Abstract
Microvesicles are generated by the outward budding and fission of membrane vesicles from the cell surface. Recent studies suggest that microvesicle shedding is a highly regulated process that occurs in a spectrum of cell types and, more frequently, in tumor cells. Microvesicles have been widely detected in various biological fluids including peripheral blood, urine and ascitic fluids, and their function and composition depend on the cells from which they originate. By facilitating the horizontal transfer of bioactive molecules such as proteins, RNAs and microRNAs, they are now thought to have vital roles in tumor invasion and metastases, inflammation, coagulation, and stem-cell renewal and expansion. This Commentary summarizes recent literature on the properties and biogenesis of microvesicles and their potential role in cancer progression.
Keywords: Microvesicles, Signaling, Tumor invasion
Introduction
Microvesicles are small membrane-enclosed sacs that are thought to be shed from a variety of cell types. Growing evidence demonstrating their bona fide presence in body fluids, such as blood, urine and ascites (Graves et al., 2004; Piccin et al., 2007; Smalley et al., 2008; Taylor and Gercel-Taylor, 2008), and their potential to serve as indicators in the diagnosis, prognosis and surveillance of a variety of health conditions, has heightened the level of interest in these structures. The function of microvesicles appears to be dependent on the cargo they carry. This, in turn, is dependent on the cell type from which they originate. For example, microvesicles secreted by skeletal cells initiate bone mineralization (Anderson et al., 2005), whereas those secreted by normal endothelial cells have been implicated in angiogenesis (Morel et al., 2004). Microvesicles shed from various tumor-cell lines have been thought to facilitate extracellular matrix (ECM) invasion and evasion of the immune response (Dolo et al., 1999; Ginestra et al., 1998; Valenti et al., 2007). Studies over the past few years have shown that microvesicles can contain bioactive molecules, nucleic acids and/or proteins (Cocucci et al., 2009). Microvesicles packaged with microRNAs (miRNAs) or mRNAs have been shown to be released mainly from progenitors of differentiated cells and tumor cells (Mytar et al., 2008; Ratajczak et al., 2006a).
Although often categorized as – or grouped together with – exosomes, which are also shed by normal and diseased cells, microvesicles are a unique population of structures that are distinct from exosomes. As discussed below, whereas exosomes originate predominantly from preformed multivesicular bodies that are released upon fusion with the plasma membrane, microvesicles are formed by the outward budding and fission of the plasma membrane. Once shed, microvesicles can cover some distance, thus enabling the horizontal transfer of bioactive molecules and deposition of packaged bioactive effectors at distal sites.
Cargo contained within microvesicles may be released into the extracellular milieu with consequences for the surrounding environment. For example, microvesicles derived from neutrophils are packed with cytokines that first release anti-inflammatory molecules and then, at later time points, can function as pro-inflammatory mediators (Koppler et al., 2006; Mack et al., 2000). The release of metalloproteases (MMPs) from microvesicles shed by tumor cells promotes tumor invasion and metastases (Dolo et al., 1999; Ginestra et al., 1998). Microvesicles shed from neurons and astrocytes contain growth factors and promote paracrine responses (Proia et al., 2008; Schiera et al., 2007). In addition, membrane proteins on microvesicles have been shown to interact specifically with molecules on target cells to promote signaling responses (Eken et al., 2008; Gasser et al., 2003; Losche et al., 2004; Pluskota et al., 2008). In some cases, direct binding results in fusion of the microvesicle with the target cell or endocytosis of the microvesicle. The roles of microvesicles have been best studied in processes such as inflammation and coagulation, and have been described in some excellent reviews (Cocucci et al., 2009; Hugel et al., 2005; Ratajczak et al., 2006b). In this Commentary, we focus on recent advances and speculations about how microvesicles are generated, and also their role in cancer progression.
Microvesicle structure and biogenesis
Microvesicles and exosomes are distinct vesicle populations
Eukaryotic cells have been known for some time to release heterogeneous populations of membrane-enclosed vesicles both in vivo and in vitro by using unconventional secretory mechanisms that do not engage the classic signal-peptide secretory transport pathway (Nickel, 2005). The release of exosomes and microvesicles are two mechanisms of unconventional exocytosis that have received much attention over the past few years. Microvesicles (elsewhere in the literature also referred to as microparticles, particles or ectosomes) are plasma-membrane-derived particles that are released into the extracellular space by outward budding and fission of the plasma membrane (Cocucci et al., 2009). Microvesicles and exosomes are morphologically distinct. A recent study reveals that protease-containing microvesicles shed from tumor-cell lines appear to be rather heterogeneous in size, ranging from 200 nm to greater than 1 μm in diameter, and shape as opposed to exosomes, which range from 50 nm to 80 nm in diameter and are a more uniform population of vesicles (Muralidharan-Chari et al., 2009a). The same study also showed that microvesicles sediment at lower speeds relative to exosomes, which pellet at 100,000 g. It is important to note such differences between shed vesicle populations because many reports on the characterization of one or more of these vesicles have used membranes that sediment at 100,000 g and are, therefore, likely to contain a mixture of microvesicles and exosomes.
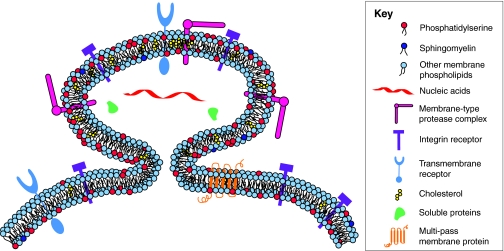
The distinction between shed microvesicles and exosomes subsists through biogenesis and release. Exosomes are formed intracellularly via endocytic invagination and are released into a structure known as a multivesicular body (MVB). The MVB then fuses with the plasma membrane, releasing its cargo of exosomes into the extracellular space. Comprehensive reviews of exosome structure and function have recently been published (Schorey and Bhatnagar, 2008; Simons and Raposo, 2009). The composition of microvesicles, however, depends largely on the cell type from which they originate, although the membrane composition of microvesicles remains distinct from that of the parental cell – often with significant remodeling, enabling specialized functions. In this regard, not all plasma-membrane proteins are incorporated into shed vesicles (Muralidharan-Chari et al., 2009a). Phosphatidylserine is relocated to the outer membrane leaflet, specifically at sites on the cell surface where microvesicle shedding occurs, while the topology of membrane proteins remains intact (Hugel et al., 2005; Lima et al., 2009; Muralidharan-Chari et al., 2009a) (see Fig. 1). As recently shown in tumor cells, phosphatidylserine externalization occurs presumably in an effort to quell an immune response and promote tumor-cell survival (Johnstone, 2006).
