Abstract
Background
Prevalence rates of Hepatitis C Virus (HCV) co-infection, the distribution of HCV genotypes, and the frequency of spontaneous resolution of hepatitis C in patients infected with the Human Immunodeficiency Virus (HIV) have a worldwide disparity. The purpose of this study is to investigate the prevalence of HCV antibodies (anti-HCV) in patients with HIV, the proportion and correlates of infection by different HCV genotypes, and rates of spontaneous resolution of HCV infection.
Methods
A cross-sectional study was conducted among 1143 HIV patients under follow-up in a HIV/AIDS outpatient reference center of the Brazilian public health system. From 357 anti-HCV positive patients, a consecutive sample of 227 individuals HCV treatment-naïve was interviewed and 207 was tested for HCV-RNA and genotypes.
Results
Anti-HCV was detected in 357 patients (31.2%). HCV-RNA was undetectable in 16.4% of 207 anti-HCV positive individuals. Genotype 1 was diagnosed in 81.5% of the sample, genotype 2 in 1.7% and genotype 3 in 16.2%. Male gender was the unique characteristic associated with higher prevalence of genotype 1 HCV.
Conclusions
Co-infection by HCV is frequent among patients with HIV in our State, and it is particularly high the infection by HCV genotype 1. Further investigation is necessary to explain the important regional variation in the proportion of infection by the different HCV genotypes and to better understand rates of spontaneous HCV clearance.
Introduction
It has been estimated that 38.6 million people are infected by the human immunodeficiency virus (HIV) worldwide, and 4–5 million are also infected with hepatitis C virus (HCV) [1]. In Brazil, about 0.5% of the adult population is infected with HIV [1] and among those attending the public health services, 16% HIV-HCV co-infection rate had been reported in outpatient care centers [2], [3], 42% in HIV-HCV anonymous testing centers [4], and 54% for patients HIV infected in infectious diseases clinics [5].
The survival of HIV infected patients has markedly improved since the introduction of highly active antiretroviral treatment (HAART). [6] While treatment of HIV-HCV co-infected patients has lower rates of sustained virological response (SVR), it might also be complicated by adverse effects and drug interaction. Therefore, the selection of patients with a favorable risk/benefit ratio to respond to treatment is necessary. The best predictor of treatment effectiveness is the HCV genotype and, consequently, it becomes a key factor to support therapeutic decisions. [7]–[11] Besides the treatment issues, geographical distribution of HCV genotypes might help to elucidate new routes of infection among HIV infected patients.
This study investigated the prevalence of HCV antibodies (anti-HCV) in patients with HIV, the proportion and correlates of infection by different HCV genotypes, and rates of undetectable HCV-RNA in anti-HCV positive patients treatment-naïve (hepatitis C spontaneous resolution).
Methods
Study design
This cross-sectional study was conducted in a public reference center for HIV testing and treatment in Porto Alegre, southern Brazil. Patients were referred by primary care doctors or anonymous testing centers.
The routine protocol for evaluation of patients included complete medical history and physical examination, basic hematological and biochemical tests, toxoplasmosis screening test, PPD skin test, chest X-ray, CD4, HIV viral load, hepatitis B surface antigen (HBsAg) and hepatitis C antibodies (anti-HCV). Anti-HCV was performed using third-generation enzyme-linked immunosorbent assays (ELISA-3 Hepanostika® - BioMerrie).
Participants
A registry of 3490 patients with HIV infection was launched in 1996 in our center, comprising all patients with at least one appointment with the infectious diseases doctor. All records were retrieved and the follow-up of surviving participants was updated until 2006. The eligibility criteria included patients aged 18 years or older, who had at least one appointment in the previous 12 months and were co-infected by HCV. The final sample comprised 1143 HIV patients actively under follow-up.
All patients with an anti-HCV positive test were consecutively interviewed from March-2005 to September-2006, during the routine visit to the attending physician. Patients who did not complete the study protocol at that day were re-scheduled to prevent losses.
