Abstract
Angiotensin II (Ang II) is a key proapoptotic factor in fibrotic tissue diseases. However, the mechanism of Ang-II-induced cell death in endothelial cells has not been previously elucidated. Using the neutral comet assay and specific receptor antagonists and agonists, we found that Ang-II-mediated apoptosis in primary pulmonary endothelial cells required the AT2 receptor. Ang II caused cytochrome c release from the mitochondria concurrent with caspase-3 activation and DNA fragmentation, and apoptosis was suppressed by an inhibitor of Bax-protein channel formation, implicating mitochondrial-mediated apoptosis. There was no evidence that the extrinsic apoptotic pathway was involved, because caspase-9, but not caspase-8, was activated by Ang-II treatment. Apoptosis required phosphoprotein phosphatase activation, and inhibition of the SHP-2 phosphatase (encoded by Ptpn11) blocked cell death. Reduced levels of anti-apoptotic Bcl-2-family members can initiate intrinsic apoptosis, and we found that Ang-II treatment lowered cytosolic Bcl-xL protein levels. Because the protein nucleolin has been demonstrated to bind Bcl-xL mRNA and prevent its degradation, we investigated the role of nucleolin in Ang-II-induced loss of Bcl-xL. RNA-immunoprecipitation experiments revealed that Ang II reduced the binding of nucleolin to Bcl-xL mRNA in an AU-rich region implicated in instability of Bcl-xL mRNA. Inhibition of SHP-2 prevented Ang-II-induced degradation of Bcl-xL mRNA. Taken together, our findings suggest that nucleolin is a primary target of Ang-II signaling, and that Ang-II-activated SHP-2 inhibits nucleolin binding to Bcl-xL mRNA, thus affecting the equilibrium between pro- and anti-apoptotic members of the Bcl-2 family.
Keywords: Intrinsic apoptosis, mRNA half-life, Bcl-xL, Nucleolin, SHP-2, Ang II
Introduction
The vasoactive peptide angiotensin II (Ang II) was originally studied for its role in blood-pressure homeostasis, but abnormal expression of Ang II has been implicated in the development and progression of fibrotic organ diseases (Wynn, 2008). Blockade of Ang-II signaling, either by using inhibitors of angiotensin-converting enzyme to prevent Ang-II maturation or using antagonists of Ang-II cellular receptors, can abrogate the development of fibrosis in animal models in the lung, liver, kidney and heart (Bataller et al., 2005; Dendorfer et al., 2005; Konigshoff et al., 2007; Sun, 2009; Wolf, 2008). In a number of fibrotic diseases, local synthesis of Ang II has been observed, and fibroblasts from the diseased tissues of human patients were found to generate Ang II (Bader, 2002; Sun et al., 2000; Wang et al., 1999). Two primary events are associated with the development and progression of fibrosis: (1) the transdifferentiation of fibroblasts into the activated fibroblast (myofibroblast) phenotype that secretes abnormal extracellular-matrix proteins such as collagens I and III (Wynn, 2008); and (2) apoptosis of the normal epithelial and endothelial cells of the tissues (Marshall, 2003; Wang et al., 2000). Although much research has focused on apoptosis of epithelial cells in fibrotic remodeling, several studies provided evidence for endothelial cell apoptosis in pulmonary fibrosis. Endothelial cell apoptosis has been identified in animal models of lung fibrosis (Zhang et al., 2007), and in samples from patients with idiopathic pulmonary fibrosis (Yoshida et al., 2002). Importantly, the use of VEGF to inhibit TGF-β1-induced endothelial apoptosis mitigated fibrotic remodeling in a rat model of pulmonary fibrosis (Farkas et al., 2009).
Ang II has been demonstrated to play a dual role in the development of fibrosis, paradoxically inducing both the proliferation of scar-tissue-producing myofibroblasts as well as the apoptosis of the primary epithelial and endothelial cells (Bataller et al., 2005; Uhal, 2002; Wolf, 2008). The contradictory actions of Ang II on the induction of growth versus apoptosis have been shown to be receptor-subtype- and cell-type-dependent. Ang II exerts its biological effects via the activation of two receptors known as angiotensin type 1 (AT1) and type 2 (AT2) receptors, both of which are members of the seven-transmembrane-domain receptor family (Mogi et al., 2007). Ang-II-induced apoptosis in primary and transformed epithelial cells and coronary artery endothelial cells was shown to be mediated by the AT1 receptor (Li et al., 1999; Papp et al., 2002). By contrast, AT2-receptor activation was shown to induce apoptosis in fibroblasts, smooth muscle cells, HUVECs and PC12W cells, whereas AT1 activation resulted in proliferative and anti-apoptotic cellular responses in these cells (Cui et al., 2001; Dimmeler et al., 1997; Horiuchi et al., 1998; Yamada et al., 1996). Although many cells respond to AT2-receptor activation with decreased cell growth, AT2 receptor was shown to mediate proliferation in cardiomyocytes (D'Amore et al., 2005).
Ang-II-induced signaling has also been demonstrated to be cell-type dependent, and the signaling specificity is believed to determine the biological response. Four major signal transduction pathways are activated by the AT receptors: (1) protein phosphatase activation; (2) nitric oxide (NO)-cGMP activation; (3) phospholipase-A2 activation, with subsequent release of arachidonic acid; and (4) protein kinase C (PKC) activation. Three specific phosphatases can be activated downstream of AT2: (1) mitogen-activated protein kinase phosphatases (MKPs), which dephosphorylate threonine residues (Horiuchi et al., 1997); (2) SH2-domain-containing phosphatases (SHP-1 and SHP-2; encoded by Ptpn6 and Ptpn11, respectively), which is a small family of tyrosine phosphatases (Nouet and Nahmias, 2000); and (3) protein phosphatase 2A (PP2A), an okadaic acid-sensitive serine/threonine phosphatase (Brechler et al., 1994). In A549 or primary alveolar epithelial cells, AT1-mediated apoptosis was inhibited by a PKC inhibitor, but not by tyrosine-phosphatase inhibitors (Papp et al., 2002). By contrast, in PC12W cells and HUVECs, AT2-mediated apoptosis required MKPs and subsequent Bcl-2 inactivation by dephosphorylation (Horiuchi et al., 1997; Rossig et al., 2002).
The mechanism of Ang-II-induced apoptosis has not been previously determined in pulmonary endothelial cells. Here, we examined the mechanism of apoptosis by Ang II in primary cultures of bovine pulmonary artery endothelial cells (PAECs). Ang-II-induced apoptosis was activated by the intrinsic (mitochondria-dependent) apoptotic pathway. Moreover, we found that Ang II caused the destabilization and decay of Bcl-xL mRNA by disassociation of the mRNA from the stabilizing protein nucleolin in a signaling pathway that required SHP-2.
Results
Ang-II-induced apoptosis requires the AT2 receptor
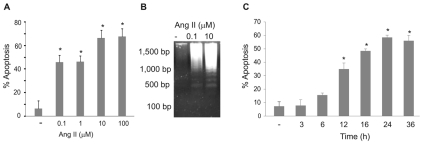
The local synthesis of Ang II has been demonstrated in lung fibrotic plaques, where it is produced by activated myofibroblasts and probably impacts the survival of other neighboring cells (Uhal, 2002; Wang et al., 1999). We investigated the effect of Ang II on bovine PAECs using the neutral comet assay, which detects chromosomal breakdown as a function of apoptosis. Ang II (100 nM and 1 μM) induced 40-50% apoptosis within 24 hours, whereas 10 μM induced 60-70% apoptosis (Fig. 1A). Higher concentrations of Ang II (100 μM) did not induce higher levels of apoptosis at 24 hours (Fig. 1A). These findings were confirmed by monitoring DNA laddering induced by 100 nM and 10 μM Ang II at 24 hours (Fig. 1B). A time course of Ang-II activity showed that significant apoptosis was detectable within 12 hours of treatment with 10 μM Ang II (Fig. 1C).
Fig. 1.
Ang II induces apoptosis in PAECs. (A) PAECs were treated with the indicated concentrations of Ang II for 24 hours before apoptosis detection using the neutral comet assay. (B) PAECs were treated with Ang II (0.1 or 10 μM) for 24 hours. DNA was used in DNA laddering assays. Base-pair standards for DNA fragment sizes are indicated. (C) PAECs were treated with Ang II (10 μM) for the indicated times before assaying apoptosis using the neutral comet assay. Bar graphs show means ± s.d. *Statistical significance from control, P<0.05, n=4. All experiments were repeated at least four times.
