Abstract
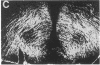
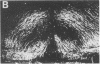
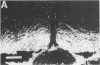
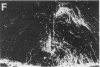
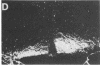
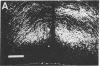
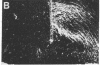
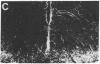
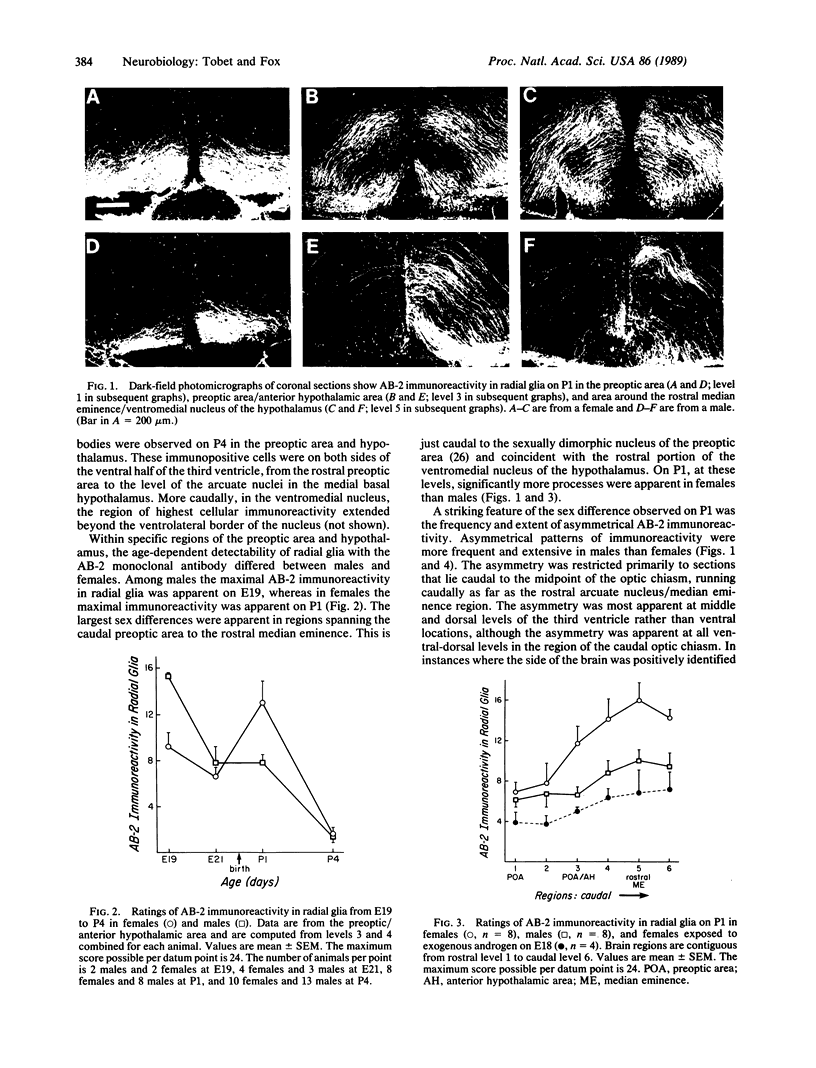
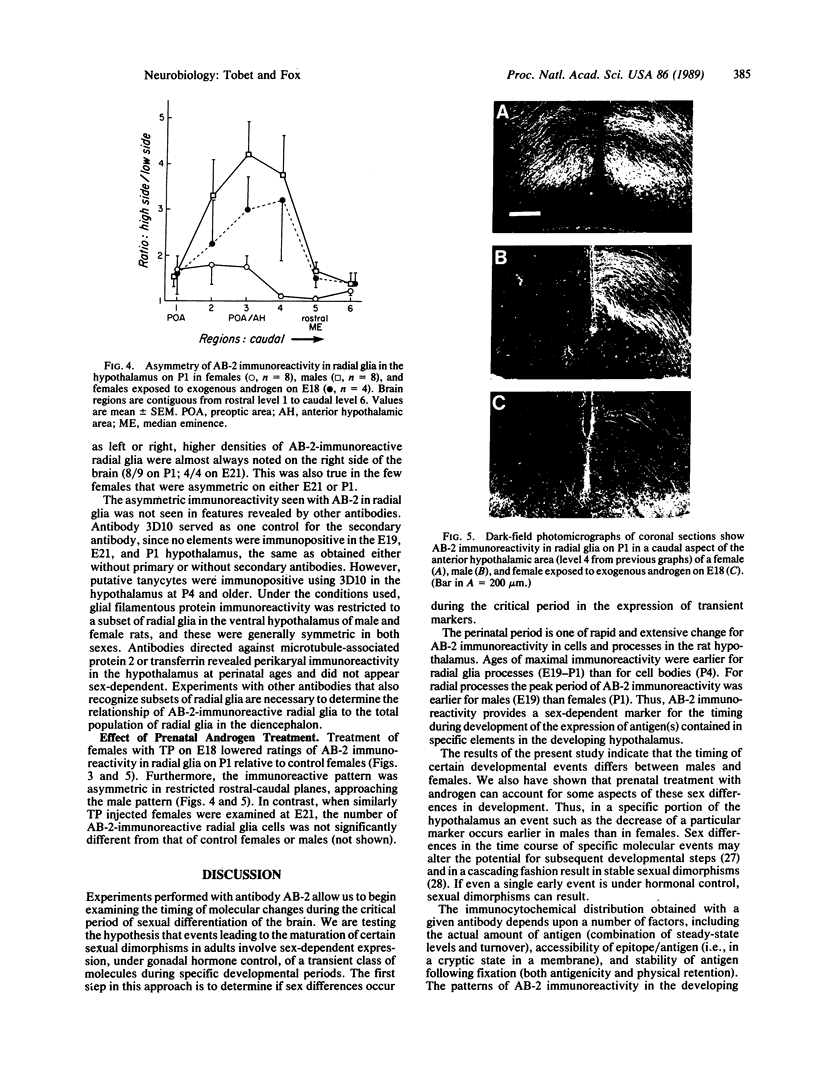
Morphological sex differences in adults can result from differential gonadal steroid exposure during critical perinatal periods. This study describes the use of a monoclonal antibody we have developed to study mechanisms of sexual differentiation of brain structure and function. Used as a marker in immunocytochemistry, antibody AB-2 revealed subsets of cells, including radial glia, transiently during the perinatal period. Peak reactivity in radial glia was on embryonic day 19 in males and on postnatal day 1 in females. On postnatal day 1, AB-2 immunoreactivity in radial glia was 2-fold greater in females than in males. Greater activity was detected in males on one side of the brain than the other (2- to 4-fold, depending on the region). To test the hormone dependence of this sex difference, pregnant rats were injected with testosterone propionate to expose fetal females to androgen on embryonic day 18. This resulted in lower levels of AB-2 immunoreactivity in radial glia of the treated female offspring on postnatal day 1 relative to control females, and the pattern was bilaterally asymmetric, approaching that of males. Thus the difference between sexes in immunoreactivity with AB-2 as a marker was hormone dependent in a predictable manner. Whether this marker is revealing a sex difference in accessibility of antigen by immunocytochemistry or a sex difference in intrinsic antigen levels is not yet resolved. In either case these results support the hypothesis that certain hormone-dependent molecular events occur transiently during development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Bayer S. A. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- Alvarez-Buylla A., Buskirk D. R., Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987 Oct 8;264(2):159–170. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- Anthony E. L., King J. C. Combined light and electron microscope immunocytochemical localization of scattered peptidergic neurons in the central nervous system. Am J Anat. 1986 Feb-Mar;175(2-3):179-95, 353. doi: 10.1002/aja.1001750207. [DOI] [PubMed] [Google Scholar]
- Arnold A. P., Gorski R. A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Ayoub D. M., Greenough W. T., Juraska J. M. Sex differences in dendritic structure in the preoptic area of the juvenile macaque monkey brain. Science. 1983 Jan 14;219(4581):197–198. doi: 10.1126/science.6849133. [DOI] [PubMed] [Google Scholar]
- Bleier R., Byne W., Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982 Dec 1;212(2):118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Immunogenic properties of the glial fibrillary acidic protein. Brain Res. 1976 Oct 29;116(1):150–157. doi: 10.1016/0006-8993(76)90257-2. [DOI] [PubMed] [Google Scholar]
