Abstract
Previous work demonstrated that rats subjected to multiple withdrawals from chronic ethanol exhibit a sensitization of anxiety-like behavior compared to animals withdrawn from treatment with an equal but continuous amount of ethanol. This study sought to examine whether corticotropin-releasing factor (CRF) could modulate this ethanol-withdrawal-induced anxiety-like behavior. Initially, rats were administered with CRF (1 μg) or vehicle intraventricularly on two occasions 5 days apart while on control diet (CD) followed by exposure to 7% ethanol diet (ED) for 5 days, with social interaction assessed 5 h into withdrawal. Social interaction was significantly reduced in the CRF-treated animals compared to vehicle-treated rats and vehicle-and CRF-treated rats maintained on CD, indicative that CRF given before ethanol exposure was capable of inducing an adaptive change that sensitized withdrawal-induced anxiety-like behavior. Next, the CRF1 receptor antagonist CRA1000 (3 mg/kg, systemically), the CRF2 receptor antagonist antisauvagine-30 (20 μg intraventricularly), or vehicle was injected 4 h after the ethanol was removed following the first and second cycles of chronic ethanol exposure and the effect on the multiple-withdrawal-induced anxiety-like behavior determined after the third withdrawal cycle. The CRF1 receptor antagonist blocked the reduced social interaction behavior, whereas the CRF2 receptor antagonist was without effect. Similar pretreatment with another CRF1 receptor antagonist CP-154,526 (10 mg/kg systemically) during the first and second withdrawals also counteracted anxiety-like behavior. These findings indicate that the CRF system and CRF1 receptors play key roles in the adaptive change responsible for the anxiety-like behavior induced by repeated withdrawals from chronic ethanol.
Keywords: Repeated ethanol withdrawal; CRF; CRA1000; Anxiety; Social interaction test; CP-154,526; CRF1 receptors
1. Introduction
Repeated ethanol exposures and withdrawals induce long-lasting adaptive changes in the brain that are reflected by behavioral consequences (e.g., Holter et al., 1998; Malcolm et al., 2000; McCown and Breese, 1990). In this respect, a recent investigation showed that anxiety-like behavior, as indexed by the social interaction test, increased in rats repeatedly withdrawn from exposure to ethanol (Overstreet et al., 2002). Rats exposed continuously for 15 days to a diet containing 4.5% ethanol exhibited a normal level of social interaction upon withdrawal, indicative that the ethanol alone was not responsible for the sensitization of the anxiety-like behavior associated with the repeated withdrawals (Overstreet et al., 2002).
Several investigators have reported alterations in the hypothalamo–pituitary–adrenal (HPA) axis after chronic ethanol treatment (e.g., Rasmussen et al., 2000; Rivier and Lee, 2001). Antagonists of corticotropin-releasing factor (CRF) have been reported to reduce anxiety-like behavior observed in ethanol-withdrawn rats (Koob et al., 1998; Rassnick et al., 1993) and attenuate foot shock-induced reinstatement of ethanol-seeking behavior (Le et al., 2000). While CRF, by driving the HPA axis, could be a key factor in the adaptive changes associated with chronic ethanol, a recent study demonstrated that adrenalectomy does not modulate foot shock-induced reinstatement of ethanol-seeking behavior (Le et al., 2000). Based upon this background, it is hypothesized that CRF contributes to the sensitized anxiety-like behavior observed in rats repeatedly withdrawn from chronic ethanol diet (ED).
To examine the role of CRF in the multiple-withdrawal-induced sensitization, it was tested whether central administration of CRF would substitute for the initial two withdrawals at 6 and 11 days of the multiple withdrawal protocol to induce anxiety-like behavior. Subsequently, it was determined if selective antagonists for CRF1 and CRF2 receptors would prevent the anxiety-like behavior seen with repeated withdrawals. These studies will support the proposed hypothesis that CRF acting on CRF1 receptors contributes to the anxiety-like behavior observed during repeated ethanol withdrawals.
2. Methods
2.1. Animals
Male Sprague–Dawley rats (Charles-River, Raleigh) were purchased at 40 days of age (160–180 g). After giving 5 days to adapt to local conditions (22°C, 50% humidity, 12:12-h light–dark cycle with lights on between 0900 and 2100 h), they were placed on a nutritionally complete diet used previously in our laboratory (e.g., Frye et al., 1983; Moy et al., 2000; Overstreet et al., 2002). Intakes of the liquid diet were recorded daily, and body weights were measured weekly. These experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and were approved by the UNC Institutional Animal Care and Use Committee.
2.2. Liquid diet
Briefly, the diet was a lactalbumin/dextrose-based, nutritionally complete diet (with concentrations of vitamins, minerals, and other nutrients derived from ICN Research Diets). Dextrose calories in the control diet (CD) were equated with ethanol calories in the ED (7% w/v).
A modified pair-feeding design was used in all of the diet studies. The rats maintained on the CD were given a volume of diet equivalent to the average volume consumed the previous day by the rats maintained on ED. The rats were weighed at weekly intervals, and the volumes of diet were adjusted to insure that the groups had similar body weights. In general, behavioral assessments were conducted after 15 days of exposure to the ED, between 5 and 6 h after the removal of the ethanol. This time point was selected on the basis of previous observations of anxiety-like behavior in our laboratory (e.g., Knapp et al., 1998; Moy et al., 1997, 2000).