Data collection
Interviewers were trained and certified to conduct the data collection, and quality control was carried out at random by one of the investigators. Patients were interviewed using a standardized questionnaire, which included questions pertaining demographic (gender, age, skin color) and behavioral (alcohol consumption, tattooing, sharing personal hygiene objects - tooth brushes, shaving blades, cuticle nippers -, acupuncture) characteristics, socioeconomic status (family income, schooling), medical history (blood transfusion, accidental exposure to biological material), sexual behavior (age at first sexual intercourse, sexual orientation, anal sex, use of condom, number of sexual partners), and use of illicit drugs (injecting drugs, snorting cocaine, crack cocaine, marijuana, solvents/inhalants). Variables associated with HIV infection (CD4 lymphocyte count, CD4/CD8 ratio, use of antiretroviral drugs, duration of HIV diagnosis, opportunistic infections) were also investigated.
After the interview, blood samples were collected and processed for HCV RNA detection and HCV genotype determination. The laboratory was certified by the Brazilian Society of Clinical Pathology and Laboratory Medicine.
HCV RNA detection and genotyping
The real-time polymerase chain reaction (RT-PCR) technique was used for the qualitative detection of HCV RNA as described elsewhere [12]–[14]. Genotypes were identified using the restriction fragment length polymorphism (RFLP) [15], adapted as described elsewhere [16]. The lower limit of detection of the test is 50 UI/mL. All tests were performed in duplicate, and reliability of HCV RNA was tested in a random sampling of 10% of the patients. Two thirds of undetectable HCV-RNA patients were retested.
Study variables
The main study outcomes were the detection of HCV antibodies, defined as a positive result in the anti-HCV test, and the prevalence of HCV infection according to the genotypes. In addition, we performed an exploratory analysis on characteristics associated with spontaneous resolution of HCV infection, characterized as an undetectable HCV-RNA in an anti-HCV positive patients who have never been submitted to an interferon based therapy.
The self-report of skin color was categorized into white or non-white. Schooling was measured by the number of years at school. Heterosexual orientation was established for those who reported sexual intercourse with partners of the opposite sex; man who have sex with men, as well as women who have sex with women, were categorized as homosexuals, and bisexuals were defined when they reported having sex with men and women [17]. Consumption of alcoholic beverages, smoking and use of illicit drugs were investigated using standardized questions. CD4, CD8 counts and HIV viral load, determined closest to the appointment date, were used in the analysis. Antiretroviral treatment was defined as taking any medicine at the time of the interview. The duration of HIV infection was calculated from the date of the diagnosis to the date of the interview as reported by the patient and confirmed with medical records.
Statistical analysis
Data were entered in duplicate and checked for consistency and reliability using the Epinfo 3.3.2 software. The Statistical Package for the Social Sciences (SPSS, version 14.0, Chicago, Il, USA) was used for statistical analyses. Pearson chi-square test and analysis of variance were used to analyze characteristics associated with HCV RNA and genotypes. P value <0.05 was considered statistically significant and a trend toward association was established for p value >0.05 and <0.15. Point prevalence and 95% confidence interval (CI) were presented for HIV-HCV coinfection, HCV RNA and genotypes. The Institutional Review Board and Ethical Committee approved the protocol, and all participants gave informed consent.
Results
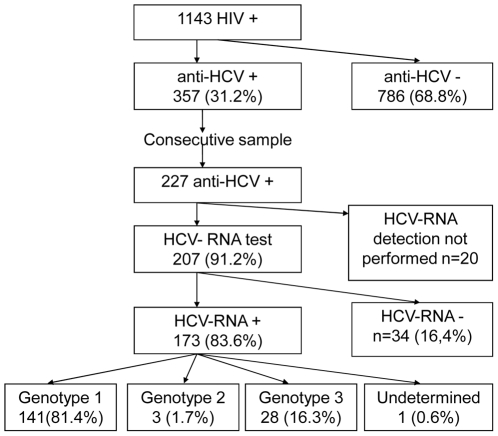
Anti-HCV testing was performed in 1143 patients. A positive anti-HCV test was detected for 357 (31.2%) individuals. From March-2005 to September-2006, all patients who went to the routine evaluation were interviewed, resulting in the enrollment of a consecutive sample of 227 co-infected patients. RNA HCV testing was performed in 207 patients (Figure 1), as 20 patients didn't come to the blood collecting laboratory to be tested. The comparison between patients with and without HCV-RNA testing results available did not reveal differences regarding demographic characteristics.