Ang-II receptors AT1 and AT2 are G-protein-coupled receptors, and are the primary transducers of Ang-II signaling. Pretreatment of PAECs with the AT2 antagonist PD123319 prior to exposure to 10 μM Ang II inhibited apoptosis as determined by neutral comet assay (Fig. 2A). By contrast, no inhibition of Ang-II-induced apoptosis was observed when cells were pretreated with telmisartan, an AT1-receptor antagonist (Fig. 2B). The AT2 agonist CGP42112A also induced apoptosis as determined by the neutral comet assay and DNA laddering assay (Fig. 2C,D). The apoptotic effects of Ang II and CGP42112A were reversed by the AT2 antagonist PD123319 (Fig. 2E). Activation of caspase-3, a common effector caspase for both the intrinsic and extrinsic apoptotic pathways, was examined next. Results show that concentrations of Ang II as low as 0.1 μM induce the activation of caspase-3; this activation was blocked using the AT2 antagonist PD123319 (Fig. 2F). These results indicate that Ang-II-induced apoptosis is mediated by the AT2 receptor.
Fig. 2.
Ang-II-induced apoptosis in PAECs requires the type 2 receptor. (A,B) PAECs were treated with (A) the AT2 antagonist PD123319 (50 μM) or (B) the AT1 antagonist telmisartan (1 μM) for 20 minutes prior to the addition of Ang II (10 μM) for 24 hours. Apoptosis was determined using the neutral comet assay. (C) PAECs were treated with either Ang II (0.1 or 10 μM) or AT2 agonist CGP-42112A (CGP; 0.01 or 10 μM) for 24 hours before determining apoptosis using the neutral comet assay. (D) PAECs were either untreated (control; C) or treated with CGP-42112A (CGP; 0.01 or 10 μM) for 16 hours before purification of DNA for DNA laddering analysis. Representative results are shown from three experiments. (E) PAECs were treated ± the AT2 antagonist PD123319 (50 μM) for 20 minutes prior to treatment with Ang II (0.1 or 10 μM) or the AT2 agonist CGP-42112A (CGP; 0.01 or 10 μM). (F) PAECs were treated with Ang II (0.1 μM) ± pretreatment with the AT2 antagonist PD123319 (50 μM) for 20 minutes. After 16 hours, cell lysates were prepared and western blotted for the activated form of caspase-3. Blots were stripped and probed for β-actin as a loading control. Graph to the bottom shows the average results of caspase-3 band densitometry, normalized to β-actin, from three experiments. For all bar graphs, data show means ± s.d. *Statistical significance from control, P=0.05, n=3. †Statistical significance from Ang-II treatment alone. Experiments were repeated at least three times.
Ang II induces apoptosis via the intrinsic apoptotic pathway
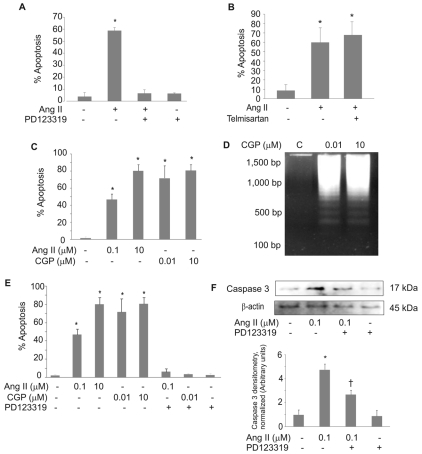
The two canonical pathways of apoptosis in eukaryotic cells are the intrinsic (mitochondria-dependent) pathway and the extrinsic (death-receptor-mediated) pathway. Mitochondrial outer membrane permeabilization (MOMP) is a key event of the intrinsic apoptotic pathway. Western blots of mitochondria-free cell lysates showed the release of cytochrome c, as an indicator of MOMP, within 16 hours of Ang-II or CGP42112A treatment (Fig. 3A). Also at this time point, Ang II or CGP42112A activated caspase-3, the common effector caspase for both the intrinsic and extrinsic apoptotic pathways (Fig. 3A). Mitochondria-free cytosolic fractions contained increased levels of activated caspase-3 and increased cytochrome c; however, in the mitochondria, cytochrome c was only present in the untreated group, and both CGP42112A and Ang-II treatment caused cytochrome c release. The mobilization of the proapoptotic protein Bax to the mitochondria was monitored. Upon Ang-II treatment, the level of Bax protein increased in the mitochondria, whereas the mitochondria-free cytosolic fraction showed a significant decrease in the levels of Bax protein (supplementary material Fig. S1). We therefore investigated the requirement of Bax activation for Ang-II-induced apoptosis. Cell death was completely inhibited by either a Bax-channel blocker (BCB) or a Bax-inhibiting peptide (V-5), whereas a control peptide had no effect (Fig. 3B).
Fig. 3.
Ang II induces the intrinsic pathway of apoptosis in PAECs. (A) PAECs were treated with the indicated concentrations of Ang II or CGP-42112A (CGP) for 16 hours before the preparation of mitochondria-free cell fractions (cytosol) or mitochondria. Lysates were western blotted for cytochrome c or activated caspase-3. Blots were stripped and reprobed for β-actin as a loading control for cytosol or for COX4, a marker for mitochondria. Densitometry results normalized to β-actin or COX4 are shown in lower panels. (B) PAECs were treated with the Bax-channel blocker (BCB, 10 μM, left panel), the Bax-inhibitory peptide V5 (100 μM, right panel) or a Bax control peptide (100 μM) for 20 minutes prior to the addition of Ang II (10 μM) for 16 hours. Neutral comet assays were then performed and the percent apoptosis was determined. (C) PAECs were pretreated with the caspase-3 inhibitor Z-DEVD-FMK (10 μM) before treatment with 10 μM Ang II for 16 hours. Neutral comet assays were performed and the percent apoptosis was determined. (D,E) PAECs were treated with 10 μM Ang II or AT2-receptor agonist CGP-42112A (CGP, 10 μM) for 16 hours and assayed for caspase-9 (D) and caspase-8 (E). TNFα (1 μg/ml) treatment for 16 hours was used as a positive control for caspase-8 activation; cytochrome c (10 μg/ml) was used as a positive control for activation of caspase-9. For all bar graphs, data show means ± s.d. *Statistical significance from control, P<0.05, n=3. All experiments were repeated at least three times.
We found that cell-permeable inhibitor of caspase-3 (Z-DEVD-FMK) also completely blocked Ang-II-induced apoptosis (Fig. 3C). Caspase-3 is common to both the intrinsic and extrinsic pathways so, to further differentiate between the two apoptotic pathways, we examined the activation of caspase-9, the initiator caspase for the intrinsic pathway, and the activation of caspase-8, the initiator caspase for the extrinsic pathway. Caspase-9 was activated by both Ang II and the AT2 agonist CGP42112A when compared with the control (Fig. 3D), whereas caspase-8 was not activated by either treatment (Fig. 3E).
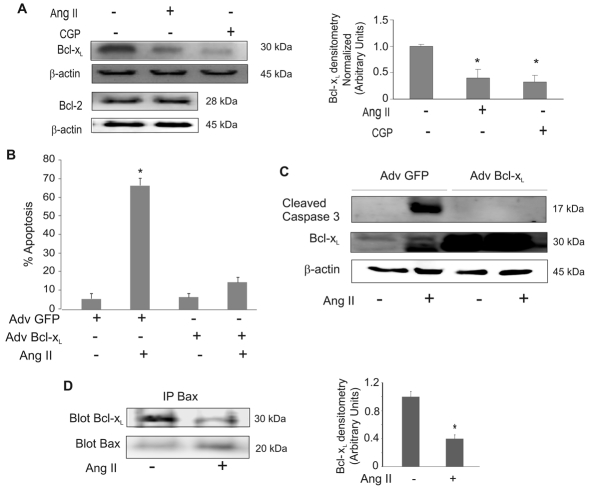
Changes in MOMP can occur if the protein levels of anti-apoptotic members of the Bcl-2 family, especially Bcl-2 or Bcl-xL, are reduced, allowing mitochondrial pore formation by proapoptotic Bcl-2-family members. A decrease in the ratio of Bcl-xL:Bax (anti- to proapoptotic protein), which is sufficient to induce apoptosis (Zhang et al., 2000). Treatment with Ang II and the AT2 agonist CGP42112A significantly decreased Bcl-xL protein levels (Fig. 4A, upper panel). We did not detect any changes in Bcl-2 protein levels with Ang-II treatment (Fig. 4A, lower panel). To determine whether the reduction of Bcl-xL was crucial for Ang-II-induced apoptosis, we ectopically expressed Bcl-xL protein (Suzuki et al., 2007). Cells infected with an adenoviral vector encoding Bcl-xL were protected from Ang-II-induced apoptosis (Fig. 4B) and the activation of caspase-3 was prevented (Fig. 4C). Infection with a GFP-expressing adenovirus had no effect on Ang-II-induced apoptosis or caspase-3 activation. Activated Bax permeabilizes the outer mitochondrial membrane, thereby committing cells to apoptosis. Bcl-xL inhibits this process by binding directly to activated Bax. Co-immunoprecipitation assays were performed to detect the amount of Bcl-xL bound to Bax. The results demonstrated that Ang-II treatment reduces the interaction of Bcl-xL with Bax (Fig. 4D). The input (whole cell lysates) was western blotted for Bcl-xL with Bax as a control (supplementary material Fig. S2A). This control shows that the level of Bcl-xL was reduced in the lysate in response to Ang II, but Bax levels were unchanged. The unbound fractions after immunoprecipitation of Bax were also western blotted for Bcl-xL and Bax to show remaining unbound protein levels (supplementary material Fig. S2B). These data are consistent with Ang-II-induced apoptosis through a mechanism that involves the downregulation of Bcl-xL.
