Abstract
Multiple studies, involving distinct clinical populations, implicate contactin associated protein-like 2 (CNTNAP2) in aspects of language development and performance. While CNTNAP2 is broadly distributed in developing rodent brain, it shows a striking gradient of frontal cortical enrichment in developing human brain, consistent with a role in patterning circuits that subserve higher cognition and language. To test the hypothesis that CNTNAP2 may be important for learned vocal communication in additional species, we employed in situ hybridization to characterize transcript distribution in the zebra finch, an experimentally tractable songbird for which the neural substrate of this behavior is well-established. Consistent with an important role in learned vocalization, Cntnap2 was enriched or diminished in key song control nuclei relative to adjacent brain tissue. Importantly, this punctuated expression was observed in males, but not females, in accord with the sexual dimorphism of neural circuitry and vocal learning in this species. Ongoing functional work will provide important insights into the relationship between Cntnap2 and vocal communication in songbirds and thereby clarify mechanisms at play in disorders of human cognition and language.
Keywords: autism, avian, birdsong, CASPR2, contactin, neurexin, zebra finch
INTRODUCTION
Multiple independent lines of evidence have recently converged to identify contactin associated protein-like 2 (CNTNAP2) as an important modulator of diverse clinical phenotypes involving impaired language performance. Early linkage scans in families with autistic children, a disorder in which language impairment is a defining feature, highlighted the distal portion of chromosome 7q (Alarcón et al., 2002; Badner and Gershon, 2002; Trikalinos et al., 2006). Subsequent fine mapping in these and other families led to the identification of associations between common variation in CNTNAP2 and both autism diagnosis (Arking et al., 2008) and language onset in autistic probands (Alarcón et al., 2008). Remarkably, work by Vernes et al. (2008) provided additional support for the relationship between common variation in CNTNAP2 and language performance outside of autism. Genetic variants overlapping with those studied previously (Alarcón et al., 2008) were found to be associated with non-word repetition in individuals with specific language impairment. Importantly, in the same study, chromatin immunoprecipitation followed by DNA sequencing identified CNTNAP2 as a direct target of the transcription factor Forkhead box 2 (FOXP2). Mutations in this latter molecule result in a severe speech and language disorder known as developmental verbal dyspraxia (Lai et al., 2001). Demonstration of an interaction between these two molecules bolsters the role of each in language development and begins to define a molecular network which may subserve vocal communication. Further support for involvement of CNTNAP2 in language comes from the identification of rare variants in children with either autism or cortical dysplasia focal epilepsy (CDFE). The latter is a disorder characterized by abnormal brain development, seizures, and behavioral abnormalities that include language regression. In autism, rare non-synonymous coding variants (Bakkaloglu et al., 2008) or structural rearrangements (Alarcón et al., 2008; Bucan et al., 2009; Poot et al., 2009) in CNTNAP2 have been observed in cases and family members. Importantly, within CDFE, study of patients and their families from the Old Order Amish suggests that homozygosity for a truncating mutation in CNTNAP2 may be sufficient to cause disease in a Mendelian fashion (Strauss et al., 2006), a result which has since been replicated in an independently ascertained case (Jackman et al., 2009).
CNTNAP2 was discovered almost a decade ago and determined, on the basis of sequence homology, to be a member of the neurexin superfamily (Poliak et al., 1999). At the same time, relatively little is known about its function, particularly in the central nervous system. Work in the peripheral nervous system has shown that the Cntnap2 protein is localized at the juxtaparanodal (JPN) region of myelinated axons where it interacts with transient axonal protein 1 (TAG-1), an interaction that mediates axon-glia contact and is required to promote the local clustering of voltage-gated potassium channels at JPN (Poliak et al., 2001; Poliak et al., 2003; Traka et al., 2003).
Of particular importance given the seizures described in the Old Order Amish patients homozygous for truncating mutations (Strauss et al., 2006), is the finding that Cntnap2 localizes to the axon initial segment, the key functional domain involved in generation of action potentials (Inda et al., 2006). Consistent with the idea that CNTNAP2 plays multiple developmental roles is the observation of defects in neuronal migration in cortical material resected from CDFE patients (Strauss et al., 2006). Characterization of transcript distribution in human fetal brain prior to the onset of axonal myelination (Abrahams et al., 2007) provides further support for this notion. In mid-gestation brain tissue, CNTNAP2 transcript is highly enriched in frontal cortex, striatum, and dorsal thalamus suggesting that the gene product is functionally specialized in these regions. Although a variety of structures have been implicated in different aspects of human cognition (Lieberman, 2002), the fact that CNTNAP2 marks cortico-striato-thalamic circuits thought to be central to aspects of executive function and language is intriguing, particularly when considered together with the genetic evidence outlined above. Interestingly, these results from human stand in sharp contrast to the broad transcript distribution observed in the developing brains of rodents (Abrahams et al., 2007). Yet, detailed knowledge about the manner by which apparent language-related circuits act to subserve vocal communication is lacking, necessitating work in other vocal learners in which more is known about the neural substrate of this behavior.
Towards functional work in songbirds, we undertook a comprehensive analysis of neural expression of Cntnap2 in the zebra finch (Taeniopygia guttata), an important animal model for study of the neural and social bases of vocal learning (Doupe and Kuhl, 1999; Jarvis, 2004). Like humans, but unlike non-human primates or rodents, songbirds have the ability to modify vocal communication signals in a process that involves implicit learning and sensorimotor integration. Like humans, zebra finches learn their vocalizations (i.e. song) during critical developmental stages and learning is dependent on social interactions (Nelson, 1997; Doupe and Kuhl, 1999; Kuhl, 2003) and auditory experience (Price, 1979; Marler, 1991; Iyengar and Bottjer, 2002b). In this species, song learning and its underlying neural circuitry are sexually dimorphic (Nottebohm and Arnold, 1976). During development, groups of neurons dedicated to song learning and production, referred to as song control nuclei, emerge as subregions of the cortical-like pallium, striatum and thalamus and become synaptically connected in males (Fig. 1) but not in females, who do not learn to produce song (Nottebohm and Arnold, 1976). These include pallial subregions known as HVC (abbreviation used as proper name) and the robust nucleus of the arcopallium (RA), which comprise the posterior vocal pathway controlling song production (Nottebohm et al., 1976) via innervation of motor neurons for the syrinx and respiratory muscles used in singing (Wild, 1997).
Fig. 1.
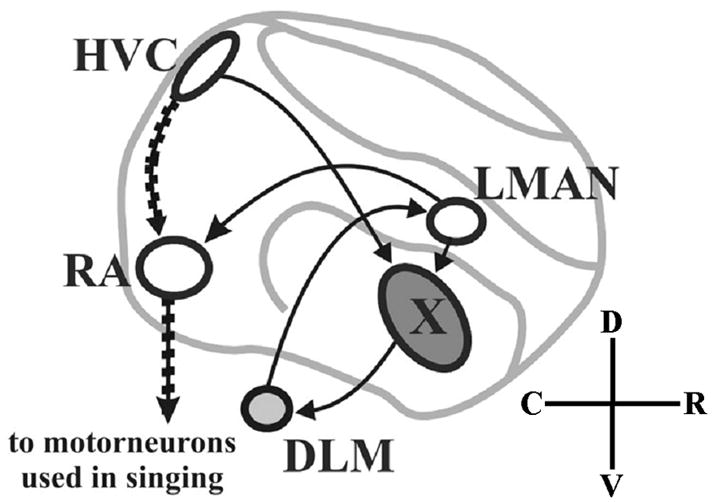
Schematic of connections within the male song circuit in pseudo-sagittal section of zebra finch brain. Cortical-like pallial structures appear in white, striatal in dark gray, and thalamic in light gray. Auditory input (not shown) enters the song circuit at HVC. In the posterior vocal pathway (stippled arrows), a subset of HVC neurons project to RA which projects to brainstem motor neurons that control the syrinx, or song organ, and respiratory muscles used in singing. A separate group of HVC neurons projects to the anterior forebrain pathway (AFP; plain arrows) which connects HVC, area X, DLM and LMAN and rejoins the posterior vocal pathway via the connection of LMAN to RA. C (caudal), D (dorsal), R (rostral), V (ventral). See Table 1 for abbreviations of brain regions. Adapted from Teramitsu et al. (2004).
A second pathway in the anterior forebrain (AFP) is required for song modification during development (Bottjer et al., 1984; Sohrabji et al., 1990; Scharff and Nottebohm, 1991). In the AFP, a subset of HVC neurons project to area X of the striatum. Area X projection neurons innervate the medial part of the dorsolateral thalamic nucleus (DLM). DLM innervates the lateral magnocellular nucleus of the anterior nidopallium (LMAN) whose neurons project back to area X and also rejoin the vocal motor pathway by innervating RA (Bottjer et al., 1989; Vates and Nottebohm, 1995; Vates et al, 1997). This pallio-striato-thalamic AFP loop is analogous to the human cortico-striato-thalamic circuits used for planning and executing complex sequential movements and involved in implicit learning, including speech and language (Bottjer and Johnson, 1997; Doupe and Kuhl, 1999; Jarvis, 2004). An additional thalamo-pallial pathway (not depicted in Fig. 1) connects the medial magnocellular nucleus of the anterior nidopallium (MMAN), HVC and the posterior part of the dorsomedial thalamic nucleus, DMP (Foster et al., 1997; Vates et al., 1997). This pathway is hypothesized to function as a reafferent loop for relaying information between the motor pathway and components of the AFP (Vates et al., 1997). Song nuclei HVC and RA are larger in the adult male zebra finch than in the female whereas striatal area X is absent in females (Nottebohm and Arnold, 1976). For a complete list of abbreviations of brain structures see Table 1.
Table 1.
List of abbreviations for brain structures in this study.
| A | arcopallium | LSt | lateral striatum |
| Cb | cerebellum | M | mesopallium |
| DLM | medial part of the dorsolateral thalamic nucleus | MLd | dorsal part of the lateral mesencephalic nucleus |
| DMP | posterior part of the dorsomedial thalamic nucleus | MMAN | medial magnocellular nucleus of the anterior nidopallium |
| DTZ | dorsal thalamic zone | MSt | medial striatum |
| GP | globus pallidus | N | nidopallium |
| HA | apical part of the hyperpallium | NC | caudal nidopallium |
| Hb | habenula | RA | robust nucleus of the arcopallium |
| HD | densocellular part of the hyperpallium | SpL | lateral spiriform nucleus |
| HVC | nucleus HVC of the nidopallium | SpM | medial spiriform nucleus |
| ICo | intercollicular nucleus | St | striatum |
| IM | magnocellular isthmus nucleus | TeO | optic tectum |
| IPC | parvocellular isthmus nucleus | X | nucleus area X of the striatum |
| LMAN | lateral magnocellular nucleus of the anterior nidopallium |
We hypothesized that CNTNAP2 might be important for the formation and function of neural circuits necessary for vocal learning in the songbird, similar to its predicted role in patterning language-related areas in the human. While the subset of neurons specialized for language within developing human cortico-striato-thalamo-cortical circuitry is not precisely localized, zebra finch neural circuitry for learned vocalizations is (see above). As a first test, we conducted a comprehensive analysis of Cntnap2 expression in zebra finch brain in the adult and at key stages during post-hatch development (see Methods, below). In support of a role in the establishment and function of song circuitry, we observed sexually dimorphic transcript distribution for Cntnap2 which mapped both temporally and spatially to the development and functional specialization of song control nuclei.