Figure 1. HCV-HIV prevalence and genotype distribution.
Flowchart of inclusion in the different stages of the study, and patient's prevalence of anti-HCV positive, HCV-RNA positive and HCV genotypes.
Table 1 shows that most participants were men, aged on average 40 years, had white skin color, injecting cocaine users, and approximately 19% were bisexuals.
Table 1. Characteristics of HIV infected patients with anti-HCV positive test (N = 227).
| N (%) or mean ±SD | ||
| Male gender (%) | 143 (63%) | |
| Age (years) | 40.3±8.7 | |
| White skin color | 129 (56.8) | |
| Schooling (years) | 6,7±3.4 | |
| Sexual orientation | ||
| Heterosexual | 97 (76.3) | |
| Homosexual | 6 (4.8) | |
| Bisexual | 24 (18.9) | |
| Transfusion before 1993 | 24 (10.6) | |
| Use of illicit drugs | ||
| Marijuana | 148 (65.2) | |
| Inhaled cocaine | 138 (60.8) | |
| Injecting | 127 (55.9) | |
| Crack cocaine | 62 (27.3) | |
| CD4 (cells/mm3) | 387±217 | |
| Duration of HIV diagnosis (years) | 6,0±4.5 |
Prevalence of HIV-HCV coinfection and HCV genotypes
Figure 1 shows that among 207 HIV-HCV co-infected patients, according to the anti-HCV test, the infection was confirmed based on a positive HCV-RNA for 173 (83.6%) participants. No of the included patients had been submitted to antiviral therapy for HCV.
Genotype 1 HCV was the most prevalent (81.5%; 95%CI 75.7–87.3), followed by genotype 3 (16.2%; 95%CI 10.7–21.7), and only two cases had genotype 2 (1.7%; 95%CI 0–3.6). Genotypes 4, 5 and 6 HCV were not detected and virus C genotype could not be determined in one patient.
Characteristics associated with HCV genotypes
Table 2 shows that most characteristics were not significantly associated to the genotype, except higher prevalence of genotype 1 among men. There was a trend toward an association with transfusions in the past. Patients with genotype 1 were more likely to be on antiretroviral treatment and have longer duration of HIV infection than genotype 3. Also, a trend for a lower HIV viral load in patients infected with HCV genotype 3 compared to 1 was found. (Table 3)
Table 2. Characteristics of patients coinfected with HIV-HCV according to HCV genotype [N (%) or mean ±SD].
| Total* | Genotype 1 | Genotype 3 | P | ||
| N = 173 | N = 141 | N = 28 | Value | ||
| Male sex | 111 (64.2) | 97 (68.8) | 12 (42.9) | 0.009 | |
| Age (years) | 40.1±8.9 | 40.1±8.7 | 40.3±10.2 | 0.9 | |
| White skin color | 96 (55.5) | 78 (55.3) | 18 (64.3) | 0.4 | |
| Schooling (years) | 6.6±3.5 | 6.6±3.4 | 6.7±3.6 | 0.3 | |
| Sexual orientation | 0.6 | ||||
| Heterosexual | 131 (75.6) | 104 (73.8) | 23 (82.1) | ||
| Homosexual | 6 (3.5) | 5 (3.5) | 1 (3.6) | ||
| Bisexual | 36 (20.9) | 32 (22.7) | 4 (14.3) | ||
| Transfusion before 1993 | 20 (11.6) | 19 (13.5) | 1 (3.6) | 0.14 | |
| Illicit drug use | |||||
| Marijuana | 119 (68.8) | 102 (72.3) | 15 (53.6) | 0.5 | |
| Snorting cocaine | 112 (64.7) | 94 (66.7) | 16 (57.1) | 0.3 | |
| Injecting | 103 (59.5) | 86 (61.0) | 15 (53.6) | 0.5 | |
| Crack | 50 (28.9) | 40 (28.4) | 8 (28.6) | 1.0 |
*total number of patients includes 3 cases of genotype 2 and 1 of undetermined genotype.
