Abstract
Prior studies have demonstrated influences of leptin on hunger and satiety, the processing of food reward, and taste and palatability perception. This pilot study tested whether leptin accounts for variability in stress-induced changes in snack intake, and explores potential mechanisms underlying this effect. Thirty-four normal weight and class I obese women were exposed to a 30-minute mental stressor and a non-stressful control task in counterbalanced order on consecutive days. Higher serum leptin concentrations predicted decreases in snack intake following the stressor. Leptin was not a significant predictor of overall hunger or stress-induced changes in hunger, but was associated with greater perceived palatability of one of the four snacks. Overall, findings suggest that leptin may moderate the effect of stress on energy intake through non-homeostatic mechanisms.
Keywords: Leptin, Stress, Obesity, Eating behavior, Reward, Palatability
1. Introduction
Exposure to stress can have a potent influence on eating behavior and food reward processing. In animals fed a chow diet, stress is usually linked to decreased energy intake and weight loss. However, stress will stimulate eating in rats exposed to high-fat, high-sucrose food. In humans studies, where food choices generally consist of more palatable items, stress has been shown to both increase and decrease food intake (Adam & Epel, 2007; Greeno & Wing, 1994; Torres & Nowson, 2007). Several factors have been examined as possible explanations for the variability in the effects of stress on eating in humans. For example, research has consistently shown that women who report chronic dietary restraint are particularly vulnerable to increased energy intake following stress (Torres & Nowson, 2007). However, restrained eaters have an orexigenic hormonal profile characterized by higher ghrelin and lower leptin levels which may explain their predisposition to “disinhibited eating” (Laessle, Wurmser, & Pirke, 2000; Schur, Cummings, Callahan, & Foster-Schubert, 2008). Others have shown that stress-induced activation of the hypothalamic-pituitary-adrenocortical axis, reflected in cortisol secretion, is associated with post-stress energy intake (Dallman, Pecoraro, & la Fleur, 2005; Epel, Lapidus, McEwen, & Brownell, 2001; Newman, O'Connor, & Conner, 2007). This research suggests that physiologic factors may account for the significant variability between individuals in the effects of stress on eating.
Leptin is an adipocyte hormone that is secreted in proportion to total fat mass (Frederich et al., 1995; Maffei et al., 1995). Leptin serves as a peripheral signal of medium- and long-term energy balance (Kolaczynski, Ohannesian, Considine, Marco, & Caro, 1996), and organisms lacking the leptin protein or its receptor show profound hyperphagia and severe obesity (Clement et al., 1998; Farooqi & O'Rahilly, 2009). Until more recently, the role of leptin signaling in the control of eating behavior was understood solely in terms of its influence on hypothalamic control of hunger and satiety (Morrison, 2009; Williams, Scott, & Elmquist, 2009). However, there is growing evidence that leptin reduces the reward value of palatable food through its actions in the mesolimbic dopamine pathway (Fulton et al., 2006; Hommel et al., 2006). For example, intracerebroventricular leptin administration attenuates motivation to work for food reward in operant responding tasks (Figlewicz, Bennett, Naleid, Davis, & Grimm, 2006), impairs the ability to develop food-conditioned place preferences (Figlewicz et al., 2004), and inhibits self-stimulation of hypothalamic areas involved in food reward (Fulton, Woodside, & Shizgal, 2000) in animal studies. Finally, leptin appears to modulate perceptions of taste and food palatability1 (Farooqi & O'Rahilly, 2009; Karhunen et al., 1998). Leptin receptors are present in taste cells (Shigemura, Miura, Kusakabe, Hino, & Ninomiya, 2003), and polymorphisms of the leptin protein and leptin receptor are associated with sweet preference (Mizuta et al., 2008). In humans, higher circulating leptin has correlated with greater perceived palatability of a carbohydrate-rich meal (Raynaud et al., 1999) and liking of sweet beverages (Belzer, Smulian, Lu, & Tepper, 2009).