Fig. 4.
Ang II induces apoptosis in PAECs by suppressing Bcl-xL protein levels. (A) PAECs were treated with Ang II (10 μM) or AT2 agonist CGP-42112A (CGP, 10 μM) for 16 hours. Mitochondria-free cytosolic extracts were used for western blot of Bcl-xL. Blots were stripped and reprobed for β-actin. Densitometry results normalized to β-actin are shown to the right. (B,C) PAECs were infected with adenovirus (Adv) GFP or Bcl-xL for 48 hours, followed by 10 μM Ang-II treatment for 24 hours. (B) Neutral comet assays were performed to detect apoptotic cells. Percentage of apoptotic cells were then determined. (C) Lysates were used for western blots with the antibodies against active caspase-3, Bcl-xL and β-actin. Representative results are shown. (D) PAECs, control or treated with Ang II (10 μM) for 16 hours, were used to prepare whole cell lysate. Equal amounts of protein lysates were immunoprecipitated with anti-Bax antibody followed by western blotting for Bcl-xL or Bax. Densitometries are shown to the right showing the ratio of Bcl-xL to Bax. All bar graphs show means ± s.d. *Statistical significance from control, P<0.05, n=3. All experiments were repeated at least three times.
SHP-2 activation is required for Ang-II-induced apoptosis
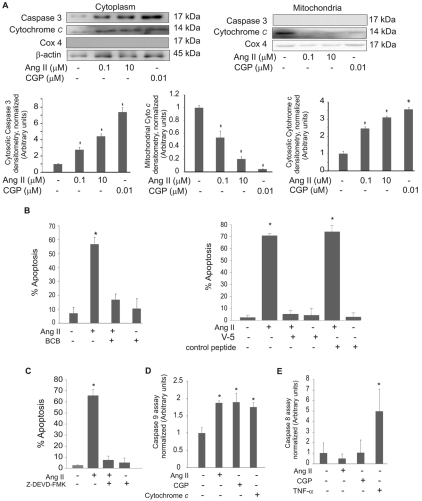
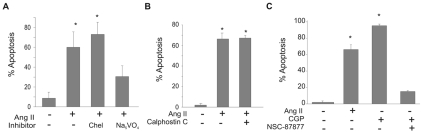
The signaling pathways activated by Ang II through its receptors have been demonstrated to be cell-type specific. Pharmacological inhibitors were used to identify signaling pathways originating from the AT2 receptor leading to apoptosis in PAECs. Ang-II-induced apoptosis was blocked by the non-specific inhibitor of tyrosine phosphatases sodium orthovanadate (Fig. 5A), whereas inhibitors of PKC (chelerythrine chloride and calphostin C) had no effect (Fig. 5A,B). An inhibitor of the SH2-domain-containing protein tyrosine phosphatases (SHP-1 and SHP-2) (NSC-87877) blocked Ang-II-induced apoptosis (Fig. 5C).
Fig. 5.
Ang-II-induced apoptosis requires SHP phosphatase. PAECs were pretreated with the following inhibitors for 20 minutes prior to Ang II (10 μM) or AT2-agonist CGP-42112A (CGP; 10 μM) treatment for 16 hours. Neutral comet assays were performed and percentages of apoptotic cells were determined. (A) PKC-inhibitor chelerythrine chloride (Chel; 1 μM) or sodium vanadate (Na3VO4; 20 μM), a broad-spectrum tyrosine phosphatase inhibitor; (B) PKC-inhibitor calphostin C (50 nM); (C) SHP-1/2 phosphatase inhibitor (NSC-87877; 50 μM). Data show means ± s.d. *Statistical significance from control, P<0.05, n=3. All experiments were repeated at least three times.
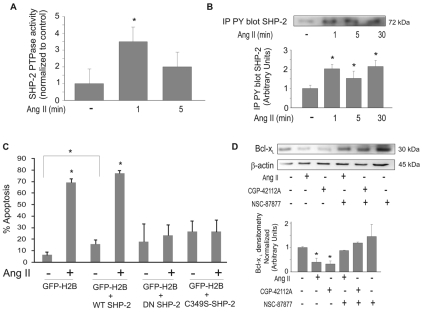
Specific inhibitors are not available to differentiate between SHP-1 and SHP-2 isoforms (Yip et al., 2000). Previous studies showed that SHP-1 is primarily expressed in hematopoietic and epithelial cells (Chong and Maiese, 2007; Sankarshanan et al., 2007; Valencia et al., 1997; Yang et al., 1998; Yi et al., 1992). By contrast, SHP-2 is ubiquitously expressed (Bennett et al., 1996). To verify the SHP-isoform expression profile in PAECs, reverse transcriptase (RT)-PCR was performed using primers specific for SHP-1 or SHP-2. Bovine pulmonary epithelial cells (PEpCs) express both SHP-1 and SHP-2, but we found that bovine PAECs only express SHP-2 (supplementary material Fig. S3). The activation of SHP-2 in PAECs was confirmed by immunoprecipitation of SHP-2 followed by phosphatase assays. SHP-2 was activated within 1 minute of Ang-II treatment (Fig. 6A). Vogel et al. (Vogel et al., 1993) and Pazdrak et al. (Pazdrak et al., 1997) previously demonstrated that SHP-2 is tyrosine phosphorylated upon activation. Immunoprecipitation with anti-phospho-tyrosine antibodies followed by western blotting for SHP-2 showed that SHP-2 was tyrosine phosphorylated in PAECs within 1 minute following Ang-II treatment (Fig. 6B).
Fig. 6.
SHP-2 is activated by Ang II. (A) PAECs were treated with 10 μM Ang II for the indicated times. Cell lysates were immunoprecipitated with anti-SHP-2. Immunoprecipitated SHP-2 protein was then subjected to phosphatase assays. (B) SHP-2 phosphorylation in response to Ang II. PAECs were treated with 10 μM Ang II for the indicated times. Cell lysates were prepared and equal amounts of protein were immunoprecipitated for phospho-tyrosine and blotted for SHP-2. Graph shows densitometry of phospho-SHP-2 bands. (C) PAECs were transfected with wild-type (WT) SHP-2, dominant negative (DN) SHP-2 or the C459S-mutant phosphatase-inactive SHP-2 (C459S-SHP-2). An expression vector for EGFP-tagged H2B histone (GFP) was co-transfected as a marker. Cells were untreated or treated with 10 μM Ang II for 24 hours and used for neutral comet assays. GFP-H2B was used to visualize transfected cells and propidium iodide was added to stain comet tails. (D) Cells were treated ± SHP-1/2 phosphatase inhibitor (NSC-87877) prior to treatment with 10 μM Ang II or 10 μM AT2 agonist (CGP-42112A). Cell lysates were prepared and equal amounts of protein were used for western blotting for Bcl-xL. Blots were stripped and probed for β-actin as a loading control. Graph shows densitometry analysis of Bcl-xL normalized to β-actin. All data show means ± s.d. *Statistical significance from control, P<0.05, n=3. Experiments were repeated at least three times.
