Abstract
Background
The MetSyn has been implicated in the development of AF; however, knowledge of this association among blacks is limited.
Methods
We determined the risk of incident AF through December 2005 in relation to baseline (1987-1989) MetSyn status in 15,094 participants of the Atherosclerosis Risk in Communities study.
Results
Over a mean follow-up of 15.4 years, 1238 incident AF events were identified. The hazard ratio (HR) for AF among individuals with, compared to those without, the MetSyn was 1.67 (95% CI, 1.49-1.87), and associations did not differ by race (p for interaction=0.73). The population attributable risk of AF from the MetSyn was 22%. The multivariable-adjusted HRs (95% CI) for each MetSyn component were 1.95 (1.72-2.21) (elevated blood pressure), 1.40 (1.23-1.59) (elevated waist circumference), 1.20 (1.06-1.37) (low HDL cholesterol), 1.16 (1.03-1.31) (impaired fasting glucose), and 0.95 (0.84-1.09) (elevated triglycerides). A monotonically increasing risk of AF with increasing number of MetSyn components was observed, with a HR of 4.40 (95% CI, 3.25-5.94) for those with all 5 MetSyn components compared to those with 0 components.
Conclusion
In this large cohort, the MetSyn and most of its components were associated with a higher risk of AF in both blacks and whites. Given the high prevalence of the MetSyn, strategies to prevent its development or to control individual components may reduce the burden of AF.
Introduction
The metabolic syndrome (MetSyn) is defined as a clustering of three or more of the following atherosclerotic risk factors: abdominal obesity, elevated triglycerides (TG), low high-density lipoprotein cholesterol (HDL-c), elevated blood pressure, and impaired glucose tolerance. Although the significance of the MetSyn as a well-defined clinical entity is uncertain, previous research has implicated this disorder and some of its individual components in the development of atrial fibrillation (AF).
The mechanisms relating the MetSyn to increased risk of developing AF are not fully understood. The MetSyn affects atrial anatomy by increasing atrial size, possibly increasing AF risk as a consequence. Alternatively, the MetSyn may predispose to the development of coronary heart disease (CHD) or heart failure (HF), in turn increasing the risk of AF.
Knowledge on the association of the MetSyn with AF risk among blacks is limited. Previous studies have suggested that atrial fibrillation is less common in blacks than whites. However, it is well known that blacks have a higher prevalence of the MetSyn and most of its components than do whites. Therefore, our study aimed to determine the association of the MetSyn and its individual components with the risk of incident AF in a population-based cohort of blacks and whites.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective investigation aimed to identify risk factors for atherosclerosis and cardiovascular disease. ARIC recruited adults aged 45-64 years from four U.S. communities: Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington County, MD. A total of 15,792 participants (8710 women) were enrolled from 1987-1989, and completed a home interview and clinic visit. Three triennial follow-up clinic visits were conducted. In addition, participants are being followed-up by annual telephone interviews and active surveillance of the ARIC community hospitals. The ARIC study was approved by institutional review boards at each participating center, and written informed consent was obtained from all participants.
Atrial Fibrillation Ascertainment
Electrocardiograms (ECGs) during the baseline visit were used to exclude individuals with prevalent AF or atrial flutter. Incident AF diagnoses through December 31, 2005 were identified from ECGs performed during study follow-up visits, hospital discharge records, and death certificates.
All ARIC examination ECGs were recorded using MAC PC Personal Cardiographs (Marquette Electronics, Inc., Milwaukee, WI). A standard supine 12-lead resting ECG was recorded at each clinic visit and was transmitted by modem to the ARIC ECG Reading Center for computer coding. ECG recordings during follow-up that were computer coded as AF were visually re-checked by a cardiologist.
Annual follow-up telephone calls were placed to cohort participants to identify hospitalizations and deaths. In addition, local hospitals were surveyed for potential cardiovascular events. Hospital discharge records were gathered from all hospitalizations, and AF was identified by an ICD-9 discharge code of 427.31 or 427.32 among any of the discharge diagnoses. AF was also identified when any listed cause of death on a death certificate was coded as AF (ICD-9 code 427.3 or ICD-10 code I48). AF occurring simultaneously with heart revascularization surgery (ICD-9 code 36.X) or other cardiac surgery involving heart valves or septa (ICD-9 code 35.X) was not considered an incident event and follow-up was continued beyond that episode. Prior analysis within the ARIC cohort to determine the validity of hospital discharge diagnoses for AF reported 84% sensitivity and 98% specificity in the ascertainment of AF events.