The primary aim of this pilot study was to determine if circulating leptin concentration accounts for variability in the effect of stress on snack intake. Considering previous research linking leptin to the modulation of hunger and perceptions of food palatability, secondary aims were to test the relations of leptin concentration to subjective hunger and palatability ratings. Normal weight (BMI: 18.5-24.9) and class I obese (BMI: 30-34.9) women were exposed to an acute mental stressor and a non-stressful control task on two consecutive days. It was hypothesized that higher circulating leptin concentrations would be associated with 1) decreased in snack intake following stress, 2) decreased subjective hunger following stress, and 3) higher ratings of food palatability.
2. Methods
2.1. Participants
Participants were recruited for a parent study of stress physiology. Inclusion criteria were female sex, body-mass index (BMI) in the normal weight (18.5-24.9) or class I obese (30.0-34.9) range, age between 25-45 y, premenopausal status, and general good health. Exclusion criteria included pregnancy, lactation, hormonal contraceptive use, current dieting, food allergies or sensitivities, previous eating pathology, and medical conditions or use of medications affecting appetite, neuroendocrine function, or metabolism. Participants were instructed to avoid caffeine, energy drinks, alcohol, and non-prescription medication in the morning prior to participation on both study days, and to avoid nicotine within 2 h prior to participation (only 2 participants smoked >5 cigarettes per day). Of 35 women who enrolled, 34 (16 normal weight and 18 class I obese) completed both days of the study. Participants were compensated $50 (US). Study procedures were approved by the Northwestern University Institutional Review Board.
2.2. Procedure
The study had a counterbalanced crossover design in which two experimental conditions were completed on consecutive weekdays. Participants were provided in advance with a standardized 430-kcal breakfast (a nutrition shake and a granola bar) that they were instructed to consume at home between 08:30 and 09:00 on both study days. No other food or drink (other than water) was allowed between midnight and the start of experimental sessions, which began at 11:30 (+/− 30 mins). With the exception of the nature of the task completed (stress vs. control), study procedures were essentially identical on both days. Participants underwent venipuncture soon after arriving to the lab, and then spent about 45 minutes completing questionnaires. Participants then completed either a 30-min stress task or a 30-min control task. Task order was counterbalanced across the entire sample and within each BMI group. A modified Trier Social Stress Task (Kirschbaum, Pirke, & Hellhammer, 1993) consisting of serial subtraction, timed completion of challenging visuospatial puzzles, and delivering a videotaped speech, was used for the stress task. The control task consisted of viewing and evaluating different aspects of a documentary film (IMAX: The Great Barrier Reef).
Following the tasks, research assistants presented participants with four plates of snacks (light butter popcorn, 20g, 4.41 kcal/g; miniature chocolate chip cookies, 50g, 4.85 kcal/g; potato chips, 40g, 5.29 kcal/g; miniature caramel flavored rice cakes, 24g, 4.00 kcal/g), reminded participants that they had not eaten since morning, and stated that all uneaten food would be discarded. Snacks varied in their primary flavor (sweet vs. salty) and fat content (high vs. low) to appeal to diverse taste preferences. The research assistant left the room for 10 minutes while participants ate. A second venipuncture was performed prior to departure. Affect and hunger ratings were collected upon arrival, before the task, and after the task on both days.
2.3. Measures
2.3.1. Anthropometrics
BMI [weight (kg) / height2 (m)] was derived from height and weight measurements taken in light clothing during the in-person screening.
2.3.2. Serum Leptin
Blood was collected by venipuncture at four time points across the two days. Serum was extracted and stored at −80°C until analysis. Samples were analyzed in duplicate by radioimmunoassay (HL-81K; LINCO Research, St. Charles, Missouri, USA). The coefficient of variation among a subset of quality control samples, representing 7% of the total samples analyzed, was 0.036. Leptin levels were highly stable across all four time points (reported in Results). Therefore, the four leptin values were averaged into a single value for each participant.