2.3. Social interaction test
The social interaction test was first introduced by File and Hyde (1978). This test involves placing a pair of animals in an arena and measuring the amount of time engaged in such behaviors as grooming, sniffing, crawling over or under, and boxing; locomotor activity is simultaneously recorded and provides a measure that is independent of social interaction (File, 1980). Social interaction has been repeatedly validated as an index of anxiety-related behavior because it is decreased following anxiety-provoking stimuli, such as bright lights or exposure to cat odor (File, 1980; File and Hyde, 1978), after administration of anxiogenic drugs (e.g., Battacharya et al., 1997; File and Lister, 1984; Guy and Gardner, 1985; Sams-Dodd, 1995) or following withdrawal from drugs of abuse, including ethanol (Andrews et al., 1997; Costall et al., 1990; File et al., 1989; Irvine et al., 2001; Kampov-Polevoy et al., 2000; Overstreet et al., 2002). Conversely, social interaction can be increased by prior exposure to the test arena (File, 1980; File and Hyde, 1978) or the administration of anxiolytic drugs at doses that have little effect on locomotor activity (Barnes et al., 1990; File, 1980; Lightowler et al., 1994).
A modification of the standard social interaction test was used to reduce the number of animals needed for experiments. According to File (1980), the most sensitive procedure is to match up pairs of rats that have the same treatment on the basis of their body weights and then treat the total number of interactions by the pair as the unit of measure. However, for other experiments where the index rat may have an implanted cannula (Gonzalez et al., 1998; Irvine et al., 2001), an untreated dummy partner is used, and only the interactions of the index rat are recorded. In the present studies, pairs of rats with the same treatment were placed in the arena and the social interactions initiated by each member of the pair were recorded, thereby requiring fewer rats. However, the 16 cannulated rats were paired with a control, untreated partner. This design permitted a comparison of the two methods as well as provided information on whether the anxiety-like behavior of one rat influences that of its partner. Statistical analyses of several data sets revealed that using the data for individual rats provided the same statistical outcome as treating the scores of the pair as a unit (Breese et al., 2003; Overstreet et al., 2003). Furthermore, in a study of 25 pairs of rats maintained on CD and 25 on ED, the rats exhibited essentially independent behavior, as there was no significant correlation between the scores of the rat pairs in either group (.03 for CD, −.13 for ED). In other words, the time spent in social interaction of one member of the pair could be quite high (>30 s), and that of the other member quite low (< 15 s).
Experienced observers who were blind to the experimental condition carried out the social interaction test in a square open field (60 × 60 cm, with 16 squares marked out on the floor). The rats were unfamiliar with the open field and the lighting conditions were low (30 lx) to generate an intermediate level of anxiety-related behavior. Rat pairs were matched on the basis of ethanol intakes, body weights, and treatment conditions and were placed simultaneously in the open field. During the 5-min session, line crosses (by two forepaws) and time spent in social interaction (grooming, sniffing, following, and crawling over/under) were scored individually for each rat (Kampov-Polevoy et al., 2000; Overstreet et al., 2002).
2.4. Intraventricular administration of CRF
After several days on CD, 30 rats were anesthetized with pentobarbital sodium, and surgery was performed to implant guide cannulae aimed at the lateral ventricles. The rats were allowed to recover for 1 week and then CRF (Sigma, St. Louis, MO; 1 μg; 16 rats) or artificial cerebral spinal fluid (14 rats) was given using a 32-gauge needle. The rats were placed in the social interaction arena 30 min after the first injection for 5 min to observe social interaction behavior and line crosses. In about half of the rats, the pairs had the same treatment. In the other half, an untreated control rat was paired up with a cannulated rat. The treatments were repeated 5 days later, and the following day, 14 rats (8 treated with CRF, 6 treated with vehicle) were exposed for 5 days to a diet containing 7% ethanol. ED was then removed and replaced with CD, and the rats were placed by pairs in the social interaction arena 5 h later. The other subgroup of rats, were placed in the social interaction arena at about the same time as the rats maintained on ED (1300–1500 h), but they were paired again with an untreated control rat.
2.5. Systemic administration of CRA1000
Preliminary studies showed that 1 mg/kg CRA1000 (a gift from Taisho, Saitama, Japan), a CRF1 receptor antagonist (Okuyama et al., 1999), would counteract the reduction in social interaction behavior induced by withdrawal from ethanol when given 30 min before the test (Knapp et al., in press). The present experiment sought to compare the effects of acute treatment with CRA1000 versus treatment given during the first and second withdrawals in a three-cycle, repeated withdrawal protocol. Rats were maintained on CD or ED. Rats on ED were exposed to 7% ethanol for a total of 15 days, in three cycles of 5 days, with two 2-day periods of withdrawal between Cycles 1 and 2, and 2 and 3. Some rats (n = 10) were injected with CRA1000 (3 mg/kg ip) 4 h after the ethanol was removed during the first and second cycles, while others were injected with the carboxymethylcellulose (CMC) vehicle at the same time. A fourth group was also subjected to the three cycles of ethanol access and withdrawal but were injected with CRA1000 (1 mg/kg) only 30 min before the social interaction test or 4.5 h after the ethanol of the third cycle was removed. For all groups that had been maintained on ethanol, CD was given throughout the periods of withdrawal.
In a separate study, the effects of pretreatment with another CRF1 receptor antagonist was examined. Three groups of rats were either exposed to CD (n = 8) or subjected to three cycles of 5-day exposures to ED (7%; n = 16). One of the latter groups was injected with CMC vehicle during the first and second withdrawals, and the other group was injected with 10 mg/kg CP-154,526 (a gift from Pfizer, Groton, CN; Seymour et al., 2003) at comparable times. Social interaction behavior and line crossings were measured approximately 5 h after the ethanol of the third cycle was removed.