Table 3. Characteristics associated with HIV according to HCV genotype [N (%) or mean±SD].
| Total* | Genotype 1 | Genotype 3 | P value | ||
| N = 173 | N = 141 | N = 28 | |||
| CD4 (cells/µl) | 0.9 | ||||
| ≤200 | 28 (15.9) | 23 (16.3) | 4 (14.3) | ||
| 201–350 | 63 (36.5) | 52 (36.9) | 9 (32.1) | ||
| >350 | 82 (47.6) | 65 (46.1) | 14 (50.0) | ||
| CD8 (cells/µl) | 1118±903 | 1147±980 | 955±320 | 0.6 | |
| CD4/CD8 ratio | 0.48±0.47 | 0.49±0.50 | 0.48±0.29 | 0.9 | |
| HIV viral load <50 copies/ml | 69 (39.9) | 60 (42.6) | 8 (28.6) | 0.2 | |
| Use of antiretroviral drug | 141 (81.5) | 119 (84.4) | 19 (67.9) | 0.04 | |
| Time since HIV diagnosis (years) | 5.4±3.9 | 5.6±3.9 | 4.5±3.3 | 0.1 |
*total number of patients includes 3 cases of genotype 2 and 1 of undetermined genotype.
Characteristics of HCV RNA negative patients
Thirty four patients (16.9%) had negative HCV RNA test. Undetectable HCV RNA was more frequent among those who 102 individual who reported sharing of personal hygiene objects (22.0% vs.10.8%; P = 0.03). The prevalence of undetectable HCV RNA levels was high among homosexuals (45.5%), intermediate between heterosexuals (16.7%) and reduced for bisexuals (7.7%) (P = 0.01).
Discussion
This study detected high prevalence of individuals co-infected with HCV (31.2%) among patients with HIV in our State. It was similar to the described in the United States (31–36%) [18], [19] for patients under follow-up at infectious diseases clinics and to that reported for volunteers of a multicenter trial conducted mainly in Europe (31%) [20]. This prevalence was higher than those reported in public health services for HIV patients from the North (16%) and Southeast (18%) of Brazil [3]. Since HIV and HCV are transmitted by large or repeated exposures to infected blood, the difference in prevalence of co-infection among regions or countries might vary according to the distribution of risk factors, noticeably, injecting drug use [21]. In countries with no remarkable variation on IDU prevalence across regions, other risk factors such as sharing personal hygiene objects may play a major role [22].
The genotype 1 prevalence of 81.4% was higher than previously described among individuals infected (50.9%) [23] and non-infected with HIV (50%) in Brazil. [23]–[25] However, participants of those studies were referred to the specialist for evaluation and treatment of hepatitis C. Differently from studies in which an evaluation by a gastroenterologist or a clinical trial are the source of enrollment, patients from our study were withdraw from a broad spectrum of HIV infected patients, and this may account for a sample of HIV-HCV coinfected individuals that better represents the true genotype distribution in our setting. Worldwide, genotype 1 prevalence in patients co-infected by the HIV-HCV also showed a large variation: from 26% in China [26], to 53 to 75% in Spain [27], [28], and to 87% in United States [29]. The high prevalence of genotype 1 has been associated to the transmission of HCV by injecting drug use [21] which accounted for approximately 22-fold increase the risk of co-infection, in this population. [22] Genotypes 4, 5 and 6 HCV were not detected, which is in accordance with other reports. [23]–[25], [30]
The only demographic variable associated to HCV genotype was male sex. The prevalence of males was greater among genotype 1 carriers than among genotype 3 patients. The reasons for this difference are not completely understood, and, our sample are not enough large (97 males with genotype 1 and 12 with genotype 3) to explore this finding in a more detailed basis. Small epidemiological differences that didn't reach statistical significance in our study, such as rates of illicit drug use, blood transfusion, and sexual behavior, may be the link to this finding when taken together. We have no reason to believe males or females are biologically prone to contract a specific HCV genotype. Instead, we believe that epidemiological circumstances exposed man or woman to the genotype they became infected. Our data suggest small differences in characteristics associated with HIV according to HCV genotypes. The only data witch presented statistically significant difference was the higher antiretroviral use among HCV genotype 1 compared