- Diamond M. C., Johnson R. E., Young D., Singh S. S. Age-related morphologic differences in the rat cerebral cortex and hippocampus: male-female; right-left. Exp Neurol. 1983 Jul;81(1):1–13. doi: 10.1016/0014-4886(83)90153-x. [DOI] [PubMed] [Google Scholar]
- Döhler K. D., Coquelin A., Davis F., Hines M., Shryne J. E., Gorski R. A. Pre- and postnatal influence of testosterone propionate and diethylstilbestrol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res. 1984 Jun 8;302(2):291–295. doi: 10.1016/0006-8993(84)90242-7. [DOI] [PubMed] [Google Scholar]
- Fischer I., Kosik K. S., Sapirstein V. S. Heterogeneity of microtubule-associated protein (MAP2) in vertebrate brains. Brain Res. 1987 Dec 8;436(1):39–48. doi: 10.1016/0006-8993(87)91554-x. [DOI] [PubMed] [Google Scholar]
- Geschwind N., Behan P. Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5097–5100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski R. A., Gordon J. H., Shryne J. E., Southam A. M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978 Jun 16;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Harlan R. E., Jacobson C. D., Shryne J. E., Southam A. M. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980 Sep 15;193(2):529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Carter C. S., Steerman C., DeVoogd T. J. Sex differences in dentritic patterns in hamster preoptic area. Brain Res. 1977 Apr 22;126(1):63–72. doi: 10.1016/0006-8993(77)90215-3. [DOI] [PubMed] [Google Scholar]
- Gurusinghe C. J., Zappia J. V., Ehrlich D. The influence of testosterone on the sex-dependent structural asymmetry of the medial habenular nucleus in the chicken. J Comp Neurol. 1986 Nov 8;253(2):153–162. doi: 10.1002/cne.902530203. [DOI] [PubMed] [Google Scholar]
- Hines M., Davis F. C., Coquelin A., Goy R. W., Gorski R. A. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985 Jan;5(1):40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ito S., Murakami S., Yamanouchi K., Arai Y. Prenatal androgen exposure, preoptic area and reproductive functions in the female rat. Brain Dev. 1986;8(4):463–468. doi: 10.1016/s0387-7604(86)80070-5. [DOI] [PubMed] [Google Scholar]
- Jacobson C. D., Davis F. C., Gorski R. A. Formation of the sexually dimorphic nucleus of the preoptic area: neuronal growth, migration and changes in cell number. Brain Res. 1985 Jul;353(1):7–18. doi: 10.1016/0165-3806(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Jacobson C. D., Shryne J. E., Shapiro F., Gorski R. A. Ontogeny of the sexually dimorphic nucleus of the preoptic area. J Comp Neurol. 1980 Sep 15;193(2):541–548. doi: 10.1002/cne.901930215. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Levitt P., Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980 Oct 1;193(3):815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Philip A., Hurlburt C., Naftolin F. Estrogen formation in the developing rat brain: sex differences in aromatase activity during early post-natal life. Psychoneuroendocrinology. 1985;10(3):355–361. doi: 10.1016/0306-4530(85)90013-7. [DOI] [PubMed] [Google Scholar]