Metabolic Syndrome Definition
Blood collection and processing techniques for the ARIC study have been previously described. Enzymatic methods were used to measure TG. HDL cholesterol was measured enzymatically after dextran sulfate-Mg2+ precipitation of other lipoproteins. Serum glucose was determined by the hexokinase method. Waist circumference was measured at the umbilicus level. Blood pressures were measured 3 times in the sitting position after 5 minutes of rest using a random-zero sphygmomanometer, and the last 2 blood pressure measurements were averaged.
The MetSyn was defined using the American Heart Association and National Heart, Lung, and Blood Institute (AHA/NHLBI) criteria as having 3 or more of the following: 1) a waist circumference of ≥88 cm in women or ≥102 cm in men, 2) fasting TG ≥150 mg/dL (or on lipid medication), 3) HDL-c <50 mg/dL in women or <40 mg/dL in men (or on lipid medication), 4) blood pressure ≥130/≥85 mmHg and/or a history of treated hypertension, and 5) fasting glucose ≥100 mg/dL or a history of diabetes (or on diabetes medication). A sensitivity analysis was conducted setting glucose levels to missing in diabetics. Since results did not differ from models including diabetics, those with diabetes were included in the elevated fasting glucose category in all models. However, non-fasters above the cut-point for TG or fasting glucose and not taking lipid or diabetes medication were set to missing for TG and/or fasting glucose.
Additional Baseline Measurements
Race, years of education, and smoking and drinking status and amount were self-reported. The sports index for physical activity during leisure time ranged from 1 (low) to 5 (high), and was based on the questionnaire developed by Baecke et al. A prescription or self-report was used to determine cholesterol and blood pressure medication use. A fasting glucose ≥126 mg/dL (or non-fasting glucose of ≥200 mg/dL), a reported physician diagnosis of diabetes, or current use of diabetes medication categorized a participant as diabetic. Prevalent CHD at baseline included individuals with a history of myocardial infarction (MI), MI adjudicated from the baseline ECG, or history of coronary bypass or angioplasty of the coronary arteries. Prevalent HF was identified using the Gothenburg criteria or self-report of HF medication use in the past 2 weeks.
Statistical Analysis
Analyses were conducted using SAS version 8.2 (SAS Institute, Cary, NC). Of the 15,792 ARIC participants, we excluded races other than black or white (N=48), blacks from Minneapolis and Washington County (N=55), prevalent AF or atrial flutter identified by the baseline ECG (N=37), missing or unreadable ECG at baseline (N=309), and unknown MetSyn status at baseline (N=249).
Person-years of follow-up were computed from the baseline exam until a first AF diagnosis, death, loss to follow-up, or December 31, 2005. Race-specific baseline participant characteristics by the MetSyn were compared using chi-squares and t-tests. Overall and race-specific age- and sex-adjusted incidence rates for AF by the MetSyn status at baseline were calculated using Poisson regression. Multivariate-adjusted hazard ratios for AF were estimated in the full cohort and separately by race using Cox proportional hazards regression after adjusting for the following baseline characteristics: age, sex, race (full cohort analysis), center, education (less than high school, high school graduate to vocational school, any college), smoking status (current, former, never), and smoking amount (pack-years). We estimated hazard ratios for AF by baseline MetSyn status, individual components of the MetSyn after additional adjustment for the other components, and by number of MetSyn components in individuals with known values for all MetSyn components. We also modified our Cox model allowing for the MetSyn, measured during clinic visits 1-4, as a time-dependent variable. Multiplicative interactions with sex and race were tested and found to be nonsignificant; however, we also report race-specific results. We estimated the population attributable risks of AF from MetSyn and its components using standard methodology. Finally, we used a macro by Zhang and colleagues to estimate a direct adjusted cumulative incidence curve of AF by number of MetSyn components at baseline, based on a stratified Cox model. Interaction tests between the MetSyn and log of follow-up time and the log-log survival curves were plotted to show that the proportional hazards assumption was met for our Cox regression models.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by NHLBI contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This study was also supported by grants RC1HL099452 from NHLBI and 09SDG2280087 from the American Heart Association. AMC was supported by NHLBI grant T32-HL-007779. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
After exclusions, 15,094 individuals remained in our dataset. Baseline characteristics of the study sample are described in Table 1. Those with the MetSyn were less well educated, had higher mean BMI, were less physically active, and had more comorbidities than did participants without the MetSyn. At baseline, 45.7% of blacks and 39.6% of whites had the MetSyn (Table 2).