2.3.3. Snack intake
The amount of each food item consumed was determined by weighing plates before and after consumption on a laboratory balance. The weight consumed and caloric content were used to determine total energy consumed in each condition, and a difference score reflecting change in total energy intake was calculated (stress day minus control day).
2.3.4. Palatability ratings
At the end of the second study day, participants rated the taste of the four snack items presented on a 0-10 scale. Mean palatability ratings were similar for all four items (chips: M=5.9, SD=3.1; cookies: M=5.1, SD=3.3; popcorn: M=5.6, SD=3.0; rice cakes: M=5.3, SD=3.0).
2.3.5. Hunger and affect ratings
Participants completed the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) upon arrival to the lab, prior to the task, and after the task on both study days. The PANAS instructs participants to rate their current experience of 10 positive and 10 negative affect descriptors on a scale from 1 (very slightly or not at all) to 5 (extremely). To measure hunger discretely, the descriptor “hunger” was embedded among the PANAS items.
3. Results
3.1. Preliminary analyses
Sample characteristics are shown in Table 1. The stress task successfully elicited significant increases in negative affect in this sample (originally reported in Appelhans, Pagoto, Peters, & Spring, 2009). A repeated measures ANOVA revealed a significant condition X time interaction on negative affect ratings (F(2,62)=10.23, p<.001, η2=.25), such that negative affect increased over time on the stress day (linear trend: F(1,31)=11.04, p<.01, η2=.17), but decreased steadily on the control day (linear trend: F(1,33)=8.57, p<.01, η2=.16). The BMI groups showed a similar negative affect response to the stress task, indicated by a non-significant BMI group X condition X time interaction in the above model (p=.87).
Table 1.
Descriptive statistics for key study variables
| Total (N=34) | Normal weight (n=16) | Obese (n=18) | |
|---|---|---|---|
|
M (SD) |
|||
| Height (cm) | 165.2 (5.5) | 166.0 (7.0) | 164.5 (4.9) |
| Weight (kg)* | 75.5 (15.8) | 60.8 (6.1) | 88.6 (8.1) |
| Body mass index (BMI)* | 27.7 (5.6) | 22.1 (1.8) | 32.7 (1.6) |
| Age (y)* | 33.5 (6.0) | 31.2 (5.2) | 35.6 (6.1) |
| Leptin (μg/L)* | 23.4 (17.2) | 9.3 (4.5) | 36.0 (14.3) |
| Snack intake (kcal) | |||
| Control day | 136.8 (131.2) | 116.8 (103.7) | 153.4 (151.4) |
| Stress day† | 142.3 (131.8) | 128.4 (98.6) | 154.0 (156.1) |
| Δ snack intake‡ | 1.0 (46.4) | 1.5 (50.3) | 0.6 (44.7) |
| n (%) |
|||
| Ethnicity | |||
| White, non-Hispanic | 11 (32.4) | 6 (37.5) | 5 (27.8) |
| Hispanic | 5 (14.7) | 2 (12.5) | 3 (16.7) |
| African-American | 13 (38.2) | 6 (37.5) | 7 (38.9) |
| Asian | 4 (11.8) | 2 (12.5) | 2 (11.1) |
| Other | 1 (2.9) | 0 (0) | 1 (5.6) |
Significant group differences at p<0.05.
Excludes one outlying case.
Positive values reflect increased snack intake in the stress condition.
Given preliminary reports suggesting that stress may affect leptin levels (Otsuka et al., 2006; Schafroth, Godang, Ueland, & Bollerslev, 2001), a repeated measures ANCOVA was formed to test for a condition X time interaction on leptin level while controlling for BMI. The interaction term was not significant in this model (p=.12), and leptin levels at all 4 time points were very highly correlated (all r's>.98, p's<.00001). Given the stability of leptin levels across all time points, the use of participants’ mean leptin level in subsequent analyses was justified.