to genotype 3 patients. The trends of a higher time since HIV diagnosis and higher prevalence of HIV viral load <50 copies/mL in genotype 1 versus 3 patients are probably related one to another, since the longer the time since HIV diagnosis, greater are the chances of antiretroviral treatment and, once one treatment, greater the chances of achieving undetectable HIV viral load. Data regarding association between HCV genotypes and HIV characteristics are scarce. Our data are in accordance with data already published by Soriano et al. [31] The EuroSIDA study authors reported higher antiretroviral use among HCV genotype 1 than genotype 3 infected patients. They also found no CD4 cell count and HIV viral load difference according to HCV genotype. [31]
Few studies have investigated systematically the prevalence of co-infection by HCV-RNA in patients with HIV infection. In the EuroSIDA less than 10% of the individuals with anti-HCV positive results had the infection confirmed by HCV-RNA detection [20]. In this study, high rate of patients (91.2%) were tested for HCV-RNA using real time-PCR technique, and a sample of negative and positive tests (40%) were repeated in order to reassure reproducibility. In a multi-center study, conducted in Europe and Australia, only 63% of patients with anti-HCV positive results underwent to HCV-RNA test, and 82% had a positive result [32]. None of the patients included in the study had been exposed to HCV treatment previously to the interview, and a false negative PCR result is unlikely since the HCV-RNA detection technique has high sensitivity [33] and all samples were processed in duplicates. Therefore, it is likely that participants with anti-HCV without detectable HCV viremia (16%) had spontaneous resolution of the infection. Several factors have been associated with spontaneous resolution of HCV viremia, such as cell immune responses [34]–[36], Asian or African ethnicity [37], and parenteral exposure to the virus [38]. Spontaneous resolution would be lower in individuals co-infected with HIV. [34] This study was not designed with enough power to investigate factors associated to spontaneous resolution. We hypothesize that individuals who have been exposed only to small amount of inoculum, such as the transmission by sharing personal hygiene objects, were more likely of having spontaneous resolution. HCV alternative routes of transmission, like sharing personal hygiene objects, are matter of controversy. Probably, small amounts of blood present in tooth brushes, shaving blades and cuticle nippers could explain HCV transmission in patient without other major risk factors. [18], [22], [39]–[42]. This hypothesis should be better investigated in future studies designed specifically to study HCV transmission routes.
In conclusion, HCV co-infection is frequent among patients with HIV infection in our State, and the genotype 1 prevails. A small proportion of patients present spontaneous resolution of the infection by HCV. The higher proportion of infection by the difficult-to-treat HCV genotype 1 has important consequences for the public health system.
Acknowledgments
The authors thank the personnel staff, physicians and patients of the Hospital Sanatório Partenon, Therapeutic Assistance Service, for their collaboration to make this study possible; and Simbios Biotecnologia for performing the high quality molecular biology tests.
Preliminary data on this study was presented at: 14th United European Gastroenterology Week–Berlin, Germany–2006. Abst WED-G-20. Gut 2006; 55 (suppl V): A249.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by a grant from the Ministry of Health/Secretariat of Health Surveillance/Nacional STD and AIDS Programme (MOH/SHS/NAP); Coordinating Agency for Advanced Training of Graduate Personnel (CAPES); the National Council for Scientific and Technological Development (CNPq); and Graduate Program in Medical Sciences, Universidade Federal do Rio Grande do Sul. This study was supported, in part, by the National Institute of Science and Technology for Health Technology Assessment (IATS) - CNPq/Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS. Global Report 2006. Avaiable at: http://data.unaids.org/pub/GlobalReport/