2.6. Intraventricular administration of antisauvagine-30
After several days on CD, 18 rats were anesthetized with pentobarbital sodium, and surgery was performed to implant guide cannulae aimed at the lateral ventricles. The rats were allowed to recover for 1 week, and then they were subjected to three cycles of 5-day exposures to 7% ethanol, with 2-day withdrawal periods (when CD was available) after the first and second cycles. Rats received intraventricular injections of antisauvagine-30 (20 μg) or artificial cerebrospinal fluid (5 μl) 4 h into the first and second withdrawals. The dose of antisauvagine-30 was selected on the basis of published reports (Brauns et al., 2001; Radulovic et al., 1999). The rat pairs were placed in the social interaction arena 5 h after the removal of ethanol.
2.7. Statistical analyses
The data for social interaction were summarized as mean seconds and analyzed by one-way ANOVAs (for the CRF, CRA1000, and CP-154,526 data) and t tests (for the anti-sauvagine-30 data). Activity was recorded as the mean number of line crosses, and the data were analyzed by one-way ANOVAs or t tests. When the ANOVAs revealed significant group differences, follow-up Tukey’s protected t tests were carried out to test specific pairs. Superscript letters were used to identify the statistical relationship between groups. Groups with different letters were significantly different according to Tukey’s test (P < .05).
3. Results
3.1. Effect of intraventricular CRF administration on withdrawal-induced anxiety-like behavior
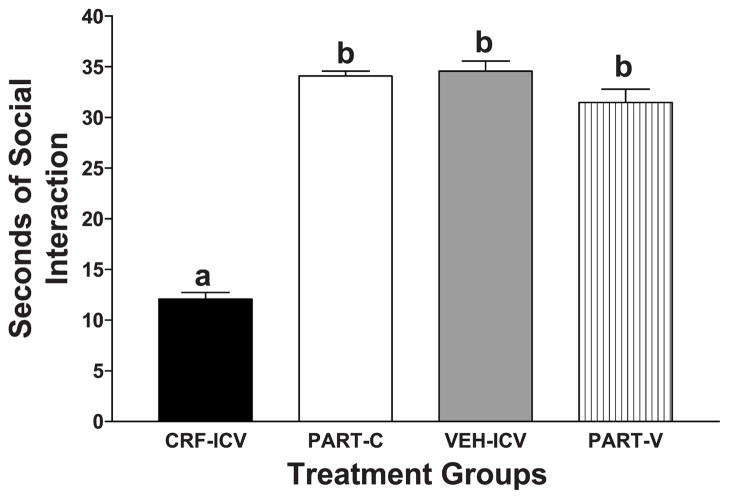
Rats that were initially treated with a single dose of CRF intraventricularly exhibited lower social interaction behavior than the rats given artificial cerebrospinal fluid, confirming the anxiogenic properties of CRF [Fig. 1; F(3,45) = 13.32, P < .001]. Interestingly, the control rats that were used as partners for the CRF-and vehicle-treated rats (PART-C and PART-V, respectively) spent as much time in social interaction as the vehicle-treated rats, although their partners differed greatly in the time they spent in social interaction (Fig. 1).
Fig. 1.
Effects of acute intraventricular injection of CRF or vehicle on social interaction behavior. One week after rats were cannulated into the cerebral ventricles, the rats were infused with 1 μg CRF (n = 16) or artificial cerebrospinal fluid (n = 14). Some pairs of rats with the same treatment were placed in the open field arena 30 min later for the measurement of social interaction behavior. Other rats (8 of each) were placed in the open field with untreated control rats as their partners. CRF-and vehicle-treated rats exhibited similar levels of social interaction behavior in the two conditions, so the data were combined. The data represent the mean seconds ± S.E.M. of time spent in social interaction. The CRF-treated group (CRF–ICV) spent significantly less time in social interaction than either the vehicle-treated group (VEH–ICV) or the two partner groups (PART-C; PART-V), according to Tukey’s protected t tests (P < .01).
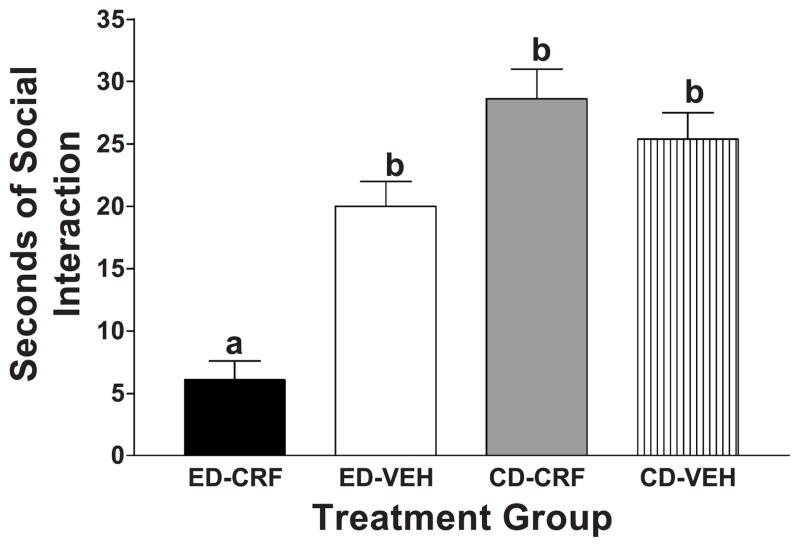
Subsequently, half of the rats were pretreated intraventricularly once more with CRF or vehicle 1 day prior to a single 5-day exposure to a 7% ED, and the other half continued to have access to CD. These multiple-CRF-treated animals exhibited a significant reduction in social interaction during withdrawal from ED compared to vehicle-treated rats [Fig. 2; F(3,26) = 23.94, P < .001]. Also shown in Fig. 2 are data for the CRF-and vehicle-treated rats that were maintained on CD; these animals exhibited normal social interaction behavior. Thus, CRF treatment interacted with ethanol exposure and withdrawal to sensitize the withdrawal-induced anxiety-like behavior. This reduction in social interaction induced by the CRF treatment was comparable to that seen with multiple withdrawals from chronic ethanol (see Fig. 3).