Fig. 1.
Microvesicle shedding. Microvesicles are formed by the outward budding of the plasma membrane, as shown. Not all plasma-membrane proteins are incorporated into shed vesicles, although the topology of membrane proteins remains intact. Membrane proteins such as oncogene and other growth-factor receptors, intergrin receptors and MHC class I molecules, soluble proteins such as proteases and cytokines, as well as nucleic acids, have been found in microvesicles. Microvesicles appear to be enriched in some lipids such as cholesterol, whereas phosphatidylserine is relocated to the outer membrane leaflet specifically at sites of microvesicle shedding.
Potential mechanisms for microvesicle formation and release
Much remains to be understood about the mechanisms by which microvesicles are formed and shed at the cell surface. It appears that the release of the microvesicle population initiated by outward budding from the surface of the plasma membrane is followed by a fission event that in many ways resembles the abscission step in cytokinesis. During abscission, contractile machinery within the cleavage furrow draws the opposing membranes together before pinching off the membrane connection and separating the daughter cells (Muralidharan-Chari et al., 2009a; Schweitzer and D'Souza-Schorey, 2004). The release of microvesicles also appears to share similarities with the events associated with viral budding (Chazal and Gerlier, 2003; Morita and Sundquist, 2004). For example, in the case of some retroviruses, newly assembled Gag molecules coalesce at the plasma membrane and cause it to distort by forming semispherical aggregates. These viral buds are eventually released when the neck of the bud is pinched behind the viral particle. Moreover, there are structural similarities between membrane-derived shed vesicles and apoptotic blebs, both of which form by outward protrusion of the plasma membrane. Unlike apoptotic bodies, however, shed vesicles do not contain cytosolic organelles and/or nuclear fragments (Taylor et al., 2008). Consistent with this understanding is the knowledge that multiple cell lines of both normal and transformed origin remain viable in culture even when stimulated to release microvesicles (Dolo et al., 1998; Ginestra et al., 1998; Muralidharan-Chari et al., 2009a; Taraboletti et al., 2002). A recurring theme, however, is that the initiating events involve both lateral and vertical redistribution of plasma-membrane constituents resulting in alterations in local membrane curvature.
Impact of membrane lipids
Previous research has shown that lipid aggregation into microdomains within the plasma membrane can result in, and act to stabilize, membrane-bending forces and interactions between constituent molecules (Corbeil et al., 2001). Although possible, the likelihood of spontaneous membrane curvature being the driving force behind de novo vesiculation remains low owing to curvature and membrane-fusion energy constraints. Proteins, however, can be used to overcome these physical limitations and alter membrane curvature through several mechanisms. First, proteins can exert a localized normal force, pushing on the membrane to generate the curvature needed to begin the budding process (Boulbitch, 1998; Farsad and Camilli, 2003). In this model, spontaneous curvature acts to lower the pushing force required from the protein. Second, proteins can bend membranes by binding to the membrane surface (adding their interactional and conformational energy to the system) or inserting amphipathic moieties into the lipid matrix – in the latter case the protein acts as a wedge and, according to the bilayer-couple mechanism, will force curvature as a result of increasing the surface area on one leaflet (Sheetz et al., 1976; Chou et al., 2001). Third, contractile proteins can conceivably add tensile or contractile forces to one leaflet of the membrane, creating a structural asymmetry that would lead to bending (Huttner and Zimmerberg, 2001; Zimmerberg and Kozlov, 2006). Fourth, proteins can act to regulate the lipid composition and asymmetry that exists in the plasma membrane (Daleke, 2003). This lipid asymmetry can be generated by using several mechanisms. Initial variation in lipid distribution occurs owing to asymmetric lipid synthesis (Bell et al., 1981). Furthermore, aminophospholipid translocases known as flippases and floppases regulate the movement of phospholipids from one leaflet to the other in an ATP-dependent manner (Seigneuret and Devaux, 1987; Martin and Pagano, 1987; Sune et al., 1987). Similarly, Ca2+-dependent scramblase will allow for the randomization of plasma-membrane phospholipids, removing lipid-generated local curvature constraints. In a related mechanism, asymmetric activity of sphingomyelinase can generate ceramide gradients across the plasma membrane resulting in vectorial budding of vesicles towards sphingomyelinase activity (Holopainen et al., 2000).
Consistent with the idea that de novo vesicle shedding does not occur as a result of spontaneous membrane curvature, studies have demonstrated that shedding requires energy input, RNA synthesis and protein translation (Dainiak and Sorba, 1991). Microvesicle shedding has also been shown to take place at specific locations on the cell membrane that are enriched in assorted lipids and proteins, with the exact composition reflecting the cellular origin. Common among these lipid requirements is cholesterol, which is a key component of membrane lipid rafts. Lipid rafts were previously hypothesized to have a role in the initial ‘pinching’ events, because the microvesicles released from activated neutrophils contained high levels of cholesterol and pharmacological depletion of cellular cholesterol inhibits microvesicle shedding (Del Conde et al., 2005; Pilzer et al., 2005). Similarly, as stated above, phosphatidylserine has been found to be exposed on the extracellular leaflet of shed vesicles (Lima et al., 2009; Muralidharan-Chari et al., 2009a). This topological reversal may serve several purposes. First, the packing defects that result from the addition of the aminophospholipid to the extracellular leaflet can cause shape changes in the plasma membrane. Second, as mentioned below, it could promote detachment from the underlying cytoskeleton. Functionally, exposure of phosphatidylserine on the outer leaflet also allows the shed vesicle to become a target for the immune system.