MATERIALS AND METHODS
Animals and tissue preparation
All animal use and experimental procedures were in accordance with NIH guidelines for experiments involving vertebrate animals and approved by the UCLA Chancellor’s Institutional Animal Care and Use Committee. Zebra finches (n=18 males and 15 females) used in this study were obtained from our breeding colony. Following decapitation, brain tissue was rapidly extracted and flash frozen on aluminum weigh boats floated on liquid nitrogen, then stored at −80°C until use. For post-hatch day 1 (1d) and 6d time points, brains were not dissected but left encased in the skull to preserve tissue integrity during sectioning. Coronal or sagittal sections were cut at 20 μm thickness on a cryostat (Leica Microsystems, Wetzlar, Germany) and thaw-mounted onto microscope slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA), to produce several replicate sets of adjacent or near-adjacent sections. One set was used for Nissl staining to allow for identification of neuroanatomical structures. At 25d, the emergence of sexually dimorphic plumage enables differentiation of male from female zebra finches. To determine the sex of younger birds (1–15d), we performed polymerase chain reaction (PCR) using their genomic DNA as template to amplify regions corresponding to sex-specific gene isoforms present on Z (male) or Z and W (female) chromosomes, as described in Soderstrom et al. (2007).
Reverse Transcription-PCR and cDNA Cloning
With the cDNA for human CNTNAP2 (AF319045.1) as a query, blastn was used to search the zebra finch Traces-WGS database (http://www.ncbi.nlm.nih.gov/genome,taxid=59729) for sequence fragments potentially corresponding to the zebra finch homolog. To obtain a complete open reading frame (ORF), primers against and between sequences recovered in this fashion were then designed for use in PCR. Total RNA was isolated from whole adult brain using RNeasy Mini Kit (Qiagen, Valencia, CA), and was random primed and reverse transcribed with Superscript III to generate cDNA according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Sequencing of PCR products resulted in the recovery of a partial ORF. Missing upstream material was obtained by 5′ RACE (GeneRacer, Invitrogen) using the manufacturer provided 5′ primer (5′-CGACTGGAGCACGAGGACACTGA-3′) in combination with a gene-specific primer (oBSA829, 5′-AGGCTTCCAGTTTCTCCCTGTATC-3′). Missing 3′ material was obtained by PCR with nested forward primers (oBSA834 and oBSA835, 5′-CTTCAGCTTCAGCACCACCAAGTCC-3′ and 5′-CCGGATTTCATGGCTGTCCTCGT-3′, respectively) in conjunction with a degenerate reverse primer (oBSA836, 5′-rATrArCCAyTCCTTyTTGCT-3′), obtained through alignment of cDNAs corresponding to the CNTNAP2 homologs from human, mouse, chicken and fugu.
Probe design and validation
Public databases for human, chicken and zebra finch genomes were employed as above to identify conserved regions within the CNTNAP2 transcript against which to generate in situ hybridization probes. Two non-overlapping regions within the 3′ untranslated region (UTR) were identified as both conserved across species and unique to CNTNAP2 (Fig. 2A). Standard PCR was then employed to amplify material from cDNA, followed by cloning of amplified products into pCR4-TOPO vector (Invitrogen) and insert verification by sequencing. Probe 1 corresponds to a 618 bp product amplified by primers 1F (5′-CTGGGATGGGATACAAGCTG-3′) and 1R (5′-CCTGCCAGGATTGACAAGAG-3′) from chicken cDNA. Probe 2 corresponds to a 601 bp product amplified by primers 2F (5′-TGTATCTTTAACTCGTACTGGAGCA-3′) and 2R (5′-CCCCCTCCTCTTTATTTATCA-3′) from zebra finch cDNA and its entire sequence is included within the GenBank expressed sequence tag EE054214.1. Validation of probe specificity confirmed that non-overlapping Cntnap2 3′UTR riboprobes yielded identical hybridization patterns in adult zebra finch brain sections (Fig. 2B). In contrast, sense probes failed to produce any significant signal on the brain sections (Fig. 2C). Unless otherwise noted, all in situ hybridization analyses performed in this study used Probe 2 (hereon designated as ‘Probe’).
Fig. 2.
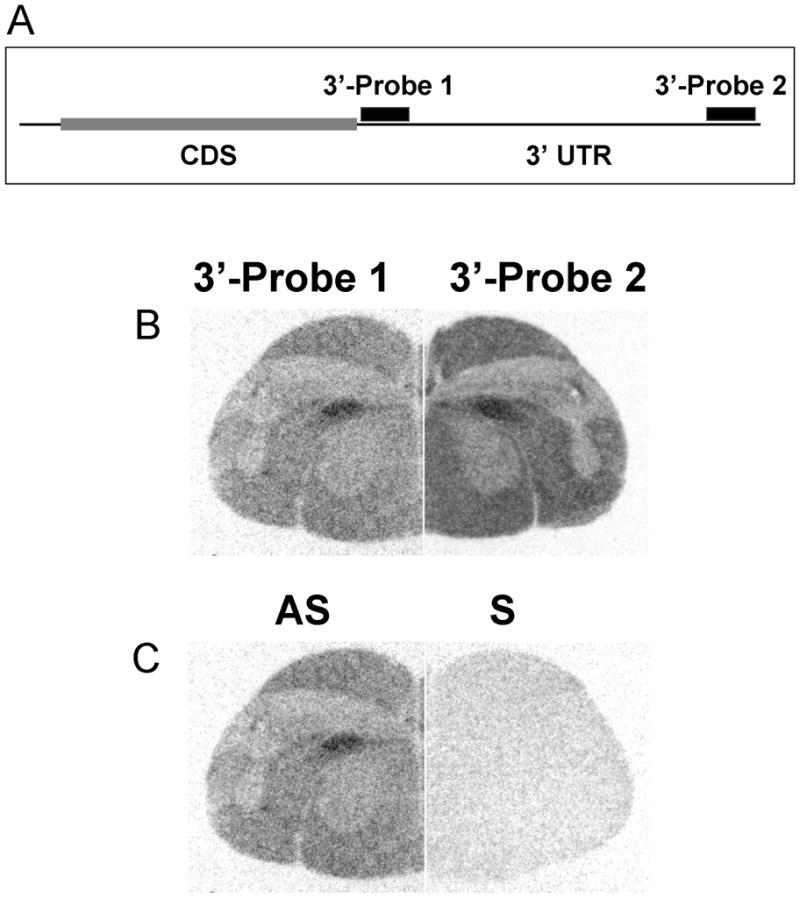
Schematic of zebra finch Cntnap2-specific riboprobes and their validation by in situ hybridization. Riboprobes were designed to hybridize to non-overlapping portions of the 3′ untranslated region (UTR) of Cntnap2, downstream of the coding sequence (CDS) (A). Both antisense (AS) riboprobes yielded identical hybridization patterns as exemplified in the hemicoronal section shown here (B). Hybridization of sense probes (S) gave no significant signal (C).
In situ hybridization
In situ hybridization analyses were performed largely as previously described in Perlman et al. (2003) with the following modifications: brain sections were hybridized with 8×106 cpm in 125 μl per slide of 33P-UTP-labeled (PerkinElmer, Waltham, MA) riboprobe and the last post-hybridization wash was in 0.25X SSC. For darkfield autoradiography, the protocol described in Teramitsu et al. (2004) was followed, with the exception that slides were incubated at 4°C for 3 weeks and after developing, sections were fixed in CitriSolv and coverslipped with Permount mounting media (Fisher Scientific, Pittsburgh, PA). To examine Cntnap2 expression during development and in the adult, hybridization signals were evaluated at several key time points: 1d (n=1 each sex), 6d (n=2 males, 1 female), 15d (n=2 each sex), 25d (n=2 each sex), 35d (n=2 each sex), 40d (n=1 each sex), 50d (n=2 males, 1 female), 75d (n=2 males, 1 female) and adult (>120d; n=4 each sex). These time points were selected to mark key events during post hatch brain development with an emphasis on the song learning process, comprising the stages prior to or at the onset of sensory acquisition (15 and 25d, respectively) and during sensorimotor practice (35, 40, 50 and 75d), leading to the production of crystallized song (>120d). Hybridization patterns described in Results were consistently observed at each time point and within each sex, unless otherwise noted.
Image processing
Photomicrographs of Nissl-stained or film autoradiography sections were acquired with an AxioCam HRm digital camera mounted on a Discovery.V12 stereomicroscope (Carl Zeiss Inc., Oberkochen, GE). Emulsion radiography images were digitized from snapshots taken with the same camera mounted on an AxioImager.V1 microscope (Carl Zeiss Inc.) capable of darkfield epi-illumination (Darklite; Nikon, Melville, NY). All digitized images were processed in Adobe Photoshop 7.0 (Adobe, San Jose, CA) to remove background and adjust grayscale levels to improve brightness and contrast. Adjustments were made to the entire image and not to selective subregions. Prior to adjustment of levels, darkfield images were black-white inverted to be consistent with the interpretation of signals from film. Thus, dark areas reflect high transcript levels and light areas reflect low transcript levels in images from both emulsion-dipped slides and film autoradiograms. Schematic drawings of neuroanatomical structures revealed by Nissl staining were traced using the freehand tool in CorelDRAW 12 (Corel, Ottawa, Canada). Identification of neural structures was guided by the use of several published atlases of songbird brain, including that available for canary (Serinus canaria; Stokes et al., 1974) and zebra finch (http://www.ncbi.nlm.nih.gov/bookshelf/picrender.fcgi?book=atlas&part=A24&blobname=&blobtype=pdf, courtesy of Dr. Barbara Nixdorf-Bergweiler), and employed the revised avian brain nomenclature described in Reiner et al. (2004).
RESULTS
Cloning of zebra finch Cntnap2
Prior to characterization of Cntnap2 transcript distribution in zebra finch, we sought to determine whether a homologous transcript was present in this species, and if so, the extent to which the open reading frame (ORF) was conserved in human and songbird. We used blastn to identify sequence fragments in the zebra finch Traces-WGS database (http://www.ncbi.nlm.nih.gov/genome, taxid=59729) with high similarity to the human mRNA (AF319045.1). This search resulted in identification of multiple sequence reads, including TGAB-apl06a12.g1, TGAB-ajb23e04.b1, TGAB-apl06a12.b1, TGAB-aec18g03.b1. Comparison of the human protein against 6 frame translations of these sequence fragments with tblastn indicated a conserved ORF across each, unlikely to occur as a result of chance alone (Eval between 3e-17 and 1e-28). PCR with multiple primer sets against cDNA confirmed the presence of zebra finch Cntnap2 transcript in adult brain. In support of an expressed transcript, each gave rise to signal in experimental but not negative control conditions (not shown).
PCR on this same cDNA was then employed to obtain internal sequence, estimated to correspond to approximately 70% of the expected ORF. Missing 5′ and 3′ ends were obtained via RACE-PCR with a degenerate reverse primer as described in Methods, and assembled with internal sequence to generate a cDNA 8935 bp in length (GenBank accession No. GU290551). Alignment of this cDNA to the July 2009 assembly of zebra finch genome defined a 1 Mb locus spanning 24 exons (Table 2). Both exon number (n=24) and identity of gene neighbors (TPK1 and CUL1) were conserved between finch and human. Available genomic sequence indicated that songbird locus is less than 50% the size of the human equivalent (Mar. 2006 Assembly), although this interpretation may be confounded by the presence of unsized gaps in the zebra finch assembly. Comparison of the predicted proteins in each species likewise highlighted more similarities than differences. In each, a protein of 148 kDa with identical domain structure was observed (Table 3). Relative to the overall average amino acid identity of 84%, percent identity across the N-terminal signal peptide and fourth and final LamG2 domain were low (55 and 66%, respectively). Further support for relatively reduced functional constraint around this LamG2 domain comes from the presence of insertion/deletion differences on either side of this region; all 9 alignment gaps observed following protein alignment flanked this motif. In contrast, 100% identity across multiple C-terminal domains (transmembrane, 4.1B binding, and PDZ binding) suggests evolutionary constraint across these intracellular portions of the protein. Strong conservation at the C-terminus is consistent with previous work demonstrating the requirement of this region in the clustering of potassium channels (Poliak and Peles, 2003) and also the fact that the premature stop codon observed in cases of CDFE eliminates this portion of the protein (Strauss et al., 2006).