Table 1. Baseline Participant Characteristics by Race Group and Metabolic Syndrome Status, ARIC 1987-89.
| Blacks (N=3,882) | Whites (N=11,212) | |||||
|---|---|---|---|---|---|---|
| No Metabolic Syndrome | Metabolic Syndrome | P-value | No Metabolic Syndrome | Metabolic Syndrome | P-value | |
| Age, years | 53.0 ± 5.9 | 54.3 ± 5.7 | <0.01 | 53.7 ± 5.7 | 55.4 ± 5.6 | <0.01 |
| Male | 953 (45.2) | 526 (29.7) | <0.01 | 3057 (45.1) | 2237 (50.4) | <0.01 |
| Education | ||||||
| < High school | 788 (37.5) | 816 (46.1) | <0.01 | 948 (14.0) | 975 (22.0) | <0.01 |
| HS to College | 594 (28.2) | 509 (28.8) | 3029 (44.8) | 2055 (46.4) | ||
| Graduate school | 721 (34.3) | 444 (25.1) | 2791 (41.2) | 1401 (31.6) | ||
| Smoking Status | ||||||
| Current | 675 (32.1) | 483 (27.2) | <0.01 | 1710 (25.3) | 1054 (23.8) | <0.01 |
| Former | 516 (24.5) | 416 (23.5) | 2295 (33.9) | 1671 (37.7) | ||
| Never | 913 (43.4) | 873 (49.3) | 2765 (40.8) | 1710 (38.5) | ||
| Smoking, pack-yrs | 12.8 ± 20.1 | 11.7 ± 20.0 | 0.12 | 16.0 ± 20.9 | 19.5 ± 23.9 | <0.01 |
| Drinking Status | ||||||
| Current | 774 (37.0) | 447 (25.5) | <0.01 | 4607 (68.1) | 2630 (59.4) | <0.01 |
| Former | 449 (21.4) | 450 (25.6) | 1056 (15.6) | 888 (20.1) | ||
| Never | 870 (41.6) | 858 (48.9) | 1101 (16.3) | 908 (20.5) | ||
| Alcohol, g/week | 39.6 ± 113.2 | 22.9 ± 73.3 | <0.01 | 46.9 ± 90.8 | 44.1 ± 98.9 | 0.12 |
| Body mass index, kg/m2 | 27.4 ± 5.5 | 32.3 ± 5.9 | <0.01 | 25.2 ± 3.8 | 29.8 ± 4.9 | <0.01 |
| Sports score | ||||||
| 1.0 to 3.0 | 1869 (88.9) | 1599 (90.5) | 0.09 | 4967 (73.5) | 3575 (80.9) | <0.01 |
| >3.0 to 5.0 | 234 (11.1) | 167 (9.5) | 1788 (26.5) | 844 (19.1) | ||
| CHD history | 53 (2.5) | 101 (5.7) | <0.01 | 196 (2.9) | 371 (8.5) | <0.01 |
| HF history | 65 (3.1) | 204 (11.6) | <0.01 | 87 (1.3) | 334 (7.7) | <0.01 |
| Waist circumference, cm | 92.9 ± 13.5 | 106.8 ± 13.8 | <0.01 | 90.8 ± 11.3 | 104.5 ± 12.2 | <0.01 |
| Triglycerides, mg/dL | 87.2 ± 35.3 | 146.6 ± 101.5 | <0.01 | 102.0 ± 46.2 | 194.6 ± 117.3 | <0.01 |
| HDL-c, mg/dL | 61.1 ± 17.5 | 47.8 ± 14.7 | <0.01 | 56.4 ± 16.6 | 41.3 ± 12.3 | <0.01 |
| Anti-lipid meds | 3 (0.1) | 52 (3.0) | <0.01 | 48 (0.7) | 336 (7.6) | <0.01 |
| Systolic BP, mmHg | 125.2 ± 20.9 | 133.4 ± 21.3 | <0.01 | 114.0 ± 15.2 | 125.3 ± 17.4 | <0.01 |
| Diastolic BP, mmHg | 78.8 ± 12.5 | 80.8 ± 11.8 | <0.01 | 69.6 ± 9.4 | 74.5 ± 10.4 | <0.01 |
| Antihypertensive meds | 581 (27.6) | 1114 (62.9) | <0.01 | 813 (12.0) | 2066 (46.6) | <0.01 |
| Glucose, mg/dL | 100.3 ± 30.7 | 139.5 ± 71.1 | <0.01 | 97.6 ± 18.0 | 117.7 ± 43.1 | <0.01 |
| Diabetes | 125 (6.0) | 640 (36.2) | <0.01 | 172 (2.5) | 847 (19.1) | <0.01 |
Values are N(%) for categorical variables and Mean ± SD for continuous variables.