Impact of the cytoskeleton
Early reports also focused on cytoskeletal disruption as a mechanism for microvesicle formation because shedding could be induced in P815 cells by colchicine, vinblastine and cold temperatures, all of which are known to disrupt the microtubule cytoskeleton (Liepins, 1983). These treatments probably cause a localized rupture of the plasma membrane from the underlying cytoskeleton as well as subsequent blebbing owing to hydrostatic pressure differences, although this sort of blebbing process has been associated with apoptosis, cytokinesis and perhaps migration. Furthermore, microvesicles (even when released) are actin positive, whereas blebs are only transiently associated with the actin cytoskeleton (during retraction) (Charras et al., 2005; Paluch et al., 2005). Separation of the plasma membrane from the cytoskeleton cannot be ruled out as a mechanism for the generation of microvesicles at the cell surface because the shift in phosphatidylserine to the outer membrane makes it unavailable for interaction with spectrins (Manno et al., 2002). Additional reports point to an active role for contractile proteins in microvesicle budding (Dainiak et al., 1988). Recent reports have also shed light on the role of contractile proteins, because phosphorylated myosin light chain kinase (MLCK2; a kinase that activates myosin II, allowing for contraction of the actin cytoskeleton) was localized to the neck of budding microvesicles (Muralidharan-Chari et al., 2009a), and myosin 1a (MYO1A) was shown to be necessary for the formation of microvesicles (McConnell et al., 2009). These data present an interesting possibility for the pinching mechanism, which ultimately releases the microvesicle from the cell surface. Unlike the endocytic mechanism in which dynamin wraps around and pinches closed the neck of the vesicle, shed vesicles seem to be contracted internally, with the edges drawn together by contractile proteins that act like a drawstring. Recent work, carried out in populations of tumor cells, presents a regulatory role for the small GTP-binding protein ARF6 in modulating the release of protease-loaded microvesicles (Muralidharan-Chari et al., 2009a). By acting through phospholipase D and extracellular signal-regulated kinases (ERKs), ARF6 regulates the activation of MLCK, and the subsequent phosphorylation of myosin light chain controls the release of protein-loaded microvesicles from invasive melanoma cells (Muralidharan-Chari et al., 2009a).
Concomitant with the assorted modes of microvesicle initiation, formation and release, there are also multiple signaling pathways that are thought to regulate their biogenesis. There are many reports that highlight a potential role for growth factors in cell activation and subsequent microvesicle release, because removal of serum from the growth medium abolishes microvesicle release (Vittorelli, 2003). There is also substantial evidence of increased shedding activity in microglia and dendritic cells when stimulated with Ca2+ (Cocucci et al., 2009). As stated previously, energy input is required for vesicle release. Although the exact points at which ATP is needed have yet to be confirmed, the steps for vesicle release in each of the models require energetic input, e.g. scramblase and flippase activity or contractile-protein activity.
Vertical redistribution of protein cargo to microvesicles
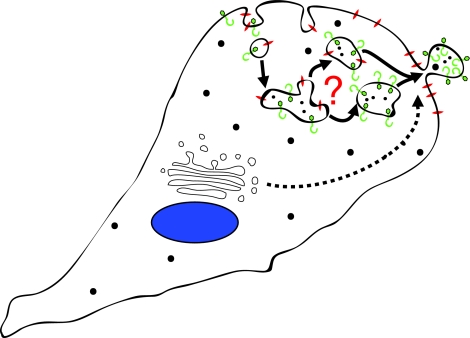
Recent studies also suggest that a variety of proteins are selectively incorporated into microvesicles, including proteins that are transported via the ARF6-regulated endosomal recycling pathway (D'Souza-Schorey and Chavrier, 2006; Donaldson, 2003). For example, MHC class I, β1 intergrin receptors and vesicle-associated membrane protein 3 (VAMP3) are delivered to microvesicles. Microvesicles are devoid of VAMP7 and Rab8, both of which have been implicated in membrane type 1 (MT1)-MMP delivery to the cell surface (Bravo-Cordero et al., 2007; Steffen et al., 2008), and lack transferrin receptors, which also traffic between the cell surface and early endosomes (D'Souza-Schorey and Chavrier, 2006). It is possible that in tumor cells, specialized recycling endosomes target cargo to the cell surface for incorporation into microvesicles (Fig. 2). Alternatively, sites of microvesicle shedding might be a convergence point for membrane trafficking pathways directing specialized cargo to these structures. At this point is it unclear how soluble cytosolic proteins or nucleic acids are targeted to microvesicles. In tumor cells, shedding appears to occur at specific sites on the plasma membrane and is designed to release selected cellular components into the surrounding environment, particularly proteins involved in cell-matrix interactions and matrix degradation. As described below, in addition to facilitating cell invasion, microvesicle-mediated horizontal transfer of bioactive molecules can impact several aspects of tumor progression.
Fig. 2.
Working model for the trafficking of cargo to sites of microvesicle shedding. Recent studies suggest that a variety of proteins, including MHC class I, β1 intergrin receptors and VAMP3 – which are trafficked via specialized early recycling endosomes – are selectively incorporated into microvesicles. It is unclear whether cargo sorting occurs in endosomes so that pre-packaged vesicles are trafficked to the cell surface, or whether sorting occurs at the plasma membrane. Proteins trafficked via other pathways (dashed line) could also be delivered to microvesicles.
Microvesicles in cancer progression
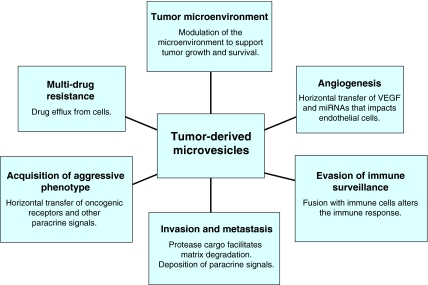
Although microvesicle shedding occurs under physiological conditions, aberrant release of microvesicles can arise in disease states. The amount of microvesicles shed by tumor cells has been shown to correlate with their invasiveness both in vitro (Ginestra et al., 1998) and in vivo (Ginestra et al., 1999). Microvesicles in cancer patients were first documented in 1978, when they were identified in cultures of spleen nodules and lymph nodes of a male patient with Hodgkin disease (Friend et al., 1978). About a decade later, it was demonstrated that plasma-membrane-derived vesicles shed spontaneously from highly metastatic B16 mouse melanoma (F10) cells and, when fused with weakly metastatic B16 mouse melanoma (F1) cells, enabled F1 cells to metastasize to the lung (Poste and Nicolson, 1980). Both of these studies set the stage for further investigations into the significance of microvesicle shedding in cancer progression. Since then, microvesicle-mediated cargo transfer to adjacent or remote cells has been shown to affect many stages of tumor progression (van Doormaal et al., 2009), including angiogenesis, escape from immune surveillance, ECM degradation and metastasis (Fig. 3). Microvesicles shed from tumor cells facilitate transfer of soluble proteins (Iero et al., 2008), nucleic acids (Skog et al., 2008), functional transmembrane proteins (Del Conde et al., 2005), chemokine receptors (Mack et al., 2000), tissue factor (Del Conde et al., 2005) and receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) (Al-Nedawi et al., 2008; Sanderson et al., 2008). Here, we summarize and highlight recent developments on the significance of microvesicles in various aspects of cancer progression.
Fig. 3.
