [Google Scholar]
- 40.Chakravarthi S. Jessop CE. Bulleid NJ. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006;7:271–275. doi: 10.1038/sj.embor.7400645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang L. Kamata H. Solinas G. Luo JL. Maeda S. Venuprasad K. Liu YC. Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Chang TS. Jeong W. Woo HA. Lee SM. Park S. Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 43.Chen J. Delannoy M. Odwin S. He P. Trush MA. Yager JD. Enhanced mitochondrial gene transcript, ATP, bcl-2 protein levels, and altered glutathione distribution in ethinyl estradiol-treated cultured female rat hepatocytes. Toxicol Sci. 2003;75:271–278. doi: 10.1093/toxsci/kfg183. [DOI] [PubMed] [Google Scholar]
- 44.Chen J. Schenker S. Henderson GI. 4-Hydroxynonenal detoxification by mitochondrial glutathione S-transferase is compromised by short-term ethanol consumption in rats. Alcohol Clin Exp Res. 2002;26:1252–1258. doi: 10.1097/01.ALC.0000024081.89523.81. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z. Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- 46.Chen Z. Putt DA. Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 47.Cheng G. Cao Z. Xu X. van Meir EG. Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 48.Chiarugi P. Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslation modifications. Antioxid Redox Signal. 2007;9:1–24. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- 49.Chiarugi P. Pani G. Giannoni E. Taddei L. Colavitti R. Raugei G. Symons M. Borrello S. Galeotti T. Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiarugi P. Src redox regulation: there is more than meets the eye. Mol Cells. 2008;26:329–337. [PubMed] [Google Scholar]
- 51.Chisolm GM. Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 52.Cho SH. Redox regulation of PTEN and protein tyrosine phosphateses in H2O2-mediated cell signaling. FEBS Lett. 2004;560:7–13. doi: 10.1016/s0014-5793(04)00112-7. [DOI] [PubMed] [Google Scholar]
- 53.Cifone MG. De Maria R. Roncaioli P. Rippo MR. Azuma M. Lanier LL. Santoni A. Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Circu ML. Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Circu ML. Rodriguez C. Maloney R. Moyer MP. Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med. 2008;44:768–778. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colell A. Coll O. García-Ruiz C. París R. Tiribelli C. Kaplowitz N. Fernández-Checa JC. Tauroursodeoxycholic acid protects hepatocytes from ethanol-fed rats against tumor necrosis factor-induced cell death by replenishing mitochondrial glutathione. Hepatology. 2001;34:964–971. doi: 10.1053/jhep.2001.28510. [DOI] [PubMed] [Google Scholar]
- 57.Colell A. Garcia-Ruiz C. Lluis JM. Coll O. Mari M. Fernández-Checa JC. Cholesterol impairs the adenine nucleotide translocator-mediated mitochondrial permeability transition through altered membrane fluidity. J Biol Chem. 2003;278:33928–33935. doi: 10.1074/jbc.M210943200. [DOI] [PubMed] [Google Scholar]
- 58.Colell A. Garcia-Ruiz C. Miranda M. Ardite E. Marí M. Morales A. Corrales F. Kaplowitz N. Fernández-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 59.Colell A. Garcia-Ruiz C. Morales A. Ballesta A. Ookhtens M. Rodés J. Kaplowitz N. Fernández-Checa JC. Transport of reduced glutathione in hepatic mitochondria and mitoplasts from ethanol-treated rats: effect of membrane physical properties and S-adenosyl-l-methionine. Hepatology. 1997;26:699–708. doi: 10.1002/hep.510260323. [DOI] [PubMed] [Google Scholar]
- 60.Colell A. García-Ruiz C. Roman J. Ballesta A. Fernández-Checa JC. Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kappa B-dependent survival pathway. FASEB J. 2001;15:1068–1070. doi: 10.1096/fj.00-0574fje. [DOI] [PubMed] [Google Scholar]
- 61.Coll O. Colell A. Garcia-Ruiz C. Kaplowitz N. Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 62.Commoner B. Townsend J. Pake GE. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 63.Conde de la Rosa L. Schoemaker MH. Vrenken TE. Buist-Homan M. Havinga R. Jansen PL. Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44:918–929. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 64.Conde de la Rosa L. Vrenken TE. Hannivoort RA. Buist-Homan M. Havinga R. Slebos DJ. Kauffman HF. Faber KN. Jansen PL. Moshage H. Carbon monoxide blocks oxidative stress-induced hepatocyte apoptosis via inhibition of the p54 JNK isoform. Free Radic Biol Med. 2008;44:1323–1233. doi: 10.1016/j.freeradbiomed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Cotgreave IA. Analytical developments in the assay of intra- and extracellular GSH homeostasis: specific protein S-glutathionylation, cellular GSH and mixed disulphide compartmentalisation and interstitial GSH redox balance. Biofactors. 2003;17:269–277. doi: 10.1002/biof.5520170126. [DOI] [PubMed] [Google Scholar]
- 66.Cremesti AE. Goñi FM. Kolesnick RN. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 67.Crespo J. Cayón A. Fernández-Gil P. Hernández-Guerra M. Mayorga M. Domínguez-Díez A. Fernández-Escalante JC. Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 68.Cristofanon S. Morceau F. Scovassi AI. Dicato M. Ghibelli L. Diederich M. Oxidative, multistep activation of the noncanonical NF-kappa B pathways via disulfide Bcl-3/p50 complex. FASEB J. 2009;23:45–57. doi: 10.1096/fj.07-104109. [DOI] [PubMed] [Google Scholar]
- 69.Czaja MJ. Liu H. Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK-1 and AP-1. Hepatology. 2003;37:1405–1413. doi: 10.1053/jhep.2003.50233. [DOI] [PubMed] [Google Scholar]
- 70.Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal. 2002;4:759–767. doi: 10.1089/152308602760598909. [DOI] [PubMed] [Google Scholar]
- 71.Dalle-Donne I. Milzni A. Gagliano N. Colombo R. Giustarini D. Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 72.Das M. Sabio G. Jiang F. Rincon M. Flavell RA. Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Bilbao F. Arsenijevic D. Vallet P. Hjelle PO. Ottersen PO. Bouras C. Raffin Y. Abou K. Langhans W. Collins S. Plamondon J. Alves-Guerra MC. Haguenauer A. Garcia I. Richard D. Ricquier D. Giannakopoulos P. Resistance to cerebral ischemic injury in UCP2 knockout mice: evidence for a role of UCP2 as a regulator of mitochondrial glutathione levels. J Neurochem. 2004;89:1283–1292. doi: 10.1111/j.1471-4159.2004.02432.x. [DOI] [PubMed] [Google Scholar]