Obese women had substantially higher leptin levels compared to normal weight women (unadjusted means: 36.0 vs. 9.3 μg/L, F(1,32)=50.54, p<.00001, η2=.61), which resulted in a bimodal distribution of leptin level. Importantly, BMI group did not interact with leptin level in any of the analyses reported, and the residuals in all statistical models were normally distributed, independent of BMI group, and homoscedastic, which are key assumptions of regression-based analytic techniques (Cohen, Cohen, Aiken, & West, 2002). Therefore, it was appropriate to test relations between leptin and snack intake across the entire sample while controlling for BMI, rather than duplicating each analysis within the normal weight and obese groups. Snack intake data were lost for one lean participant who took food from the lab on the stress day. Another lean participant's stress day snack intake was more than 3 SD above the mean and approximately 1 SD above than the next highest value. These participants were excluded from analyses involving snack intake.
3.2. Primary analyses
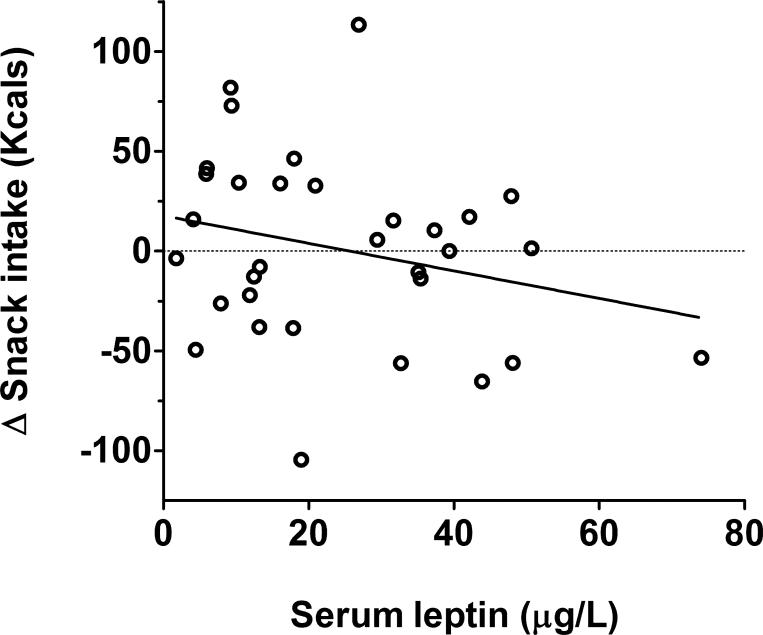
The association between circulating leptin concentration and stress-induced changes in snack intake was tested in a linear regression model predicting the snack intake change score. As circulating leptin is known to be substantially elevated in obesity (Frederich et al., 1995; Maffei et al., 1995), BMI was included as a covariate to examine the influence of circulating leptin on changes in snack intake independent of body mass. The model indicated that higher leptin concentration was associated with a decrease in snack intake due to stress (β=−.59, t(29)=2.12, p=.04; η2=.13, Figure 1). This effect remained significant with age included as a covariate in the model. Leptin was not associated with overall snack intake across the two study days, and the main effects of condition and BMI were not significant in these models.
Figure 1.
Association between mean leptin level and change in snack intake.
Repeated measures ANCOVA was used to model change in hunger ratings from leptin level, condition (stress vs. control), time (baseline, pre-task, post-task), and the condition X time interaction, while controlling for BMI. Leptin was not a significant predictor of changes in hunger in this model (p=.84), and partial correlations between leptin levels and hunger ratings controlling for BMI were non-significant at all time points.
Associations between leptin levels and the set of four food palatability ratings were evaluated through a MANCOVA model that included BMI as a covariate. Leptin was not a significant predictor of the multivariate set of taste ratings (p=.27). However, there was a positive association between leptin and cookie palatability ratings in a univariate test (β=.62, t(30)=2.08, p<.05, η2=.12).