Table 2. Prevalence of Metabolic Syndrome, Individual Metabolic Syndrome Components, and Number of Metabolic Syndrome Components Present at Baseline by Race, ARIC 1987-89.
| Blacks | Whites | |
|---|---|---|
| Metabolic Syndrome | 1774 (45.7) | 4437 (39.6) |
| Individual Components | ||
| Elevated waist circumference | 2373 (61.1) | 5630 (50.2) |
| Elevated blood pressure | 2609 (67.2) | 4600 (41.0) |
| Elevated triglycerides | 653 (17.4) | 3641 (32.7) |
| Low HDL cholesterol | 1298 (33.9) | 4801 (42.9) |
| Impaired fasting glucose | 2086 (54.1) | 5223 (46.8) |
| Number of Components* | ||
| 0 | 333 (9.0) | 1628 (14.7) |
| 1 | 745 (20.0) | 2640 (23.8) |
| 2 | 997 (26.8) | 2441 (22.0) |
| 3 | 932 (25.0) | 2129 (19.2) |
| 4 | 533 (14.3) | 1500 (13.5) |
| 5 | 182 (4.9) | 756 (6.8) |
Values are N(%).
Individuals with missing values for any component of the metabolic syndrome were excluded from this analysis, N=14,816.
Over a mean follow-up of 15.4 years, 1238 incident cases of AF were identified. The age- and sex-adjusted incidence rates for AF were 60 and 36 per 10,000 person-years in participants with and without the MetSyn at baseline, respectively (Table 3). The hazard ratio for AF among individuals with, compared to those without, the MetSyn was 1.67 (95% CI, 1.49-1.87) overall, and associations did not statistically significantly differ by race (p for interaction=0.73). The corresponding population attributable risk (PAR) was 22.0%, indicating that 22% of AF events could have been prevented with the elimination of the MetSyn, if the association was causal. Analyses adjusting for heart rate or using MetSyn status as a time-dependent variable did not appreciably change the associations (data not shown). Further adjustment for BMI as a potential confounder of the MetSyn and AF association attenuated the estimates (overall HR, 1.42; 95% CI, 1.25-1.61). Considering only AF events identified in ECGs done at study visits (121 AF events), the hazard ratio of AF in those with MetSyn compared to those without was 1.96 (95% CI, 1.36-2.83).
Table 3. Overall and Race-Specific Incidence Rates and Hazard Ratios for Atrial Fibrillation by the Metabolic Syndrome, ARIC 1987-2005.
| Overall | Blacks | Whites | ||||
|---|---|---|---|---|---|---|
| No Metabolic Syndrome | Metabolic Syndrome | No Metabolic Syndrome | Metabolic Syndrome | No Metabolic Syndrome | Metabolic Syndrome | |
| Entire Cohort | ||||||
| N Events | 558 | 680 | 100 | 144 | 458 | 536 |
| Person-years | 140,655 | 92,109 | 32,306 | 25,982 | 108,349 | 66,126 |
| Incidence Rate* | 36 | 60 | 30 | 52 | 38 | 62 |
| Hazard Ratio† | 1.00 | 1.67 | 1.00 | 1.76 | 1.00 | 1.64 |
| 95% CI | (ref.) | (1.49-1.87) | (ref.) | (1.35-2.29) | (ref.) | (1.45-1.87) |
| Without CHD/HF‡ | ||||||
| N Events | 482 | 493 | 81 | 111 | 401 | 382 |
| Person-years | 133,046 | 78,789 | 30,496 | 22,170 | 102,550 | 56,619 |
| Incidence Rate* | 33 | 51 | 26 | 48 | 35 | 53 |
| Hazard Ratio† | 1.00 | 1.53 | 1.00 | 1.91 | 1.00 | 1.46 |
| 95% CI | (ref.) | (1.35-1.74) | (ref.) | (1.42-2.57) | (ref.) | (1.26-1.68) |
| With CHD/HF§ | ||||||
| N Events | 66 | 163 | 16 | 29 | 50 | 134 |
| Person-years | 5,037 | 11,462 | 1,345 | 3,588 | 3,691 | 7,875 |
| Incidence Rate* | 99 | 117 | 112 | 78 | 98 | 133 |
| Hazard Ratio† | 1.00 | 1.28 | 1.00 | 0.62 | 1.00 | 1.52 |
| 95% CI | (ref.) | (0.95-1.71) | (ref.) | (0.34-1.16) | (ref.) | (1.08-2.12) |
Age- and sex-adjusted incidence rate per 10,000 person-years.