Tumor-derived microvesicles influence many aspects of cancer progression. By their ability to harness select bioactive molecules and propagate the horizontal transfer of these cargoes, tumor-derived microvesicles can affect a variety of cellular events to have an enormous impact on tumor progression.
Acquisition of aggressive cancerous phenotypes
A recent report showed that the oncogenic receptor EGFRvIII, which is found exclusively in a subset of aggressive glioma tumors, was transferred to a non-aggressive population of tumor cells through microvesicles (Al-Nedawi et al., 2008). As a consequence, the recipient cells exhibited the activation of two signaling pathways [mitogen-activated protein kinase (MAPK) and Akt] and changes in the expression of EGFRvIII-regulated genes [vascular endothelial growth factor (VEGF), Bcl-XL, p27], leading to morphological transformation and an increase in anchorage-independent growth. Notably, treatment of the microvesicles with EGFRvIII kinase inhibitor diminished the aforementioned signaling responses in recipient cells, as did masking of their exposed phosphatidylserine residues with annexin V, which points towards a linear link between the downstream effects in the recipient cells and the acquisition of EGFRvIII from microvesicles.
Microvesicles and tumor angiogenesis
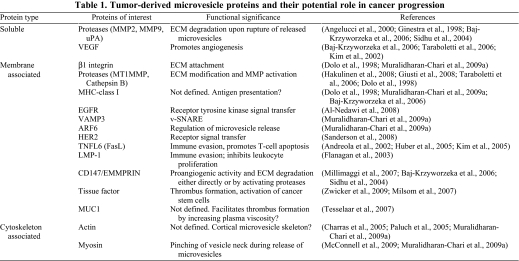
Angiogenesis is vital for tumor survival and tumor growth, and occurs by proliferation of endothelial cells to form a mesh of blood vessels that infiltrate into the tumor, facilitating the supply of nutrients and oxygen for tumor growth as well as removal of waste products (Carmeliet, 2005). As discussed below, several reports indicate that tumor-derived microvesicles stimulate secretion of several pro-angiogenic factors by stromal fibroblasts, and chemoattract and facilitate the proliferation of endothelial cells to promote angiogenesis and enable tumor growth (Table 1).
Table 1.
Tumor-derived microvesicle proteins and their potential role in cancer progression
Matrix reorganization by endothelial cells – a cellular process that is facilitated by matrix-degrading proteases, particularly MMPs – is crucial for the process of vascularization under normal conditions and also in cancer. Dolo and colleagues showed that microvesicles shed by endothelial cells contain MMPs, such as MMP2, MMP9 and MT1-MMP, that facilitate autocrine stimulation of endothelial-cell invasion into Matrigel and result in cord formation (Taraboletti et al., 2002). In a follow-up study, the same group demonstrated that microvesicles isolated from ovarian-cell lines such as CABA1 and A2780, stimulated the motility and invasiveness of endothelial cells in vitro and also reported the presence of VEGF in microvesicles together with MMPs (Taraboletti et al., 2006). Interestingly, Al-Nedawi and colleagues showed that the onset of VEGF expression and its receptor VEGFR in endothelial cells ensues following the transfer of EGFR via microvesicles shed by human cancer-cell lines that harbor the activated EGFR mutation (Al-Nedawi et al., 2009). Beside growth factors and proteases, microvesicle-mediated transfer of miRNAs has also been shown to stimulate tubule formation in endothelial cells by modifying the translational profile of these cells and, thereby, promoting acquisition of the angiogenic phenotype (Skog et al., 2008).
Lipids from microvesicles can impact endothelial-cell migration and angiogenesis. In this regard, sphingomyelin, a major component identified in microvesicles shed from the fibrosarcoma cell line HT1080, together with VEGF, was shown to confer migratory and angiogenesis-inducing properties to endothelial cells (Kim et al., 2002). Whereas purified sphingomyelin elicited similar migratory and angiogenic effects to that of lipid extracts from microvesicles, sphingomyelinase-treated lipid extracts lost their migration-promoting activity. Further, CD147/extracellular matrix metalloprotease inducer (EMMPRIN), a plasma-membrane glycoprotein and an ECM metalloproteinase, has been demonstrated to have a crucial role in the progression of malignancies by regulating expression of VEGF and MMPs in stromal cells (Biswas et al., 1995; Tang et al., 2004).
Thus, microvesicles secreted by tumor cells induce endothelial cells to release microvesicles that contain VEGF and sphingomyelin in order to promote angiogenesis. It is interesting that in lung cancer models, hypoxia induces an increased release of microvesicles (Wysoczynski and Ratajczak, 2009). Thus, the adverse tumor microenvironment somehow triggers tumor cells to release microvesicles, which in turn facilitates angiogenesis by bringing nutrients and oxygen to the rescue of cancer cells.
Microvesicles and tumor-triggered blood coagulation
A range of hematological complications broadly categorized as ‘thromboembolism’ is associated with cancer-related mortality (Zwicker et al., 2007). Tissue factor (TF) is emerging to be the single most-responsible factor for hypercoagulation and related disorders in cancer patients. TF forms complexes with coagulation factors VII and/or VIIIa to activate thrombin, leading to fibrin deposition (Giesen et al., 1999). Although the presence of TF in microvesicles is generally accepted, the source of microvesicles carrying TF is controversial because – in addition to tumor cells – endothelial cells, monocytes and platelets can also be triggered to release microvesicles (Dvorak et al., 1983; Mezzano et al., 2008; Edwards et al., 1979). A recent study showed that most of the TF-bearing microvesicles were tumor derived (Zwicker et al., 2009). The group further confirmed the association between the presence of TF-bearing microvesicles and an increased risk of thromboembolic disease in malignancy (Zwicker et al., 2009). Additionally, activation of the coagulation system and TF signaling has also been suggested to deliver growth-promoting stimuli to dormant cancer stem cells (Milsom et al., 2007).
Impact on the tumor microenvironment
Cancer cells interact with the stroma and actively modify the microenvironment to favor their own progression (Fidler and Poste, 2008). Accordingly, a recent study from Castellana and colleagues highlights a mechanism of reciprocal communication between cancer cells and microvesicles. In this study, microvesicles released by PC3 cells, an invasive prostate cancer cell line, triggered ERK phosphorylation, MMP9 upregulation, increased motility and resistance to apoptosis in fibroblasts in the surrounding microenvironment. In turn, the activated fibroblasts shed microvesicles to facilitate the migration and invasion of the prostate cancer line (Castellana et al., 2009). A similar feedback phenomenon was reported in yet another study, confirming the role of prostate-tumor-derived microvesicles in the ‘activation’ of stromal cells in the tumor microenvironment (Di Vizio et al., 2009). This study also identified increased chromosomal loss of the DIAPH3 locus in a cohort of prostate cancer patients, which encodes the diaphanous homolog 3 (DIAPH3) gene, suggesting that DIAPH3 is a physiologically relevant protein involved in this process. Microvesicles released by lung cancer cells also activate and chemoattract stromal fibroblasts as well as endothelial cells to facilitate tumor cell growth (Wysoczynski and Ratajczak, 2009).