Table 2.
Comparison of CNTNAP2 gene, cDNA, and protein structure in human and zebra finch.
| Ensembl human locus (Mar. 2006 Assembly) | Ensembl zebra finch locus (Jul. 2009 Assembly) | GenBank acession No. GU290551 | |
| Locus Start (bps) | chr7: 145,444,386 | Chr2:30,635,452 | chr2:31,216,806 |
| Locus End (bps) | chr7:147,749,019 | Chr2:30,184,705 | chr2:30,180,291 |
| Locus Size (bps) | 2,304,634 | 450,748 | 1,036,385 |
| Exon number | 24 | 16 | 24 |
| Upstream gene | TPK1 | Tpkl | Tpkl |
| Downstream gene | CUL1 | Cull | Cull |
| Refseq human cDNA (NM014141.4) | Ensembl zebra finch cDNA (ENSTGUT00000001874)1 | GenBank acession No. GU290551 | |
| bps 5′ UTR | 516 | 0 | 537 |
| bps Coding | 3993 | 2647 | 3987 |
| bps 3′ UTR | 5381 | 0 | 4411 |
| bps mRNA | 9890 | 2647 | 8935 |
| Refseq human protein (NP_054860) | Ensembl zebra finch protein (ENSTGUP00000001856)1 | GenBank acession No. GU290551 | |
| Number of aminoacids | 1331 | 882 | 1329 |
| Weight (Daltons) | 148116 | 97407 | 147661 |
| Number of Motifs | 11 | 7 | 11 |
| Identical to Human | NA | 735/885 (83%) | 1126/1334 (84%) |
| Positive vs. Human | NA | 799/885 (90%) | 1213/1334 (90%) |
| Gaps vs. Human | NA | 6/885 (<1%) | 9/1334 (<1%) |
Both cDNA and inferred protein were the output of a modified version of the standard EnsEMBL genebuild pipeline, relying on alignment of EnsEMBL chicken, other avian and mammalian proteins, as well as evidence from zebra finch. For the taeGut3.2.4 assembly, the predicted gene set contains 18,447 genes, of which 17,475 are protein coding (Potter et al., 2004).
Table 3.
Identity and conservation of CNTNAP2 protein domains in human and zebra finch.
| Motif | Amino Acid Position | Number of Zebra Finch-Human Differences | Domain Length | Percent Identity |
|---|---|---|---|---|
| Signal Peptide | 1–25 | 11 | 25 | 56 |
| FA58C | 35–181 | 13 | 147 | 91 |
| LamG2 | 215–344 | 13 | 130 | 90 |
| LamG2 | 400–528 | 23 | 129 | 82 |
| EGF3 | 553–590 | 4 | 38 | 89 |
| LamG2 | 826–994 | 27 | 169 | 84 |
| EGF3 | 963–1001 | 7 | 39 | 82 |
| LamG2 | 1057–1189 | 45 | 133 | 66 |
| TM | 1258–1277 | 0 | 20 | 100 |
| 4.1B | 1278–1296 | 0 | 19 | 100 |
| PDZ Binding | 1328–1331 | 0 | 4 | 100 |
Sexually dimorphic Cntnap2 expression in the adult
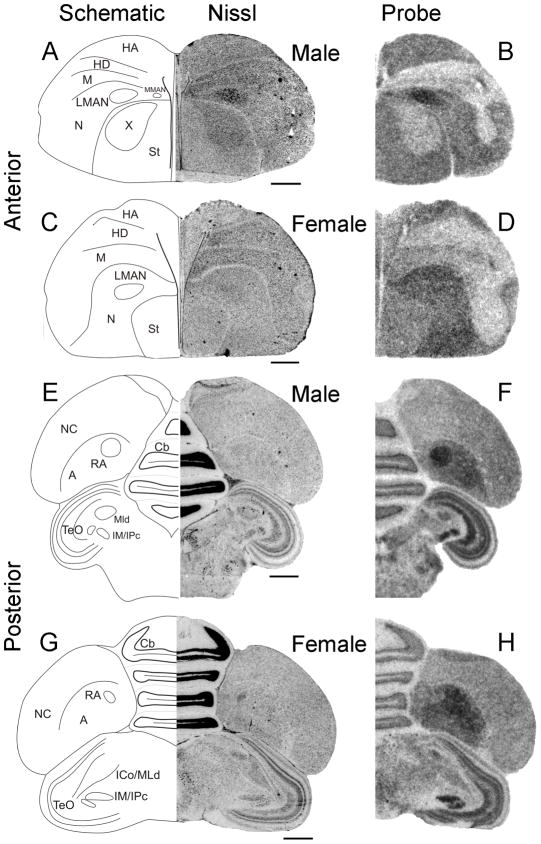
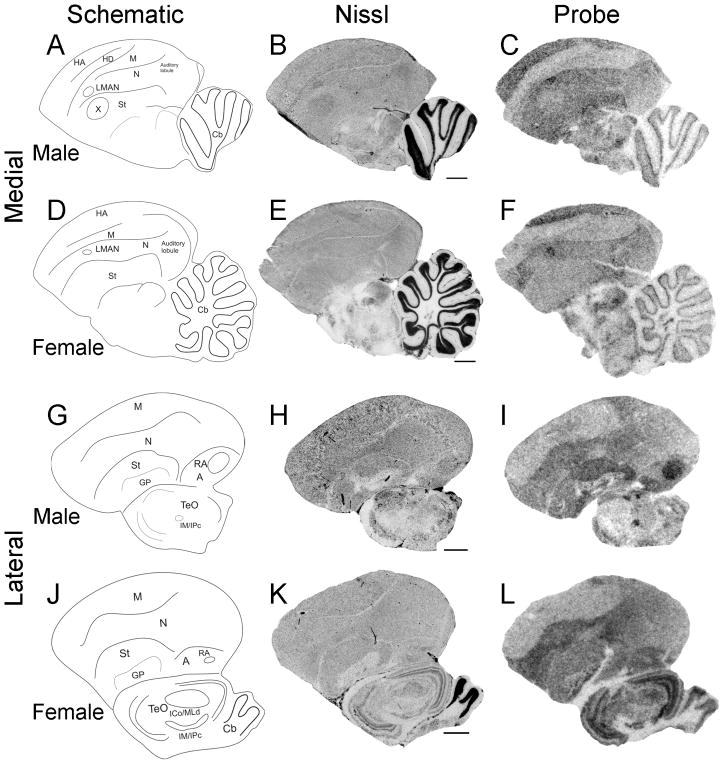
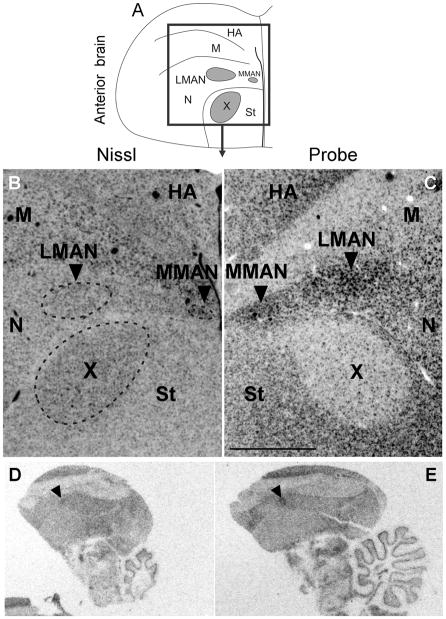
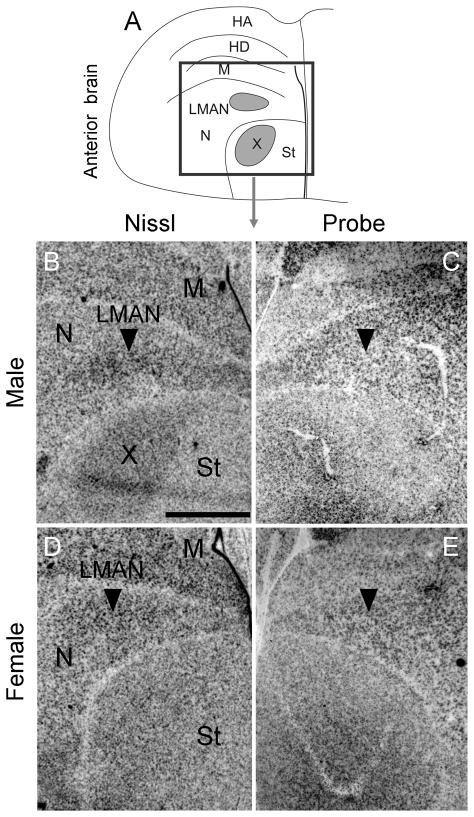
To begin to test the hypothesis that Cntnap2 is important for the formation and functional specialization of zebra finch song circuitry, we compared the pattern of Cntnap2 expression between adult (>120d) male and female zebra finch brains. Using hemicoronal (Fig. 3) and parasagittal (Fig. 4) sections, we uncovered differential Cntnap2 transcript distribution within several subregions dedicated to song control, consistent with the known sexual dimorphism of the zebra finch song circuit in these areas (Nottebohm and Arnold, 1976). Within male brains, Cntnap2 expression was enriched bilaterally in both the lateral and medial magnocellular nuclei of the anterior nidopallium (LMAN, MMAN, respectively; Figs. 3B, 4C), relative to the surrounding nidopallium (N), and in the robust nucleus of the arcopallium (RA; Figs. 3F, 4I), compared to the surrounding arcopallium (A). In male area X, low levels of Cntnap2 were in sharp contrast to transcript abundance in the surrounding striatum (St; Figs. 3B, 4C). Emulsion autoradiography of an additional male brain confirmed this expression pattern, revealing strong transcript enrichment in both male LMAN and MMAN and diminished levels in area X (Fig. 5C).
Fig. 3.
Cntnap2 transcript distribution marks song nuclei in the adult male zebra finch. In anterior forebrain, enriched Cntnap2 signals were detected in both LMAN and MMAN of hemicoronal (B) sections of male brain. A similar enrichment could not be detected in hemicoronal sections of female brain (D). Low Cntnap2 levels in male area X (B) stood in contrast to high transcript levels in surrounding St and uniform levels in the corresponding area of female St (D). In posterior brain, strong signals were detected in male (F) but not female (H) RA. Outside the song circuitry, robust Cntnap2 levels were detected in HA, N and St (B,D) in anterior brain, as well as in the NC and the subpallial areas of TeO, Cb and IM/IPc in posterior brain (F,H) of both sexes. For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 4.
Cntnap2 expression in parasagittal sections of adult male and female brain. Cntnap2 transcript is enriched in male song nuclei LMAN (C) and RA (I) and reduced in area X (C) compared to adjacent tissue. While elevated Cntnap2 hybridization signal could also be discerned in adult female LMAN (F), there was no corresponding enrichment in RA (L) and transcript levels appeared uniform in female striatum which lacks area X (F). Outside song circuitry transcript abundance was high in HA, N, St, TeO and Cb of both sexes. For abbreviations see Table 1. Scale bars = 1 mm.
