Abstract
Objective
We previously demonstrated that adherence of endometrial epithelial (EECs) and stromal cells (ESCs) to peritoneal mesothelial cells (PMCs) is partly regulated by ESC/EEC CD44 interactions with PMC associated hyaluronan. CD44, a transmembrane glycoprotein and major ligand for hyaluronan, has numerous splice variants which may impact hyaluronan binding. Here, we assessed whether ESCs and EECs from women with endometriosis demonstrate increased adherence to PMCs and examined CD44 splice variants’ potential role in this process.
Design
In vitro study.
Setting
Academic medical Center
Patient(s)
Fertility patients with and without endometriosis
Intervention(s)
Menstrual endometrium was collected from women with and without endometriosis confirmed surgically. The adherence of ESC/EECs to PMCs was measured. ESC/EEC CD44 splice variants were assessed using dot blot analysis.
Results
ESCs and EECs from women with endometriosis demonstrated increased adherence to PMCs. The predominant CD44 splice variants expressed by ESCs and EECs from women with and without endometriosis were v3, v6, v7, v8, v9, and v10. ESCs and EECs from women with endometriosis were more likely to express v6, v7, v8 or v9.
Conclusions
Increased eutopic endometrial-PMC adherence and CD44 splice variant expression may contribute to the histogenesis of endometriotic lesions. Elucidation of factors controlling this expression may lead to novel endometriosis therapies.
Keywords: Endometriosis, endometrium, CD44, hyaluronan, splice variant, adherence
INTRODUCTION
Sampson’s theory of retrograde menstruation is supported by the available scientific evidence to explain the pathogenesis of endometriosis (1). Despite retrograde menstruation in nearly all reproductive age women, only 10 % develop endometriosis (2). It has been postulated that the eutopic endometrium from women with endometriosis may differ in terms of ability to adhere and invade the mesothelium, resistance to apoptosis, differential gene and protein expression, hormone production and responsiveness, and cytokine milieu (3).
We have developed an in vitro model using explants of peritoneum or peritoneal mesothelial cells (PMCs) in monolayer culture and were able to demonstrate that endometrial epithelial cell (EEC) and stromal cell (ESC) adherence is rapid and depends on the source of ESCs rather than PMCs (4). These studies confirmed that endometrial fragments and dispersed endometrial cells rapidly adhere to PMCs. These studies also demonstrated that both endometrial epithelial (EECs) and stromal cells (ESCs) adhere to peritoneal mesothelium, but the mechanism is poorly defined. Our previous studies have demonstrated significant variability in the rate of endometrial-PMC adherence that is dependent on the source of ESCs rather than the source of PMCs (4).
Hyaluronan (or hyaluronic acid; (HA)) is a linear polymer consisting of repeating disaccharides of D-glucuronate and N-acetyl-D-glucosamine that is produced by many cell types including PMCs (5,6). The function of HA is mediated through hyaluronan-binding proteins called hyaladherins. There are a growing number of hyaladherins, but the best characterized is CD44 (5,7). CD44 is a transmembrane glycoprotein involved in cell-cell and cell-extracellular matrix interactions (8). Twenty exons are involved in the genomic organization of CD44 with the first five and last five exons being constant. The smallest CD44 isoform lacks the entire variable region and is known as “standard” CD44 (CD44s). The ten intervening exons are subject to alternative splicing, resulting in the generation of a variable region. The variant exons are called v1-v10 and the proteins containing the sequences are identified by the specific exon used (e.g. CD44v6, CD44v3, CD44v8-10). Differential utilization of the ten variable region exons, as well as variations in N-linked glycosylation, O-linked glycosylation, and glycosaminoglycanation generate multiple isoforms (5,7). Modifications of the CD44 isoforms have been shown to alter a cell’s ability to bind HA.