To determine the requirement of SHP-2 for Ang-II-induced apoptosis, we ectopically expressed either dominant-negative (DN) SHP-2 or the SHP-2 mutant C459S (C459S-SHP-2), in which the phosphatase is inactive (Berchtold et al., 1998; Noguchi et al., 1994). PAECs were transiently transfected with wild-type SHP-2, DN SHP-2 or C459S-SHP-2 constructs, or empty-vector control. Co-transfection of EGFP-tagged histone (H2BC-EGFP) was used for identification of transfected cells. Cells were treated with Ang II for 16 hours and apoptotic cells were identified using the neutral comet assay. Green fluorescent cells (cells positive for H2BC-EGFP expression) were scored for red comet tails stained with propidium iodide. Co-transfection of an empty vector confirmed that expression of histone H2BC-EGFP had no effect on Ang-II-induced apoptosis (Fig. 6C). DN SHP-2 and C459S-SHP-2 expression significantly reduced apoptosis induced by Ang II (Fig. 6C). Because SHP-2 is probably activated by association with AT2, overexpression of DN or mutant SHP-2 probably competes with endogenous wild-type SHP-2 for binding with the receptor, thus preventing activation of the endogenous SHP-2. We also observed a significant increase in the level of basal apoptosis in cells overexpressing wild-type SHP-2 (Fig. 6C), consistent with a proapoptotic role of SHP-2 in PAECs.
To determine the link between SHP-2 and the reduction in Bcl-xL protein, PAECs were pretreated with an SHP phosphatase inhibitor (NSC-87877) prior to treatment with either Ang II or the AT2 agonist CGP42112A for 16 hours. Inhibition of SHP-2 preserved Bcl-xL protein at basal levels (Fig. 6D).
Ang-II-induced apoptosis requires the Gαs protein as an adaptor
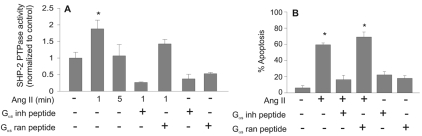
In transformed epithelial cells, SHP-1 is activated by the AT2 receptor in a mechanism that requires the physical association of Gαs protein (α-subunit of stimulatory heterotrimeric G protein) (Feng et al., 2002). The activation of the G protein is not required for SHP-1 activation, suggesting that Gαs functions as an adaptor molecule in this process. We investigated the requirement of Gαs for SHP-2 activation by AT2. A peptide corresponding to the last 11 amino acids of Gαs was shown by Feng et al. (Feng et al., 2002) to inhibit the interaction between Gαs and AT2. The Gαs-inhibiting peptide blocked activation of SHP-2 (Fig. 7A) and reduced Ang-II-induced apoptosis (Fig. 7B). Feng et al. also showed that SHP-1 activation by cholera toxin A dissociated AT2-Gαs coupling by constitutively activating the Gαs protein (Feng et al., 2002). Activation of Gαs via cholera toxin A in PAECs also prevented both activation and tyrosine phosphorylation of SHP-2 protein by Ang II (supplementary material Fig. S4A,B). Cholera toxin A also reduced Ang-II-induced apoptosis by 70% (supplementary material Fig. S4C, left panel). Gαs signaling activates the cAMP-dependent protein kinase (PKA). To determine whether activation of PKA affected Ang-II-induced apoptosis, PAECs were treated with forskolin, to directly upregulate cAMP and activate PKA, or with H89, to inhibit PKA. Forskolin treatment had no effect on Ang-II-induced apoptosis (supplementary material Fig. S4C, middle panel), suggesting that the activation of adenylate cyclase, upregulation of cAMP and PKA activation were not the mechanism(s) by which cholera toxin A inhibited Ang-II-induced apoptosis. H89 inhibition of PKA also failed to block Ang-II-induced apoptosis (supplementary material Fig. S4C, right panel), suggesting that PKA is not required for apoptosis. Together, these findings suggest that Gαs acts as an adaptor for AT2-mediated activation of SHP-2 in endothelial cells, in a mechanism parallel to the activation of SHP-1 by AT2 in epithelial cells.
Fig. 7.
Activation of SHP-2 and induction of apoptosis by Ang II require Gαs protein as an adaptor. (A,B) PAECs were treated with Gαs-inhibiting peptide or random peptide (0.5 μM) prior to treatment with Ang II (10 μM). (A) PAEC lysates were prepared after 1 minute of Ang-II exposure, and 100 μg of protein was used to immunoprecipitate SHP-2. Phosphatase assays were performed as described in the Materials and Methods. (B) Neutral comet assays were performed, and the percentage of apoptotic cells determined after Ang-II treatment (10 μM, 24 hours).
Ang II reduces both the mRNA half-life of Bcl-xL and the binding of nucleolin to the 3′UTR of Bcl-xL mRNA
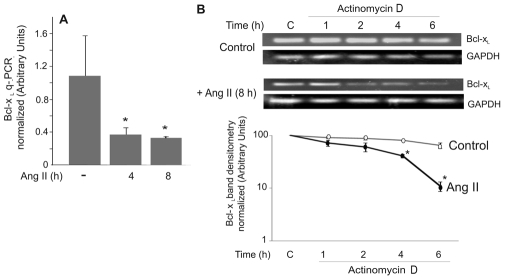
Our data indicated that Ang II reduced cytoplasmic Bcl-xL protein levels within 16 hours of Ang-II treatment. We investigated the effect of Ang II on Bcl-xL mRNA, and found that mRNA levels were reduced within 4 hours of treatment (Fig. 8A). Bcl-xL mRNA levels are controlled by promoter regulation, by alternative splicing and by the mRNA half-life (Boise et al., 1993; Kren et al., 1996; Li et al., 2004; Suzuki et al., 2007). Examination of the effect of Ang II on Bcl-xL-promoter activation showed no differences from basal levels, and alternatively spliced Bcl-xL was not detected in our cells (data not shown). We therefore investigated the effects of Ang II on the half-life of Bcl-xL mRNA. Ang II caused a reduction in the half-life of Bcl-xL mRNA, from ~7 hours in control cells to ~3 hours in Ang-II-treated cells (Fig. 8B).
Fig. 8.
Ang II destabilizes Bcl-xL mRNA in PAECs. (A) PAECs were untreated (−) or treated with Ang II (10 μM) for the indicated time. mRNA was prepared and qPCR was performed to determine Bcl-xL mRNA levels normalized to the internal GAPDH mRNA level. Results show mean ± s.d.; n=4. *P<0.05. (B) PAECs were treated with 10 μM Ang II for 8 hours, followed by treatment with 5 μg/ml actinomycin D for the indicated times. PCR was performed for Bcl-xL. White circles, controls; black circles, Ang II. Densitometry data was normalized to the zero time point for each condition. Data show means ± s.d. *Statistical significance from control, P<0.05, n=3. Experiments were repeated three times.
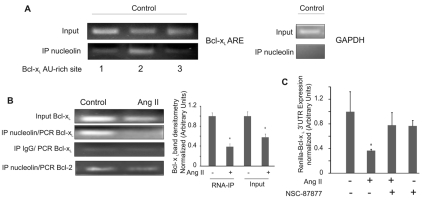
Bcl-xL mRNA stability is regulated by the 3′UTR, which contains AU-rich elements (AREs) that target the mRNA for degradation (Bachelor and Bowden, 2004). Nucleolin, a ubiquitously expressed 110-kDa multifunctional protein, has been shown to bind to the Bcl-xL 3′UTR and stabilize the mRNA in the cytoplasm (Srivastava and Pollard, 1999; Zhang et al., 2008). We hypothesized that the Ang-II-induced decrease in Bcl-xL mRNA half-life could be associated with decreased binding of nucleolin to the 3′UTR. RNA-immunoprecipitation (RNA-IP) experiments were performed to identify the nucleolin-binding site and to determine the effect of Ang-II signaling on nucleolin binding. We isolated Bcl-xL mRNA fragments bound to nucleolin and used specific PCR primers to individually amplify the three AREs in the Bcl-xL 3′UTR. The first ARE is located 164 nucleotides downstream of the stop codon, whereas the second and the third AU-rich regions start at 317 and 1268 nucleotides downstream of the stop codon, respectively. We detected the highest binding affinity to the second ARE (Fig. 9A). GAPDH, used as a negative control, is present in the input RNA sample but is not present after immunoprecipitation with nucleolin (Fig. 9A). Ang-II treatment of the PAECs significantly decreased nucleolin binding to the second ARE (Fig. 9B). This data suggests that Ang-II regulation of the Bcl-xL mRNA half-live involves nucleolin binding to the second ARE of the 3′UTR.
Fig. 9.