Fig. 5.
Inverted darkfield images of emulsion autoradiography of coronal sections of anterior adult male brain confirmed strong signals in LMAN and MMAN and low signals in area X compared to adjacent tissue (C). In situ hybridization of parasagittal sections of adult female brain indicate that there is considerable variability in Cntnap2 levels in LMAN, as identified by arrowheads in D and E. Unprocessed images in D and E, representing sections from different individuals, were taken under similar illumination conditions in order to emphasize the inter-individual variability in hybridization signals in female LMAN. For abbreviations see Table 1. Scale bar = 1 mm in C (applies to B and C).
In contrast, Cntnap2 levels in adult female LMAN appeared to vary. While we could not detect enhanced expression in female LMAN in any coronal sections hybridized to probe (Fig. 3D), in parasagittal sections of two additional female brains, Cntnap2 levels in LMAN appeared increased above those in surrounding N (Fig. 4F), more so in sections from one of the female brains, compared to another (Fig. 5D vs E). In adult female RA, Cntnap2 hybridization signal was similar to or diminished compared to surrounding A (Figs. 3H, 4L). Moreover, Cntnap2 transcript was uniformly distributed in female St, which lacks area X (Figs. 3D, 4F). For a summary of relative Cntnap2 expression levels in song nuclei and adjacent brain regions in young and adult birds see Table 4.
Table 4.
Summary of relative levels1 of Cntnap2 expression in selected song-related regions and adjacent tissue of male and female zebra finch brain.
| Age (days posthatch) | 15d | 25d | 35d | 50d | 75d | Adult | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | M | F | M | F | M | F | M | F | M | F |
| Telencephalon | ||||||||||||
| HA | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| HD | + | + | + | + | + | + | + | + | + | + | + | + |
| M | + | + | + | + | + | + | + | + | + | + | + | + |
| LMAN | +++ | ++ | +++ | ++ | +++ | +++ | ++++ | ++++ | ++++ | +++ | ++++ | +++ |
| N | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| X | ++ | - | ++ | - | ++ | - | ++ | - | ++ | - | ++ | - |
| St | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| NC | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| A | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| RA | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++++ | +++ | ++++ | ++ |
| HVC | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Diencephalon | ||||||||||||
| DTZ | ++ | ++ | ||||||||||
| SpM | ++++ | ++++ | ||||||||||
| SpL | ++++ | ++++ | ||||||||||
| Midbrain | ||||||||||||
| TeO (layer 13) | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| ICo/MLd | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| IM/IPc | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Hindbrain | ||||||||||||
| Cb | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
Transcript levels were semi-quantitatively estimated by densitometric measurements of in situ film hybridization signals in single sections of individual birds using AxioVision software. Levels of relative expression were then classified according to a subjective scale as ++++ (very high), +++ (high), ++ (moderate), + (low) or – (not detectable or structures not present). For abbreviations of brain structures, see Table 1.
Developmental expression of Cntnap2
Compelled by the finding of site-specific sexual dimorphism of Cntnap2 expression in the adult songbird brain, we proceeded to characterize gene expression throughout post-hatch brain development, at key stages in the ontogeny of song nuclei and vocal learning. Thus, to best capture whether changes in Cntnap2 transcript distribution may be linked to neuroanatomical changes and functional specialization associated with the formation of song circuitry, we conducted in situ hybridization analyses at post-hatch day (d) 1, 6, 15, 25, 35, 50 and 75. For a summary of changes in the relative expression levels of Cntnap2 during male and female post-hatch development see Table 4. Notably, both during the formation and maturation of song learning circuitry, changes in Cntnap2 transcript levels appear to track the structural and functional changes occurring within the song nuclei themselves, albeit with some distinctions, noted below.
Lateral magnocellular nucleus of the anterior nidopallium
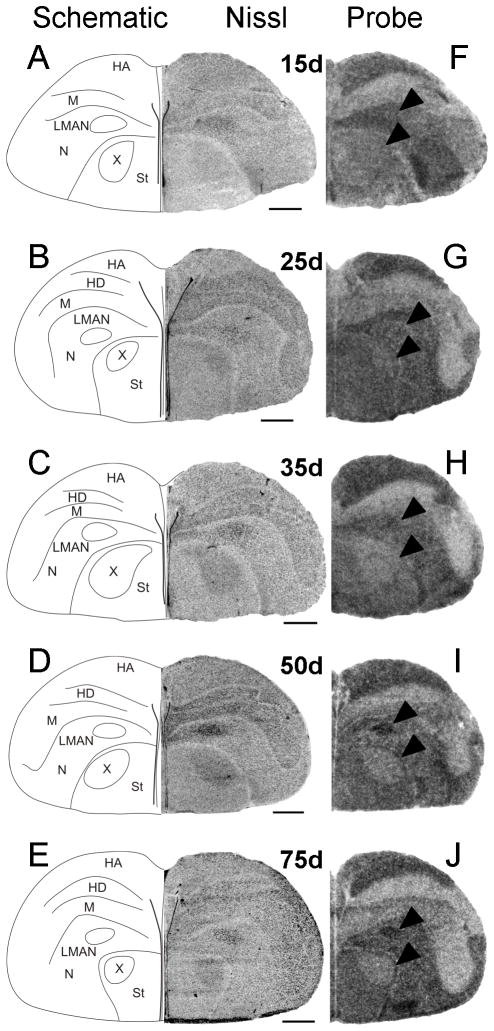
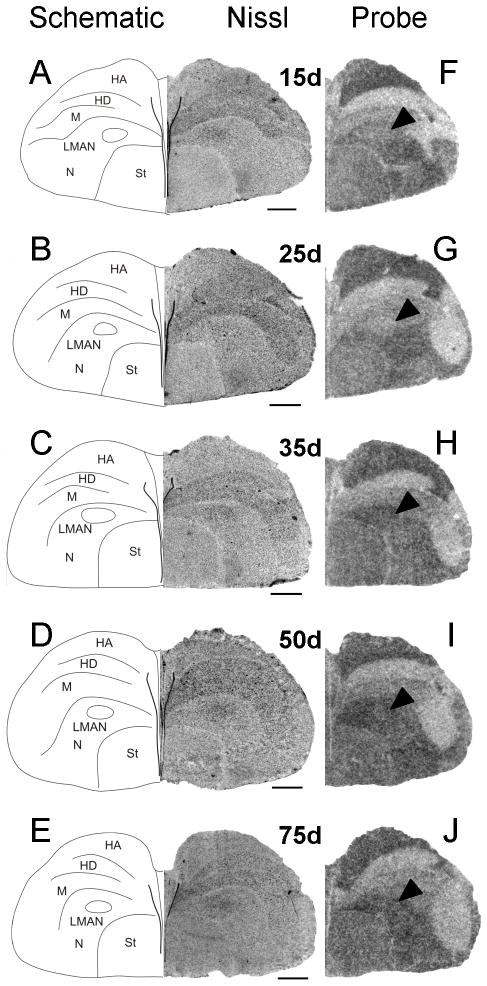
Between 15 and 35d, Cntnap2 hybridization signals in both male (Fig. 6F–H) and female (Fig. 7F–H) LMAN were indistinguishable from or appeared slightly lower (Fig. 9G) than those in the surrounding N which was robustly labeled in both sexes. However, at 50 and 75d, Cntnap2 transcript levels in LMAN were enriched in males, with hybridization signals becoming even stronger than the surrounding N (Fig. 6I,J). In contrast, Cntnap2 signals in female LMAN were virtually indistinguishable from surrounding N, indicating an absence of comparable transcript enrichment at the same time points (Fig. 7I,J). To increase the resolution of hybridization signal and potentially detect more subtle changes in Cntnap2 transcript levels in male and female LMAN at 35d, we performed emulsion autoradiography analyses. As with the film autoradiograms, no differences in emulsion grain labeling were detected when comparing hemicoronal sections that included male (Fig. 8C) and female (Fig. 8E) LMAN at this age.
Fig. 6.
Developmental pattern of Cntnap2 expression in hemicoronal sections of male anterior forebrain. Prior to 50d, Cntnap2 signals in LMAN (F,G,H) were similar to those in the surrounding N. From 50–75d, Cntnap2 signals in LMAN were higher than those in the robustly labeled region of N. Transcript levels in area X of developing males were lower than in the surrounding St. This contrast was particularly evident at 50 (I) and 75d (J). Robust expression was also detected in HA, N and St throughout development. Arrowheads point to male LMAN and area X as identified in A–E. For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 7.
Developmental pattern of Cntnap2 expression in hemicoronal sections of female anterior forebrain. From 15 to 75d, Cntnap2 signals in female LMAN (F–J) were comparable or slightly decreased to the abundant transcript levels present in the surrounding N. Female St was robustly labeled by the Cntnap2 probe (F–J) and no attenuation of transcript hybridization signal was detected in the striatal region corresponding to male area X (which females lack). Arrowheads point to female LMAN as identified in A-E. For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 9.
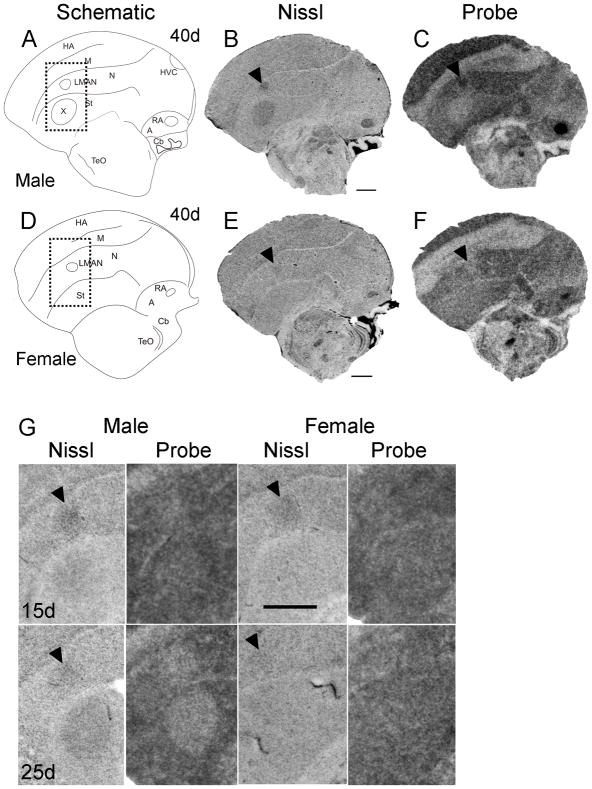
Onset of sexual dimorphism in Cntnap2 expression in zebra finch song circuitry. At ~40d Cntnap2 probe hybridization signals in male LMAN and RA (C) were enriched above those in the surrounding pallial regions, which were also robustly labeled. In male area X (B), low Cntnap2 hybridization signal was in contrast to transcript abundance in surrounding St (C). Of note, although transcript was present in this region, Cntnap2 levels were not enriched in male HVC (B) compared to surrounding pallial area (C). Cntnap2 levels did not appear enriched in LMAN (F) of ~40d female and were uniformly distributed in the St which lacks area X (F). Probe signal was enriched in the smaller female RA (F) compared to surrounding A.