4. Discussion
The primary aim of this pilot study was to determine whether leptin concentrations explain variability in the effect of stress on snack intake independent of body mass. Higher circulating leptin, adjusting for BMI, was associated with a decrease in snack intake following an acute stress task. This finding suggests that the effect of stress on food intake is sensitive to signals of long-term energy balance, which nicely complements a proposed teleological explanation for stress eating articulated by Sapolsky (1998). The physical stressors commonly faced in the human ancestral environment (e.g., predators, warfare) necessitated vigorous behavioral “fight-or-flight” responses involving significant energy expenditure. Initial appetite suppression would allow an organism to focus energy and attention on preparing for and responding to the challenge, whereas a later increase in appetite following stress would promote repletion of the energy expended while responding to the stressor. The current findings suggest that elevated leptin concentrations, reflecting a state of positive energy balance, would signal a diminished need to replenish expended energy following stress.
Despite the appeal of this explanation, it seemingly conflicts with reports that normal weight and obese individuals do not differ in the effect of stress on eating (Torres & Nowson, 2007). It is worth noting that the associations reported herein reflect the unique contribution of leptin to stress-related changes in snack intake independent of body mass. It is possible that circulating leptin concentration, and not the amount of energy stored as fat, is the more proximal influence on stress eating. This notion makes sense given that most obese individuals are leptin resistant (i.e., demonstrate hyperphagia and obesity in the presence of elevated leptin; Morrison, 2008), and circulating leptin is clearly less effective in regulating energy intake in accordance with energy stores in the obese state. Future studies aimed at identifying the specific leptin pathways that are downregulated in the obese state (and those which are not) would be valuable for interpreting how leptin might differentially affect stress eating across the spectrum of body mass.
Leptin levels were not associated with stress-induced changes in hunger or with hunger levels at any time point. Similarly, leptin was not associated with overall snack intake during the study. Leptin is known to be involved in hypothalamic controls of energy balance, and has previously been shown to reduce meal frequency and size (Zorrilla, Inoue, Valdez, Tabarin, & Koob, 2005). The lack of association between leptin concentration and hunger and overall snack intake in this study may be due to the fact that all participants consumed identical breakfasts about 3 hrs prior to participation, which likely reduced variability in hunger. It is also worth noting that the small sample size limited statistical power to detect associations between leptin and hunger and overall snack intake.
Higher leptin was modestly associated with greater perceived palatability of one of the four snacks (cookies). Previous research has linked leptin to perceived food palatability and sweet taste perception (Mizuta et al., 2008; Raynaud et al., 1999), but the mechanisms underlying leptin's effect on palatability have not yet been elucidated. Palatability was assessed only once at the conclusion of participation in order to keep participants blind to the fact that snack intake was a focus of the study. Therefore, it can not be determined whether leptin is related to changes in food palatability with stress. Further research is needed to determine if leptin causally influences food palatability and whether such modulation might account for the association between leptin and changes in energy intake with stress.
The strengths of this study include the use of fasting and a standardized breakfast to control for energy intake outside of the lab, application of relatively stringent eligibility criteria to eliminate potential confounds in the sample, and restricting data collection to a narrow time window to minimize the potential impact of diurnal rhythms in leptin and food intake. Study limitations include the use of a quasi-experimental study design that precludes the ability to draw causal inferences, and a small sample size which limited statistical power. Additionally, the fact that the sample was composed of healthy, normal weight and class I obese women limits the generalization of findings to other populations. Replication in a larger, more heterogeneous sample is justified.
Taken at face value, findings indicate that leptin moderates the effect of stress on snack intake through mechanisms independent of hunger. Future research on the integration between brain systems that govern energy balance, stress responses, and food reward and palatability may shed light on the etiology and potential treatment of obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Though taste and palatability are related to a food's reward value, these terms have distinct meanings and reflect different neurobiological process (see Barbano & Cador, 2007).