Ang II reduces nucleolin binding to the 3′UTR of Bcl-xL mRNA. (A) Total RNA of untreated PAECs was extracted and PCR amplified for all three Bcl-xL AU-rich sites and for GAPDH as a control (top left and right images). RNA-IP of nucleolin was also performed on the untreated cells (bottom left). Cells were fixed with 4% paraformaldehyde, sonicated and immunoprecipitated with anti-nucleolin antibody. RNA-protein complex was reverse-crosslinked for 2 hours, and proteinase K and DNase were added. Phenol/choroform RNA extraction was performed for cDNA synthesis. PCR was performed for all three AU-rich region of the 3′UTR of Bcl-xL. (B) After PAECs had been treated with 10 μM Ang II for 16 hours, RNA-IP for nucleolin and IgG was done. PCR was performed for the second AU-rich region of the 3′UTR of Bcl-xL and for Bcl-2. Right panel shows densitometry of Bcl-xL mRNA band. (C) Cells were transfected with Renilla–Bcl-xL 3′UTR and co-transfected with a luciferase vector RSV-Luc construct to normalize for transfection efficiency. 6 hours after transfection, cells were placed in serum-free medium. Cells were treated with the SHP-1/2 phosphatase inhibitor (NSC-87877) prior to 10 μM Ang-II exposure for 16 hours. Luciferase and Renilla assays were performed on cell lysates. All data show means ± s.d. *Statistical significance from control, P<0.05, n=3. Experiments were repeated at least three times.
Finally, we investigated the effect of Ang II on the stability of a reporter gene containing the Bcl-xL mRNA 3′UTR. PAECs were transfected with a Renilla-luciferase (Renilla)–Bcl-xL-3′UTR reporter construct (Bachelor and Bowden, 2004), and cells were either untreated or treated with Ang II. Renilla expression decreased significantly upon Ang-II treatment (Fig. 9C). We also found that inhibition of SHP-2 by NSC-87877 prior to Ang-II treatment restored Renilla expression to basal levels (Fig. 9C). These results suggest that Ang II destabilizes Bcl-xL mRNA by reducing nucleolin binding to the second ARE in the 3′UTR, via a signaling pathway that requires SHP-2.
Discussion
The major finding of this study is that Ang II induces mitochondria-dependent apoptosis of primary pulmonary artery endothelial cells through the AT2 receptor in a signal transduction pathway leading to the downregulation of Bcl-xL protein through the destabilization of Bcl-xL mRNA. The balance between the levels of pro- and anti-apoptotic proteins of the Bcl-2 family can determine mitochondrial membrane permeability (Zhang et al., 2000). Consistent with this, we found that Ang-II treatment reduced Bcl-xL–Bax binding and caused translocation of Bax to the mitochondria. We also determined that the decreased half-life of Bcl-xL mRNA is associated with decreased nucleolin binding to the second ARE of the 3′UTR in a signaling pathway that requires SHP-2 phosphatase activation.
Ang-II-induced apoptosis in PAECs required AT2, but not AT1, activation. Published findings provide conflicting evidence for the roles of AT1 and AT2 in cell proliferation and apoptosis. The contradictory effect of Ang II on cellular proliferation versus apoptosis has been hypothesized to be determined by cell-type-specific expression of the receptors as well as differences in downstream signaling. The activation of SHP-1 and SHP-2 protein phosphatases is considered one of the most crucial downstream effects of Ang II, and is believed to mediate negative crosstalk between AT2 with AT1 and possibly with other growth-factor receptors (Alvarez et al., 2008; Bedecs et al., 1997; Cui et al., 2001; Li et al., 2007; Marrero et al., 1998; Matsubara et al., 2001; Wu et al., 2004). In some cells, it has been demonstrated that Ang-II treatment activates opposing signals through simultaneous AT1 and AT2 activation. As an example of this, AT1 activation of ERK MAPK and Pyk kinase for cell survival is attenuated by AT2 activation of SHP-1 phosphatase in vascular smooth muscle cells (Cui et al., 2001; Matsubara et al., 2001). Our data indicate that SHP-2 is required for the Ang-II-induced decrease of Bcl-xL protein, and loss of anti-apoptotic Bcl-2-family members might also be the mechanism for Ang-II-mediated inhibition of proliferative signaling by other receptors. Our data demonstrate that AT2 activation of SHP-2 also requires the presence of the inactive Gαs protein. In this complex containing AT2 and the Gαs protein, SHP-2 is rapidly phosphorylated with a time course (within 1 minute) that matches the enhancement of its phosphatase activity by Ang II. This suggests that the complex of the AT2 receptor, SHP-2 and Gαs might also contain a protein kinase. The identity of this kinase is currently under investigation in our laboratory.
We demonstrate that downregulation of Bcl-xL by Ang II is associated with decreased binding of nucleolin to the 3′UTR of Bcl-xL mRNA. Nucleolin is a ubiquitously expressed, multifunctional RNA- and DNA-binding protein (Srivastava and Pollard, 1999). In the cytoplasm, nucleolin plays a role in cell survival through the stabilization of both Bcl-2 and Bcl-xL mRNA (Otake et al., 2007; Sengupta et al., 2004). Nucleolin binding to the 3′UTR of some mRNAs prevents AU-targeting of the mRNA for degradation (Zhang et al., 2008). However, upon dephosphorylation, nucleolin is translocated from the cytoplasm to the nucleus, where it is no longer available for mRNA stabilization (Schwab and Dreyer, 1997). Downregulation of nucleolin, by siRNA or by agents that cause its degradation, is sufficient to induce growth arrest and apoptosis (Kito et al., 2003; Ugrinova et al., 2007). Our data indicate that SHP-2 phosphatase activity is necessary for Ang-II-induced degradation of Bcl-xL, and we hypothesize that SHP-2 might directly affect the phosphorylation state of nucleolin to either cause its translocation to the nucleus or to affect its stability. The phosphorylation site on nucleolin that is affected by SHP-2 has yet to be identified. Ang-II-induced alterations in nucleolin protein levels, phosphorylation and subcellular localization are currently under investigation in our laboratory.
Fibrotic remodeling involves both the transdifferentiation of normal fibroblasts to myofibroblasts and the induction of apoptosis in epithelial and endothelial cells (Friedman, 2004; Strieter, 2008; Tomasek et al., 2002; Wynn, 2008). Here we have identified a novel mechanism for Ang-II-induced apoptosis in endothelial cells that involves SHP-2-induced destabilization of Bcl-xL mRNA through reduction of nucleolin binding. The identification of specific pathways and mechanisms for apoptosis in fibrotic remodeling could provide novel targets for the mitigation of fibrotic diseases.
Materials and Methods
Reagents
Ang II was purchased from Bachem (Torrance, CA). Antibodies against cytochrome c, Bcl-2 and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against normal rabbit IgG, anti-cleaved caspase-3 and SHP-2 were from Cell Signaling Technology (Danvers, MA). DN SHP-2, mutant C295S-SHP-2 and wild-type SHP-2 expression vectors, and Gαs-inhibiting peptides and random peptides were gifts of Ying H. Feng (Uniformed Services University, Bethesda, MD) (Berchtold et al., 1998; Noguchi et al., 1994). The Bax-channel blocker, Bax-inhibiting peptide V5 and control peptide were purchased from Tocris Bioscience (Ellisville, MO). The adenovirus Bcl-xL expression vector, the GFP-nucleolin expression vector pEGF-C1 and Renilla–Bcl-xL construct were gifts of Yuichiro J. Suzuki (Georgetown University, Washington, DC), Michael Kastan (St Jude Children's Research Hospital, Memphis, TN) (Takagi et al., 2005) and George Tim Bowden (University of Arizona, Tucson, AZ) (Zhang et al., 2008), respectively. Immortalized bovine PEpCs were a gift of David McClenahan (University of Northern Iowa, Cedar Falls, IA) (McClenahan et al., 2008).
Cell culture
Bovine PAECs were from American Type Culture Collection (Manassas, VA). Passage 2-8 cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Gemini Bioproducts, Woodland, CA), 1% penicillin/streptomycin and 0.5% fungizone (Invitrogen). Immortalized bovine PEpCs were cultured in Dulbecco's modified Eagle's medium (Invitrogen), 10% FBS (Gemini), 1% glutamine, 0.005% epithelial growth factor, 0.01% insulin, 1% penicillin/streptomycin and 0.5% fungizone (Invitrogen). Cells were grown in 5% CO2 at 37°C in a humidified atmosphere culture incubator. PAECs were grown to 80% confluence and placed in 0.1% FBS/RPMI/1% penicillin/streptomycin and 0.5% fungizone overnight before treatment with agents.