At 15 and 25d no sex differences in Cntnap2 expression were detected in LMAN (G). In male song nucleus area X there is indication of downregulation of Cntnap2 transcript at 15d which becomes more apparent at 25d (G). Rectangles outlined with a dotted line in schematic drawings A and D indicate the region of higher magnification Nissl and autoradiography parasagittal images of developing male and female brain depicted in G. Arrowheads in B,C,E,F and G point to male and female LMAN. For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 8.
Inverted darkfield images of emulsion autoradiography sections confirm brightfield findings of Cntnap2 hybridization in LMAN and area X. At 35d, Cntnap2 emulsion grain signals in both male (C) and female (E) LMAN appeared slightly diminished or similar compared to transcript levels in the surrounding N. Attenuated labeling in male area X (C), a song nucleus that females lack (D), stood in contrast to transcript abundance in both male (C) and female (E) St. Arrowheads point to the location of male (C) and female (E) LMAN, as identified on adjacent Nissl-stained sections (B,D). For abbreviations, see Table 1. Scale bar = 1 mm.
To better define the age at which Cntnap2 signals become enriched in males, we performed additional in situ hybridization analyses of parasagittal sections of ~40d male brain. By this time point, Cntnap2 labeling of male LMAN had increased above the levels of surrounding N (Fig. 9C). This enrichment was not observed in parasagittal sections containing LMAN of a comparably aged female (Fig. 9F), providing evidence for an even earlier onset of sexual dimorphism of Cntnap2 expression in LMAN than we had initially detected (i.e. 40 vs. 50d). This finding contrasted with the lack of sex differences in LMAN at earlier time points (15 and 25d), seen in analyses of parasagittal sections of developing male and female brain (Fig. 9G).
Area X of striatum
From 15 to 75d, Cntnap2 levels in male area X appeared attenuated compared to the robust expression in the surrounding St (Figs. 6F–J, 9G). The reduced Cntnap2 hybridization signals within area X were apparent as early as 15 and 25d (Fig. 9G) and were especially evident after 35d, rendering the contour of this song nucleus clearly discernible from the rest of St (Fig. 6I,J). In contrast, from 15 to 75d, females (who lack area X) exhibited uniform and robust Cntnap2 transcript labeling throughout St (Fig. 7F–J). As expected from film autoradiograms, emulsion grain signals reconfirmed the presence of low transcript levels in male area X, compared to surrounding St (Fig. 8C), and uniform transcript distribution within female St (Fig. 8E).
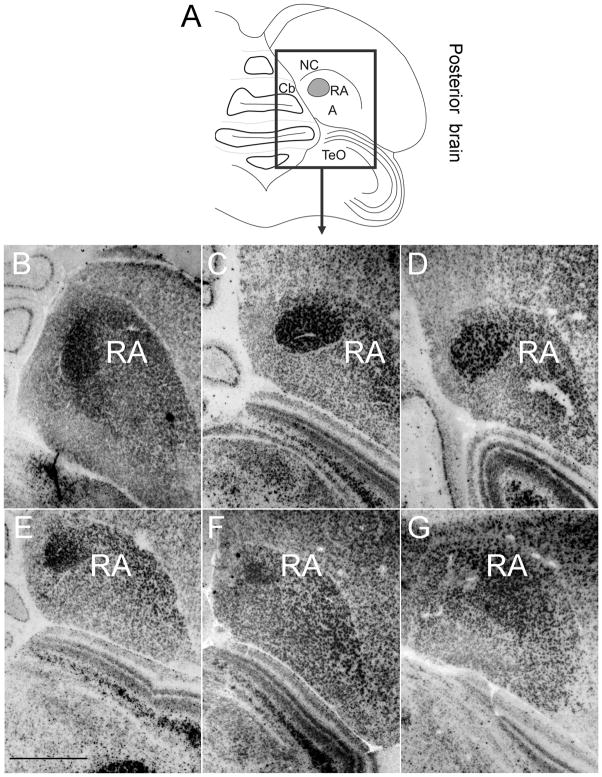
Robust nucleus of the arcopallium
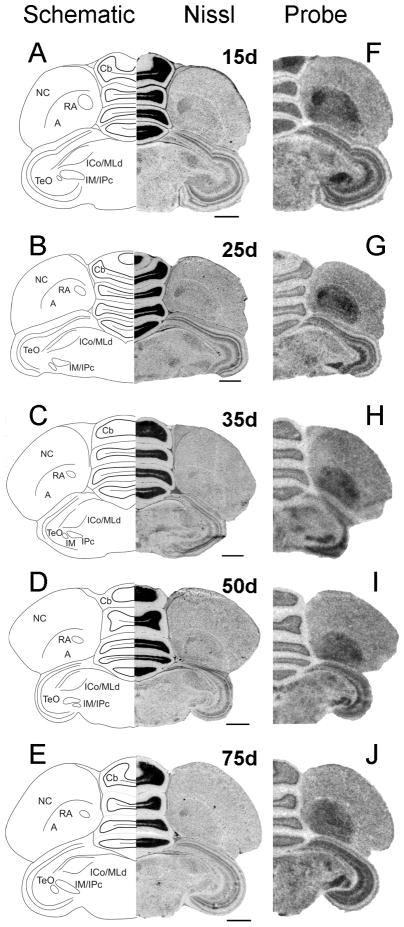
Nissl-staining of coronal sections of male (Fig. 10A) and female (Fig. 10B) posterior brain allowed us to identify song nucleus RA as early as 6d. At this age, Cntnap2 signal in RA was elevated in both sexes compared to surrounding A (Fig. 10C,D), representing the earliest sign of Cntnap2 enrichment detected within any song nucleus. At 15 and 25d, Cntnap2 hybridization signals were strong in both male (Fig. 11F,G) and female (Fig. 12F,G) RA. After 35d, however, Cntnap2 labeling of RA began to differ markedly between the two sexes. Between 35 and 75d, strong enrichment of Cntnap2 transcript persisted in male RA (Fig. 11H–J), in contrast to attenuation of Cntnap2 levels in female RA (Fig. 12H–J), resulting in hybridization signals that were indistinguishable from those in the surrounding A. Emulsion autoradiographic signals confirmed these findings. At 15 and 35d, and in the adult, strong hybridization signals were present in the enlarging male RA (Fig. 13B–D), in striking contrast to diminishing signals exhibited by the regressing female RA at the same time points (Fig. 13E–G).
Fig. 10.
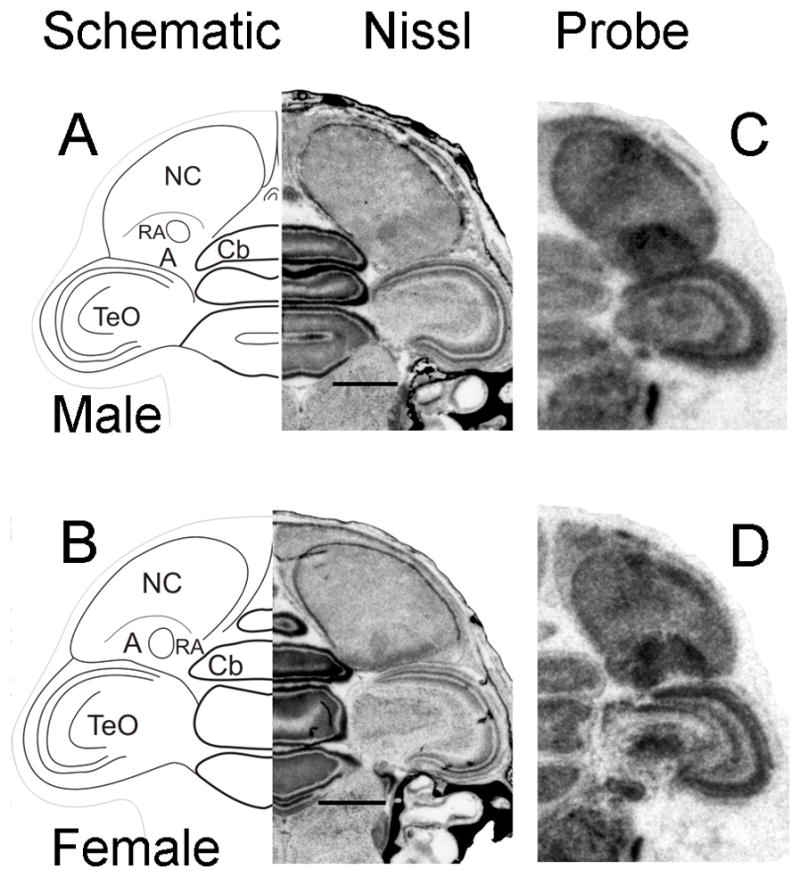
Cntnap2 transcript is detected early in the development of song nucleus RA in zebra finch brain. At 6d, Cntnap2 hybridization signals in RA were enriched above those in A in hemicoronal sections of both male (C) and female (D) posterior brain, as identified on adjacent Nissl-stained sections (A,B). For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 11.
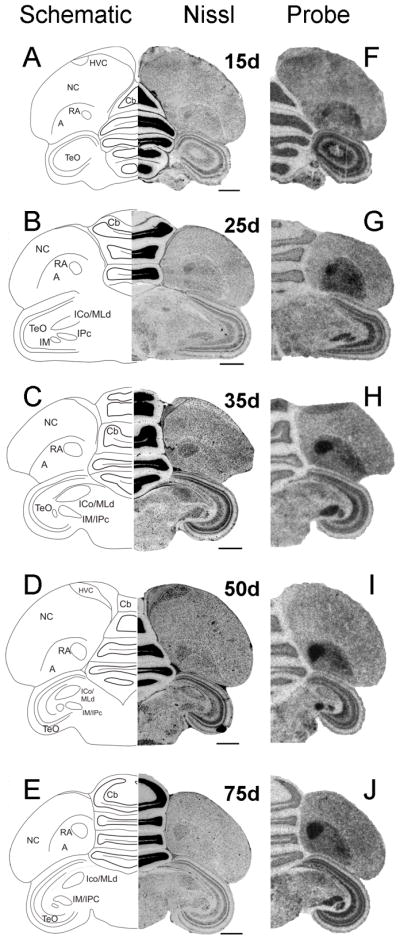
Developmental pattern of Cntnap2 expression in male posterior brain. Throughout development, Cntnap2 transcript distribution revealed a consistently robust hybridization signal in hemicoronal sections of male RA (F–J), as identified based on adjacent Nissl-stained sections (A–E). Strong Cntnap2 signals were also present in Cb, TeO and tectal nuclei IM/IPc (F–J). For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 12.
Developmental pattern of Cntnap2 expression in female posterior brain. Cntnap2 probe strongly labeled female RA from 15d until ~35d (F–H). Thereafter, Cntnap2 signals were diminished (I,J), rendering female RA indistinguishable from surrounding A, as identified based on adjacent Nissl-stained sections (A–E). Robust signals were also detected in the Cb, TeO and tectal nuclei IM/IPc throughout development (F–J). For abbreviations, see Table 1. Scale bars = 1 mm.
Fig. 13.
Inverted darkfield images of emulsion radiography signals confirm brightfield findings. In male RA, robust Cntnap2 hybridization signal was detected at 15 (B) and 35d (C) and in the adult brain (D). In contrast, in the female RA, hybridization signal diminished between 15 (E) and 35d (F), with further attenuation in the adult brain (G). For abbreviations, see Table 1. Scale bar = 1 mm.