Multivariate model adjusted for the following covariates at baseline: age, sex, center, educational attainment, smoking status and cigarette-years of smoking. Overall model additionally adjusted for race.
Analysis among those without prevalent coronary heart disease or heart failure at baseline, N=13,289.
Analysis among those with prevalent coronary heart disease or heart failure at baseline, N=1,252.
We also stratified our analysis by prevalent CHD and HF at baseline to investigate potential mediation of the MetSyn and AF association through CHD and HF (Table 3). The prevalence of the MetSyn was much higher in those with CHD or HF at baseline compared to those without (70.3% and 38.3%, respectively). For those free of CHD or HF, the association between the MetSyn and incident AF was similar to that of the entire cohort (HR, 1.53; 95% CI, 1.35-1.74). The incidence rate of AF among those with prevalent CHD or HF at baseline was higher, but the hazard ratio for the association of MetSyn with AF was attenuated in comparison to the entire cohort or the subgroup of participants without prevalent CHD or HF. In a model excluding those with prevalent CHD or HF and censoring incident CHD or HF during follow-up, the hazard ratio for AF was also attenuated (HR, 1.30; 95% CI, 1.10-1.54).
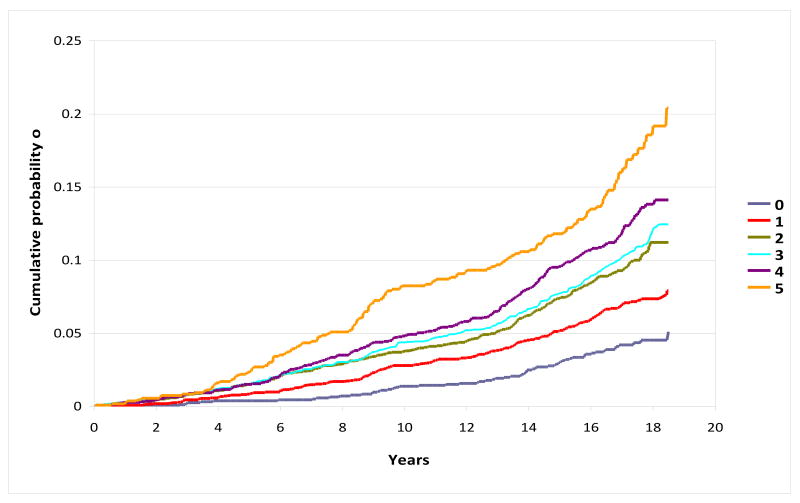
Of the individual MetSyn components, elevated blood pressure appeared to contribute most to AF risk, corresponding to a two-fold increased risk of AF (Table 4). Elevated waist circumference conferred a 40% (95% CI, 23%-59%) increased risk, low HDL-c a 20% (95% CI, 6%-37%) increased risk, and impaired fasting glucose a 16% (95% CI, 3%-31%) increased risk of incident AF, whereas elevated TG was not associated with AF (HR, 0.95; 95% CI, 0.84-1.09). Elevated blood pressure also had the highest PAR of all MetSyn components at 31.9%. Elevated waist circumference had a PAR of 17.6%, and both low HDL-c and impaired fasting glucose were 8.1%. A monotonically increasing risk of AF with increasing number of MetSyn components was observed, with a hazard ratio of 4.40 for those with 5 MetSyn components compared to those with 0 components. Figure 1 depicts, in the full cohort, higher cumulative probabilities of incident AF with greater numbers of MetSyn components, with a cumulative risk of 5.1% among those with 0 components and 20.4% among those with 5 components at baseline.