Evasion of immune surveillance
Although most tumor antigens originate from the tumor, spontaneous cancer immunity occurs via immune surveillance in the host to contain cancer growth in its early phases of progression (Dunn et al., 2004; Valenti et al., 2007). However, this housekeeping mechanism usually fails with disease progression when escape mechanisms adopted by tumor cells that silence their immunogenic profile prevail, and immunosuppressive pathways are activated (Zou, 2005). To outlast immune surveillance mechanisms, cancer cells can either alter cross-priming by antigen-presenting cells to switch off T-cell responses or eliminate the anti-tumor effector cells. Accordingly, direct fusion of microvesicles produced by human melanoma or colorectal carcinoma cells with monocytes inhibited the differentiation of monocytes to antigen-presenting cells both in vitro and in vivo (Valenti et al., 2006). Instead, monocytes released immunosuppressive cytokines that inhibited cytolytic T-cell activation and function. In addition, studies have shown that cancer-cell-released microvesicles with exposed TNFL6 (also known as FasL or CD95 ligand), a ligand of TNR6 (also known as FAS or CD95) can induce apoptosis in activated anti-tumor T cells to abrogate the potential of these effector cells to kill tumor cells (Andreola et al., 2002; Huber et al., 2005; Wysoczynski and Ratajczak, 2009). In oral squamous cell carcinoma, a modest correlation was identified between tumor burden (measured by lymph-node infiltration) and the numbers of circulating FasL-exposed microvesicles in the blood (Kim et al., 2005). Also, microvesicles from lymphoblastoma cells have been shown to expose latent membrane protein (LMP-1), another immune-suppressing transmembrane protein, which inhibits leukocyte proliferation (Flanagan et al., 2003).
Hypothetically, cancer cells can fuse with microvesicles derived from non-cancer cells to camouflage behind the lipids and membrane-specific proteins of non-transformed cells. A study by Tesselaar and colleagues identified a low number of circulating microvesicles from cancer patients that stained for both MUC1, a cancer-cell marker, and glycoprotein IIIa, a protein that is exclusively present on platelets (Tesselaar et al., 2007). It could be argued that such microvesicles are released by tumor cells after they have fused with microvesicles released by platelets. Additional studies are warranted to confirm this hypothesis. All of the above suggest that the horizontal transfer of microvesicle cargo can successfully divert immune cells to altered phenotypes, thereby facilitating cancer-cell evasion of the immune response.
Impact on tumor invasion and metastasis
Matrix degradation is essential for promoting tumor growth and metastasis (Hotary et al., 2006). As indicated above, microvesicles that are shed by tumor cells are loaded with proteases and provide an additional means of matrix degradation, creating a path of least resistance for invading tumor cells. Accordingly, studies report the presence of MMP2, MMP9, MT1-MMP and their zymogens urokinase-type plasminogen activator (uPA) and EMMPRIN, within tumor-derived microvesicles (Angelucci et al., 2000; Ginestra et al., 1998; Baj-Krzyworzeka et al., 2006; Hakulinen et al., 2008). MMPs degrade basement collagens, whereas uPA catalyzes the conversion of plasminogen into plasmin, a serine protease that facilitates the conversion of MMP zymogens into their active forms as well as the degradation of matrix components such as fibrin (Angelucci et al., 2000). In addition to uPA, cathepsin B, which is also present within the microvesicles, gets activated at low pH – typical of the acidic environment of solid tumors – and facilitates activation of MMPs within microvesicles (Giusti et al., 2008; Taraboletti et al., 2006).
Given the importance of matrix degradation in tumor metastases, it is logical to hypothesize that there is a direct correlation between the number of invasive microvesicles and tumor progression. Indeed, protease-loaded membrane vesicles with invasive properties have been observed in malignant ovarian ascites that are derived from women with stage-I to -IV ovarian cancer (Graves et al., 2004). This study also showed that late-stage ascites contained substantially more vesicles than those in early-stage disease, although the invasive ability of the vesicles was approximately the same, irrespective of disease stage. Similarly, in breast cancer-cell lines, the number and proteolytic capacity of shed microvesicles correlate with their in vitro invasive capacity (Ginestra et al., 1998). Both inhibition of proteases and inhibition of microvesicle adhesion to the ECM abolished the ability of these microvesicles to promote tumor invasiveness, supporting the relevance of this pathway. As mentioned earlier, recent work has shown that the small GTP-binding protein ARF6 localizes to protease-containing microvesicles shed from invasive tumor-cell lines. Consistent with its role in regulating tumor invasion in cell and animal models (D'Souza-Schorey and Chavrier, 2006; Hu et al., 2009; Muralidharan-Chari et al., 2009b), ARF6 activation promotes microvesicle shedding, whereas dominant inhibition of ARF6 activation attenuates microvesicle shedding (Muralidharan-Chari et al., 2009a). Thus, the release of invasive microvesicles might serve in part as a mechanism by which ARF6 regulates tumor invasion. It should be noted that microvesicle-mediated ECM degradation appears to be distinct from matrix degradation by invadopodia, another type of invasive structure that is formed at the adherent surface of tumor cells and the formation of which is also linked to the activation of ARF6. In the relevant study, cortactin – a bona fide component of invadopodia – was shown to be absent in microvesicles (Muralidharan-Chari et al., 2009a). Although proteases at the surface of invadopodia might represent a mechanism for local pericellular proteolysis at the leading or invading membrane edge, microvesicle release probably promotes more distant focal proteolysis and creation of an invasion path.
Microvesicles and multi-drug resistance
An example for the direct involvement of microvesicles in facilitating tumor-cell survival comes from the demonstrated expulsion of therapeutic drugs from tumor cells through microvesicles. Tumor cells treated with doxyrubicin accumulated and released the drug in shed microvesicles, implying microvesicle shedding as a drug-efflux mechanism involved in drug resistance (Shedden et al., 2003). Another study documented that microvesicles of cisplatin-insensitive cancer cells contained 2.6-fold more cisplatin than cisplatin-sensitive cells that release microvesicles (Safaei et al., 2005). Therefore, by virtue of their ability to harness select bioactive molecules and propagate the horizontal transfer of these cargoes, shed microvesicles can have an enormous impact on tumor growth, survival and spread.