Dorsal thalamic zone
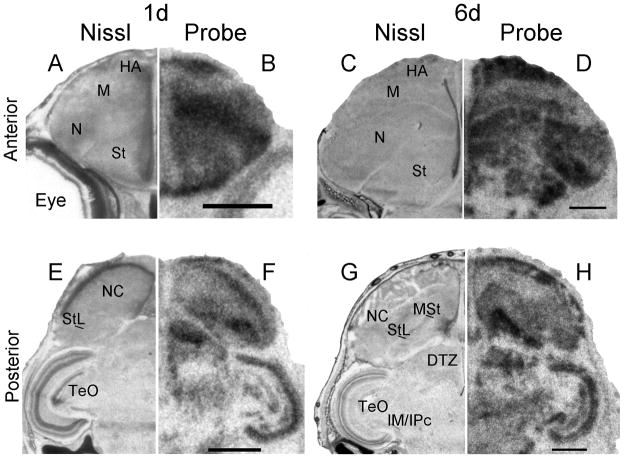
In the diencephalon, Cntnap2 transcript levels appeared relatively increased in the dorsal thalamic zone (DTZ) which contains the medial part of the dorsolateral thalamic nucleus (DLM), a key component of the song circuit. While specific enrichment could not be attributed to DLM itself (Fig. 14J), elevated signals were detected within DTZ, both in the developing brain, at 1 (Fig. 14F) and 6d (Fig. 14H), and in the adult (Fig. 14L).
Fig. 14.
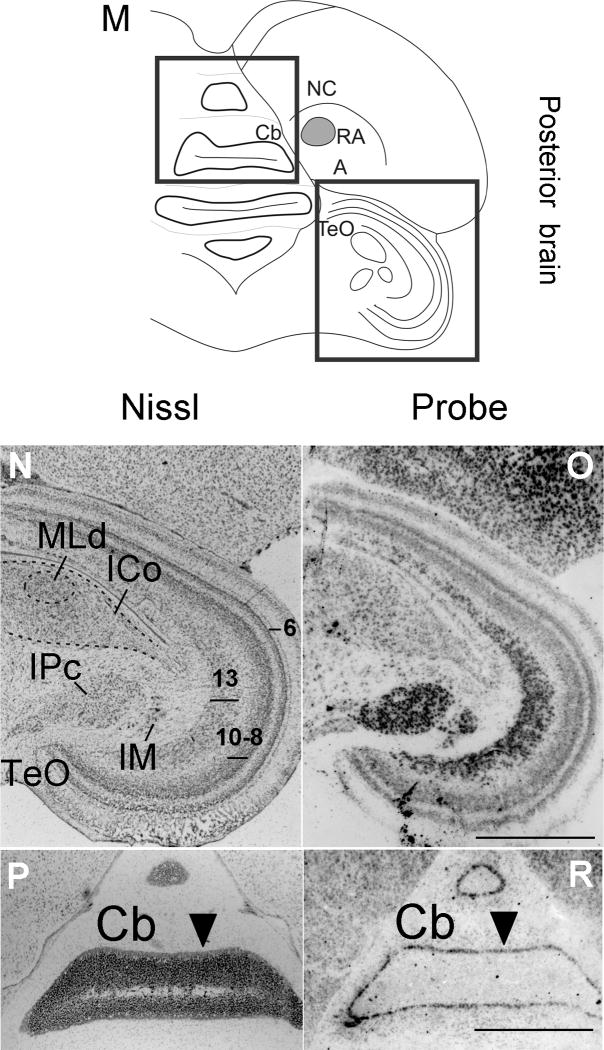
Cntnap2 expression outside the song system in developing and adult male zebra finch brain. High levels of Cntnap2 were detected early in development, in both anterior (B) and posterior (F) hatchling (1d) brain, highlighting robust expression in HA, N, NC, St (including MSt and StL), DTZ and TeO. A similar Cntnap2 expression pattern was conserved in anterior (D) and posterior (H) male brain at 6d. The same structures were labeled by Cntnap2 probe in female brain (not shown). In the adult, Cntnap2 hybridization signals were enriched in NC, MSt, StL, SpL (J), Hb, DTZ and SpM (L), TeO and IM/IPc (J,L). Inverted darkfield images of emulsion autoradiograhy of posterior male brain revealed robust labeling of TeO layers 6, 8–10 and 13 and tectal nuclei IM and IPc (O), as well as in the Purkinje cell layer of Cb (R). Arrowheads in P and R point to the Purkinje cell layer. More attenuated emulsion grain signals were detected in the midbrain nuclei ICo/MLd (O). For abbreviations, see Table 1. Scale bars = 1 mm.
Song nucleus HVC
While Cntnap2 signals were present in HVC, interestingly, there was no enrichment within this region in males, and transcript levels were similar to those of the surrounding N throughout development (Figs. 9C, 11F,I). This represents a notable exception to the punctuated expression of Cntnap2 in the other male song nuclei. No enrichment was detected in the female HVC in either the developing or adult brain.
Cntnap2 expression outside the song system
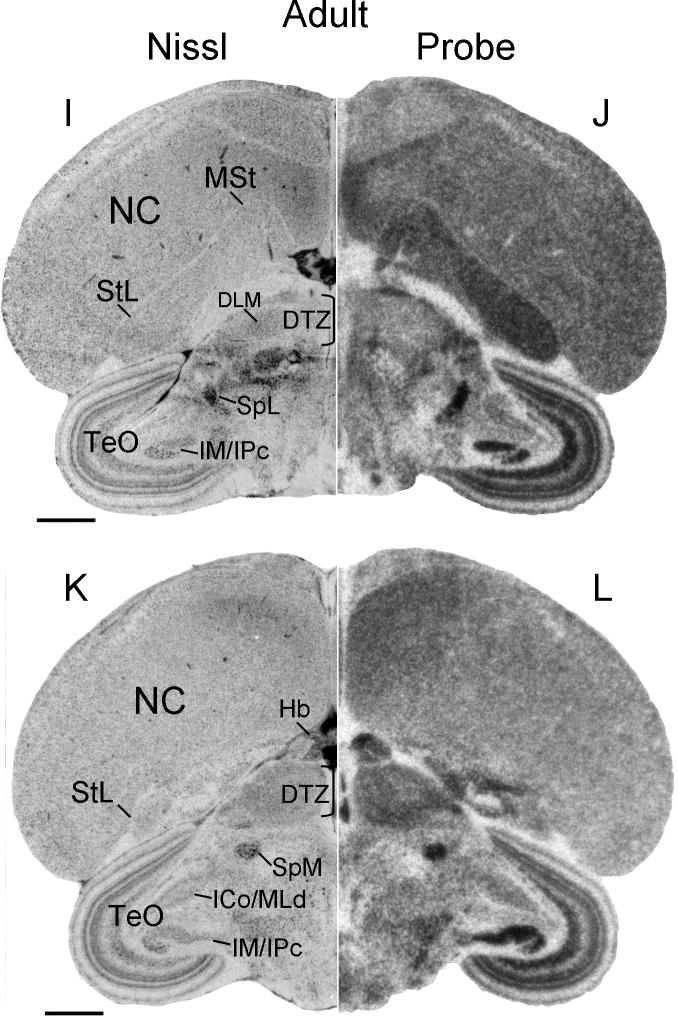
Outside the song system, both during development and in the adult, Cntnap2 transcript distribution in both sexes was widespread, showing regional differences, but lacking focal enrichment or attenuation like that observed for the song system. In anterior brain, robust Cntnap2 hybridization signals were detected in the apical part of the hyperpallium (HA), N and St (including medial and lateral St), in both developing (Figs. 6F–J, 7F–J, 9C,F, 14B,D) and adult (Figs. 3B,D, 4C,F) birds. In posterior brain, Cntnap2 transcript was robustly expressed in caudal N and A, in both developing (10C,D, 11F–J, 12F–J, 14F,H) and adult (Figs. 3F,H, 4I,L, 14J,L) birds. Transcript enrichment was also detected in habenula (Fig. 14L), cerebellum (Figs. 3F,H, 10C,D, 11F–J, 12F–J) – with strong signals in the Purkinje cell layer (Fig. 14R) – and optic tectum (Figs. 3F,H, 10C,D, 11F–J, 12F–J), with highest expression in layers 6, 8–10 and 13 (Fig. 14O).
Cntnap2 transcript enrichment was also detected in several midbrain structures dedicated to processing visual and auditory information, such as diencephalic lateral (SpL; Fig. 14J) and medial (SpM; Fig. 14L) spiriform nuclei, tectal nuclei isthmus magnocellular/parvocellular (IM/IPc; Figs. 3F,H, 11G–J, 12F–J, 14J,L,N) and, at lower levels, in the dorsal lateral mesencephalic nucleus within the nucleus intercollicularis (ICo/MLd; Figs. 3F,H, 11G–J, 12F–J). Posterior brain areas implicated in processing auditory input and feedback during song learning and production that comprise the auditory lobule did not appear to show altered Cntnap2 signal compared to surrounding pallial areas (Fig. 4C,F).
DISCUSSION
Here we report an extensive analysis of Cntnap2 transcript distribution in the adult zebra finch brain and during the development of the song control system. Consistent with genetic data from human providing support for a role in language performance and disorders in which language is compromised (Abrahams et al., 2007; Alarcón et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008; Vernes et al., 2008), Cntnap2 shows punctuated expression within songbird brain subregions specialized for song learning and production.
Sexual dimorphism of Cntnap2 expression in the adult
In the adult zebra finch, Cntnap2 is differentially expressed in three of the male song control nuclei, consistent with the sexual dimorphism of these regions and of vocal learning (Nottebohm and Arnold, 1976; Gurney, 1982; Wade and Arnold, 2004). Cntnap2 transcript distribution results in a sharp delineation of song control circuitry, highlighting both a song nucleus-specific enrichment (male lateral magnocellular nucleus of the anterior nidopallium, LMAN, and male robust nucleus of the arcopallium, RA) and attenuation (male area X) of transcript levels within the telencephalic components of the song control system. The functional connectivity and contribution to song learning and production of these nuclei has been studied extensively. While LMAN and area X are part of the anterior forebrain pathway (AFP), a circuit required for song modification, RA is a key component of the posterior vocal pathway controlling song production (Nottebohm and Arnold, 1976). In young male zebra finches, lesions of male LMAN result in premature stereotypy of song, indicating a critical role for LMAN in song learning during development (Bottjer et al., 1984; Scharff and Nottebohm, 1991), while lesions of area X lead to highly variable and unstable song (Sohrabji et al., 1990; Scharff and Nottebohm, 1991). In contrast, ablation of area X in adults seems to have little effect on song production (Sohrabji et al., 1990; Scharff and Nottebohm, 1991), while lesions of LMAN prevent adult song plasticity (Williams and Mehta, 1999; Brainard and Doupe, 2000; Kao et al., 2005). Lesions of the posterior vocal pathway at either HVC or RA, at any time in life, cause severe disruption or even complete cessation of song (Nottebohm et al., 1976; Simpson and Vicario, 1990).
Enhanced Cntnap2 expression is also detected in the male medial magnocellular nucleus of the anterior nidopallium, MMAN, a small nucleus medially adjacent to LMAN (Fig. 3B) but characterized by different connectivity via efferent projections to HVC (Nottebohm et al., 1982; Bottjer, 1989; Vates et al., 1997) and afferent input from the posterior part of the dorsomedial thalamic nucleus (DMP; Foster et al., 1997; Vates et al., 1997). Similar to LMAN, efferent projections to area X have also been described for MMAN (Vates and Nottebohm, 1995). Connections to pallial, striatal and thalamic subregions implicated in song control, suggest that MMAN plays a role in vocal behavior, as supported by lesion studies (Foster and Bottjer, 2001), but the exact function of this subregion has yet to be determined. In the adult female, the variability in Cntnap2 levels observed in LMAN is puzzling (Fig. 3D vs 5D,E), given that during development, transcript levels in female LMAN appeared consistent across the time points sampled here (see below). The possibility that elevated transcript levels in certain individuals may reflect an adult function for the female LMAN, e.g. in song perception related to mate choice (Hamilton et al., 1997), remains to be tested.