Fig. 2.
Effects of prior treatment with CRF or vehicle on social interaction behavior in rats maintained on CD or withdrawn from a 5-day exposure to 7% ethanol. Artificial cerebrospinal fluid or CRF (1 μg) were infused 1 and 6 days before exposure to 5 consecutive days of 7% ED. The social interaction test was carried out 5 h after withdrawal from ethanol or at the same time in the afternoon 5 days after the last CRF treatment in the rats maintained on CD. The data represent the mean seconds ± S.E.M. of time spent in social interaction. The group pretreated with CRF and subsequently exposed to ethanol (ED – CRF) engaged in significantly less social interaction behavior than the group pretreated with vehicle and exposed to ethanol (ED–VEH) or the groups maintained throughout on CD (CD–CRF; CD–VEH), according to Tukey’s protected t tests (P < .01).
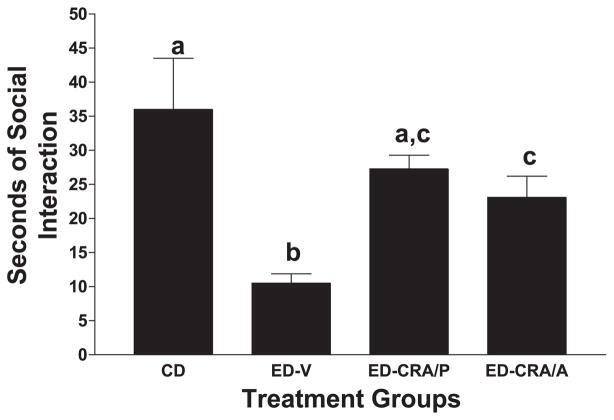
Fig. 3.
Effects of CRA1000 on social interaction behavior of rats subjected to repeated withdrawals from ethanol. Rats were exposed to CD throughout (n = 8) or three cycles of 5 days of an ED (7% w/v). The rats were maintained on CD during the 2 days of withdrawal between the first and second, and the second and third cycles, and between ethanol withdrawal and behavior testing after the third cycle. One group was injected with CMC vehicle at 4 h into the first and second withdrawal (ED –VEH); one was pretreated with 3 mg/kg CRA1000 at the same times (ED–CRA/P); the final group was injected acutely with 1 mg/kg CRA1000 30 min before the social interaction test on the third withdrawal, 4.5 h after the ethanol was removed (ED–CRA/A). The other groups exposed to ED were also tested in the social interaction arena 5 h after removal of ethanol. The data represent the mean seconds ± S.E.M. of time spent in social interaction for eight rats per group. A one-way ANOVA revealed significant group differences (P < .01). Groups with different letters are significantly different according to Tukey’s test (P < .01).
In contrast to the dramatic decrease in social interaction behavior, CRF treatment slightly, but significantly, reduced line crosses after its intraventricular administration (132 ± 8.9 for vehicle vs. 103 ± 11 for CRF; t = 2.21, P=.035). Rats that were treated with vehicle or CRF and maintained on CD did not differ in line crosses (91.8 ± 11.2 vs. 105.8 ± 11.0 for vehicle-and CRF-treated, respectively; t = 0.34, P>.05). There were no differences in line crosses during withdrawal from chronic ethanol exposure (76.5 ± 13.7 vs. 72.6 ± 10.5 for vehicle-and CRF-treated rats, respectively; t = 0.23, P>.05). However, note that withdrawal from ethanol resulted in decreased activity, as reported earlier (Overstreet et al., 2002). Thus, the suppression of locomotor activity as a consequence of ethanol withdrawal does not respond to manipulations of the CRF system, while the reduction in social interaction behavior does.
3.2. Effects of CRF1 receptor antagonists, on withdrawal-induced anxiety associated with multiple withdrawals from chronic ethanol
Following the demonstration that CRF pretreatment sensitized ethanol withdrawal-induced anxiety-like behavior, attention turned to determining if blockade of CRF receptors would minimize the reduction in social interaction observed with repeated withdrawals. In Fig. 3, the effect of the CRF1 receptor antagonist CRA1000 on social interaction behavior induced by repeated withdrawals is illustrated. There were significant differences among the four treatment groups [F(3,29) = 30.89, P < .001], with the group given CD exhibiting significantly more social interaction behavior than the group given the repeated exposures to ED and treated with vehicle (ED–VEH). Treatment with CRA1000 significantly increased social interaction behavior in rats exposed to ED whether given into the third withdrawal or given during the initial two withdrawals and not the final third withdrawal (ED–CRA/A and ED–CRA/P, respectively). The group that received the CRA1000 pretreatment at 4 h into the first and second withdrawals (ED–CRA/P) was not significantly different from the CD group—a particularly important finding. Social interaction behaviors were not affected by acute treatment of CRA1000 (1 mg/kg) to control rats, nor was it altered when two injections of 3 mg/kg CRA1000 was given to control rats 10 and 5 days before exposure to the social interaction arena (data not shown; Knapp et al., in press). Therefore, CRA1000 counteracts the anxiogenic involvement of CRF related to withdrawal from chronic ethanol but does not have a direct anxiolytic effect by itself (see Harro et al., 2001).