Concluding remarks and future perspectives
The main role of microvesicles is to promote communication between the cells from which they are derived and their surrounding environments. Whereas the biogenesis and roles of microvesicles have been burgeoning areas of research in the recent past, several pertinent issues require further investigation to better understand the significance and the therapeutic potential of these structures. For example, it would be of interest to know whether microvesicles serve as a general mechanism for intercellular transfer of oncogenic receptors. Another question that needs to be addressed is whether tumor cells simultaneously release distinct populations of microvesicles that contain discrete sets of molecules. Alternatively, is the composition of shed microvesicles and the nature of the cargo packaged within these structures determined by disease stage? In addition, the cellular mechanisms involved in microvesicle formation and release, as well as the targeting of molecules to these sites promise to be a new and exciting area of investigation. Molecules that regulate microvesicle shedding and proteins on circulating microvesicles that are responsible for tumor growth, progression and survival will be effective targets for anti-cancer therapeutics.
As microvesicles can be detected in biological fluids such as blood, urine and ascites they could potentially serve as prognostic and predictive biomarkers for cancer progression. Tumor-specific markers that are exposed on circulating microvesicles might be particularly useful as potential biomarkers. The protein composition of microvesicles might reflect molecular changes in tumor cells from which they are derived and, therefore, can potentially serve as a prognostic indicator of disease stage and efficacy of treatment. Microvesicle biogenesis and shedding is an important but relatively understudied area of tumor-cell biology. Accumulating evidence, as outlined above, demonstrates that they are important mediators of cell communication and underappreciated but vital components of the tumor microenvironment niche.
Acknowledgments
We apologize to authors whose work we have not cited or only cited indirectly owing to space constraints. Research in the D'Souza-Schorey laboratory on the mechanisms of tumor-cell invasion has been supported by the Department of Defense-CDMRP, the National Institutes of Health and the Walther Cancer Foundation. Deposited in PMC for release after 12 months.
References
- Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619-624 [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K., Meehan B., Kerbel R. S., Allison A. C., Rak J. (2009). Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. USA 106, 3794-3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. C., Garimella R., Tague S. E. (2005). The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 10, 822-837 [DOI] [PubMed] [Google Scholar]
- Andreola G., Rivoltini L., Castelli C., Huber V., Perego P., Deho P., Squarcina P., Accornero P., Lozupone F., Lugini L., et al. (2002). Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J. Exp. Med. 195, 1303-1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci A., D'Ascenzo S., Festuccia C., Gravina G. L., Bologna M., Dolo V., Pavan A. (2000). Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin. Exp. Metastasis 18, 163-170 [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M., Szatanek R., Weglarczyk K., Baran J., Urbanowicz B., Branski P., Ratajczak M. Z., Zembala M. (2006). Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol. Immunother. 55, 808-818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R., Ballas L., Coleman R. (1981). Lipid topogenesis. J. Lipid Res. 22, 391-403 [PubMed] [Google Scholar]
- Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. (1995). The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 55, 434-439 [PubMed] [Google Scholar]
- Boulbitch A. A. (1998). Deflection of a cell membrane under application of a local force. Phys. Rev. E 57, 2123-2128 [Google Scholar]
- Bravo-Cordero J. J., Marrero-Diaz R., Megias D., Genis L., Garcia-Grande A., Garcia M. A., Arroyo A. G., Montoya M. C. (2007). MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 26, 1499-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. (2005). Angiogenesis in life, disease and medicine. Nature 438, 932-936 [DOI] [PubMed] [Google Scholar]
- Castellana D., Zobairi F., Martinez M. C., Panaro M. A., Mitolo V., Freyssinet J. M., Kunzelmann C. (2009). Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 69, 785-793 [DOI] [PubMed] [Google Scholar]
- Charras G. T., Yarrow J. C., Horton M. A., Mahadevan L., Mitchison T. J. (2005). Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal N., Gerlier D. (2003). Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67, 226-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T., Kim K. S., Oster G. (2001). Statistical thermodynamics of membrane bending-mediated protein-protein attractions. Biophys. J. 80, 1075-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E., Racchetti G., Meldolesi J. (2009). Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43-51 [DOI] [PubMed] [Google Scholar]
- Corbeil D., Roper K., Fargeas C. A., Joester A., Huttner W. B. (2001). Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2, 82-91 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. (2006). ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347-358 [DOI] [PubMed] [Google Scholar]
- Dainiak N., Sorba S. (1991). Intracellular regulation of the production and release of human erythroid-directed lymphokines. J. Clin. Invest. 87, 213-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N., Riordan M. A., Strauss P. R., Feldman L., Kreczko S. (1988). Contractile proteins participate in release of erythroid growth regulators from mononuclear cells. Blood 72, 165-171 [PubMed] [Google Scholar]
- Daleke D. L. (2003). Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 44, 233-242 [DOI] [PubMed] [Google Scholar]
- Del Conde I., Shrimpton C. N., Thiagarajan P., Lopez J. A. (2005). Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106, 1604-1611 [DOI] [PubMed] [Google Scholar]
- Di Vizio D., Kim J., Hager M. H., Morello M., Yang W., Lafargue C. J., True L. D., Rubin M. A., Adam R. M., Beroukhim R., et al. (2009). Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 69, 5601-5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolo V., Ginestra A., Cassara D., Violini S., Lucania G., Torrisi M. R., Nagase H., Canevari S., Pavan A., Vittorelli M. L. (1998). Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 58, 4468-4474 [PubMed] [Google Scholar]
- Dolo V., D'Ascenzo S., Violini S., Pompucci L., Festuccia C., Ginestra A., Vittorelli M. L., Canevari S., Pavan A. (1999). Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin. Exp. Metastasis 17, 131-140 [DOI] [PubMed] [Google Scholar]
- Donaldson J. G. (2003). Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 278, 41573-41576 [DOI] [PubMed] [Google Scholar]