Numerous studies have demonstrated that HA is the principal ligand for CD44. HA and CD44 are involved in the adherence of ovarian cancer and gastric cancer cell lines to mesothelium. In vitro studies demonstrate that these cells express a variety of adhesion molecules. Anti-CD44 antibodies, or treatment with hyaluronidase, reduces the rate of adherence to mesothelium (9,10,11). Similarly, we demonstrated that treatment of PMCs with hyaluronidase significantly reduced adherence of EECs and ESCs to PMCs (12).
The purpose of this study was to determine if menstrual endometrial cells from women with endometriosis have an increased ability to adhere to PMCs. We further sought to characterize CD44 isoform expression to determine its relationship to adherence.
MATERIALS AND METHODS
Approval for this study and collection of endometrium was obtained from the Institutional Review Board of the University of Texas Health Science Center at San Antonio. The authors report no conflict of interest in the performance of this study.
Endometrial Cell Culture
Samples were obtained by aspiration biopsy on cycle days 1–2 using a Pipelle (Unimar Inc., Prodimed, Neuilly-En-Thelle, France) from normally cycling women with (n=21) and without (n=8) endometriosis (Controls) not taking hormonal medication in the previous three menstrual cycles. Endometriosis had been identified or excluded by previous laparoscopy or laparotomy in the 12 months preceding the biopsy. The surgeries were performed to address fertility limitations. Tissue samples were placed in Cellgro® (Mediatech, Herndon, VA) complete serum-free medium for transport to the laboratory Monolayer culture of ESCs and EECs were established as previously described (12,13,14).
Peritoneal Mesothelial Cells
Previous studies have demonstrated similar rates of endometrial cell binding to commercially available LP9 PMCs (NIH Aging Cell Repository, Coriell Institute for Medical Research, Camden NJ) and PMCs derived from parietal peritoneum and ovarian surface epithelium (4). The LP9 PMCs were grown in MCDB-131/Medium 199 (1:1) (Sigma) supplemented with epidermal growth factor (20 ng/ml), L-glutamine (2 mM), hydrocortisone (400 ng/ml), 1% antibiotics and antimycotics, HEPES buffer, and 15% fetal calf serum.
Endometrial-PMC Adherence Assay
A previously described adherence assay was used to evaluate the rate of ESC and EEC adherence to LP9 PMCs (4).
Expression of CD44 Variants in Endometrial Tissue
Expression of different CD44 variants was determined with an exon/variant-specific dot-blot hybridization using a randomly selected subset of cells from patients with and without endometriosis (ESCs from patients with endometriosis, n = 9; ESCs from controls, n=5; EECs from patients with endometriosis, n = 8; EECs from controls, n=5). Specific oligonucleotide probes for each exon/variant of human CD44 were designed by using OligoPicker software (http://pga.mgh.harvard.edu/oligopicker/) and sequences are presented in Table 1.
TABLE 1.