- 74.Deevska GM. Rozenova KA. Giltiay NV. Chambers MA. White J. Boyanovsky BB. Wei J. Daugherty A. Smart EJ. Reid MB. Merrill AH Jr. Nikolova-Karakashian M. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J Biol Chem. 2009;284:8359–8368. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Haan JB. Francesca C. Ianello R. Bladier C. Kelner MJ. Kola I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996;5:283–291. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 76.De la Asunción JG. Millan A. Pla R. Bruseghini L. Esteras A. Pallardo FV. Sastre J. Viña J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- 77.Delettre C. Yuste VJ. Moubarak RS. Bras M. Robert N. Susin SA. Identification and characterization of AIFsh2, a mitochondrial apoptosis-inducing factor (AIF) isoform with NADH oxidase activity. J Biol Chem. 2006;281:18507–18518. doi: 10.1074/jbc.M601751200. [DOI] [PubMed] [Google Scholar]
- 78.DeLeve LD. Kaplowitz N. Importance and regulation of hepatic GSH. Semin Liver Dis. 1990;10:251–266. doi: 10.1055/s-2008-1040481. [DOI] [PubMed] [Google Scholar]
- 79.De Minicis S. Bataller R. Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology. 2006;131:272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 80.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumor. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 81.de Pablo MA. Susin SA. Jacotot E. Larochette N. Costantinie P. Ravagnan L. Zamzami N. Kroemer G. Palmitate induces apoptosis via a direct effect on mitochondria. Apoptosis. 1999;4:81–87. doi: 10.1023/a:1009694124241. [DOI] [PubMed] [Google Scholar]
- 82.Ding Y. Choi SJ. Kim JH. Han X. Piao Y. Jeong JH. Choe W. Kang I. Ha J. Forman JH. Lee J. Yoon KS. Kim SS. Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation. Am J Pathol. 2008;172:1529–1541. doi: 10.2353/ajpath.2008.070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dixon BM. Heath SH. Kim R. Suh JH. Hagen TM. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid Redox Signal. 2008;10:963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dobson AW. Kelley MR. Wilson GL. Ledoux SP. Targeting DNA repair proteins to mitochondria. Methods Mol Biol. 2002;197:351–362. doi: 10.1385/1-59259-284-8:351. [DOI] [PubMed] [Google Scholar]
- 85.Droge W. Schulze-Osthoff K. Mihm S. Galter D. Schenk H. Eck HP. Roth S. Gmunder H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994;8:1131–1138. [PubMed] [Google Scholar]
- 86.Droge W. Free radicals in the physiological control of cell functions. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 87.Dumitru CA. Gulbins E. TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25:5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- 88.Echtay KS. Murphy MP. Smith RAJ. Talbot DA. Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side: studies using targeted antioxidants. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 89.Esterbauer H. Schaur RJ. Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 90.Facundo HT. de Paula JG. Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42:1039–1048. doi: 10.1016/j.freeradbiomed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Feldstein AE. Canbay A. Giucciardi ME. Higuchi H. Bronk SF. Gores GJ. Diet associated steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 92.Feldstein AE. Werneburg NW. Canbay A. Guicciardi ME. Bronk SF. Rydzewski R. Burgart LJ. Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 93.Fernandes AP. Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antiox Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 94.Fernandez A. Colell A. Caballero F. Matias N. Garcia-Ruiz C. Fernandez-Checa JC. Mitochondrial S-adenosyl-L-methionine transport is insensitive to alcohol-mediated changes in membrane dynamics. Alcohol Clin Exp Res. 2009;33:1–12. doi: 10.1111/j.1530-0277.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 95.Fernández-Checa JC. Kaplowitz N. García-Ruiz C. Colell A. Miranda M. Mari M. Ardite E. Morales A. Glutathione transport in mitochondria: defense against TNF-induced oxidative stress and alcohol. Am J Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 96.Fernandez-Checa JC. Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez-Checa JC. Redox regulation and signaling lipids in mitochondrial apoptosis. Biochim Biophys Res Commun. 2003;304:471–479. doi: 10.1016/s0006-291x(03)00619-3. [DOI] [PubMed] [Google Scholar]
- 98.Ferri KF. Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 99.Frezza C. Cipolat S. Martins de Brito O. Micaroni M. Beznoussenko GV. Rudka T. Bartoli D. Polishuck RS. Danial NN. De Strooper B. Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 100.Galle PR. Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18:141–151. doi: 10.1055/s-2007-1007150. [DOI] [PubMed] [Google Scholar]
- 101.Gallogly MM. Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Gao F. Kinnula VL. Myllarniemi M. Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal. 2008;10:343–354. doi: 10.1089/ars.2007.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Ruiz C. Colell A. Mari M. Morales A. Calvo M. Enrich C. Fernandez-Checa JC. Defective TNF-α-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.García-Ruiz C. Colell A. Morales A. Calvo M. Enrich C. Fernández-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J Biol Chem. 2002;277:36443–36448. doi: 10.1074/jbc.M206021200. [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Ruiz C. Colell A. Morales A. Kaplowitz N. Fernández-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial GSH status in loss of mitochondrial function and activation of transcription factor NF-κB: studies in isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825–834. [PubMed] [Google Scholar]
- 106.García-Ruiz C. Colell A. París R. Fernández-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000;14:847–858. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- 107.Garcia-Ruiz C. Colell Mari Morales A. Fernández-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Ruiz C. Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21:S3–S6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 109.Garcia-Ruiz C. Mari M. Colell A. Morales A. Caballero F. Montero F. Terrones O. Basañez G. Fernandez-Checa JC. Mitochondrial cholesterol in health and disease. Histol Histopathol. 2009;24:117–132. doi: 10.14670/HH-24.117. [DOI] [PubMed] [Google Scholar]
- 110.Garcia-Ruiz C. Morales A. Ballesta A. Rodes J. Kaplowitz N. Fernandez-Checa Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J Clin Invest. 1994;94:193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Ruiz C. Morales A. Colell A. Ballesta A. Rodes J. Kaplowitz N. Fernandez-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- 112.Garofalo T. Giammaroli AM. Misasi R. Tinari A. Manganelli V. Gambardella L. Pavan A. Malorni W. Sorice M. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death Differ. 2005;12:1378–1389. doi: 10.1038/sj.cdd.4401672. [DOI] [PubMed] [Google Scholar]