Cell lysate
Cells were washed with ice-cold PBS and lysed in 50 mM HEPES, pH 7.4; 1% (v/v) Triton X-100; 4 mM EDTA; 1 mM sodium fluoride; 0.1 mM sodium orthovanadate; 1 mM tetrasodium pyrophosphate; 2 mM phenylmethylsulfonyl fluoride; 10 μg/ml leupeptin; and 10 μg/ml aprotinin. Lysates were incubated for 15 minutes on ice, vortexed and insoluble materials were removed by centrifugation (14,000 g, 10 minutes, 4°C). For immunoprecipitation of Bax protein, cells were washed with cold PBS and lysed in CHAPS buffer [50 mM Tris-HCl; 1 mM EGTA; 1% (w/v) CHAPS; 10% glycerol; 50 mM sodium fluoride; 1 mM sodium orthovanadate; 2 mM phenylmethylsulfonyl fluoride; 10 μg/ml leupeptin; and 10 μg/ml aprotinin]. Equal concentrations of protein from cell lysates were incubated with primary antibody (1:1000 dilution). GammaBind Plus beads (1:100 dilution; Amersham Biosciences, Piscataway, NJ) were added and samples were rotated at 4°C overnight. The beads were centrifuged at 10,000 g for 10 minutes at 4°C then washed twice with lysis buffer. To elute, beads were resuspended in 25 μl Laemmli buffer and incubated for 5 minutes at 95°C.
Phosphatase assay
SHP-2 immunoprecipitates on GammaBind beads were washed three times with cold lysis buffer (without Na3VO4) and three times with phosphatase buffer (50 mM HEPES, pH 7.4; 5 μg/ml aprotinin; and 1 μg/ml leupeptin). Phosphatase activity was assayed by 50 μl of reaction buffer [phosphatase buffer, pH 5.5; 1 mg/ml bovine serum albumin (BSA); 5 mM EDTA; and 10 mM dithiothreitol). 50 μl of paranitrophenyl phosphate (10 nM final concentration) was added at 37°C for 30 minutes. The reaction was stopped by the addition of 17 μl of 5 N NaOH and the absorbance was measured at 405 nm.
Western blots
Whole cell lysates (10 μg of total protein) were subjected to SDS polyacrylamide gel electrophoresis and electroblotted onto PVDF membrane (0.2-μm pore size). Membranes were blocked with 5% BSA in Tween-20/Tris-buffered saline (TBS) (TTBS; Tris-buffered saline, 0.1% Tween-20) for 1 hour at ambient temperature. Membranes were then incubated overnight at 4°C with primary antibody (1:1000 dilution) in TTBS containing 0.5% BSA. Membranes were washed three times with TTBS for 10 minutes, then incubated with horseradish peroxidase-labeled secondary antibody (1:1000 in TTBS) for 1 hour at ambient temperature. For protein detection, membranes were washed for 3 hours with TBS, incubated in ECL (Amersham) and analyzed on a FujiFilm Image Reader LAS-1000Pro (FujiFilm USA, Valhalla, NY).
Mitochondria and mitochondria-free cytosolic protein extraction
Mitochondria extraction was performed using the Mitochondria Isolation Kit for Cultured Cells according to the manufacturer's protocol (Pierce, Rockford, IL).
Neutral comet assay
The neutral comet assay was used to measure double-stranded DNA breaks as an indication of apoptosis (Kitta et al., 2001). After treatment, cells were embedded in 1% low-melting agarose (Sigma, St Louis, MO) and placed on comet slides (Trevigen, Gaithersburg, MD). Slides were placed in lysis solution (2.5 M NaCl, 1% Na-lauryl sarcosinate, 100 mM EDTA, 10 mM Tris base, 0.01% Triton X-100) for 30 minutes, then washed in 1×TBE buffer (0.089 M Tris; 0.089 M Boric acid; and 0.003 M EDTA, pH 8.0). Nuclei were electrophoresed for 10 minutes at 18 V in 1×TBE. Cells were then fixed with 75% ethanol for 10 minutes, air-dried overnight, stained with 1×Sybr Green (Molecular Probes, Eugene, OR) or propidium iodide (Sigma-Aldrich, St Louis, MO), and visualized with an Olympus FV500 confocal laser scanning microscope (Olympus Imaging America, Center Valley, PA) using 20× magnification at 478-nm excitation, 507-nm emission wavelengths for EGFP and Sybr Green and at 535-nm excitation, 617-nm emission wavelengths for propidium iodide. Cells were randomly selected per treatment group and assigned into type A, B or C comet categories, based on their tail moments. Type C comets were defined as apoptotic cells (Krown et al., 1996).
DNA laddering assay
Cells were harvested in medium and pelleted at 1000 g. Pellets were resuspended and incubated on ice in lysis buffer (10 mM Tris, 1 mM EDTA, 0.2% Triton at pH 8.0) for 15 minutes. Resuspended pellet was centrifuged (14,000 g, 10 minutes at 4°C) and supernatant containing the fragmented DNA was collected. RNase A (final concentration of 60 mg/ml) was added and incubated for 30 minutes at 37°C. SDS was added to a final concentration of 0.5% along with 150 μg/ml of proteinase K and incubated for 2 hours at 50°C. 0.1 volume of 5 M NaCl and 1 volume of ice-cold isopropanol was added and samples were incubated on ice for 10 minutes. The samples were centrifuged at 13,000 g for 15 minutes at 4°C. The DNA pellet was briefly dried and dissolved in 20 μl of TE buffer, followed by electrophoresis (~2 hours, 20 V) in 1.5% agarose.
Caspase assays
Caspase-8 and caspase-9 activities were measured using the Caspase-Glo Assay according to the Cell-Based Assay protocol (Promega, Madison, WI). PAECs were grown in a 96-well white-walled cell-culture plate (30,000-50,000 cells/well). Following treatments, 0.2 ml of Caspase-Glo Assay Reagent, including the MG-132 inhibitor, was added to each well containing 0.2 ml culture media, and incubated for 2 hours at room temperature on a rotating shaker. Luminescent signals were collected using a Dynex MLX Microtiter Plate Luminometer (Dynex Technologies, Chantilly, VA).
RNA isolation and reverse transcription
Total RNA was obtained from bovine PAEC using the RNeasy kit (Qiagen, Valencia, CA). Genomic DNA was removed using the RNase-Free DNAse Set (Qiagen, Valencia, CA). RNA concentrations were determined spectroscopically at 260 nm (ND-1000 Spectrophotometer, NanoDrop, Wilmington, DE). RNA (1.0 μg) was subjected to reverse transcription with GeneAmp RNA PCR kit according to the manufacturer's protocol (Applied Biosystems, Foster City, CA).
Semi-quantitative RT-PCR
1 μl of cDNA from the RT reaction was used for PCR reaction containing 0.4 μM each forward and reverse primer, 200 μM each dNTP, 1 U of iTaq DNA polymerase, and 1× PCR buffer (Bio-Rad Laboratories, Hercules, CA). PCR reactions were optimized for annealing temperatures using a temperature gradient in a Bio-Rad iCycler. Reactions were carried out for 25 cycles using the following conditions: 95°C 1 minute; 60°C 45 seconds; 72°C 1.5 minutes. The last cycle extension was for 10 minutes at 65°C. PCR reactions were analyzed on a 1.5% agarose gel in Tris EDTA buffer and bands were visualized using ethidium bromide. All primer sequences are available upon request.
Quantitative real-time RT-PCR (qPCR)
0.2 μl of cDNA from the RT reaction was used in 20 μl qPCR with the Bcl-xL primer(s) (sequences available upon request). qPCR was performed as recommended by the manufacturer (Applied Biosystems) in triplicate using 6 μM of each primer and 10 μl of Sybr Green PCR master mix (Applied Biosystems, Foster City, CA). PCR was run under the following conditions: activation of AmpliTaq Gold polymerase for 10 minutes at 95°C followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Absence of non-specific amplification was confirmed by 2% agarose gel electrophoresis. As an internal control of mRNA level, α-tubulin was used. The comparative threshold cycle (Ct) method was used to assess relative changes in mRNA levels. Data were collected from four to six experiments.
Cloning of EGFP-tagged histone H2B
Total mRNA was prepared from cultured primary human bronchial epithelial cells (Cell Applications) and used for RT as described above. 2 μl cDNA was subjected to 50 μl PCR using the GC-Rich System (Roche Applied Science, Indianapolis, IN), according to the manufacturer's instructions. PCR cycles were: 1×94°C, 3 minutes; 5×94°C 30 seconds, 55°C 30 seconds, 72°C 2 minutes; 22×95°C 30 seconds, 55°C 30 seconds, 72°C 1 minute; and 1×72°C 7 minutes. PCR product was analyzed by 1.5% agarose gel electrophoresis, purified from the gel, and cloned into the BamHI and KpnI restriction sites of the pEGFP-N1 vector (BD Biosciences, San Jose, CA). Primer sequences available upon request.