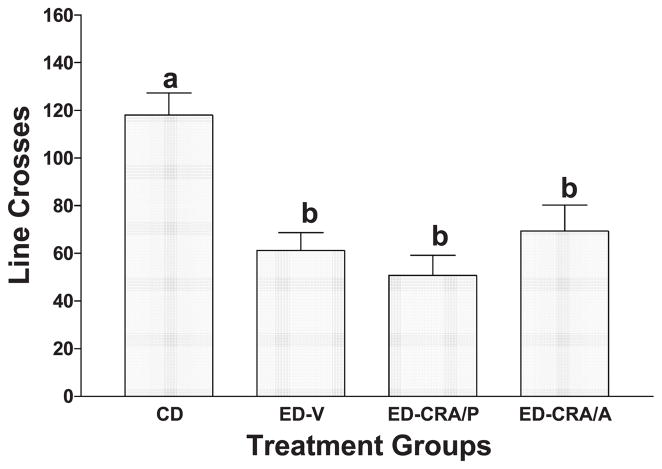
The findings for locomotor activity are summarized in Fig. 4. There were significant differences among the groups [F(3,29) = 7.45, P < .001]. However, the reduced line crosses observed in the ethanol-withdrawn rats were not influenced by the injections of CRA1000, regardless of the mode of treatment (see Fig. 4). All groups that received ED were significantly less active than the rats maintained on CD.
Fig. 4.
Effects of CRA1000 on line crosses of rats subjected to repeated withdrawals from ethanol. See legend of Fig. 3 for description of procedure. The data represent the mean ± S.E.M. line crosses for eight rats per group. A one-way ANOVA revealed significant group differences (P < .001). Groups with different letters are significantly different according to Tukey’s test (P < .01).
Importantly, another orally active nonpeptide CRF1 receptor antagonist, CP-154,526 (10 mg/kg), administered during the first two withdrawal periods but not the third, induced just as much time in social interaction (35.0 ± 3.6 s) as did exposure to CD (29.4 ± 4.1 s). Inasmuch as the group repeatedly withdrawn from ethanol and given vehicle exhibited a decrease in social interaction behavior (14.1 ± 4.1 s), these data confirm that the CRF1 receptor is involved in the repeated-withdrawal-induced anxiety-like behavior. A one-way ANOVA [F(2,19) = 6.57, P < .01] and subsequent Tukey’s tests confirmed that the ethanol-withdrawn, vehicle-treated group was significantly different from the other two groups (P < .01).
3.3. Effects of antisauvagine-30, CRF2 receptor antagonist, on withdrawal-induced anxiety-like behavior
To examine the potential role of CRF2 receptors in the withdrawal-induced anxiety, antisauvagine-30 was tested. In contrast to the CRF1 receptor antagonist, intraventricular pretreatment with antisauvagine-30 during the first and second withdrawals of a three-cycle exposure to chronic ethanol did not counteract the anxiogenic behavior exhibited by repeated ethanol withdrawals. The antisauvagine-30-treated group exhibited reduced time spent in social interaction, similar to the time demonstrated by the vehicle-treated group in Fig. 3, and there was no difference between the groups (11.1 ± 1.2 s of social interaction for control and 12.0 ± 1.9 s for antisauvagine-30; t = 0.39, NS). There were also no significant differences in locomotor activity (t = 0.32, NS). The control group had 75.3 ± 7.1 line crosses and the group treated with antisauvagine-30 had 71.3 ± 10.1. Thus, an intraventricular dose of 20 μg antisauvagine-30 did not modify the reduced social interaction or locomotor activity associated with repeated ethanol withdrawals.
3.4. Body weights
As indicated above, a modified pair-feeding method was used in which the volume of CD received by the control animals was the average volume ingested on the previous day by the rats on ED. As can be seen in Table 1, this procedure resulted in adequate control over body weight, with no differences being observed between groups.
Table 1.
Body weights (g) of rats used in the studies
| Treatment group (n) | Initial weight | Final weight |
|---|---|---|
| Experiment 1 | ||
| Cannulated vehicle (6) | 382.8 ± 13.9 | 397.3 ± 5.9 |
| Cannulated CRF (8) | 386.8 ± 17.1 | 393.7 ± 14.5 |
| Experiment 2 | ||
| CD (8) | 195.9 ± 3.2 | 296.4 ± 3.2 |
| ED–VEH (12) | 198.1 ± 1.7 | 292.2 ± 4.9 |
| ED–CRA/A (10) | 195.7 ± 2.6 | 297.2 ± 5.4 |
| ED–CRA/P (10) | 201.1 ± 2.3 | 306.3 ± 10.5 |
| Experiment 3 | ||
| Cannulated vehicle (8) | 351.1 ± 7.7 | 356.8 ± 6.4 |
| Antisauvagine-30 (10) | 358.3 ± 8.6 | 372.2 ± 9.6 |
3.5. Ethanol intake
The average daily intakes of ethanol for the ED treatment groups in the CRA1000 study were 11.18 ± 0.37, 11.62 ± 0.34, and 11.35 ± 0.31 g/kg/day for the vehicle, acute CRA1000 and pretreatment CRA1000 groups. The intakes of the groups in the antisauvagine-30 study were somewhat less (10.3 ± 0.6 and 10.3 ± 0.3 g/kg/day for control and antisauvagine-30 groups, respectively), but these intakes were not different from each other. The intakes of the groups in the CRF study were substantially less than those in the CRA1000 study most likely because they only had 5 days of access to ethanol. Nevertheless, the cannulated vehicle group (7.16 ± 0.33 g/kg/day) did not differ from the cannulated CRF group (7.88 ± 0.24 g/kg/day). Thus, neither CRA1000, antisauvagine, nor CRF affected the intake of ED.