- 113.Garrido N. Griparic L. Jokitalo E. Wartiovaara J. van der Bliek AM. Spelbrink JN. Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell. 2003;14:1583–1596. doi: 10.1091/mbc.E02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gilot D. Loyer P. Corlu A. Glaise D. Lagdic-Gossmann D. Atfi A. Morel F. Ichijo H. Guguen-Guillouzo C. Liver protection from apoptosis requires both blockade of initiator caspase activities and inhibition of ASK1/JNK pathway via GST regulation. J Biol Chem. 2002;277:49220–49229. doi: 10.1074/jbc.M207325200. [DOI] [PubMed] [Google Scholar]
- 115.Giorgio M. Migliaccio E. Orsini F. Paolucci D. Moroni M. Contursi C. Pelliccia G. Luzi L. Minucci S. Marcaccio M. Pinton P. Rizzuto R. Bernardi P. Paolucci F. Pelicci PG. Electron transfer between cytochrome c and p66Shc generates hydrogen peroxide species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Giorgio M. Trinei M. Migliaccio E. Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalvez F. Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 118.Gonzalvez F. Schug ZT. Houtkooper RH. MacKenzie ED. Brooks DG. Wanders RJA. Petit PX. Vaz FM. Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Green DR. Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 120.Griffith OW. Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Griffith OW. Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 122.Grune T. Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 123.Gudz TI. Tserng KY. Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 124.Gujral JS. Hinson JA. Farhood A. Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;287:G243–G252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- 125.Gunawan BK. Liu ZX. Han D. Hanawa N. Gaarde WA. Kaplowitz N. c-Jun N-terminal kinase plays a major role in murin acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 126.Guzy RD. Hoyos B. Robin E. Chen H. Liu L. Mansfield KD. Simon MC. Hammerling U. Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 127.Ha KN. Chen Y. Cai J. Sternberg , Jr Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: implication for protection against oxidative stress. Invest Ophthalmol Vis Sci. 2006;47:2709–2715. doi: 10.1167/iovs.05-1322. [DOI] [PubMed] [Google Scholar]
- 128.Halliwell B. Aruoma OI. DNA damage by oxygen-derived species: its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 129.Hampton MB. Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- 130.Han D. Canali R. Rettori D. Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol. 2003;64:1136–1144. doi: 10.1124/mol.64.5.1136. [DOI] [PubMed] [Google Scholar]
- 131.Han MS. Park SY. Shinzawa K. Kim S. Chung KW. Lee JH. Kwon CH. Lee KW. Lee JH. Park CK. Chung WJ. Hwang JS. Yan JJ. Song DK. Tsujimoto Y. Lee MS. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 132.Handy DE. Lubos E. Yang Y. Galbraith JD. Kelly N. Zhang YY. Leopold JA. Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular response. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hannun YA. Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 134.Hansen JM. Zhang H. Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 135.He Q. Suzuki H. Sharma N. Sharma RP. Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse Liver. Toxicol Sci. 2006;94:388–397. doi: 10.1093/toxsci/kfl102. [DOI] [PubMed] [Google Scholar]
- 136.Heinrich M. Neumeyer J. Jakob M. Hallas C. Tchikov V. Winoto-Morbach S. Wickel M. Schneider-Brachert W. Trauzold A. Hethke A. Schütze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and −3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 137.Heinrich M. Wickel M. Schneider-Brachert W. Sandberg C. Gahr J. Schwandner R. Weber T. Saftig P. Peters C. Brunner J. Krönke M. Schütze S. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18:5252–5263. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hentze H. Latta M. Kunsle G. Lucas R. Wendel A. Redox control of hepatic cell death. Toxicol Lett. 2003;139:111–118. doi: 10.1016/s0378-4274(02)00425-3. [DOI] [PubMed] [Google Scholar]
- 139.Ho YF. Guenthner TM. Isolation of liver nuclei that retain functional trans-membrane transport. J Pharmacol Toxicol Methods. 1997;38:163–168. doi: 10.1016/s1056-8719(97)00082-8. [DOI] [PubMed] [Google Scholar]
- 140.Holland WL. Brozinick JT. Wang LP. Hawkins ED. Sargent KM. Liu Y. Narra K. Hoehn KL. Knotts TA. Siesky A. Nelson DH. Karathanasis SK. Fontenot GK. Birnbaum MJ. Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 141.Holme JA. Hongslo JK. Bjorge C. Nelson SD. Comparative cytotoxic effects of acetaminophen (N-acetyl-p-aminophenol), a non-hepatotoxic regioisomer acetyl-m-aminophenol and their postulated reactive hydroquinone and quinone metabolites in monolayer cultures of mouse hepatocytes. Biochem Pharmacol. 1991;42:1137–1142. doi: 10.1016/0006-2952(91)90299-k. [DOI] [PubMed] [Google Scholar]
- 142.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 143.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 144.Hwang C. Sinskey AJ. Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 145.Iimuro Y. Gallucci RM. Luster MI. Kono H. Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 146.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 147.Imai H. Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 148.Iwata JW. Lee K. Okada KK. Lee M. Iwata R. Rasmussen TA. Link P. Ramaswamy S. Jap BK. Complete structure of the 11-subunit of bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 149.Jaeschke H. Ho YS. Fisher MA. Lawson JA. Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–450. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- 150.Jaeschke H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology. 2008;48:699–701. doi: 10.1002/hep.22556. [DOI] [PubMed] [Google Scholar]
- 151.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 152.Jang JH. Moritz W. Graf R. Clavien PA. Preconditioning with death ligands FasL and TNF-a protects the cirrhotic mouse liver against ischemic injury. Gut. 2008;57:492–499. doi: 10.1136/gut.2007.137703. [DOI] [PubMed] [Google Scholar]
- 153.Jessop CE. Bulleid NJ. Glutathione directly reduces an oxidoreductase in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2004;279:55341–55347. doi: 10.1074/jbc.M411409200. [DOI] [PubMed] [Google Scholar]
- 154.Jocelyn PC. Kamminga A. The non-protein thiol of rat liver mitochondria. Biochim Biophys Acta. 1974;343:356–362. doi: 10.1016/0304-4165(74)90099-3. [DOI] [PubMed] [Google Scholar]