Sequences of CD44 Exon/variant-specific oligonucleotide DNA probes
| CD44 Probe | Sequence |
|---|---|
| Exon 5 | ctattgttaaccgtgatggcacccgctatgtccagaaaggagaatacagaacgaatcctgaagacatcta |
| V2 | actagtgctacagcaactgagacagcaaccaagaggcaagaaacctgggattggttttcatggttgtttc |
| V3 | gtacgtcttcaaataccatctcagcaggctgggagccaaatgaagaaaatgaagatgaaagagacagaca |
| V4 | tttcaaccacaccacgggcttttgaccacacaaaacagaaccaggactggacccagtggaacccaagcca |
| V5 | atgtagacagaaatggcaccactgcttatgaaggaaactggaacccagaagcacaccctcccctcattca |
| V6 | tccaggcaactcctagtagtacaacggaagaaacagctacccagaaggaacagtggtttggcaacagatg |
| V7 | cagcctcagctcataccagccatccaatgcaaggaaggacaacaccaagcccagaggacagttcctggac |
| V8 | atatggactccagtcatagtacaacgcttcagcctactgcaaatccaaacacaggtttggtggaagattt |
| V9 | agcagagtaattctcagagcttctctacatcacatgaaggcttggaagaagataaagaccatccaacaac |
| V10 | ataggaatgatgtcacaggtggaagaagagacccaaatcattctgaaggctcaactactttactggaagg |
| Exon16-17 | gagaccaagacacattccaccccagtggggggtcccataccactcatggatctgaatcagatggacactc |
Equimolar concentrations (20 μM) of each exon/variant specific oligonucleotide were applied to nylon membranes (Magnagraph 0.22 μM; Osmonics, Minnetonka, USA) to prepare an exon/variant-specific microarray. Negative controls consisted of non-specific oligonucleotide sequences of equal length. After cross-linking the oligonucleotide to the nylon membrane using a UV cross-linker (UVC500, Hoefer Pharmacia Biotech, San Francisco, CA), the membrane was used to hybridize a 32P-labled PCR generated CD44S cDNA probe employing standard methodology. To prepare the CD44S cDNA probe, total RNA was isolated from EECs or ESCs from women with and without endometriosis using TRI Reagent (Sigma) according to the manufacturer’s protocol. Total complimentary DNA (cDNA) was synthesized using 200ng total RNA by reverse transcription employing the murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) according to the manufacturer protocol. The primer set used for generating human CD44S cDNA are sense primer 5′-AACCGTGATGGCACCCGCTATGTC-3′ and antisense primer 5′-GGACCAGAGGTTGTGTTTGCTCCA-3′, flanking the variable region (i.e. exon 5 and exon 16). After removing any free isotope, the cDNA probe was used for hybridization. Densitometric values after normalizing CD44S and internal controls were used to determine the expression of the specific exon/variants in various tissue samples. To ensure that passage of cells did not change the fidelity of variant expression, three samples were examined at collection and after passage. The expression of CD 44 splice variants before and after passage was the same.
Statistical Analysis
The rate of adherence of ESCs or EECs to PMCs from women with and without endometriosis was compared with a Student’s t test. The rate of expression of CD 44 splice variants by ESCs and EECs from women with and without endometriosis was evaluated with a Fisher Exact Test.
RESULTS
ESCs from women with endometriosis demonstrated increased adherence to LP9 PMCs compared with ESCs from women without endometriosis (p<0.002; Figure 1). EECs from women with endometriosis also demonstrated an increased adherence to PMCs that approached statistical significance when compared to EECs from without endometriosis (p=0.07; Figure 1).
Figure 1.
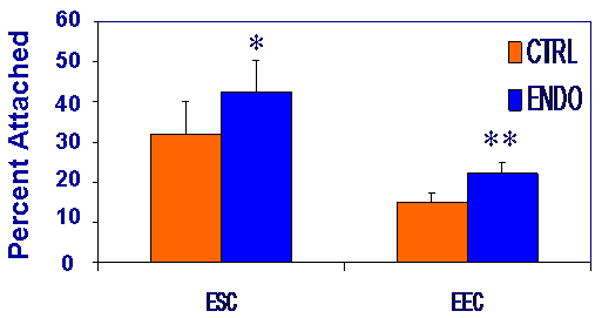
An increased proportion of ESCs and EECs from women with endometriosis adhered to LP9 PMCs compared with ESCs and EECs from controls (43 % versus 32 and 23% versus 15%, respectively) (* p<0.002, ** p =0.07).
The predominant CD44 splice variants expressed by ESCs and EECs from women with and without endometriosis were v3, v6, v7, v8, v9, and v10 (Table 2). ESCs from women with endometriosis were more likely to express v6, v7, v8, or v9 than ESCs from women without endometriosis (69% versus 45%, p<0.05). Increased expression of these variants by EECs from women with endometriosis approached statistical significance when compared to controls (93% versus 75%, p=0.067). A characteristic dot blot is shown in Figure 2.
TABLE 2.