- 2.Monteiro MR, do Nascimento MM, Passos AD, Figueiredo JF. Hepatitis C: prevalence and risk factors among patients with HIV/AIDS in Belem Para, in Brazilian Amazon. Rev Soc Bras Med Trop. 2004;37(Suppl 2):40–6. doi: 10.1590/s0037-86822004000700006. [DOI] [PubMed] [Google Scholar]
- 3.Mendes-Correa MC, Barone AA, Guastini C. Hepatitis C virus seroprevalence and risk factors among patients with HIV infection. Rev Inst Med Trop Sao Paulo. 2001;43:15–9. doi: 10.1590/s0036-46652001000100003. [DOI] [PubMed] [Google Scholar]
- 4.Pereira GA, Stefani MM, Martelli CM, Turchi MD, Siqueira EM, et al. Human immunodeficiency virus type 1 and hepatitis C virus co-infection and viral subtypes at an HIV testing center in Brazil. J Med Virol. 2006;78:719–23. doi: 10.1002/jmv.20613. [DOI] [PubMed] [Google Scholar]
- 5.Pavan MH, Aoki FH, Monteiro DT, Gonçales NS, Escanhoela CA, et al. Viral hepatitis in patients infected with human immunodeficiency virus. Braz J Infect Dis. 2003;7:253–61. doi: 10.1590/s1413-86702003000400005. [DOI] [PubMed] [Google Scholar]
- 6.Merchante N, Giron-Gonzalez JA, Gonzalez-Serrano M, Torre-Cisneros J, García-García JA, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20:49–57. doi: 10.1097/01.aids.0000198087.47454.e1. [DOI] [PubMed] [Google Scholar]
- 7.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, et al. Pegylated interferon alpha-2b vs standard interferon alpha-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 8.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 9.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIVcoinfected persons. N Engl J Med 2004; 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laguno M, Murillas J, Blanco JL, Martínez E, Miquel R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18:F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–54. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boom R, Sol CJ, Salimans MM, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmonds P, Alberti A, Alter HJ, Bonino F, Bradley DW, et al. A proposed system for the nomenclatute of hepatitis C viral genotypes. Hepatology. 1994;19:1321–4. [PubMed] [Google Scholar]
- 14.Sanguinetti CJ, Dias Neto E, Simpson AJ. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques. 1994;17:914–21. [PubMed] [Google Scholar]
- 15.McOmish F, Yap PL, Dow BC, Follett EA, Seed C, et al. Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative survey. J Clin Microbiol. 1994;32:884–92. doi: 10.1128/jcm.32.4.884-892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca ASK, Lunge VR, Ikuta N. New genotype of hepatitis C virus (HCV) in Brazil. 1999. pp. 69–74. In: XX World Congress of Pathology and Laboratory Medicine, São Paulo.
- 17.Barcellos NT, Fuchs SC, Fuchs FD. Prevalence of and risk factors for HIV infection in individuals testing for HIV at counseling centers in Brazil. Sex Transm Dis. 2003;30:166–73. doi: 10.1097/00007435-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Bollepalli S, Mathieson K, Bay C, Hillier A, Post J, et al. Prevalence of Risk Factors for Hepatitis C Virus in HIV-Infected and HIV/Hepatitis C Virus-Coinfected Patients. Sex Transm. 2007;Dis34:367–70. doi: 10.1097/01.olq.0000240295.35457.b1. [DOI] [PubMed] [Google Scholar]
- 19.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus Pravalence among Patients Infected with Human Immunodeficiency Virus: A Cross-Sectional Analysis of the US Adult AIDS Clinical Trial Group. Clin Infec Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 20.Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 21.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Wolff FH, Fuchs SC, Barcellos NT, Falavigna M, Cohen M, et al. Risk factors for hepatitis C virus infection in individuals infected with the HIV. Dig Liver Dis. 2008;40:460–7. doi: 10.1016/j.dld.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Valle Tovo C, Alves de Mattos A, Ribeiro de Souza A, Ferrari de Oliveira Rigo J, Lerias de Almeida PR, et al. Impact of human immunodeficiency virus infection in patients infected with the hepatitis C virus. Liver International. 2007;27:40–6. doi: 10.1111/j.1478-3231.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 24.Krug LP, Lunge VR, Ikuta N, Fonseca AS, Cheinquer H, et al. Hepatitis C virus genotypes in Southern Brazil. Braz J Med Biol Res. 1996;29:1629–32. [PubMed] [Google Scholar]