Table 4. Overall and Race-Specific Hazard Ratios and 95% Confidence Intervals for Atrial Fibrillation by the Individual Components of the Metabolic Syndrome and by Number of Components Fulfilled, ARIC 1987-2005.
| Overall | Blacks | Whites | |
|---|---|---|---|
| Metabolic Syndrome Components* | |||
| Elevated waist circumference | 1.40 (1.23-1.59) | 1.50 (1.08-2.09) | 1.37 (1.19-1.58) |
| Elevated blood pressure | 1.95 (1.72-2.21) | 1.60 (1.15-2.23) | 2.02 (1.76-2.32) |
| Elevated triglycerides | 0.95 (0.84-1.09) | 1.17 (0.84-1.62) | 0.93 (0.80-1.07) |
| Low HDL cholesterol | 1.20 (1.06-1.37) | 1.53 (1.14-2.05) | 1.14 (0.99-1.32) |
| Impaired fasting glucose | 1.16 (1.03-1.31) | 1.04 (0.78-1.38) | 1.18 (1.03-1.35) |
| Number of Components† | |||
| 0 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
| 1 | 1.69 (1.27-2.25) | 1.63 (0.70-3.75) | 1.71 (1.26-2.33) |
| 2 | 2.45 (1.86-3.23) | 2.97 (1.35-6.51) | 2.37 (1.77-3.19) |
| 3 | 2.66 (2.02-3.51) | 3.12 (1.42-6.89) | 2.60 (1.93-3.50) |
| 4 | 3.13 (2.36-4.16) | 4.61 (2.07-10.28) | 2.90 (2.14-3.94) |
| 5 | 4.40 (3.25-5.94) | 4.83 (2.00-11.66) | 4.30 (3.12-5.94) |
Multivariate model adjusted for the following covariates at baseline: age, sex, center, educational attainment, smoking status, cigarette-years of smoking, and the other metabolic syndrome components. Overall model additionally adjusted for race.
Multivariate model among individuals without any missing values for metabolic syndrome components. Multivariate model adjusted for the following covariates at baseline: age, sex, center, educational attainment, smoking status and cigarette-years of smoking. Overall model additionally adjusted for race.
Figure 1. Cumulative Probability of Atrial Fibrillation by Number of Metabolic Syndrome Components at Baseline, ARIC 1987-2005.
Figure adjusted for the following covariates at baseline: age (45 to < 50, 50 to <55, 55 to<60, ≥60), sex, race, center, educational attainment, smoking status and cigarette-years of smoking (quartiles).
Discussion
In this population-based prospective study with up to 19 years of follow-up, individuals with the MetSyn at baseline had a 67% increased risk of incident AF, with similar results in blacks and whites. The risk of AF increased with each MetSyn component present, and of the individual components of the MetSyn, elevated blood pressure was the most strongly associated with AF risk.
Our results are consistent with a prospective study based on annual health checkups in a Japanese population, which reported a 61% increased risk of AF among individuals with the MetSyn according to AHA/NHLBI guidelines. They also found all components, except TG, to increase the risk of incident AF in age- and sex-adjusted analyses. We also found an increased risk for all components, except TG, even after adjustment for additional confounders and the other components of the MetSyn. Another small prospective study of patients admitted to a cardiovascular care center in Japan (32 AF cases, 560 controls) reported a 2.8-fold increased odds of AF among those with the MetSyn. Obesity/overweight was associated with a 3-fold greater odds of AF.
Several studies have reported the associations of high blood pressure, diabetes, and obesity alone with the risk of AF, providing additional evidence that individual components of the MetSyn are risk factors for AF. The Cardiovascular Health Study reported an 11% increased risk of AF for each 10 mmHg increase in systolic blood pressure, and a linear trend of increasing AF risk with increasing systolic and diastolic blood pressure was found in the Women's Health Study. In the Framingham Heart Study, the odds of developing AF among those with hypertension was 1.5 in men and 1.4 in women, and the population attributable risk for AF resulting from hypertension was 14%. In the same study, diabetes was associated with a 1.4 and 1.6-fold increased odds of AF in men and women, respectively. Prospective studies in other settings have also found an increased risk of AF in diabetics compared to non-diabetics. Finally, obesity has been implicated in the development of AF: a meta-analysis pooling 5 population-based cohort studies reported a 49% increased risk of developing AF among obese participants (BMI≥30) compared to nonobese (BMI<30) individuals.