- 155.Jones DP. Carlson JL. Mody VC. Cai J. Lynn MJ. Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 156.Kagan VE. Tyurin VA. Jiang J. Tyurina YY. Ritov VB. Amoscato AA. Osipov AN. Belikova NA. Kapralov AA. Kini V. Vlasova II. Zhao Q. Zou M. Di P. Svistunenko DA. Kurnikov IV. Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 157.Kalanxhi E. Wallace CJ. Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models. Biochem J. 2007;407:179–187. doi: 10.1042/BJ20070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kamata H. Honda S. Maeda S. Chang L. Hirata H. Karin M. Reactive oxygen species promote TNF-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 159.Kaplowitz N. Aw TY. Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 160.Kaplowitz N. Than TA. Shinohara M. Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 161.Kerr JFR. Willie AH. Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kienhofer J. Haussler DJF. Ruckelshausen F. Muessig E. Weber K. Pimentel D. Ullrich V. Burkle A. Bachschmid M. Association of mitochondrial antioxidant enzymes with mitochondrial DNA as integral nucleoid constituents. FASEB J. 2009;23:2034–2044. doi: 10.1096/fj.08-113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Klungland A. Rosewell I. Hollenbach S. Larsen E. Daly G. Epe B. Seeberg E. Lindahl T. Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kodama Y. Brenner DA. c-Jun N-terminal kinase signaling in the pathogenesis of nonalcoholic fatty liver disease: multiple roles in multiple steps. Hepatology. 2009;49:6–8. doi: 10.1002/hep.22710. [DOI] [PubMed] [Google Scholar]
- 165.Kodama Y. Taura K. Miura K. Schnabl B. Osawa Y. Brenner DA. Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 166.Kolesnick RN. Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 167.Krähenbühl S. Krähenbühl-Glauser S. Stucki J. Gehr P. Reichen J. Stereological and functional analysis of liver mitochondria from rats with secondary biliary cirrhosis: impaired mitochondrial metabolism and increased mitochondrial content per hepatocyte. Hepatology. 1992;15:1167–1172. doi: 10.1002/hep.1840150631. [DOI] [PubMed] [Google Scholar]
- 168.Krähenbühl S. Stucki J. Reichen J. Reduced activity of the electron transport chain in liver mitochondria isolated from rats with secondary biliary cirrhosis. Hepatology. 1992;15:1160–1166. doi: 10.1002/hep.1840150630. [DOI] [PubMed] [Google Scholar]
- 169.Krähenbühl S. Talos C. Lauterburg BH. Reichen J. Reduced antioxidative capacity in liver mitochondria from bile duct ligated rats. Hepatology. 1995;22:607–612. doi: 10.1002/hep.1840220234. [DOI] [PubMed] [Google Scholar]
- 170.Kriska T. Korytowski W. Girotti AW. Role of mitochondrial cardiolipin peroxidation in apoptotic photokilling of 5-aminolevulinate-treated tumor cells. Arch Biochem Biophys. 2005;433:435–446. doi: 10.1016/j.abb.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 171.Kroemer G. Galluzi L. Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 172.Kuboki S. Okaya T. Schuster R. Blanchard J. Denenberg A. Wong HR. Lentsch AB. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol. 2007;292:G201–G207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 173.Kuo PC. Abe KY. Schroeder RA. Interleukin-1-induced nitric oxide production modulates glutathione synthesis in cultured rat hepatocytes. Am J Physiol. 1996;271:C851–C862. doi: 10.1152/ajpcell.1996.271.3.C851. [DOI] [PubMed] [Google Scholar]
- 174.Kuwana T. Mackey MR. Perkins G. Ellisman MH. Latterich M. Schneiter R. Green DR. Newmeyer DD. Bid, Bax and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 175.Kwong M. Kan YW. Chan JY. The CNC basic leucine zipper factor, Nrf1, is essential for cell survival in response to oxidative stress-inducing agents. J Biol Chem. 1999;274:37491–37498. doi: 10.1074/jbc.274.52.37491. [DOI] [PubMed] [Google Scholar]
- 176.Lamlé J. Marhenke S. Borlak J. von Wasielewski R. Ericsson CJ. Geffers R. Manns MP. Yamamoto M. Vogel A. Nuclear factor-erythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–1168. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 177.Laurent A. Nicco C. Chereau C. Goulvestre C. Alexandre J. Alves A. Levy E. Goldwasser F. Panis Y. Soubrane O. Weill B. Batteaux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–956. [PubMed] [Google Scholar]
- 178.Lee AH. Scapa EF. Cohen DE. Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP-1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee JY. Jung GY. Heo HJ. Yun MR. Park JY. Bae SS. Hong KW. Lee WS. Kim CD. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- 180.Lee Y. Hirose H. Ohneda M. Johnson JH. McGarry JD. Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Linke K. Jakob U. Not every disulfide lasts forever: disulfide bond formation as a redox switch. Antioxid Redox Signal. 2003;5:425–434. doi: 10.1089/152308603768295168. [DOI] [PubMed] [Google Scholar]
- 182.Lippman SM. Klein EA. Goodman PJ. Lucia MS. Thompson IM. Ford LG. Parnes HL. Minasian LM. Gaziano JM. Hartline JA. Parsons JK. Bearden JD., 3rd Crawford ED. Goodman GE. Claudio J. Winquist E. Cook ED. Karp DD. Walther P. Lieber MM. Kristal AR. Darke AK. Arnold KB. Ganz PA. Santella RM. Albanes D. Taylor PR. Probstfield JL. Jagpal TJ. Crowley JJ. Meyskens FL Jr. Baker LH. Colfman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Little C. Olinescu R. Reid KG. O'Brien PJ. Properties and regulation of glutathione peroxidase. J Biol Chem. 1970;245:3632–3636. [PubMed] [Google Scholar]
- 184.Liu Y. Adachi M. Zhao S. Hareyama M. Koong AC. Luo D. Rando TA. Imai K. Shimomura Y. Preventing oxidative stress: a new role for XBP-1. Cell Death Differ. 2009;16:847–857. doi: 10.1038/cdd.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu Y. Fiskum G. Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 186.Llacuna L. Mari M. Garcia-Ruiz C. Fernandez-Checa JC. Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 187.Llacuna L. Mari M. Lluis JM. Garcia-Ruiz C. Fernandez-Checa JC. Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1–10. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lluis JM. Buricchi F. Chiarugi P. Morales A. Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor κB via c-Src and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 189.Lluis JM. Colell A. Garcia-Ruiz C. Kaplowitz N. Fernandez-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708–724. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 190.Lluis JM. Morales A. Blasco C. Colell A. Mari M. Garcia-Ruiz C. Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–3232. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- 191.Lou H. Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase dependent and independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- 192.Lu C. Armstrong JS. Role of calcium and cyclophilin D in the regulation of mitochondrial permeabilization induced by glutathione depletion. Biochem Biophys Res Commun. 2007;363:572–577. doi: 10.1016/j.bbrc.2007.08.196. [DOI] [PubMed] [Google Scholar]