- Dunn G. P., Old L. J., Schreiber R. D. (2004). The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137-148 [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Van DeWater L., Bitzer A. M., Dvorak A. M., Anderson D., Harvey V. S., Bach R., Davis G. L., DeWolf W., Carvalho A. C. (1983). Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res. 43, 4434-4442 [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R., Bobrove A. M. (1979). Mononuclear cell tissue factor: cell of origin and requirements for activation. Blood 54, 359-370 [PubMed] [Google Scholar]
- Eken C., Gasser O., Zenhaeusern G., Oehri I., Hess C., Schifferli J. A. (2008). Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J. Immunol. 180, 817-824 [DOI] [PubMed] [Google Scholar]
- Farsad K., Camilli P. D. (2003). Mechanisms of membrane deformation. Curr. Opin. Cell Biol. 15, 372-381 [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Poste G. (2008). The “seed and soil” hypothesis revisited. Lancet Oncol. 9, 808 [DOI] [PubMed] [Google Scholar]
- Flanagan J., Middeldorp J., Sculley T. (2003). Localization of the Epstein-Barr virus protein LMP 1 to exosomes. J. Gen. Virol. 84, 1871-1879 [DOI] [PubMed] [Google Scholar]
- Friend C., Marovitz W., Henie G., Henie W., Tsuei D., Hirschhorn K., Holland J. G., Cuttner J. (1978). Observations on cell lines derived from a patient with Hodgkin's disease. Cancer Res. 38, 2581-2591 [PubMed] [Google Scholar]
- Gasser O., Hess C., Miot S., Deon C., Sanchez J. C., Schifferli J. A. (2003). Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 285, 243-257 [DOI] [PubMed] [Google Scholar]
- Giesen P. L., Rauch U., Bohrmann B., Kling D., Roque M., Fallon J. T., Badimon J. J., Himber J., Riederer M. A., Nemerson Y. (1999). Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. USA 96, 2311-2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestra A., La Placa M. D., Saladino F., Cassara D., Nagase H., Vittorelli M. L. (1998). The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 18, 3433-3437 [PubMed] [Google Scholar]
- Ginestra A., Miceli D., Dolo V., Romano F. M., Vittorelli M. L. (1999). Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res. 19, 3439-3445 [PubMed] [Google Scholar]
- Giusti I., D'Ascenzo S., Millimaggi D., Taraboletti G., Carta G., Franceschini N., Pavan A., Dolo V. (2008). Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia 10, 481-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L. E., Ariztia E. V., Navari J. R., Matzel H. J., Stack M. S., Fishman D. A. (2004). Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 64, 7045-7049 [DOI] [PubMed] [Google Scholar]
- Hakulinen J., Sankkila L., Sugiyama N., Lehti K., Keski-Oja J. (2008). Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J. Cell Biochem. 105, 1211-1218 [DOI] [PubMed] [Google Scholar]
- Holopainen J. M., Angelova M. I., Kinnunen P. K. (2000). Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys. J. 78, 830-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K., Li X. Y., Allen E., Stevens S. L., Weiss S. J. (2006). A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 20, 2673-2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Shi B., Jarzynka M. J., Yiin J. J., D'Souza-Schorey C., Cheng S. Y. (2009). ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway. Cancer Res. 69, 794-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber V., Fais S., Iero M., Lugini L., Canese P., Squarcina P., Zaccheddu A., Colone M., Arancia G., Gentile M., et al. (2005). Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology 128, 1796-1804 [DOI] [PubMed] [Google Scholar]
- Hugel B., Martinez M. C., Kunzelmann C., Freyssinet J. M. (2005). Membrane microparticles: two sides of the coin. Physiology (Bethesda) 20, 22-27 [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Zimmerberg J. (2001). Implications of lipid microdomains for membrane curvature, budding and fission. Curr. Opin. Cell Biol. 13, 478-484 [DOI] [PubMed] [Google Scholar]
- Iero M., Valenti R., Huber V., Filipazzi P., Parmiani G., Fais S., Rivoltini L. (2008). Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 15, 80-88 [DOI] [PubMed] [Google Scholar]
- Johnstone R. M. (2006). Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 36, 315-321 [DOI] [PubMed] [Google Scholar]
- Kim C. W., Lee H. M., Lee T. H., Kang C., Kleinman H. K., Gho Y. S. (2002). Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 62, 6312-6317 [PubMed] [Google Scholar]
- Kim J. W., Wieckowski E., Taylor D. D., Reichert T. E., Watkins S., Whiteside T. L. (2005). Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. 11, 1010-1020 [PubMed] [Google Scholar]
- Koppler B., Cohen C., Schlondorff D., Mack M. (2006). Differential mechanisms of microparticle transfer toB cells and monocytes: anti-inflammatory propertiesof microparticles. Eur J. Immunol. 36, 648-660 [DOI] [PubMed] [Google Scholar]
- Liepins A. (1983). Possible role of microtubules in tumor cell surface membrane shedding, permeability, and lympholysis. Cell Immunol. 76, 120-128 [DOI] [PubMed] [Google Scholar]
- Lima L. G., Chammas R., Monteiro R. Q., Moreira M. E., Barcinski M. A. (2009). Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 283, 168-175 [DOI] [PubMed] [Google Scholar]
- Losche W., Scholz T., Temmler U., Oberle V., Claus R. A. (2004). Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils. Platelets 15, 109-115 [DOI] [PubMed] [Google Scholar]
- Mack M., Kleinschmidt A., Bruhl H., Klier C., Nelson P. J., Cihak J., Plachy J., Stangassinger M., Erfle V., Schlondorff D. (2000). Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6, 769-775 [DOI] [PubMed] [Google Scholar]
- Manno S., Takakuwa Y., Mohandas N. (2002). Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA 99, 1943-1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O., Pagano R. E. (1987). Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process(es). J. Biol. Chem. 262, 5890-5898 [PubMed] [Google Scholar]
- Mezzano D., Matus V., Saez C. G., Pereira J., Panes O. (2008). Tissue factor storage, synthesis and function in normal and activated human platelets. Thromb. Res. 122, S31-S36 [DOI] [PubMed] [Google Scholar]
- McConnell R. E., Higginbotham J. N., Shifrin D. A., Jr, Tabb D. L., Coffey R. J., Tyska M. J. (2009). The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185, 1285-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milsom C., Yu J., May L., Meehan B., Magnus N., Al-Nedawi K., Luyendyk J., Weitz J., Klement P., Broze G., et al. (2007). The role of tumor-and host-related tissue factor pools in oncogene-driven tumor progression. Thromb. Res. 120, S82-S91 [DOI] [PubMed] [Google Scholar]
- Morel O., Toti F., Hugel B., Freyssinet J. M. (2004). Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr. Opin. Hematol. 11, 156-164 [DOI] [PubMed] [Google Scholar]