The percentage of ESCs and EECs from patients with endometriosis (Endo) and without endometriosis (Ctrl) expressing the specific CD44 splice variants.
| V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | V6-V9* | V10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| ESC Endo | 0 | 100 | 0 | 0 | 89 | 33 | 78 | 78 | 69 | 100 |
| ESC Ctrl | 0 | 100 | 0 | 0 | 40 | 20 | 60 | 60 | 45 | 100 |
| EEC Endo | 0 | 100 | 0 | 0 | 100 | 75 | 100 | 100 | 93 | 100 |
| EEC Ctrl | 0 | 100 | 20 | 20 | 80 | 80 | 80 | 60 | 75 | 100 |
The percentage of ESCs and EECs expressing the four variants of V6, V7, V8, V9.
Figure 2.
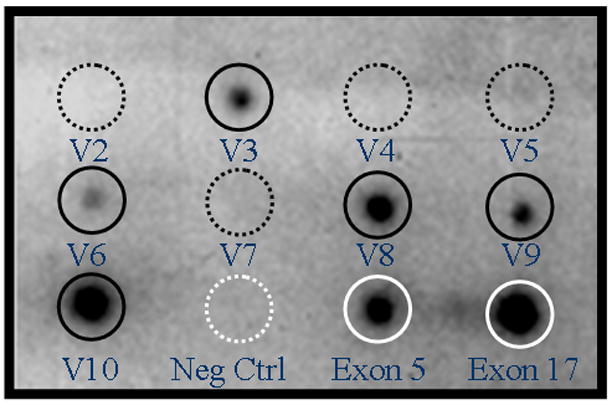
Representative dot blot array hybridization of endometrial epithelial cells from a woman with endometriosis. Hybridization with specific splice variants showing v3, v6, v8, v9, and v10 (dark solid circles). Splice variants v2, v4, v5, and v7 are not expressed (dark dashed circles). Negative controls consisted of non-specific oligonucleotide sequences (white dashed circle). Positive controls are exon 5 and 17 from the non-variable region of CD44 (white solid circles).
DISCUSSION
Here we provide the first evidence that menstrual endometrial cells from women with endometriosis have an increased ability to adhere to PMCs. The adherence of ESCs from women with endometriosis was significantly greater than that of ESCs obtained from women without endometriosis. Menstrual EECs from women with endometriosis also demonstrated a greater ability to adhere to PMC when compared to EECs from women without endometriosis, but this observation only approached statistical significance (p=0.07). The lack of statistical significance was likely attributable to the large variability in EEC adherence to PMCs, regardless of the source of endometrium. These observations serve as additional evidence that menstrual endometrium from women with endometriosis is indeed different from women without the disease.
CD44 isoform expression in human endometrium has not been systematically studied. Several investigators have used reverse transcription PCR and immunocytochemistry to assess endometrial CD44 expression in different phases of the menstrual cycle. The results are somewhat confusing and contradictory (15–20). It appears that both EECs and ESCs express CD44s and endometrial tissue from some women express CD44v3, CD44v6, CD44v7, Cd44v8, and CD44v8-10. CD44 expression is increased in the late secretory phase with peak expression in the late secretory/menstrual phase, but no consistent pattern has been demonstrated for CD44 splice variants.
Our study focused on menstrual endometrium because of our interest in retrograde menstruation vis-à-vis the pathogenesis of endometriosis. We did not identify a specific isoform that was unique to women with endometriosis. However, eutopic endometrium from women with endometriosis was more likely to express a combination of CD44v6, CD44v7, CD44v8, and CD44v9 when compared with eutopic endometrium of controls. This is a novel observation that may have a role in the initial adherence of EECs and ESCs to PMCs.