- 193.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 194.Lucken-Ardjomande S. Montessuit S. Martinou JC. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ. 2008;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- 195.Malhi H. Bronk SF. Werneburg NW. Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 196.Malhi H. Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mari M. Caballero F. Colell A. Morales A. Caballeria J. Fernandez A. Enrich C. Fernandez-Checa JC. Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 198.Mari M. Colell A. Morales A. Caballero F. Moles A. Fernandez A. Terrones O. Basañez G. Antonsson B. Garcia-Ruiz C. Fernandez-Checa JC. Mechanisms of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kB activation. Gastroenterology. 2008;134:1507–1520. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 199.Mari M. Colell A. Morales A. Pañeda C. Varela-Nieto I. García-Ruiz C. Fernández-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J Clin Invest. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mari M. Fernandez-Checa JC. Sphingolipids signaling and liver diseases. Liver Intl. 2007;27:440–450. doi: 10.1111/j.1478-3231.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 201.Mari M. Morales A. Colell A. Garcia-Ruiz C. Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antiox Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Markovic J. Borras C. Ortega A. Sastre J. Vina J. Pallardo FV. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem. 2007;282:20416–20424. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 203.Marra F. Gastaldelli A. Svegliati Baroni G. Tell G. Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 204.Martínez-Ruiz A. Lamas S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res. 2007;75:220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 205.Mato JM. Alvarez L. Ortiz P. Pajares A. S-adenosyl-L-methionine: molecular mechanisms and clinical implications. Pharmacol Ther. 1997;73:265–280. doi: 10.1016/s0163-7258(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 206.Matsumaru K. Ji C. Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 207.Matsuzawa N. Takamura T. Kurita S. Misu H. Ota T. Ando H. Yokoyama M. Honda M. Zen Y. Nakanuma Y. Miyamoto K. Kaneko S. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 208.Matthews JR. Wakasugi N. Virelizier JL. Yodoi J. Hay RT. Thioredoxin regulates the DNA binding activity of NF-κB by reduction of a disulphide bond involving cysteine62. Nuclei Acids Res. 1992;20:3821–3880. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McClung JP. Roneker CA. Mu W. Lisk DJ. Langlais P. Liu F. Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McCord JM. Fridovich I. Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 211.Meissner F. Molawi K. Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2009;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 212.Meister A. Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 213.Meng TC. Buckley DA. Galic S. Tiganis T. Tonos NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 214.Micheau O. Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 215.Miramar MD. Costantini P. Ravagnan L. Saraiva LM. Haouzi D. Brothers G. Penninger JM. Peleato ML. Kroemer G. Susin SA. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J Biol Chem. 2001;276:16391–16398. doi: 10.1074/jbc.M010498200. [DOI] [PubMed] [Google Scholar]
- 216.Mitchell JR. Jollow DJ. Potter WZ. Davis DC. Gillette JR. Brodie BB. Acetaminophen-induced hepatic necrosis, I: role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 217.Mitsuyoshi H. Nakashima T. Sumida Y. Yoh T. Nakajima Y. Ishikawa H. Inaba K. Sakamoto Y. Okanoue T. Kashima K. Ursodeoxycholic acids protects hepatocytes against oxidative injury via induction of antioxidants. Biochem Biophys Res Commun. 1999;263:537–542. doi: 10.1006/bbrc.1999.1403. [DOI] [PubMed] [Google Scholar]
- 218.Moellering D. McAndrew J. Patel RP. Cornwell T. Lincoln T. Cao X. Messina JL. Forman HJ. Jo H. Darley-Usmar VM. Nitric oxide-dependent induction of glutathione synthesis through increased expression of gamma-glutamylcysteine synthetase. Arch Biochem Biophys. 1998;358:74–82. doi: 10.1006/abbi.1998.0854. [DOI] [PubMed] [Google Scholar]
- 219.Mohr S. Zech B. Lapetina EG. Brüne B. Inhibition of caspase-3 by S-nitrosation and oxidation caused by nitric oxide. Biochem Biophys Res Commun. 1997;238:387–391. doi: 10.1006/bbrc.1997.7304. [DOI] [PubMed] [Google Scholar]
- 220.Monetti M. Levin MC. Watt MJ. Sajan MP. Marmor S. Hubbard BK. Stevens RD. Bain JR. Newgard CB. Farese RV Sr. Hevener AL. Farese RV., Jr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 221.Montalvo-Jave E. Pina E. Montalvo-Arenas C. Urrutia R. Benavente-Chenhalls L. Pena-Sanchez J. Geller DA. Role of ischemic preconditioning in liver surgery and hepatic transplantation. J Gastrointest Surg. 2009 Apr 30; doi: 10.1007/s11605-009-0878-7. Epub ahead of print. [DOI] [PubMed]
- 222.Montero J. Morales A. Llacuna L. Lluis JM. Terrones O. Basañez G. Antonsson B. Prieto J. García-Ruiz C. Colell A. Fernández-Checa JC. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68:5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 223.Morales A. García-Ruiz C. Miranda M. Marí M. Colell A. Ardite E. Fernández-Checa JC. Tumor necrosis factor increases hepatocellular glutathione by transcriptional regulation of the heavy subunit chain of γ-glutamylcysteine synthetase. J Biol Chem. 1997;272:30371–30379. doi: 10.1074/jbc.272.48.30371. [DOI] [PubMed] [Google Scholar]
- 224.Morales A. Lee H. Goñi F. Kolesnick RN. Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 225.Morales A. Miranda M. Sanchez-Reyes A. Biete A. Fernandez-Checa JC. Oxidative damage of mitochondrial and nuclear DNA induced by ionizing radiation in human hepatoblastoma cells. Int J Radiat Oncol Biol Phys. 1998;42:191–203. doi: 10.1016/s0360-3016(98)00185-0. [DOI] [PubMed] [Google Scholar]
- 226.Mulcahy RT. Wartman MA. Bailey HH. Gipp JJ. Constitutive and β-naphthoflavone-induced expression of the human γ-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 227.Muppidi JR. Tschopp J. Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–446. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 228.Nagai H. Matsumaru K. Feng G. Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 229.Nakagawa T. Shimizu S. Watanabe T. Yamaguchi O. Otsu K. Yamagata H. Inohara H. Kubo T. Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 230.Nakamura S. Takamura T. Matsuzawa-Nagata N. Takayama H. Misu H. Noda H. Nabemoto S. Kurita S. Ota T. Ando H. Miyamoto KI. Kaneko S. Palmitate Induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Negre-Salvayre A. Coatrieux C. Ingueneau C. Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins: potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 233.Neuschwander-Tetri BA. Nicholson C. Wells LD. Tracy TF., Jr Cholestatic liver injury down-regulates hepatic glutathione synthesis. J Surg Res. 1996;63:447–451. doi: 10.1006/jsre.1996.0290. [DOI] [PubMed] [Google Scholar]