4. Discussion
Previous studies have demonstrated that restraint stress applied at weekly intervals prior to 5 days of 4.5% ED resulted in sensitization of a withdrawal-induced reduction in social interaction behavior (Breese et al., 2003). Inasmuch as the present findings confirm that a single withdrawal from 7% ED does not induce anxiety-like behavior (Overstreet et al., 2002), we were able to examine whether CRF would substitute for multiple stresses to sensitize anxiety.
It was initially demonstrated that CRF administered intraventricularly resulted in an acute decrease in social interaction behavior 30 min later, confirming the anxiogenic effect of CRF found in other tasks (e.g., Spina et al., 2002). Subsequently, to determine if CRF would substitute for two stresses to sensitize withdrawal-induced anxiety-like behavior, rats on CD were treated intraventricularly with CRF on two occasions 5 days apart to substitute for the initial two withdrawals of the multiple withdrawal protocol. When these rats were withdrawn from a single 5-day chronic ethanol exposure, a sensitization of anxiety was observed, to a degree like that seen with multiple withdrawals (Figs. 2 and 3; Overstreet et al., 2002). However, rats that were only exposed to intraventricular CRF twice (Fig. 2) or to a single 5-day exposure to 7% ED (Fig. 2; Overstreet et al., 2002, 2003) exhibited normal social interaction behavior. Thus, this finding supports the important role of the CRF system in withdrawal from ethanol exposure reported by Menzaghi et al. (1994) and Knapp et al. (in press). Of interest was that control cannulated animals did not differ from the partners (Fig. 1) or cannulated rats that were given vehicle and were exposed to ethanol for 5 days (Fig. 2). Only the animals given two injections of CRF and exposed to 5 days of ethanol, which by itself does not affect social interaction behavior (Overstreet et al., 2002, 2003), exhibited a decrease in social interaction behavior.
With confirmation that CRF is involved in the sensitization of withdrawal-induced anxiety-like behavior arising from a single 5-day exposure to ethanol, the next approach was to determine if antagonism of CRF receptors during the initial two withdrawals of the multiple withdrawal protocol would antagonize the withdrawal-induced reduction in social interaction observed with the 7% ethanol liquid diet. In this respect, the CRF1 receptor antagonists, CRA1000 and CP-154,526, blocked the reduced social interaction associated with withdrawals from repeated chronic ethanol exposures. This latter finding is consistent with the hypothesis that CRF also participates in the increased anxiety-like behavior induced by repeated withdrawals from chronic ethanol. Such a finding would be consistent with a number of other previous reports linking anxiety-like behavior to CRF and other components of the HPA system (Koob et al., 1998; Menzaghi et al., 1994).
A novel approach in the present investigation was administering the CRF1 receptor antagonists during the first and second withdrawals but not during the third withdrawal to examine the role of CRF in the repeated-withdrawal-induced anxiety. This strategy gave results comparable to those obtained when the CRF1 receptor antagonist was given 30 min before the behavioral test during the third withdrawal from the multiple withdrawal protocol (see Fig. 3). This outcome suggests that an adaptive mechanism(s), passed from one withdrawal to the next, contributes to the anxiety-like behavior associated with repeated withdrawals. Thus, CRA1000 and CP-154,526 counteracted the adaptive changes in some system impacted by CRF during the repeated withdrawals from ethanol. A number of earlier reports implicated CRF1 receptors in the anxiogenic effects of CRF or stress (e.g., Heinrichs et al., 1997; Landgraf, 2001) and in the anxiolytic effects of CRF receptor antagonists (Brauns et al., 2001; Keck et al., 2001; Radulovic et al., 1999; Seymour et al., 2003). The present results are consistent with these reports but add a new dimension. By pretreatment during the earlier repeated withdrawal periods, the CRF1 receptor antagonists can prevent the reduction of social interaction seen upon the final withdrawal.
Two other recent reports have provided evidence consistent with the hypothesis that CRF1 receptors play a key role in the anxiety associated with ethanol withdrawal. Using a design similar to that employed in this study, Breese et al. (2003) showed that the application of two periods of restraint stress (1 h) could induce anxiety-like behavior in rats following a single 5-day exposure to ethanol and that the CRF1 receptor antagonist CRA1000 blocked this effect. In a complementary study, Valdez et al. (2003) subjected rats to a brief (15-min) restraint stress and examined anxiety-like behavior in the elevated plus maze. Only the rats that had a previous history of alcohol exposure exhibited anxiety-like behavior; treatment with a CRF1 receptor antagonist prevented this response.