Several studies have shown that HA-binding capacity is regulated, in part, by the specific CD44 isoforms expressed (21–25). Hyaluronan binding by the various isoforms is clearly cell-type dependent. Some cell types demonstrate enhanced binding with CD44s while others show enhanced binding with CD44 variant isoforms. While the expression of CD44 on the cell surface influences binding, some CD44-expressing cells do not bind HA constitutively. Neither does the amount of CD44 cell-surface expression always correlate with the ability to bind HA. Recent studies indicate that the functional heterogeneity of CD44 is due to differences in cell-type specific glycosylation and glycosaminoglycanation (22,23,26–29). The degree of glycosylation is consistently increased in CD44 splice variants as extra N- and O-linked sites are provided by the additional exons.
Our observation of increased expression of variant isoforms in the endometrium of women with endometriosis suggests a greater degree of glycosylation. CD44 glycosylation can either increase or decrease binding depending on the isoform and specific cell type studied (22,23,26,27,29). We recently demonstrated that treatment of endometrial cells with an inhibitor of glycosylation decreased adherence of EECs and ESCs to PMCs (Nair A, Witz CA, Nair HB, Tekmal RR, Schenken RS; unpublished). These findings raise the possibility that the additional glycosylation sites present on the CD44 variants in endometrial cells may be partly responsible for the increased ability of the endometrial cells to adhere to PMCs.
In summary, the present study is the first to demonstrate that menstrual endometrial cells from women with endometriosis have an increased rate of adherence to PMCs when compared to endometrial cells from controls. The increased expression rates of CD44v6, CD44v7, CD44v8, or CD44v9 by endometrial cells from women with endometriosis suggest that qualitative differences in CD44 isoform expression may contribute to the pathogenesis of endometriosis. Studies are in progress to further clarify the role of variable CD44 isoform expression in the genesis of the early endometriotic lesion and its potential for future targeted therapies.
Acknowledgments
Supported by National Institutes of Health 1R01 HD044135-01
Footnotes
Presented, in part, at the 63rd Annual Meeting of the American Society of Reproductive Medicine, Washington D.C. October 13–17th, 2007
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–69. [Google Scholar]
- 2.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obst Gynecol Clin N Am. 1997;24:235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 3.Witz CA. Pathogenesis of endometriosis. In: Olive DL, editor. Endometriosis in Clinical Practice. Andover, Hampshire, UK: Taylor & Francis; 2005. pp. 61–78. [Google Scholar]
- 4.Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84:16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 6.Laurent TC, Fraser JR. Hyaluronan. FASEB Journal. 1992;6:2397–404. [PubMed] [Google Scholar]
- 7.Culty M, Nguyen HA, Underhill CB. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992;116:1055–62. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naot D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- 9.Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–37. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannistra SA, Kansas GS, Niloff J, Defranzo B, Kim Y, Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53:3830–8. [PubMed] [Google Scholar]
- 11.Gardner MJ, Catterall JB, Jones LM, Turner GA. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis. 1996;14:325–34. doi: 10.1007/BF00123391. [DOI] [PubMed] [Google Scholar]
- 12.Dechaud H, Witz CA, Montoya-Rodriguez IA, Degraffenried L, Schenken RS. Mesothelial Cell-Associated Hyaluronic Acid Facilitates Endometrial Stromal and Epithelial Cell Binding to Mesothelium. Fertil Steril. 2001;76:1012–8. doi: 10.1016/s0015-0282(01)02839-4. [DOI] [PubMed] [Google Scholar]