The mechanisms underlying the association between the MetSyn and AF are unclear. The MetSyn and obesity may increase atrial size, and obesity has also been associated with left ventricular hypertrophy. Hypertension is associated with left ventricular hypertrophy, impaired ventricular filling, left atrial enlargement, and slowing of atrial conduction velocity. Finally, diabetes may cause metabolic stress on the atrium or irritability of the atrium through its association with systemic illnesses, such as infection or renal failure. In addition, the MetSyn and its individual components may increase the risk of AF through the development of CHD or HF. We found that among individuals with prevalent CHD or HF at the baseline exam, and also among individuals free of CHD or HF and censoring CHD or HF after the baseline exam, the associations between the MetSyn and AF were attenuated, suggesting that CHD and HF may be mediators of the MetSyn and AF association.
The present study has certain limitations that need to be taken into account when reading the results. First, some incident AF events may have been missed among individuals without symptoms or who were not hospitalized. Most AF events were ascertained by hospital discharge records; therefore, underascertainment of events could have led to misclassification of the events. However, our estimates of AF incidence are similar to those reported by other cohorts. Further, in a sensitivity analysis including only AF events identified during exam ECGs, the hazard ratio for AF was similar to the association found when considering all AF cases. Finally, associations of genetic risk factors and AF in ARIC were similar to other cohort studies whose identification of incident AF events relied more on study exam ECGs. These observations suggest that the possible misclassification of the events due to the method of AF ascertainment was minimal. Second, although we attempted to control for any misclassification in exposure status by conducting time-dependent analyses, the last exposure measurement occurred during study visit 4 (1996-98), almost a decade prior to the end of follow-up for AF. Finally, we did not have information to classify AF as paroxysmal, persistent, or permanent, eliminating the possibility of conducting sub-group analyses on type of AF. Some strengths of our study also deserve mention, including the large sample size, long follow-up, high response rates, racial diversity, and availability of information in a host of potential confounders.
In conclusion, a 67% increased risk of incident AF was seen among individuals with, compared to those without, the MetSyn during the baseline exam, and the risk of AF increased with greater number of MetSyn components. Elevated blood pressure appeared to contribute most to AF risk, although all components except TG were independently associated with an increased risk of AF. The population attributable risk of AF associated with the MetSyn was 22%. Given these findings, strategies to reduce the development of the MetSyn or to control its individual components might reduce the burden of AF.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Tanabe N, Watanabe T, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117(10):1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetani K, Kodama Y, Nakamura T, et al. High prevalence of paroxysmal atrial fibrillation and/or atrial flutter in metabolic syndrome. Circulation Journal. 2007;71(2):252–255. doi: 10.1253/circj.71.252. [DOI] [PubMed] [Google Scholar]
- Nicolaou VN, Papadakis JE, Karatzis EN, Dermitzaki SI, Tsakiris AK, Skoufas PD. Impact of the metabolic syndrome on atrial size in patients with new-onset atrial fibrillation. Angiology. 2007;58(1):21–25. doi: 10.1177/0003319706297913. [DOI] [PubMed] [Google Scholar]
- Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116(11 Suppl):213–219. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Manolio TAMHS, Kuller LHDH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- Smith SC, Jr, Clark LT, Cooper RS, Daniels SR, Kumanyika SK, Ofili E, Quinones MA, Sanchez EJ, Saunders E, Tiukinhoy SD. American Heart Association Obesity, Metabolic Syndrome,and Hypertension Writing Group. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: report of the Obesity, Metabolic Syndrome, and Hypertension Writing Group. Circulation. 2005;111(10):e134–9. doi: 10.1161/01.CIR.0000157743.54710.04. [DOI] [PubMed] [Google Scholar]
- The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(4):1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thrombosis & Haemostasis. 1989;61(1):15–19. [PubMed] [Google Scholar]
- Nagele U, Hagele EO, Sauer G, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22(2):165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28(6):1379–1388. [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Caidaul K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Loberiza FR, Klein JP, Zhang M. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119(16):2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- Movahed M, Hashemzadeh M, Mazen Jamal M. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105(3):315–318. doi: 10.1016/j.ijcard.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of new-onset diabetes mellitus on development of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial) Am J Cardiol. 2008;101(5):634–638. doi: 10.1016/j.amjcard.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155(2):310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(10A):9G–14G. doi: 10.1016/s0002-9149(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86(5):516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Rice KM, Arking DE, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41(8):879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]