- 25.Galperim B, Cheinquer H, Stein A, Fonseca A, Lunge V, et al. Prevalence of hepatitis C virus in alcoholic patients: role of parenteral risk factors. Arq Gastroenterol. 2006;43:81–84. doi: 10.1590/s0004-28032006000200004. [DOI] [PubMed] [Google Scholar]
- 26.Garten RJ, Zhang J, Lai S, Liu W, Chen J, et al. Coinfection with HIV and hepatitis C virus among injection drug users in southern China. Clin Infect Dis. 2005;41 (S1):S18–24. doi: 10.1086/429491. [DOI] [PubMed] [Google Scholar]
- 27.Rubio Caballero M, Rubio Rivas C, Nogues Biau A, Manonelles Fernandez A. Epidemiology of chronic hepatitis C virus in patients infected by human immunodeficiency virus. Study of 767 patients. Med Clin (Barc) 2005;125:56–8. doi: 10.1157/13076466. [DOI] [PubMed] [Google Scholar]
- 28.Ramos B, Nunez M, Toro C, Sheldon J, Garcia-Samaniego J, et al. Changes in the distribution of hepatitis C virus (HCV) genotypes over time in Spain according to HIV serostatus: implications for HCV therapy in HCV/HIV-coinfected patients. J Infect. 2007;54:173–9. doi: 10.1016/j.jinf.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez SA, Liu RC, Edlin BR, Jacobson IM, Talal AH. HIV/hepatitis C virus-coinfected patients with normal alanine aminotransferase levels. J Acquir Immune Defic Syndr. 2006;41:582–9. doi: 10.1097/01.qai.0000214806.90841.c8. [DOI] [PubMed] [Google Scholar]
- 30.Zocratto KB, Caiaffa WT, Proietti FA, Carneiro-Proietti AB, Mingoti SA, et al. HCV and HIV infection and co-infection: injecting drug use and sexual behavior, AjUDE-Brasil I Project. Cad Saude Publica. 2006;22:839–48. doi: 10.1590/s0102-311x2006000400022. [DOI] [PubMed] [Google Scholar]
- 31.Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, et al. EuroSIDA Study Group. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect. 2008;Dis1:1337–44. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]
- 32.Amin J, Kaye M, Skidmore S, Pillay D, Cooper DA, et al. HIV and hepatitis C coinfection within the CAESAR study. HIV Med. 2004;5:174–9. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 33.Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonák J, et al. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Kim AY, zur Wiesch JS, Kuntzen T. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 2006;3:e492. doi: 10.1371/journal.pmed.0030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schott E, Witt H, Hinrichsen H, Neumann K, Weich V, et al. Gender-dependent association of CTLA4 polymorphisms with resolution of hepatitis C virus infection. J Hepatol. 2007;46:372–80. doi: 10.1016/j.jhep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, et al. Acute Hepatitis C Italian Study Group. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–9. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busch MP, Glynn SA, Stramer SL, Orland J, Murphy EL, et al. Correlates of hepatitis C virus (HCV) RNA negativity among HCV-seropositive blood donors. Transfusion. 2006;46:469–75. doi: 10.1111/j.1537-2995.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 38.Quinn PG, Jamal MM, Carey JD, Arora S, Harris T, et al. A case-control study of the factors associated with spontaneous resolution of hepatitis C viremia. Am J Gastroenterol. 1999;94:668–73. doi: 10.1111/j.1572-0241.1999.00934.x. [DOI] [PubMed] [Google Scholar]
- 39.Karmochkine M, Carrat F, Dos Santos O, Cacoub P, Raguin G, et al. A case-control study of risk factors for hepatitis C infection in patients with unexplained routes of infection. J Viral Hepat. 2006;13:775–82. doi: 10.1111/j.1365-2893.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 40.Hyder Q, Burhan-ul-Haq M, Rashid R, Qazi S, Mehmood S, et al. Non-conventional transmission of hepatitis C: a true possibility ignored. J Pak Med Assoc. 2009;59:430–3. [PubMed] [Google Scholar]
- 41.Zylberberg H, Thiers V, Lagorce D, Squadrito G, Leone F, et al. Epidemiological and virological analysis of couples infected with hepatitis C virus. Gut. 1999;45:112–6. doi: 10.1136/gut.45.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lock G, Dirscher M, Obermeier F, Gelbmann CM, Hellerbrand C, et al. Hepatitis C–contamination of toothbrushes: myth or reality? J Viral Hep. 2006;13:571–3. doi: 10.1111/j.1365-2893.2006.00735.x. [DOI] [PubMed] [Google Scholar]