- Malsbury C. W., McKay K. A sex difference in the pattern of substance P-like immunoreactivity in the bed nucleus of the stria terminalis. Brain Res. 1987 Sep 15;420(2):365–370. doi: 10.1016/0006-8993(87)91258-3. [DOI] [PubMed] [Google Scholar]
- Martinez J. L., Jr, Koda L. Penetration of fluorescein into the brain: a sex difference. Brain Res. 1988 May 31;450(1-2):81–85. doi: 10.1016/0006-8993(88)91546-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983 Jun;30(3):277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Sandrock A. W., Jr Cyclophosphamide treatment used to manipulate the immune response for the production of monoclonal antibodies. J Immunol Methods. 1987 Jun 26;100(1-2):73–82. doi: 10.1016/0022-1759(87)90174-8. [DOI] [PubMed] [Google Scholar]
- Nordeen E. J., Yahr P. Hemispheric asymmetries in the behavioral and hormonal effects of sexually differentiating mammalian brain. Science. 1982 Oct 22;218(4570):391–394. doi: 10.1126/science.7123240. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972 May;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Roy E. J., Lynn D. M. Asymmetry in responsiveness of the hypothalamus of the female rat to estradiol. Physiol Behav. 1987;40(2):267–269. doi: 10.1016/0031-9384(87)90219-8. [DOI] [PubMed] [Google Scholar]
- Silver J., Lorenz S. E., Wahlsten D., Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982 Sep 1;210(1):10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Gorski R. A. Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. J Comp Neurol. 1984 May 10;225(2):151–166. doi: 10.1002/cne.902250202. [DOI] [PubMed] [Google Scholar]
- Tobet S. A., Baum M. J., Tang H. B., Shim J. H., Canick J. A. Aromatase activity in the perinatal rat forebrain: effects of age, sex and intrauterine position. Brain Res. 1985 Dec;355(2):171–178. doi: 10.1016/0165-3806(85)90038-0. [DOI] [PubMed] [Google Scholar]
- Tobet S. A., Zahniser D. J., Baum M. J. Differentiation in male ferrets of a sexually dimorphic nucleus of the preoptic/anterior hypothalamic area requires prenatal estrogen. Neuroendocrinology. 1986;44(3):299–308. doi: 10.1159/000124660. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D. On the genesis of sexual differentiation of the general nervous system: morphogenetic consequences of steroidal exposure and possible role of alpha-fetoprotein. Prog Brain Res. 1984;61:63–98. doi: 10.1016/s0079-6123(08)64429-5. [DOI] [PubMed] [Google Scholar]
- Van Eden C. G., Uylings H. B., Van Pelt J. Sex-difference and left-right asymmetries in the prefrontal cortex during postnatal development in the rat. Brain Res. 1984 Jan;314(1):146–153. doi: 10.1016/0165-3806(84)90186-x. [DOI] [PubMed] [Google Scholar]
- Weisz J., Ward I. L. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980 Jan;106(1):306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- del Abril A., Segovia S., Guillamón A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987 Apr;429(2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]