- 13.Witz CA, Cho S, Montoya-Rodriguez IA, Schenken RS. The alpha(2)beta(1) and alpha(3)beta(1) integrins do not mediate attachment of endometrial cells to peritoneal mesothelium. Fertil Steril. 2002;78:796–803. doi: 10.1016/s0015-0282(02)03340-x. [DOI] [PubMed] [Google Scholar]
- 14.Kirk D, Irwin JC. Normal human endometrium in cell culture. Methods Cell Biol. 1980;21B:51–77. doi: 10.1016/s0091-679x(08)60678-0. [DOI] [PubMed] [Google Scholar]
- 15.Albers A, Thie M, Hohn HP, Denker HW. Differential expression and localization of integrins and CD44 in the membrane domains of human uterine epithelial cells during the menstrual cycle. Acta Anatomica. 1995;153:12–9. doi: 10.1159/000147710. [DOI] [PubMed] [Google Scholar]
- 16.Behzad F, Seif MW, Campbell S, Aplin JD. Expression of two isoforms of CD44 in human endometrium. Biol Reprod. 1994;51:739–47. doi: 10.1095/biolreprod51.4.739. [DOI] [PubMed] [Google Scholar]
- 17.Fujita N, Yaegashi N, Ide Y, Sato S, Nakamura M, Ishiwata I, et al. Expression of CD44 in normal human versus tumor endometrial tissues: possible implication of reduced expression of CD44 in lymph-vascular space involvement of cancer cells. Cancer Res. 1994;54:3922–8. [PubMed] [Google Scholar]
- 18.Prifti S, Sillem M, Arslic T, Monga B, Rehberger S, Runnebaum B. In vitro expression of soluble and cell surface-associated CD44 on endometrial cells from women with and without endometriosis. Eur J Clin Invest. 1998;28:1055–60. doi: 10.1046/j.1365-2362.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 19.Saegusa M, Hashimura M, Okayasu I. CD44 expression in normal, hyperplastic, and malignant endometrium. J Pathol. 1998;184:297–306. doi: 10.1002/(SICI)1096-9896(199803)184:3<297::AID-PATH995>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Yaegashi N, Fujita N, Yajima A, Nakamura M. Menstrual cycle dependent expression of CD44 in normal human endometrium. Hum Pathol. 1995;26:862–5. doi: 10.1016/0046-8177(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 21.Dougherty GJ, Cooper DL, Memory JF, Chiu RK. Ligand binding specificity of alternatively spliced CD44 isoforms. Recognition and binding of hyaluronan by CD44R1. J Biol Chem. 1994;269:9074–8. [PubMed] [Google Scholar]
- 22.English NM, Lesley JF, Hyman R. Site-specific de-N-glycosylation of CD44 can activate hyaluronan binding, and CD44 activation states show distinct threshold densities for hyaluronan binding. Cancer Res. 1998;58:3736–42. [PubMed] [Google Scholar]
- 23.Lesley J, English N, Perschl A, Gregoroff J, Hyman R. Variant cell lines selected for alterations in the function of the hyaluronan receptor CD44 show differences in glycosylation. J Exp Med. 1995;182:431–7. doi: 10.1084/jem.182.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamenkovic I, Aruffo A, Amiot M, Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991;10:343–8. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Der Voort R, Manten-Horst E, Smit L, Ostermann E, Van Den Berg F, Pals ST. Binding of cell-surface expressed CD44 to hyaluronate is dependent on splicing and cell type. Biochem Biophys Res Comm. 1995;214:137–44. doi: 10.1006/bbrc.1995.2267. [DOI] [PubMed] [Google Scholar]
- 26.Bartolazzi A, Nocks A, Aruffo A, Spring F, Stamenkovic I, Bartolazzi A, et al. Glycosylation of CD44 is implicated in CD44-mediated cell adhesion to hyaluronan Regulation of growth and dissemination of a human lymphoma by CD44 splice variants. J Cell Biol. 1996;132:1199–208. doi: 10.1083/jcb.132.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett KL, Modrell B, Greenfield B, Bartolazzi A, Stamenkovic I, Peach R, et al. Regulation of CD44 binding to hyaluronan by glycosylation of variably spliced exons. J Cell Biol. 1995;131:1623–33. doi: 10.1083/jcb.131.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunthert U. CD44 in malignant disorders. Cur Topics Microbiol Immunol. 1996;213:271–85. [PubMed] [Google Scholar]
- 29.Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995;182:419–29. doi: 10.1084/jem.182.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]