- 234.Nguyen AD. McDonald JG. Bruick RK. DeBose-Boyd RA. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor (HIF)-mediated induction of Insigs. J Biol Chem. 2007;282:27436–27446. doi: 10.1074/jbc.M704976200. [DOI] [PubMed] [Google Scholar]
- 235.Nobel CS. Burgess DH. Zhivotovsky B. Burkitt MJ. Orrenius S. Slater AF. Mechanism of dithiocarbamate inhibition of apoptosis: thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem Res Toxicol. 1997;10:636–643. doi: 10.1021/tx970006a. [DOI] [PubMed] [Google Scholar]
- 236.Noh YH. Baek JY. Jeong W. Rhee SG. Chang TS. Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. J Biol Chem. 2009;284:8470–8477. doi: 10.1074/jbc.M808981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.O'Brien PJ. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 238.O'Brien PJ. Radical formation during the peroxidase catalyzed metabolism of carcinogens and xenobiotics: the reactivity of these radicals with GSH, DNA, and unsaturated lipid. Free Radic Biol Med. 1988;4:169–183. doi: 10.1016/0891-5849(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 239.Olmos Y. Valle I. Borniquel S. Tierrez A. Soria E. Lamas S. Monsalve M. Mutual dependence of FOXO3A and PGC-1a in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Orr WC. Sohal RS. Effects of Cu,Zn superoxide dismutase overexpression of life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch Biochem Biophys. 1993;301:34–40. doi: 10.1006/abbi.1993.1111. [DOI] [PubMed] [Google Scholar]
- 241.Ozcan U. Cao Q. Yilmaz E. Lee AH. Iwakoshi NN. Ozdelen E. Tuncman G. Görgün C. Glimcher LH. Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 242.Page S. Fischer C. Baumgartner B. Haas M. Kreusel U. Loidl G. Hayn M. Ziegler-Heitbrock HW. Neumeier D. Brand K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- 243.Paik JH. Kollipara R. Chu G. Ji H. Xiao Y. Ding Z. Miao L. Tothova Z. Horner JW. Carrasco DR. Jiang S. Gilliland DG. Chin L. Wong WH. Castrillon DH. DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pallardó FV. Markovic J. García JL. Viña J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspects Med. 2009 Jan 9; doi: 10.1016/j.mam.2009.01.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 245.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim Biophys Acta. 2008;1777:1249–1262. doi: 10.1016/j.bbabio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 246.Pastorino JG. Hoek JW. Ethanol potentiates tumor necrosis factor cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 247.Payabvash S. Ghahremani MH. Goliaei A. Mandegary A. Shafaroodi H. Amanlou M. Dehpour AR. Nitric oxide modulates glutathione synthesis during endotoxemia. Free Radic Biol Med. 2006;41:1817–1828. doi: 10.1016/j.freeradbiomed.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 248.Petersen DR. Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 249.Pham CG. Bubici C. Zazzeroni F. Papa S. Jones J. Alvarez K. Jayawardena S. De Smaele E. Cong R. Beaumont C. Torti FM. Torti SV. Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 250.Piantadosi CA. Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 251.Poli G. Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 252.Poole LB. Karplus PA. Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 253.Rabilloud T. Heller M. Gasnier F. Luche S. Rey C. Aebersold R. Benahmed M. Louisot P. Lunardi J. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J Biol Chem. 2002;277:19396–19401. doi: 10.1074/jbc.M106585200. [DOI] [PubMed] [Google Scholar]
- 254.Ray R. Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 255.Raza H. John A. 4-Hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 256.Rhee SG. Chae HZ. Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 257.Ricci JE. Gottlieb RA. Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Richter C. Park JW. Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1998;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rimkunas VM. Graham MJ. Crooke RM. Liscum L. TNF plays a role in hepatocyte apoptosis in Niemann-Pick type C liver disease. J Lipid Res. 2009;50:327–333. doi: 10.1194/jlr.M800415-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rippo MR. Malisan F. Ravagnan L. Tomassini B. Condo I. Costantini P. Susin SA. Rufini A. Todaro M. Kroemer G. Testi R. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J. 2000;14:2047–2054. doi: 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- 261.Roeb E. Purucker E. Gartung C. Geier A. Jansen B. Winograd R. Matern S. Effect of glutathione depletion and hydrophilic bile acids on hepatic acute phase reaction in rats with extrahepatic cholestasis. Scand J Gastroenterol. 2003;38:878–885. doi: 10.1080/00365520310003471. [DOI] [PubMed] [Google Scholar]
- 262.Roskams T. Yang SQ. Koteish A. Durnez A. DeVos R. Huang X. Achten R. Verslype C. Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and non-alcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rudiger HA. Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 264.Ruvolo PP. Deng X. Ito T. Carr BK. May WS. Ceramide induces bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 265.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Salazar JJ. Van Houten B. Preferential mitochondrial DNA injury caused by glucose oxidase as a steady generator of hydrogen peroxide in human fibroblasts. Mutat Res. 1997;385:139–149. doi: 10.1016/s0921-8777(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 267.Salmon AB. Perez VI. Bokov A. Jernigan A. Kim G. Zhao H. Levine RL. Richardson A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J epub. 2009 Jun 1; doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Salvesen GS. Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 269.Sanchez A. Factor VM. Espinoza LA. Schoreder IS. Thorgeirsson SS. In vitro differentiation of rat liver derived stem cells results in sensitization to TNF-mediated apoptosis. Hepatology. 2004;40:590–599. doi: 10.1002/hep.20363. [DOI] [PubMed] [Google Scholar]