References
- Adam T, Epel E. Stress, eating and the reward system. Physiology & Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Pagoto SL, Peters EN, Spring BJ. HPA axis response to stress predicts short-term snack intake in obese women. Appetite. doi: 10.1016/j.appet.2009.11.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Belzer LM, Smulian JC, Lu SE, Tepper BJ. Changes in sweet taste across pregnancy in mild gestational diabetes mellitus: Relationship to endocrine factors. Chemical Senses. 2009;34:595–605. doi: 10.1093/chemse/bjp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, Aiken LS, West SG. Applied multiple regression-correlation analysis for the behavioral sciences. 3rd ed. Lawrence Erlbaum; Mahwah, NJ: 2002. [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain, Behavior, and Immunity. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, O'Rahilly S. Leptin: A pivotal regulator of human energy homeostasis. The American Journal of Clinical Nutrition. 2009;89:980S–984S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behavioral Neuroscience. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiology & Behavior. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, et al. Expression of ob mRNA and its encoded protein in rodents. impact of nutrition and obesity. The Journal of Clinical Investigation. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Karhunen LJ, Lappalainen RI, Haffner SM, Valve RH, Tuorila H, Miettinen H, et al. Serum leptin, food intake and preferences for sugar and fat in obese women. International Journal of Obesity and Related Metabolic Disorders. 1998;22:819–821. doi: 10.1038/sj.ijo.0800657. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ - a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. The Journal of Clinical Endocrinology and Metabolism. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- Laessle RG, Wurmser H, Pirke KM. Restrained eating and leptin levels in overweight preadolescent girls. Physiology & Behavior. 2000;70:45–47. doi: 10.1016/s0031-9384(00)00243-2. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Mizuta E, Kokubo Y, Yamanaka I, Miyamoto Y, Okayama A, Yoshimasa Y, et al. Leptin gene and leptin receptor gene polymorphisms are associated with sweet preference and obesity. Hypertension Research. 2008;31:1069–1077. doi: 10.1291/hypres.31.1069. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin resistance and the response to positive energy balance. Physiology & Behavior. 2008;94:660–663. doi: 10.1016/j.physbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochimica et Biophysica Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Otsuka R, Yatsuya H, Tamakoshi K, Matsushita K, Wada K, Toyoshima H. Perceived psychological stress and serum leptin concentrations in Japanese men. Obesity. 2006;14:1832–1838. doi: 10.1038/oby.2006.211. [DOI] [PubMed] [Google Scholar]
- Raynaud E, Brun JF, Perez-Martin A, Sagnes C, Boularan AM, Fedou C, et al. Serum leptin is associated with the perception of palatability during a standardized high-carbohydrate breakfast test. Clinical Science. 1999;96:343–348. doi: 10.1042/cs0960343. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why Zebras Don't Get Ulcers: An Updated Guide to Stress, Stress-related Diseases, and Coping. W. H. Freeman and Co.; New York: 1998. [Google Scholar]
- Schafroth U, Godang K, Ueland T, Bollerslev J. Leptin response to endogenous acute stress is independent of pituitary function. European Journal of Endocrinology. 2001;145:295–301. doi: 10.1530/eje.0.1450295. [DOI] [PubMed] [Google Scholar]
- Schur EA, Cummings DE, Callahan HS, Foster-Schubert KE. Association of cognitive restraint with ghrelin, leptin, and insulin levels in subjects who are not weight-reduced. Physiology & Behavior. 2008;93:706–712. doi: 10.1016/j.physbeh.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemura N, Miura H, Kusakabe Y, Hino A, Ninomiya Y. Expression of leptin receptor (ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Archives of Histology and Cytology. 2003;66:253–260. doi: 10.1679/aohc.66.253. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams KW, Scott MM, Elmquist JK. From observation to experimentation: Leptin action in the mediobasal hypothalamus. The American Journal of Clinical Nutrition. 2009;89:985S–990S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety: Acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology. 2005;177:324–335. doi: 10.1007/s00213-004-1952-1. [DOI] [PubMed] [Google Scholar]