- Morita E., Sundquist W. I. (2004). Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20, 395-425 [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G., D'Souza-Schorey C. (2009a). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875-1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V., Hoover H., Clancy J., Schweitzer J., Suckow M. A., Schroeder V., Castellino F. J., Schorey J. S., D'Souza-Schorey C. (2009b). ADP-ribosylation factor 6 regulates tumorigenic and invasive properties in vivo. Cancer Res. 69, 2201-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mytar B., Baj-Krzyworzeka M., Majka M., Stankiewicz D., Zembala M. (2008). Human monocytes both enhance and inhibit the growth of human pancreatic cancer in SCID mice. Anticancer Res. 28, 187-192 [PubMed] [Google Scholar]
- Nickel W. (2005). Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic 6, 607-614 [DOI] [PubMed] [Google Scholar]
- Paluch E., Piel M., Prost J., Bornens M., Sykes C. (2005). Cortical actomyosin breakage triggers shape oscillations in cells and cell fragments. Biophys J. 89, 724-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin A., Murphy W. G., Smith O. P. (2007). Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21, 157-171 [DOI] [PubMed] [Google Scholar]
- Pilzer D., Gasser O., Moskovich O., Schifferli J. A., Fishelson Z. (2005). Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin. Immunopathol. 27, 375-387 [DOI] [PubMed] [Google Scholar]
- Pluskota E., Woody N. M., Szpak D., Ballantyne C. M., Soloviev D. A., Simon D. I., Plow E. F. (2008). Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood 112, 2327-2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G., Nicolson G. L. (1980). Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc. Natl. Acad. Sci. USA 77, 399-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia P., Schiera G., Mineo M., Ingrassia A. M., Santoro G., Savettieri G., Di Liegro I. (2008). Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 21, 63-67 [PubMed] [Google Scholar]
- Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M. Z. (2006a). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847-856 [DOI] [PubMed] [Google Scholar]
- Ratajczak J., Wysoczynski M., Hayek F., Janowska-Wieczorek A., Ratajczak M. Z. (2006b). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487-1495 [DOI] [PubMed] [Google Scholar]
- Safaei R., Larson B. J., Cheng T. C., Gibson M. A., Otani S., Naerdemann W., Howell S. B. (2005). Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 4, 1595-1604 [DOI] [PubMed] [Google Scholar]
- Sanderson M. P., Keller S., Alonso A., Riedle S., Dempsey P. J., Altevogt P. (2008). Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J. Cell Biochem. 103, 1783-1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiera G., Proia P., Alberti C., Mineo M., Savettieri G., Di Liegro I. (2007). Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J. Cell Mol. Med. 11, 1384-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey J. S., Bhatnagar S. (2008). Exosome function: from tumor immunology to pathogen biology. Traffic 9, 871-881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J. K., D'Souza-Schorey C. (2004). Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp. Cell Res. 295, 1-8 [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. (1987). ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes.. Proc. Natl. Acad. Sci. USA 81, 3751-3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedden K., Xie X. T., Chandaroy P., Chang Y. T., Rosania G. R. (2003). Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 63, 4331-4337 [PubMed] [Google Scholar]
- Sheetz M., Painter R., Singer S. (1976). Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J. Cell Biol. 70, 193-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Raposo G. (2009). Exosomes-vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575-581 [DOI] [PubMed] [Google Scholar]
- Skog J., Wurdinger T., van Rijn S., Meijer D. H., Gainche L., Sena-Esteves M., Curry W. T., Jr, Carter B. S., Krichevsky A. M., Breakefield X. O. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470-1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley D. M., Sheman N. E., Nelson K., Theodorescu D. (2008). Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J. Proteome Res. 7, 2088-2096 [DOI] [PubMed] [Google Scholar]
- Steffen A., Le Dez G., Poincloux R., Recchi C., Nassoy P., Rottner K., Galli T., Chavrier P. (2008). MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr. Biol. 18, 926-931 [DOI] [PubMed] [Google Scholar]
- Sune A., Bette-Bobillo P., Bienvenue A., Fellmann P., Devaux P. F. (1987). Selective outside-inside translocation of aminophospholipids in human platelets. Biochemistry 26, 2972-2978 [DOI] [PubMed] [Google Scholar]
- Tang Y., Kesavan P., Nakada M. T., Yan L. (2004). Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol. Cancer Res. 2, 73-80 [PubMed] [Google Scholar]
- Taraboletti G., D'Ascenzo S., Borsotti P., Giavazzi R., Pavan A., Dolo V. (2002). Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 160, 673-680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G., D'Ascenzo S., Giusti I., Marchetti D., Borsotti P., Millimaggi D., Giavazzi R., Pavan A., Dolo V. (2006). Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia 8, 96-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. D., Gercel-Taylor C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13-21 [DOI] [PubMed] [Google Scholar]
- Taylor R. C., Cullen S. P., Martin S. J. (2008). Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9, 231-241 [DOI] [PubMed] [Google Scholar]
- Tesselaar M. E., Romijn F. P., Van Der Linden I. K., Prins F. A., Bertina R. M., Osanto S. (2007). Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J. Thromb. Haemost. 5, 520-527 [DOI] [PubMed] [Google Scholar]
- Valenti R., Huber V., Filipazzi P., Pilla L., Sovena G., Villa A., Corbelli A., Fais S., Parmiani G., Rivoltini L. (2006). Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 66, 9290-9298 [DOI] [PubMed] [Google Scholar]
- Valenti R., Huber V., Iero M., Filipazzi P., Parmiani G., Rivoltini L. (2007). Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 67, 2912-2915 [DOI] [PubMed] [Google Scholar]
- van Doormaal F. F., Kleinjan A., Di Nisio M., Buller H. R., Nieuwland R. (2009). Cell-derived microvesicles and cancer. Neth. J. Med. 67, 266-273 [PubMed] [Google Scholar]
- Vittorelli M. L. (2003). Shed membrane vesicles and clustering of membrane-bound proteolytic enzymes. Curr. Top. Dev. Biol. 54, 411-432 [DOI] [PubMed] [Google Scholar]
- Wysoczynski M., Ratajczak M. Z. (2009). Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int. J. Cancer 125, 1595-1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Kozlov M. M. (2006). How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7, 9-19 [DOI] [PubMed] [Google Scholar]
- Zou W. (2005). Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263-274 [DOI] [PubMed] [Google Scholar]
- Zwicker J. I., Furie B. C., Furie B. (2007). Cancer-associated thrombosis. Crit. Rev. Oncol. Hematol. 62, 126-136 [DOI] [PubMed] [Google Scholar]
- Zwicker J. I., Liebman H. A., Neuberg D., Lacroix R., Bauer K. A., Furie B. C., Furie B. (2009). Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin. Cancer Res. 15, 6830-6840 [DOI] [PMC free article] [PubMed] [Google Scholar]