- 270.Scaffidi C. Fulda S. Srinivasan A. Friesen C. Li F. Tomaselli KJ. Debatin KM. Krammer PH. Peter ME. Two CD95 signaling pathways. EMBO J. 1998;16:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Scaloni A. Codarin E. Di Maso V. Arena S. Renzone G. Tiribelli C. Quadrifoglio F. Tell G. Modern strategies to identify new molecular targets for the treatment of liver diseases: the promising role of proteomics and redox proteomics investigations. Proteomics Clin Appl. 2009;3:242–262. doi: 10.1002/prca.200800169. [DOI] [PubMed] [Google Scholar]
- 272.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 273.Schneider-Brachert W. Tchikov V. Neumeyer J. Jakob M. Winoto-Morbach S. Held-Feindt J. Heinrich M. Merkel O. Ehrenschwender M. Adam D. Mentlein R. Kabelitz D. Schütze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 274.Schoneich C. Mechanism of protein damage induced by cysteine thiyl radical formation. Chem Res Toxicol. 2008;21:1175–1179. doi: 10.1021/tx800005u. [DOI] [PubMed] [Google Scholar]
- 275.Schulze T. Zarse K. Voigt A. Urban N. Birringer M. Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 276.Schuppe-Koistinen I. Gerdes R. Moldeus P. Cotgreave IA. Studies on the reversibility of protein S-thiolation in human endothelial cells. Arch Biochem Biophys. 1994;315:226–234. doi: 10.1006/abbi.1994.1494. [DOI] [PubMed] [Google Scholar]
- 277.Schutze S. Tchikov V. Schneider-Brachert W. Regulation of TNFR1 and CD95 signaling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 278.Scorrano L. Ashiya M. Buttle K. Weiler S. Oakes SA. Mannella CA. Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 279.Ségui B. Cuvillier O. Adam-Klages S. Garcia V. Malagarie-Cazenave S. Lévêque S. Caspar-Bauguil S. Coudert J. Salvayre R. Krönke M. Levade T. Involvement of FAN in TNF-induced apoptosis. J Clin Invest. 2001;108:143–151. doi: 10.1172/JCI11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Seo JH. Lim JC. Lee DY. Kim KS. Piszeck G. Nam HW. Kim YS. Ahn T. Yun C. Kim K. Chock B. Chae HZ. A novel protective mechanism against irreversible hyperoxidation of peroxiredoxin: N a-terminal acetylation of human peroxiredoxin II. J Biol Chem. 2009;284:13455–13465. doi: 10.1074/jbc.M900641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Seo MS. Kang SW. Kim K. Baines IC. Lee TH. Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- 282.Shen XD. Ke B. Zhai Y. Gao F. Tsuchihashi S. Lassman CR. Busuttil RW. Kupiec-Weglinski JW. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transplant. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 283.Shulga N. Hoek JB. Pastorino JG. Elevated PTEN levels account for the increased sensitivity of ethanol-exposed cells to tumor necrosis factor-induced cytotoxicity. J Biol Chem. 2005;280:9416–9424. doi: 10.1074/jbc.M409505200. [DOI] [PubMed] [Google Scholar]
- 284.Sies H. Oxidative stress: introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic Press; 1985. pp. 1–8. [Google Scholar]
- 285.Sohn J. Rudolph J. Catalytic and chemical competence of regulation of CDC25 phosphatase by oxidation/reduction. Biochemistry. 2003;42:10060–10070. doi: 10.1021/bi0345081. [DOI] [PubMed] [Google Scholar]
- 286.Srivastava S. Chan C. Hydrogen peroxide and hydroxyl radicals mediate palmitate-induced cytotoxicity to hepatoma cells: relation to mitochondrial permeability transition. Free Radic Res. 2007;41:38–49. doi: 10.1080/10715760600943900. [DOI] [PubMed] [Google Scholar]
- 287.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 288.Stocco DM. Starting to understand cholesterol transfer. Nat Struct Biol. 2000;7:445–447. doi: 10.1038/75834. [DOI] [PubMed] [Google Scholar]
- 289.Stocker R. Keanney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 290.St-Pierre J. Drori S. Uldry M. Silvaggi JM. Rhee J. Jäger S. Handschin C. Zheng K. Lin J. Yang W. Simon DK. Bachoo R. Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 291.Stranges S. Marshall JR. Natarajan R. Donahue RP. Trevisan M. Combs GF. Cappucio FP. Ceriello A. Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 292.Su GL. Goyert SM. Fan MH. Aminlari A. Gong KQ. Klein RD. Myc A. Alarcon WH. Steinstraesser L. Remick DG. Wang SC. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol. 2000;283:G640–G645. doi: 10.1152/ajpgi.00253.2001. [DOI] [PubMed] [Google Scholar]
- 293.Suetsugu H. Iimuro Y. Uehara T. Nishio T. Harada N. Yoshida M. Hatano E. Son G. Fujimoto J. Yamaoka Y. Nuclear factor {kappa}B inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut. 2005;54:835–842. doi: 10.1136/gut.2004.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sun MG. Williams J. Munoz-Pinedo C. Perkins GA. Brown JM. Ellisman MH. Green DR. Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat Cell Biol. 2007;9:1057–1072. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- 295.Sun S. Zhang H. Xue B. Wu Y. Wang J. Yin Z. Luo L. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm Res. 2006;55:504–510. doi: 10.1007/s00011-006-6037-7. [DOI] [PubMed] [Google Scholar]
- 296.Sun X. Shih AY. Johannssen HC. Erb H. Li P. Murphy TH. Two-photon imaging of glutathione levels in intact brain indicates enhanced redox buffering in developing neurons and cells at the cerebrospinal fluid and blood-brain interface. J Biol Chem. 2006;281:17420–17431. doi: 10.1074/jbc.M601567200. [DOI] [PubMed] [Google Scholar]
- 297.Susin SA. Lorenzo HK. Zamzami N. Marzo I. Snow BE. Brothers GM. Mangion J. Jacotot E. Costantini P. Loeffler M. Larochette N. Goodlett DR. Aebersold R. Siderovski DP. Penninger JM. Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 298.Tamaki N. Hatano E. Taura K. Tada M. Kodama Y. Nitta T. Iwaisako K. Seo S. Nakajima A. Ikai I. Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G498–G505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 299.Thimmulappa RK. Scollick C. Traore K. Yates M. Trush MA. Liby KT. Sporn MB. Yamamoto M. Kensler TW. Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tomita Y. Tamiya G. Ando S. Ohsumi K. Chiyo T. Mizutani A. Kitamura N. Toda K. Kaneko T. Horie Y. Han JY. Kato S. Shimoda M. Oike Y. Tomizawa M. Makino S. Ohkura T. Saito H. Kumagai N. Nagata H. Ishii H. Hibi T. Tumour necrosis factor a signaling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Traber MG. Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Trachootham D. Zhou Y. Zhang H. Demizu Y. Chen Z. Pelicano H. Chiao PJ. Achanta G. Arlinghaus RB. Liu J. Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 303.Tsavachidou D. McDonnell TJ. Wen S. Wang X. Vakar-Lopez F. Pisters LL. Pettaway CA. Wood CG. Do KA. Thall PF. Stephens C. Efstathiou E. Taylor R. Menter DG. Troncoso P. Lippman SM. Logothetis CJ. Kim J. Selenium vitamin E: cell type– and intervention-specific tissue effects in prostate cancer. J Natl Cancer Inst. 2009;101:306–320. doi: 10.1093/jnci/djn512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Uchida K. Kanematsu M. Morimitsu Y. Osawa T. Noguchi N. Niki E. Acrolein is a product of lipid peroxidation reaction: formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 305.Ueda K. Ueyama T. Yoshida K. Kimura H. Ito T. Shimizu Y. Oka M. Tsuruo Y. Ichinose M. Adaptive HNE-Nrf2-HO-1 pathway against oxidative stress is associated with acute gastric mucosal lesions. Am J Physiol Gastrointest Liver Physiol. 2008;295:G460–G469. doi: 10.1152/ajpgi.00204.2007. [DOI] [PubMed] [Google Scholar]