Because antisauvagine-30, a CRF2 receptor antagonist, was without effect, the CRF1 receptor subtype appears to be critical to the decrease in social interaction observed with withdrawal, and the CRF2 receptor is not contributing to the sensitization of withdrawal-induced anxiety-like behavior. However, this conclusion must remain tentative because only a single dose of antisauvagine-30 was used in this investigation. Furthermore, the negative result in this study does not preclude the possibility that CRF2 receptors in brain regions not reached by intraventricular injections might participate in anxiety-like behavior because there is evidence that urocortins, which interact selectively with CRF2 receptors, have anxiogenic effects (Spina et al., 2002). On the other hand, Heinrichs et al. (1997) reached a conclusion similar to ours: the CRF1 receptors are more important for anxiety-like behavior than CRF2 receptors.
Others have suggested that corticosterone may not be involved in stress-stimulated relapse in rats because relapse still occurs in adrenalectomized rats (Le et al., 2000). Data from our laboratory indicate that corticosterone administered instead of the initial withdrawals did not induce a withdrawal-induced reduction in social interaction (Breese et al., 2003). Nonetheless, several aspects concerning the role of CRF in a multiple-withdrawal-induced decrease in social interaction require comment. For example, the present findings do not permit a conclusive statement about the brain region(s) that may participate in the modulation of social interaction behavior by the CRF system(s). Previous work demonstrated that the central amygdala was involved in the anxiety induced by withdrawal from a single episode of chronic ethanol (Koob et al., 1998; Menzaghi et al., 1994; Rassnick et al., 1993). Such work emphasizes that extrahypothalamic sites are likely critical to the sensitized anxiety-like behavior induced by repeated withdrawals.
Because the CRF1 receptor antagonists were effective in selectively counteracting the affective component of the withdrawal syndrome in the rats, it is likely that they could also ameliorate affective symptoms of withdrawal in humans (Keck and Holsboer, 2001; Kehne and De Lombaert, 2002). Therefore, such CRF1 receptor antagonists might reduce the risk of relapse in alcoholics because the affective symptoms experienced during ethanol withdrawal have been implicated in the risk to relapse (Driessen et al., 2001; Sinha, 2001).
Despite the significant changes in social interaction behavior induced by prior treatment with CRA1000 or CRF, no change in the number of line crosses was observed as a measure of activity (Fig. 4). This finding confirms other studies indicating that social interaction and activity, as reflected by line crosses, are controlled by independent mechanisms (Breese et al., 2003; File, 1980; Overstreet et al., 2002, 2003). In this respect, the reduction in social interaction behavior can be sensitized by repeated ethanol withdrawals in rats maintained on a 4.5% ED without a change in line crosses (Overstreet et al., 2002). In support of this conclusion, the reduction in social interaction behavior associated with repeated ethanol withdrawals can be counteracted by injections of a 5-HT2C receptor antagonist or a 5-HT1A agonist without affecting this measure of activity (Overstreet et al., 2003).
Nonetheless, these conclusions do not preclude the possibility that reduced locomotor activity can signify an anxiety-like state in other circumstances. Indeed, there is a long history of the association of reduced locomotor activity with emotional behavior (Archer, 1973). The Maudsley Reactive and Nonreactive Rats, selectively bred for differences in open field defecation, a widely recognized index of anxiety, also differ in open-field activity (Blizard and Adams, 2002), as do rats selectively bred for differences in anxiety-related behavior in the elevated plus maze (Landgraf and Wigger, 2002). Based upon these reports, it might be possible to conclude that the reduced activity seen in ethanol-withdrawn rats is also an anxiety-like behavior. Nevertheless, the reduction in activity is not affected by the manipulations that counteract the reduction in social interaction (compare Fig. 3 with Fig. 4; Breese et al., 2003; Overstreet et al., 2002, 2003).
The present study has also provided important data regarding the methodology for the social interaction test. As indicated previously, it has been recommended that unmanipulated animals should be paired up with the basis of treatment and body weights, whereas surgically manipulated rats should be paired with an untreated, control rat (e.g., File, 1980; File and Seth, 2003). In this study, we examined the acute effects of CRF both when the animals had the same treatment and when they were paired with an untreated control partner. The degree of anxiety-like behavior was similar in the two conditions; therefore, these data were combined for the overall analysis. So, at least for this data set, the degree of anxiety-like behavior observed in one rat is not influenced by the degree of anxiety-like behavior exhibited by its partner. By the same token, the partners of the vehicle-and CRF-treated rats were not different, indicating that the behaviors of normal rats are also not influenced by the degree of anxiety-like behavior exhibited by their partner. These current data also support the approach we have used to analyze social interaction behavior, using the data from individual animals (see Breese et al., 2003; Overstreet et al., 2003).
Acknowledgments
This research was supported by funding from NIAAA (2 P60 AA-011605-06, AA-014073, AA-014284). We wish to thank Taisho Pharmaceutical (Saitama, Japan) for the generous supply of CRA1000 and Pfizer (Groton, CN) for CP-154,526. We thank Dhritiman Muckerjee, Qi Yu, and Mili Senapati for expert technical assistance.