In the posterior pathway for song production, a notable exception to the delineation of song circuitry by Cntnap2 was provided by premotor nucleus HVC, which showed no reliably detected differences in transcript levels compared to surrounding pallial areas. Homogenous expression levels across HVC and adjacent pallial regions is intriguing, given the striking modulation of hybridization signals detected for its targets, song nuclei RA and area X, and the marked sexual dimorphism of this song control region in adults (Nottebohm et al., 1976).
The restricted pattern of Cntnap2 expression within mature song control nuclei occurred against a backdrop of conserved transcript distribution in the rest of male and female brain, within the regions of the apical part of the hyperpallium (HA), nidopallium (N), caudal nidopallium (NC), arcopallium (A), striatum (St), cerebellum (Cb) and optic tectum (TeO). In both sexes, Cntnap2 transcript enrichment was also detected within several structures involved in sensory processing, such as the intercollicular nucleus (ICo), which includes the dorsal part of the lateral mesencephalic nucleus (MLd), together known as ICo/MLd, the diencephalic lateral (SpL) and medial (SpM) spiriform nuclei, and the tectal nuclei isthmus magnocellular and isthmus parvocellular (IM/IPc). MLd, a homologue of the mammalian inferior colliculus (Karten, 1967), is a main relay site for sensory information carried by avian auditory ascending and descending pathways (Karten, 1967; Wild et al., 1993). In addition, in songbirds the shell of MLd receives dense projections from the RA cup (Mello et al., 1998), suggesting a role for this nucleus in sensorimotor integration of auditory inputs and motor commands originating in song control regions. While SpL has an established role in processing visual information via connections to TeO (Toledo et al., 2002), the function of SpM, which in songbirds is known to receive afferent inputs from dorsal A (Bottjer et al., 2000) and to send projections to Cb (Karten and Finger, 1976), remains to be established. Tectal IM and IPc represent important components of the avian visual system and have reciprocal connections with TeO layers (Wang et al., 2004). Cntnap2 expression in these nuclei specialized for processing auditory and visual information, as well as relay information to and from motor command centers, seem to implicate this gene in the functional specialization of structures important for feedforward and/or feedback of sensorimotor information.
Developmental expression of Cntnap2 in song control regions
Consistent with largely monomorphic developmental changes in LMAN between 15 and 35d (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996), levels of Cntnap2 transcript in male and female LMAN were similar to or appeared slightly lower than those in the surrounding region of N. While LMAN borders can not be reliably distinguished on Nissl-stained sections before 10d (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996), between 15 and 25d, male and female LMAN appear to be comparably sized (Nixdorf-Bergweiler and Von Bohlen und Halbach, 2005) and undergo accelerated growth until they reach peak volume, at ~20 and ~30d, respectively (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996). This time interval marks the onset of the sensory acquisition phase of song learning and precedes the stage when young male zebra finches begin to produce their first learned vocalizations (Immelmann, 1969). Starting at 30–35d, males enter the vocal practice or sensorimotor stage of song learning (Immelmann, 1969; Price, 1979), during which LMAN has been shown to take on a significant role (reviewed in Brainard and Doupe, 2002), and exhibit premotor activity responsible for driving the production of plastic juvenile song (Aronov et al., 2008).
After reaching peak volume, both male and female LMAN begin to regress. Male LMAN shrinks to half of its original volume by ~50d and female LMAN regression follows with a delay of ~10d (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996). In the male, developmental changes after ~30d have been attributed to processes of cell death (Bottjer et al., 1985; Bottjer and Sengelaub, 1989; Korsia and Bottjer, 1989) and subsequent enlargement of remaining LMAN neurons (Nixdorf-Bergweiler, 1998), as well as refinement of afferent (from DLM; Iyengar et al., 1999; Iyengar and Bottjer, 2002a) and efferent (to RA; Herrmann and Arnold, 1991; Iyengar et al., 1999) connections. The final stage of regression of male LMAN also coincides with the time when lesions of this forebrain nucleus begin to lose their effectiveness in disrupting vocal learning (Bottjer et al., 1984). Given that cell loss in male LMAN appears complete by 37d (Korsia and Bottjer, 1989), the increase in Cntnap2 levels in this song nucleus at ~40d onward is likely explained by increased gene expression in surviving LMAN neurons and appears to track the developmental switch in the function of this song nucleus. In contrast, during the development of female LMAN, Cntnap2 transcript levels did not become enriched relative to surrounding N. While this is consistent with the behavioral difference in song learning between the two sexes, as noted above, there was some variability in the levels of gene expression in the adult female LMAN.
In our study, area X was first identified on Nissl-stained coronal sections of male brain at 15d and showed reduced expression of Cntnap2 at all post-hatch developmental time points investigated (15–75d). Despite the enlargement of this song nucleus from 15d until the adult size is attained at ~40–50d (Nixdorf-Bergweiler, 1996; Bottjer et al., 1985), no corresponding increases in transcript levels were detected. Attenuated Cntnap2 levels in area X throughout development contrasted sharply with transcript abundance in the surrounding region of St. The lower signal in area X (Fig. 8C) is intriguing since the cell packing density there, as evidenced by denser Nissl stain (Nottebohm et al., 1976), is higher than in the outlying striatum. Given equal expression levels per cell, one would expect increased signal in area X. Reduced expression in area X relative to surrounding regions (which contain similar cell types; Farries and Perkel 2000, 2002) suggests that low transcript levels may be important for the development of this striatal nucleus. While the sexual dimorphism in area X may be expected, given the absence of this song nucleus in female zebra finches, within males the contrast in Cntnap2 transcript between area X and adjacent tissue is striking.
As early as 6d, Cntnap2 signals were elevated in RA compared to surrounding A, and were even more robust by 15d in both sexes. Consistent with a lack of significant sex differences in volume and comparable growth rates of this song nucleus in both sexes in the first 3 weeks post-hatch (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996), strong Cntnap2 signals were detected in both male and female RA at 25d. By ~20d, female RA is expected to have reached its peak volume (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996). Following 20d, male RA continues to enlarge and reaches its maximum volume by ~50d (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996) while female RA undergoes a dramatic regression (Konishi and Akutagawa, 1985; Bottjer et al., 1985; Nixdorf-Bergweiler, 1996), culminating in shrinkage to about half of its maximum volume around the same time (Bottjer et al., 1985; Nixdorf-Bergweiler, 1996). Importantly, at ~35d, nerve fibers originating from HVC synapse onto RA neurons in male, but not female, zebra finches, resulting in the functional activation of the brain pathway specialized for song production (Konishi and Akutagawa, 1985; Mooney and Rao, 1994; Holloway and Clayton, 2001).
Reflecting these developmental differences, Cntnap2 labeling of RA began to differ markedly between the two sexes after 25d. In males, Cntnap2 levels were enriched in RA, compared to surrounding A at all time points investigated, matching the developmental enlargement (Bottjer et al., 1985; Konishi and Akutagawa, 1985; Nixdorf-Bergweiler, 1996) and predicted increase in synaptic connectivity (Konishi and Akutagawa, 1985; Mooney and Rao, 1994) of the nucleus. In contrast, Cntnap2 signals in female RA became diminished by 35d and appeared virtually indistinguishable from those of the surrounding A at 50 and 75d, likely reflecting the substantial developmental regression and associated processes of cell death (Konishi and Akutagawa, 1985) 24 occurring during this time interval. Thus, the divergent developmental trajectories of male and female RA and the accompanying changes (enrichment in male and attenuation in female) in Cntnap2 levels after 25d set in place a sexual dimorphism of gene expression that is preserved into adulthood.
Role of Cntnap2 in the development and function of song circuitry
In the zebra finch songbird species, the sexual dimorphism of song circuitry is tightly linked to behavioral sex differences (males sing a courtship song, females do not), making birdsong an advantageous model to understand both the neuroendocrine and genetic bases for the emergence of sexually-dimorphic brain structures and vocal learning (Nottebohm and Arnold, 1976; Gurney and Konishi, 1980; Gurney, 1982; Wade and Arnold, 2004). In the case of steroid-related genes, the one that encodes the androgen receptor (AR) is highly enriched in male song nuclei which are more responsive to androgens than in females, both in the developing and adult brain (Kim et al., 2004). Enrichment of AR transcript (and androgen binding; Arnold et al., 1976) within the male song system and its absence in corresponding areas in the female brain provided compelling evidence for the role of hormones in the sexual differentiation of the steroid-sensitive song circuitry.
To date, most gene expression analyses in songbird brain have focused on revealing the cellular and molecular bases of the specialized ability of song-related circuitry to subserve the capacity for learned vocalizations (see for review White, 2009). Thus, the dynamic expression in male song nuclei has been reported for “motor-driven” whose levels change during singing, first exemplified for the immediate early gene zenk (zif-268, egr-1, NGFI-A and krox-24; Jarvis and Nottebohm, 1997) and now shown for several other genes (Poopatanapong et al., 2006; Wada et al., 2006).
The investigation of language-related genes in song control circuitry, represents a powerful and complementary approach to using the specialized anatomy of the song control system to identify molecules important for vocal learning. For example, study of the transcription factor FOXP2, known to be disrupted in a severe speech and language disorder (Lai et al., 2001), as well as its downstream target CNTNAP2, in the zebra finch song circuitry (Haesler et al., 2007; this study), provide a special opportunity for dissecting the genetic bases of learned vocalization, including song and speech. We previously showed that zebra finch FoxP2 has a parallel expression pattern to human FOXP2 and overlapping distribution with FoxP1 (Teramitsu et al., 2004), encoding a related FoxP subfamily member with which FoxP2 can interact to bind DNA promoter regions (Carlsson and Mahlapuu, 2002; Li et al., 2004; Shu et al., 2001). The finding that FoxP1 is expressed in a sexually dimorphic pattern within zebra finch song circuitry (Teramitsu et al., 2004) lent support to the idea that, like FoxP2, FoxP1 may also play a role in vocal learning and could contribute to altered development and function in human language – related disorders (Teramitsu et al., 2004). This prediction has recently received support in the discovery of a de novo chromosomal deletion of only the FOXP1 gene resulting in haploinsufficiency that was associated with severe language impairment in a young patient diagnosed with Chiari I malformation and other congenital physical abnormalities (Abdul-Rahman et al., 2008).
Cntnap2 and language-related neuropathology
The mechanisms whereby disruption of Cntnap2 gene results in neural dysfunction remain largely unknown. Abnormal or common genetic variation in Cntnap2 has been uncovered in a number of neural diseases, as diverse as autism spectrum disorder (Alarcón et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008), specific language impairment (Vernes et al., 2008), Gilles de la Tourette syndrome (Verkerk et al., 2003), schizophrenia and epilepsy (Friedman et al., 2007). Cntnap2 has an identified role as a scaffold protein at the juxtaparanodal regions of myelinated axons (Poliak and Peles, 2003). However, the focal enrichment of CNTNAP2 in language-related structures and circuits in human occurs at a pre-myelination mid-gestation stage (Abrahams et al., 2007). This raises the possibility that other aspects of human brain development that precede or are unrelated to the myelination process could be modulated by this molecule. In songbirds, the only available data on this topic suggest that myelin is absent in axonal processes of HVC and LMAN neurons at 20d and that myelination starts shortly after 20d in LMAN (Nixdorf-Bergweiler and Von Bohlen und Halbach, 2004). Given the strong Cntnap2 expression before 20d in song nuclei and the rest of the brain, it appears that, as suggested in the human, there might also be additional developmental roles for Cntnap2 in songbirds.