Determination of mRNA half-life
Bovine PAECs were treated as indicated, then incubated with 5 μg/ml actinomycin D for time courses between 30 minutes and 8 hours. Total RNA was isolated and the level of Bcl-xL mRNA was monitored by qPCR or by semi-quantitative RT-PCR. The primer sequences for human histone-H2B (GenBank: AF531286.1) are available upon request. The level of GAPDH mRNA was determined by gel electrophoresis and used for normalization of the semi-quantitative RT-PCR. ImageJ software was used for densitometry (http://www.uhnresearch.ca/facilities/wcif/index.htm).
Plasmid transfection
1 day prior to transfection, cells were plated at 1.4×105 cells per well in a 12-well plate. 1 μg of DNA per well was transfected using the FuGENE 6 Transfection Reagent (Roche Applied Science), according to the manufacturer's instructions, in serum-free, antibiotic-free medium. The H2B-GFP reporter was co-transfected with the DN or mutant SHP-2 plasmids (or empty-vector control), at a ratio of 1:2 (total DNA concentration 1 μg). Cells were transfected for 6 hours and then medium was replaced with 0.01% FBS medium with antibiotics. For luciferase assays, transfection mixtures contained a ratio of Renilla–Bcl-xL reporter construct to luciferase control vector (RSV-Luc) of 6:1 to normalize transfection efficiency.
Dual luciferase assay
Transfected cells were washed twice with cold PBS, lysed with passive lysis buffer, and assayed for firefly and Renilla luciferase activities using the Dual Luciferase Assay (Promega) according to the manufacturer's instructions in a Turner TD-20/20 luminometer (Turner Designs, Sunnyvale, CA).
RNA-IP
PAECs were subjected to RNA-IP as previously described (Sun et al., 2006). The PCR primer sequences are available upon request.
Statistical analysis
Means ± standard deviations (s.d.) were calculated and statistically significant differences between two groups were determined by the Student's t-test. For three or more groups, statistical analysis was performed using one-way ANOVA, followed by the Bonferroni post-analysis, as appropriate; P<0.05 was considered statistically significant. For mRNA half-life, linear regression was calculated and confidence intervals determined. Statistical software for all analysis was SigmaStat 3.1 (Point Richmond, CA).
Supplementary Material
Acknowledgments
We thank Autumn J. Griffin for technical help and Bethanie L. Morrison for constructive comments on the manuscript. This work was supported by NIH HL-073929 and a USUHS research grant to R.M.D., and by an American Heart Association predoctoral fellowship to Y.H.L. Some of the authors are employees of the US Government. This work was prepared as part of their official duties. See Title 17 U.S.C. §105. The views in this article are those of the authors and do not necessarily reflect the views, official policy, or position of the Uniformed Services University of the Health Sciences, Department of the Navy, Department of Defense or the U.S. Federal Government. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/10/1634/DC1
References
- Alvarez S. E., Seguin L. R., Villarreal R. S., Nahmias C., Ciuffo G. M. (2008). Involvement of c-Src tyrosine kinase in SHP-1 phosphatase activation by Ang II AT2 receptors in rat fetal tissues. J. Cell Biochem. 105, 703-711 [DOI] [PubMed] [Google Scholar]
- Bachelor M. A., Bowden G. T. (2004). Ultraviolet A-induced modulation of Bcl-XL by p38 MAPK in human keratinocytes: post-transcriptional regulation through the 3′-untranslated region. J. Biol. Chem. 279, 42658-42668 [DOI] [PubMed] [Google Scholar]
- Bader M. (2002). Role of the local renin-angiotensin system in cardiac damage: a minireview focussing on transgenic animal models. J. Mol Cell. Cardiol. 34, 1455-1462 [DOI] [PubMed] [Google Scholar]
- Bataller R., Sancho-Bru P., Gines P., Brenner D. A. (2005). Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid. Redox Signal. 7, 1346-1355 [DOI] [PubMed] [Google Scholar]
- Bedecs K., Elbaz N., Sutren M., Masson M., Susini C., Strosberg A. D., Nahmias C. (1997). Angiotensin II type 2 receptors mediate inhibition of mitogen-activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem. J. 325, 449-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. M., Hausdorff S. F., O'Reilly A. M., Freeman R. M., Neel B. G. (1996). Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol. Cell. Biol. 16, 1189-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold S., Volarevic S., Moriggl R., Mercep M., Groner B. (1998). Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol. Endocrinol. 12, 556-567 [DOI] [PubMed] [Google Scholar]
- Boise L. H., Gonzalez-Garcia M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nunez G., Thompson C. B. (1993). bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597-608 [DOI] [PubMed] [Google Scholar]
- Brechler V., Reichlin S., De Gasparo M., Bottari S. P. (1994). Angiotensin II stimulates protein tyrosine phosphatase activity through a G-protein independent mechanism. Recept. Channels 2, 89-98 [PubMed] [Google Scholar]
- Chong Z. Z., Maiese K. (2007). The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol. Histopathol. 22, 1251-1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui T., Nakagami H., Iwai M., Takeda Y., Shiuchi T., Daviet L., Nahmias C., Horiuchi M. (2001). Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc. Res. 49, 863-871 [DOI] [PubMed] [Google Scholar]
- D'Amore A., Black M. J., Thomas W. G. (2005). The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension 46, 1347-1354 [DOI] [PubMed] [Google Scholar]
- Dendorfer A., Dominiak P., Schunkert H. (2005). ACE inhibitors and angiotensin II receptor antagonists. Handb. Exp. Pharmacol. 170, 407-42 [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Rippmann V., Weiland U., Haendeler J., Zeiher A. M. (1997). Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ. Res. 81, 970-976 [DOI] [PubMed] [Google Scholar]
- Farkas L., Farkas D., Ask K., Moller A., Gauldie J., Margetts P., Inman M., Kolb M. (2009). VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Invest. 119, 1298-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. H., Sun Y., Douglas J. G. (2002). Gbeta gamma-independent constitutive association of Galpha s with SHP-1 and angiotensin II receptor AT2 is essential in AT2-mediated ITIM-independent activation of SHP-1. Proc. Natl. Acad. Sci. USA 99, 12049-12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. (2004). Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastroenterol. Hepatol. 1, 98-105 [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Hayashida W., Kambe T., Yamada T., Dzau V. J. (1997). Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J. Biol. Chem. 272, 19022-19026 [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Akishita M., Dzau V. J. (1998). Molecular and cellular mechanism of angiotensin II-mediated apoptosis. Endocr. Res. 24, 307-314 [DOI] [PubMed] [Google Scholar]
- Kito S., Shimizu K., Okamura H., Yoshida K., Morimoto H., Fujita M., Morimoto Y., Ohba T., Haneji T. (2003). Cleavage of nucleolin and argyrophilic nucleolar organizer region associated proteins in apoptosis-induced cells. Biochem. Biophys. Res. Commun. 300, 950-956 [DOI] [PubMed] [Google Scholar]
- Kitta K., Day R. M., Ikeda T., Suzuki Y. J. (2001). Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic. Biol. Med. 31, 902-910 [DOI] [PubMed] [Google Scholar]
- Konigshoff M., Wilhelm A., Jahn A., Sedding D., Amarie O. V., Eul B., Seeger W., Fink L., Gunther A., Eickelberg O., et al. (2007). The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. Am. J. Respir. Cell Mol. Biol. 37, 640-650 [DOI] [PubMed] [Google Scholar]
- Kren B. T., Trembley J. H., Krajewski S., Behrens T. W., Reed J. C., Steer C. J. (1996). Modulation of apoptosis-associated genes bcl-2, bcl-x, and bax during rat liver regeneration. Cell Growth Differ. 7, 1633-1642 [PubMed] [Google Scholar]
- Krown K. A., Page M. T., Nguyen C., Zechner D., Gutierrez V., Comstock K. L., Glembotski C. G., Quintana P. J. E., Sabbadini R. A. (1996). Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes: involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Invest. 98, 2854-2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Chu J. Y., Yu J. K., Huang X. Q., Liu X. J., Shi L., Che Y. C., Xie J. Y. (2004). Regulation of alternative splicing of Bcl-x by IL-6, GM-CSF and TPA. Cell Res. 14, 473-479 [DOI] [PubMed] [Google Scholar]