- 306.Ueda S. Masutani H. Nakamura H. Tanaka T. Ueno M. Yodoi J. Redox control of caspases. Antioxid Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 307.Uthus EO. Ross S. Dietary selenium (Se) and copper (Cu) interact to affect homocysteine metabolism in rats. Biol Trace Elem Res. 2009;129:213–220. doi: 10.1007/s12011-008-8295-4. [DOI] [PubMed] [Google Scholar]
- 308.Van Kuijk FJ. Holte LL. Dratz EA. 4-Hydroxyhexenal: a lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim Biophys Acta. 1990;1043:116–118. doi: 10.1016/0005-2760(90)90118-h. [DOI] [PubMed] [Google Scholar]
- 309.Van Meer G. Voelker DR. Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Vascotto C. Cesaratto L. D'Ambrosio C. Scaloni A. Avellini C. Paron I. Baccarani U. Adani GL. Tiribelli C. Quadrifoglio F. Tell G. Proteomic analyses of liver tissues subjected to early ischemia/reperfusion injury during human orthotopic liver transplantation. Proteomics. 2006;6:3455–3465. doi: 10.1002/pmic.200500770. [DOI] [PubMed] [Google Scholar]
- 311.Vaughn AE. Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Velasquez A. Bechara RI. Lewis JF. Malloy J. McCaig L. Brown LA. Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- 313.Vendemiale G. Grattagliano I. Altomare E. Turturro N. Guerrieri F. Effect of acetaminophen administration on hepatic glutathione compartmentation and mitochondrial energy metabolism in the rat. Biochem Pharmacol. 1996;52:1147–1154. doi: 10.1016/0006-2952(96)00414-5. [DOI] [PubMed] [Google Scholar]
- 314.Venkatakrishnan P. Nakayasu ES. Almeida IC. Miller RT. Absence of nitric oxide synthase in sequentially purified rat liver. J Biol Chem. 2009;284:19843–19855. doi: 10.1074/jbc.M109.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ventura JJ. Cogwell P. Flavell RA. Baldwin AS. Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Verhagen AM. Ekert PG. Pakusch M. Silke J. Connolly LM. Reid GE. Moritz RL. Simpson RJ. Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 317.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Voehringer DW. Mcconkey DJ. Mcdonnell TJ. Brisbay S. Meyn RE. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc Natl Acad Sci USA. 1998;95:2956–2960. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.von Montfort C. Matias N. Garcia-Ruiz C. Fernandez-Checa JC. The mitochondrial GSH level is responsible for the efficiency and the protective effect of superoxide elimination. J Hepatol. 2009;50:S265. (abstract). [Google Scholar]
- 320.Wang W. Fang H. Groom L. Cheng A. Zhang W. Liu J. Wang X. Li K. Han P. Zheng M. Yin J. Wang W. Mattson MP. Kao JP. Lakatta EG. Sheu SS. Ouyang K. Chen J. Dirksen RT. Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wang X. Quinn P. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 322.Weintraub NL. Nox response to injury. Arterioscler Thromb Vasc Biol. 2002;22:4–5. [PubMed] [Google Scholar]
- 323.Wheeler MD. Nakagami M. Bradford BU. Uesugi T. Mason RP. Connor HD. Dikalova A. Kadiiska M. Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J Biol Chem. 2001;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- 324.Wild AC. Moinova HR. Mulcahy RT. Regulation of g-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 325.Wilson KP. Black JA. Thomson JA. Kim EE. Griffith JP. Navia MA. Murcko MA. Chambers SP. Aldape RA. Raybuck SA. Livingston DJ. Structure and mechanism of interleukin-1b converting enzyme. Nature. 1994;370:270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 326.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 327.Woo HA. Chae HZ. Hwang SC. Yang KS. Kang SW. Kim K. Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 328.Woo HA. Jeong W. Chang TS. Park KJ. Park SJ. Yang JS. Rhee SG. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J Biol Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- 329.Yakes FM. Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yamashita T. Hashiramoto A. Haluzik M. Mizukami H. Beck S. Norton A. Kono M. Tsuji S. Daniotti JL. Werth N. Sandhoff R. Sandhoff K. Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Yang KS. Kang SW. Woo HA. Hwang SC. Chae HZ. Kim K. Rhee SG. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J Biol Chem. 2002;277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]
- 332.Yang S. Koteish A. Lin H. Huang J. Roskams T. Dawson V. Diehl AM. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- 333.Yang Y. Dieter MZ. Chen Y. Shertzer HG. Nebert DW. Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm (–/–) knockout mouse: novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277:49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 334.Yerushalmi B. Dahl R. Devereaux MW. Gumpricht E. Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- 335.Yin M. Wheeler MD. Kono H. Bradford BU. Gallucci RM. Luster MI. Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 336.Yu CK. Li S. Whorton AR. Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 337.Zhai Y. Shen XD. O'Connell R. Gao F. Lassman C. Busuttil RW. Cheng G. Kupiec-Weglinski JW. TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor-3 dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 338.Zhang C. Walker LM. Hinson JA. Mayeux PR. Oxidant stress in rat liver after lipopolysaccharide administration: effect of inducible nitric-oxide synthase inhibition. J Pharmacol Exp Ther. 2000;293:968–972. [PubMed] [Google Scholar]
- 339.Zhang G. Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J Endotoxin Res. 2000;6:453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]
- 340.Zhao H. Przybylska M. Wu IH. Zhang J. Maniatis P. Pacheco J. Piepenhagen P. Copeland D. Arbeeny C. Shayman JA. Aerts JM. Jiang C. Cheng SH. Yew NS. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 2009;50:85–93. doi: 10.1002/hep.22970. [DOI] [PubMed] [Google Scholar]
- 341.Zhao P. Kahorn TF. Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–335. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 342.Zimmermann AK. Loucks FA. Schroeder EK. Bouchard RJ. Tyler KL. Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]