Our discovery of punctuated expression for Cntnap2 within song control regions reinforces the importance of the songbird model in exploring the biological roles of genes disrupted in language and communication disorders. Understanding the genetic bases of vocal learning may ultimately reveal both shared and unique neuromolecular networks that have emerged in the evolution of learned vocal behavior.
Acknowledgments
We are grateful to Mark Mobilia and Tim Masloski from Carl Zeiss MicroImaging, Inc., for sharing technical expertise. William Grisham provided useful assistance with identification of neuroanatomical structures. We also thank Ikuko Teramitsu and Amy Poopatanapong for technical support with tissue sectioning.
Grant Sponsors: National Institute of Health (RO1 MH070712, R21 HD06527, RO1 MH64547, U54 MH68172, P50 HD055784), Autism Speaks and The Foundation for Psychocultural Research.
References
- Abdul-Rahman O, Zimmerman H, Justice N, Lese-Martin C. Haploinsufficiency of FOXP1 is associated with Chiari I malformation and speech/language disorder. Abstract presented at the annual meeting of The American Society of Human Genetics; November 11–15, 2008; Philadelphia, PA. 2008. http://www.ashg.org/2008meeting/abstracts/fulltext/f20013.htm. [Google Scholar]
- Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci U S A. 2007;104:17849–17854. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón M, Cantor RM, Liu J, Gilliam TC, Geschwind DH the Autism Genetic Research Exchange Consortium. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genetics. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Nottebohm F, Pfaff DW. Hormone concentrating cells in vocal control and other areas of the brain of the zebra finch (Poephila guttata) J Comp Neurol. 1976;165:487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Regional meta-analysis of published data supports linkage of autism with markers on chromosome 7. Mol Psychiatry. 2002;7:56–66. doi: 10.1038/sj.mp.4000922. [DOI] [PubMed] [Google Scholar]
- Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, Tanriover G, Abrahams BS, Duvall JA, Robbins EM, Geschwind DH, Biederer T, Gunel M, Lifton RP, State MW. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Sengelaub DR. Cell death during development of a forebrain nucleus involved with vocal learning in zebra finches. J Neurobiol. 1989;20:609–618. doi: 10.1002/neu.480200702. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Glaessner SL, Arnold AP. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. J Neurosci. 1985;5:1556–1562. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol. 2000;420:244–260. [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, Alvarez Retuerto AI, Imielinski M, Hadley D, Bradfield JP, Kim C, Gidaya NB, Lindquist I, Hutman T, Sigman M, Kustanovich V, Lajonchere CM, Singleton A, Kim J, Wassink TH, McMahon WM, Owley T, Sweeney JA, Coon H, Nurnberger JI, Li M, Cantor RM, Minshew NJ, Sutcliffe JS, Cook EH, Dawson G, Buxbaum JD, Grant SF, Schellenberg GD, Geschwind DH, Hakonarson H. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5(6):e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. Electrophysiological properties of avian basal ganglia neurons recorded in vitro. J Neurophysiol. 2000;84:2502–2513. doi: 10.1152/jn.2000.84.5.2502. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22:3776–3787. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: influences on vocal behavior in juveniles and adults. J Neurobiol. 2001;46:142–165. doi: 10.1002/1097-4695(20010205)46:2<142::aid-neu60>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol. 1997;382:364–381. doi: 10.1002/cne.903820305. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BHW, Knoers NV, Cahn W, Kahn RS, Edelmann L, Davis KL, Silverman JM, Brunner HG, van Kessel AG, Wijmenga C, Ophoff RA, Veltman JA. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2007;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- Gurney ME. Behavioral correlates of sexual differentiation in the zebra finch song system. Brain Res. 1982;231:153–172. doi: 10.1016/0006-8993(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5(12):e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KS, King AP, Sengelaub DR, West MJ. A brain of her own: a neural correlate of song assessment in a female songbird. Neurobiol Learn Mem. 1997;68:325–332. doi: 10.1006/nlme.1997.3781. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Arnold AP. The development of afferent projections to the robust archistriatal nucleus in male zebra finches: a quantitative electron microscopic study. J Neurosci. 1991;11:2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird vocalizations. Cambridge: Cambridge University press; 1969. pp. 61–74. [Google Scholar]
- Inda MC, DeFelipe J, Muñoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci U S A. 2006;103:2920–2925. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. Development of individual axon arbors in a thalamocortical circuit necessary for song learning in zebra finches. J Neurosci. 2002a;22:901–911. doi: 10.1523/JNEUROSCI.22-03-00901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. The role of auditory experience in the formation of neural circuits underlying vocal learning in zebra finches. J Neurosci. 2002b;22:946–958. doi: 10.1523/JNEUROSCI.22-03-00946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Viswanathan SS, Bottjer SW. Development of topography within song control circuitry of zebra finches during the sensitive period for song learning. J Neurosci. 1999;19:6037–6057. doi: 10.1523/JNEUROSCI.19-14-06037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman C, Horn ND, Molleston JP, Sokol DK. Gene associated with seizures, autism, and hepatomegaly in an Amish girl. Pediatr Neurol. 2009;40:310–313. doi: 10.1016/j.pediatrneurol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci U S A. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Karten HJ. The organization of the ascending auditory pathway in the pigeon (Columba livia) I. Diencephalic projections of the inferior colliculus (nucleus mesencephali lateralis, pars dorsalis) Brain Res. 1967;6:409–427. doi: 10.1016/0006-8993(67)90055-8. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Finger TE. A direct thalamo-cerebellar pathway in pigeon and catfish. Brain Res. 1976;102:335–338. doi: 10.1016/0006-8993(76)90888-x. [DOI] [PubMed] [Google Scholar]
- Kim Y-H, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985;315:145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- Korsia S, Bottjer SW. Developmental changes in the cellular composition of a brain nucleus involved with song learning in zebra finches. Neuron. 1989;3:451–460. doi: 10.1016/0896-6273(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Human speech and birdsong: communication and the social brain. Proc Natl Acad Sci U S A. 2003;100:9645–9646. doi: 10.1073/pnas.1733998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Li S, Weidenfeld J, Morrisey EE. Transcriptional and DNA binding activity of the FoxP1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol Cell Biol. 2004;24:809–822. doi: 10.1128/MCB.24.2.809-822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. On the nature and evolution of the neural bases of human language. Am J Phys Anthropol Suppl. 2002;35:36–62. doi: 10.1002/ajpa.10171. [DOI] [PubMed] [Google Scholar]
- Marler P. Song-learning behavior: the interface with neuroethology. Trends Neurosci. 1991;14:199–206. doi: 10.1016/0166-2236(91)90106-5. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vates GE, Okuhata S, Nottebohm F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata) J Comp Neurol. 1998;395:137–160. [PubMed] [Google Scholar]
- Mooney R, Rao M. Waiting periods versus early innervation: the development of axonal connections in the zebra finch song system. J Neurosci. 1994;14:6532–6543. doi: 10.1523/JNEUROSCI.14-11-06532.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA. Social interaction and sensitive phases for song learning: a critical review. In: Snowdon CT, Hausberger M, editors. Social Influences on Vocal Development. Cambridge: Cambridge University Press; 1997. pp. 7–22. [Google Scholar]
- Nixdorf-Bergweiler BE. Divergent and parallel development in volume sizes of telencephalic song nuclei in male and female zebra finches. J Comp Neurol. 1996;375:445–456. doi: 10.1002/(SICI)1096-9861(19961118)375:3<445::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE. Enlargement of neuronal somata in the LMAN coincides with the onset of sensorimotor learning for song. Neurobiol Learn Mem. 1998;69:258–273. doi: 10.1006/nlme.1998.3819. [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Von Bohlen und Halbach V. Major sex differences in the development of myelination are prominent in song system nucleus HVC but not LMAN. Anim Biol. 2004;54:27–43. [Google Scholar]
- Nixdorf-Bergweiler BE, Von Bohlen und Halbach V. Neuronal number in song nucleus LMAN remains high in female zebra finches throughout development and in adulthood. Acta Zoologica Sinica. 2005;51:257–267. [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Ramachandran B, Arnold AP. Expression of androgen receptor mRNA in the late embryonic and early posthatch zebra finch brain. J Comp Neurol. 2003;455:513–530. doi: 10.1002/cne.10510. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Salomon D, Berglund EO, Ohara R, Ranscht B, Peles E. Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J Neurosci. 2001;21:7568–7575. doi: 10.1523/JNEUROSCI.21-19-07568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poopatanapong A, Teramitsu I, Byun JS, Vician LJ, Herschman HR, White SA. Singing, but not seizure, induces synaptotagmin IV in zebra finch song circuit nuclei. J Neurobiol. 2006;66:1613–1629. doi: 10.1002/neu.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M, Beyer V, Schwaab I, Damatova N, Van’t Slot R, Prothero J, Holder SE, Haaf T. Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics. 2009 doi: 10.1007/s10048-009-0205-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Potter SC, Clarke L, Curwen V, Keenan S, Mongin E, Searle SM, Stabenau A, Storey R, Clamp M. The Ensembl analysis pipeline. Genome Res. 2004;14:934–941. doi: 10.1101/gr.1859804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PH. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol. 1979;93:260–277. [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Yang H, Zhang L, Lu MM, Morrisey EE. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001;276:27488–27497. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Qin W, Leggett MH. A minimally invasive procedure for sexing young zebra finches. J Neurosci Methods. 2007;164:116–119. doi: 10.1016/j.jneumeth.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Noredeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo CA, Pezzini R, Santos RC, Britto LR. Expression of AMPA-type glutamate receptors in pretectal nuclei of the chick brain. Brain Res Bull. 2002;57:359–361. doi: 10.1016/s0361-9230(01)00722-5. [DOI] [PubMed] [Google Scholar]
- Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos TA, Karvouni A, Zintzaras E, Ylisaukko-oja T, Peltonen L, Järvelä I, Ioannidis JP. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Mol Psychiatry. 2006;11:29–36. doi: 10.1038/sj.mp.4001750. [DOI] [PubMed] [Google Scholar]
- Vates GE, Nottebohm F. Feedback circuitry within a song-learning pathway. Proc Natl Acad Sci U S A. 1995;92:5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-“cortical” loops in the song system of oscine songbirds. J Comp Neurol. 1997;380:275–290. [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA the Tourette Syndrome Association International Consortium for Genetics. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcón M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Howard JT, McConnell P, Whitney O, Lints T, Rivas MV, Horita H, Patterson MA, White SA, Scharff C, Haesler S, Zhao S, Sakaguchi H, Hagiwara M, Shiraki T, Hirozane-Kishikawa T, Skene P, Hayashizaki Y, Carninci P, Jarvis ED. A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes. Proc Natl Acad Sci U S A. 2006;103:15212–15217. doi: 10.1073/pnas.0607098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann N Y Acad Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Wang Y, Major DE, Karten HJ. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus) J Comp Neurol. 2004;469:275–297. doi: 10.1002/cne.11007. [DOI] [PubMed] [Google Scholar]
- White SA. Genes for vocal learning. Brain Lang. 2009 doi: 10.1016/j.bandl.2009.10.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. J Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]