- Li D., Yang B., Philips M. I., Mehta J. L. (1999). Proapoptotic effects of ANG II in human coronary artery endothelial cells: role of AT1 receptor and PKC activation. Am. J. Physiol. 276, H786-H792 [DOI] [PubMed] [Google Scholar]
- Li J. M., Mogi M., Tsukuda K., Tomochika H., Iwanami J., Min L. J., Nahmias C., Iwai M., Horiuchi M. (2007). Angiotensin II-induced neural differentiation via angiotensin II type 2 (AT2) receptor-MMS2 cascade involving interaction between AT2 receptor-interacting protein and Src homology 2 domain-containing protein-tyrosine phosphatase 1. Mol. Endocrinol. 21, 499-511 [DOI] [PubMed] [Google Scholar]
- Marrero M. B., Venema V. J., Ju H., Eaton D. C., Venema R. C. (1998). Regulation of angiotensin II-induced JAK2 tyrosine phosphorylation: roles of SHP-1 and SHP-2. Am. J. Physiol. 275, C1216-C1223 [DOI] [PubMed] [Google Scholar]
- Marshall R. P. (2003). The pulmonary renin-angiotensin system. Curr. Pharm. Des. 9, 715-722 [DOI] [PubMed] [Google Scholar]
- Matsubara H., Shibasaki Y., Okigaki M., Mori Y., Masaki H., Kosaki A., Tsutsumi Y., Uchiyama Y., Fujiyama S., Nose A., et al. (2001). Effect of angiotensin II type 2 receptor on tyrosine kinase Pyk2 and c-Jun NH2-terminal kinase via SHP-1 tyrosine phosphatase activity: evidence from vascular-targeted transgenic mice of AT2 receptor. Biochem. Biophys. Res. Commun. 282, 1085-1091 [DOI] [PubMed] [Google Scholar]
- McClenahan D., Hellenbrand K., Atapattu D., Aulik N., Carlton D., Kapur A., Czuprynski C. (2008). Effects of lipopolysaccharide and Mannheimia haemolytica leukotoxin on bovine lung microvascular endothelial cells and alveolar epithelial cells. Clin. Vaccine Immunol. 15, 338-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Iwai M., Horiuchi M. (2007). Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler. Thromb. Vasc. Biol. 27, 2532-2539 [DOI] [PubMed] [Google Scholar]
- Noguchi T., Matozaki T., Horita K., Fujioka Y., Kasuga M. (1994). Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 14, 6674-6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouet S., Nahmias C. (2000). Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol. Metab. 11, 1-6 [DOI] [PubMed] [Google Scholar]
- Otake Y., Soundararajan S., Sengupta T. K., Kio E. A., Smith J. C., Pineda-Roman M., Stuart R. K., Spicer E. K., Fernandes D. J. (2007). Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood 109, 3069-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M., Li X., Zhuang J., Wang R., Uhal B. D. (2002). Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J. Physiol. Lung Cell Mol. Physiol. 282, L713-L718 [DOI] [PubMed] [Google Scholar]
- Pazdrak K., Adachi T., Alam R. (1997). Src homology 2 protein tyrosine phosphatase (SHPTP2)/Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival. J. Exp. Med. 186, 561-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossig L., Hermann C., Haendeler J., Assmus B., Zeiher A. M., Dimmeler S. (2002). Angiotensin II-induced upregulation of MAP kinase phosphatase-3 mRNA levels mediates endothelial cell apoptosis. Basic Res. Cardiol. 97, 1-8 [DOI] [PubMed] [Google Scholar]
- Sankarshanan M., Ma Z., Iype T., Lorenz U. (2007). Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 1. J. Immunol. 179, 483-490 [DOI] [PubMed] [Google Scholar]
- Schwab M. S., Dreyer C. (1997). Protein phosphorylation sites regulate the function of the bipartite NLS of nucleolin. Eur J. Cell Biol. 73, 287-297 [PubMed] [Google Scholar]
- Sengupta T. K., Bandyopadhyay S., Fernandes D. J., Spicer E. K. (2004). Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J. Biol. Chem. 279, 10855-10863 [DOI] [PubMed] [Google Scholar]
- Srivastava M., Pollard H. B. (1999). Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J. 13, 1911-1922 [PubMed] [Google Scholar]
- Strieter R. M. (2008). What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc. Am. Thorac. Soc. 5, 305-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B. K., Deaton A. M., Lee J. T. (2006). A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol. Cell 21, 617-628 [DOI] [PubMed] [Google Scholar]
- Sun Y. (2009). Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J. Mol. Cell Cardiol. 48, 483-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Zhang J., Zhang J. Q., Ramires F. J. (2000). Local angiotensin II and transforming growth factor-beta1 in renal fibrosis of rats. Hypertension 35, 1078-1084 [DOI] [PubMed] [Google Scholar]
- Suzuki Y. J., Nagase H., Wong C. M., Kumar S. V., Jain V., Park A. M., Day R. M. (2007). Regulation of Bcl-xL expression in lung vascular smooth muscle. Am. J. Respir. Cell Mol. Biol. 36, 678-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Absalon M. J., McLure K. G., Kastan M. B. (2005). Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 123, 49-63 [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349-363 [DOI] [PubMed] [Google Scholar]
- Ugrinova I., Monier K., Ivaldi C., Thiry M., Storck S., Mongelard F., Bouvet P. (2007). Inactivation of nucleolin leads to nucleolar disruption, cell cycle arrest and defects in centrosome duplication. BMC Mol. Biol. 8, 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhal B. D. (2002). Apoptosis in lung fibrosis and repair. Chest 122, 293S-298S [DOI] [PubMed] [Google Scholar]
- Valencia A. M., Oliva J. L., Bodega G., Chiloeches A., Lopez-Ruiz P., Prieto J. C., Susini C., Colas B. (1997). Identification of a protein-tyrosine phosphatase (SHP1) different from that associated with acid phosphatase in rat prostate. FEBS Lett. 406, 42-48 [DOI] [PubMed] [Google Scholar]
- Vogel W., Lammers R., Huang J., Ullrich A. (1993). Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science 259, 1611-1614 [DOI] [PubMed] [Google Scholar]
- Wang R., Ramos C., Joshi I., Zagariya A., Pardo A., Selman M., Uhal B. D. (1999). Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J. Physiol. 277, L1158-L1164 [DOI] [PubMed] [Google Scholar]
- Wang R., Ibarra-Sunga O., Verlinski L., Pick R., Uhal B. D. (2000). Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L143-L151 [DOI] [PubMed] [Google Scholar]
- Wolf G. (2008). Novel aspects of the renin-angiotensin-aldosterone-system. Front Biosci. 13, 4993-5005 [DOI] [PubMed] [Google Scholar]
- Wu L., Iwai M., Li Z., Shiuchi T., Min L. J., Cui T. X., Li J. M., Okumura M., Nahmias C., Horiuchi M. (2004). Regulation of inhibitory protein-kappaB and monocyte chemoattractant protein-1 by angiotensin II type 2 receptor-activated Src homology protein tyrosine phosphatase-1 in fetal vascular smooth muscle cells. Mol. Endocrinol. 18, 666-678 [DOI] [PubMed] [Google Scholar]
- Wynn T. A. (2008). Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Horiuchi M., Dzau V. J. (1996). Angiotensin II type 2 receptor mediates programmed cell death. Proc. Natl. Acad. Sci. USA 93, 156-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liang X., Niu T., Meng W., Zhao Z., Zhou G. W. (1998). Crystal structure of the catalytic domain of protein-tyrosine phosphatase SHP-1. J. Biol. Chem. 273, 28199-28207 [DOI] [PubMed] [Google Scholar]
- Yi T. L., Cleveland J. L., Ihle J. N. (1992). Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol. Cell. Biol. 12, 836-846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S. S., Crew A. J., Gee J. M., Hui R., Blamey R. W., Robertson J. F., Nicholson R. I., Sutherland R. L., Daly R. J. (2000). Up-regulation of the protein tyrosine phosphatase SHP-1 in human breast cancer and correlation with GRB2 expression. Int. J. Cancer 88, 363-368 [PubMed] [Google Scholar]
- Yoshida K., Kuwano K., Hagimoto N., Watanabe K., Matsuba T., Fujita M., Inoshima I., Hara N. (2002). MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J. Pathol. 198, 388-396 [DOI] [PubMed] [Google Scholar]
- Zhang H., Lawson W. E., Polosukhin V. V., Pozzi A., Blackwell T. S., Litingtung Y., Chiang C. (2007). Inhibitor of differentiation 1 promotes endothelial survival in a bleomycin model of lung injury in mice. Am. J. Pathol. 171, 1113-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tsaprailis G., Bowden G. T. (2008). Nucleolin stabilizes Bcl-X L messenger RNA in response to UVA irradiation. Cancer Res. 68, 1046-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yu J., Park B. H., Kinzler K. W., Vogelstein B. (2000). Role of BAX in the apoptotic response to anticancer agents. Science 290, 989-992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.