References
- Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphe nucleus–dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology. 1997;130:228–34. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- Archer J. Tests for emotionality in rats and mice. A review Anim Behav. 1973;21:205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Costall B, Kelly ME, Onaivi ES, Naylor RH. Ketotifen and its analogues reduce aversive responding in the rodent. Pharmacol Biochem Behav. 1990;37:785–93. doi: 10.1016/0091-3057(90)90564-x. [DOI] [PubMed] [Google Scholar]
- Battacharya SK, Satyan KS, Chakraharti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–24. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Adams N. The Maudsley reactive and nonreactive strains: a new perspective. Behav Genet. 2002;32:277–99. doi: 10.1023/a:1020206120248. [DOI] [PubMed] [Google Scholar]
- Brauns O, Liepold T, Radulovic J, Spiess J. Pharmacological and chemical properties of astressin, antisauvagine-30 and alpha-helCRF: significance for behavioral experiments. Neuropharmacology. 2001;41:507–16. doi: 10.1016/s0028-3908(01)00094-6. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DK, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF1 and benzodiazepine receptor antagonists and a 5-HT1A receptor agonist. Neuropsychopharmacology. doi: 10.1038/sj.npp.1300282. online publication 10 July, 2003. Available at: http://www.acnp.org/citations/Npp07100303159/default.pdf. [DOI] [PMC free article] [PubMed]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Ondansetron inhibits a behavioural consequence of withdrawing from drugs of abuse. Pharmacol Biochem Behav. 1990;36:339–44. doi: 10.1016/0091-3057(90)90414-d. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–55. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Lister RG. Do the reductions in social interaction produced by picrotoxin and pentylenetetrazole indicate anxiogenic actions? Neuropharmacology. 1984;23:793–6. doi: 10.1016/0028-3908(84)90113-8. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcot PK. Flumazenil but not nitrendipine reverses the increased anxiety during alcohol withdrawal in the rat. Psychopharmacology. 1989;98:262–4. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of alcohol withdrawal signs in the rat to γ-aminobutyric acid (GABA) mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–5. [PubMed] [Google Scholar]
- Gonzalez LE, File SE, Overstreet DH. Selectively bred lines of rat differ in social interaction and hippocampal 5-HT1A receptor function: a link between anxiety and depression? Pharmacol Biochem Behav. 1998;59:787–92. doi: 10.1016/s0091-3057(97)00525-x. [DOI] [PubMed] [Google Scholar]
- Guy AP, Gardner CR. Pharmacological characterisation of a modified social interaction model of anxiety in the rat. Neuropsychobiology. 1985;13:194–200. doi: 10.1159/000118187. [DOI] [PubMed] [Google Scholar]
- Harro J, Tomissaar M, Eller M. The effects of CRA 1000, a non-peptide antagonist of corticotropin-releasing factor receptor type 1, on adaptive behaviour in the rat. Neuropeptides. 2001;35:100–9. doi: 10.1054/npep.2001.0851. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Lapansky J, Lovenberg TW, DeSouza EB, Chalmers DT. Corticotropin-releasing factor CRF1 but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–8. [PubMed] [Google Scholar]
- Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, et al. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology. 2001;153:315–20. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–84. [PubMed] [Google Scholar]
- Keck ME, Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–44. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Englemann M, Holsboer F, et al. The anxiolytic effect of the CRH (1) receptor antagonist R 121919 depends on innate emotionality in rats. Eur J Pharmacol. 2001;13:373–80. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kehne J, De Lombaert S. Non-peptide CRF1 receptor antagonists for the treatment of anxiety, depression and stress disorders. Curr Drug Targets CNS Neurol Disord. 2002;1:467–93. doi: 10.2174/1568007023339049. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal-induced anxiety. Alcohol Clin Exp Res. 1998;22:481–93. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal anxiety in rats. Alcohol. 2004 doi: 10.1016/j.alcohol.2003.08.007. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Landgraf R. Neuropeptides and anxiety-related behavior. Endocr J. 2001;48:517–33. doi: 10.1507/endocrj.48.517. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A. High v low anxiety-related behavior rats. An animal model of extremes in trait anxiety. Behav Genet. 2002;32:301–14. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytch W, Watchus J, Shalev U, Shamam Y. The role of corticotropin releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–24. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lightowler S, Kennett GA, Williamson U, Blackburn TP, Tulloch IF. Anxiolytic-like effect of paroxetine in the rat social interaction test. Pharmacol Biochem Behav. 1994;49:281–5. doi: 10.1016/0091-3057(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Roberts J-S, Wang W, Myrick H, Anton RF. Multiple previous detoxifications are associated with less responsive treatment and heavier drinking during an index outpatient detoxification. Alcohol. 2000;22:159–64. doi: 10.1016/s0741-8329(00)00114-2. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol ‘‘kindles’’ inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–9. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Rassnick S, Heinrich S, Baldwin H, Pich EM, Weiss F, et al. The role of corticotropin releasing factor in the anxiogenic effects of ethanol withdrawal. Ann N Y Acad Sci. 1994;739:176–84. doi: 10.1111/j.1749-6632.1994.tb19819.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology. 1997;131:354–60. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following ethanol withdrawal: effects of flumazenil. Psychopharmacology. 2000;152:208–15. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Kawashima N, Suzuki Y, Ogawa S, Nakazato A, et al. Receptor binding, behavioral, and electrophysiological profiles of non-peptide corticotropin-releasing factor subtype1 receptor antagonists CRA1000 and CRA1001. J Pharmacol Exp Ther. 1999;289:926–35. [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decreases in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–69. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2C antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–52. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Leopold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress; differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–25. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo – pituitary – adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–49. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Effect of repeated exposure to alcohol on the response of the hypothalamic – pituitary – adrenal axis of the rat: II. Role of the length and regimen of alcohol treatment. Alcohol Clin Exp Res. 2001;25:106–11. [PubMed] [Google Scholar]
- Sams-Dodd F. Automation of the social interaction test by a video-tracking system: behavioral effects of repeated phencyclidine treatment. J Neurosci Methods. 1995:157–67. doi: 10.1016/0165-0270(94)00173-e. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schultz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor. A review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, et al. Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology. 2002;160:113–21. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]