Abstract
Earlier recognition of chronic kidney disease (CKD) could slow progression, prevent complications, and reduce cardiovascular-related outcomes. However, current estimates of CKD awareness indicate that both patient- and provider-level awareness remain unacceptably low. Many of the factors that are possibly associated with CKD awareness, which could help guide implementation of awareness efforts, have yet to be fully examined. Also, little is known regarding whether increased patient or provider awareness improves clinical outcomes or whether there are possible negative consequences of awareness for CKD patients. Further research is necessary to continue to design and refine awareness campaigns aimed at both patients and providers, but there is an immediate need for dissemination of basic CKD information, given both the high prevalence of CKD and its risk factors and the low estimated awareness of CKD.
Keywords: chronic kidney disease, awareness, knowledge, patient, provider, review
Introduction
Chronic kidney disease (CKD) is a common and growing problem worldwide; in the United States, >10% of adults showing some evidence of kidney damage and/or reduced kidney function (1). Identification of CKD requires recognition of individual risk and appropriate laboratory testing (serum creatinine and/or urinary protein) (2), since symptoms generally do not manifest in earlier stages of CKD; however, earlier-stage CKD can lead to several complications, such as anemia and bone mineral metabolism disorders, and poor outcomes, including cardiovascular events, morbidity, and mortality (3), in addition to progression to end-stage renal disease (ESRD), requiring dialysis or transplant for survival. Despite these known adverse consequences of CKD, the majority of persons with the disease, especially prior to ESRD, remain unaware of their disease (4–6). Awareness of CKD remains unacceptably low, despite recent attempts to increase awareness through dissemination of clinical practice guidelines and recommendations for patients with CKD or its risk factors to providers (7–9), community awareness events such as World Kidney Day (10, 11), and free screening efforts for high-risk individuals like the Kidney Early Evaluation Program (KEEP) (12, 13). Earlier recognition of CKD could slow progression of CKD, prevent complications, and reduce cardiovascular-related outcomes; additionally, early referral to a nephrologist has been shown to improve outcomes for those who progress to end-stage renal disease (14–17).
Here, we review previous work in CKD awareness and examine the need for future research to guide the implementation of further dissemination and policymaking efforts. CKD awareness can be broadly categorized as community- or patient-level awareness and provider-level awareness, each of which is discussed in detail below. However, as shown in Figure 1, it should be noted that the two types of awareness are far from independent. Although patients may have general knowledge of CKD and be aware of their individual risk, they cannot know their individual CKD status or obtain appropriate treatment without provider awareness, testing, and communication; additionally, patient requests for testing based on perceived risk could increase provider awareness. Because CKD awareness can be defined as knowledge of CKD and its causes, risk factors, appropriate treatment, and consequences and also as knowledge of disease risk and status, the terms “awareness” and “knowledge” are used interchangeably throughout.
Figure 1.
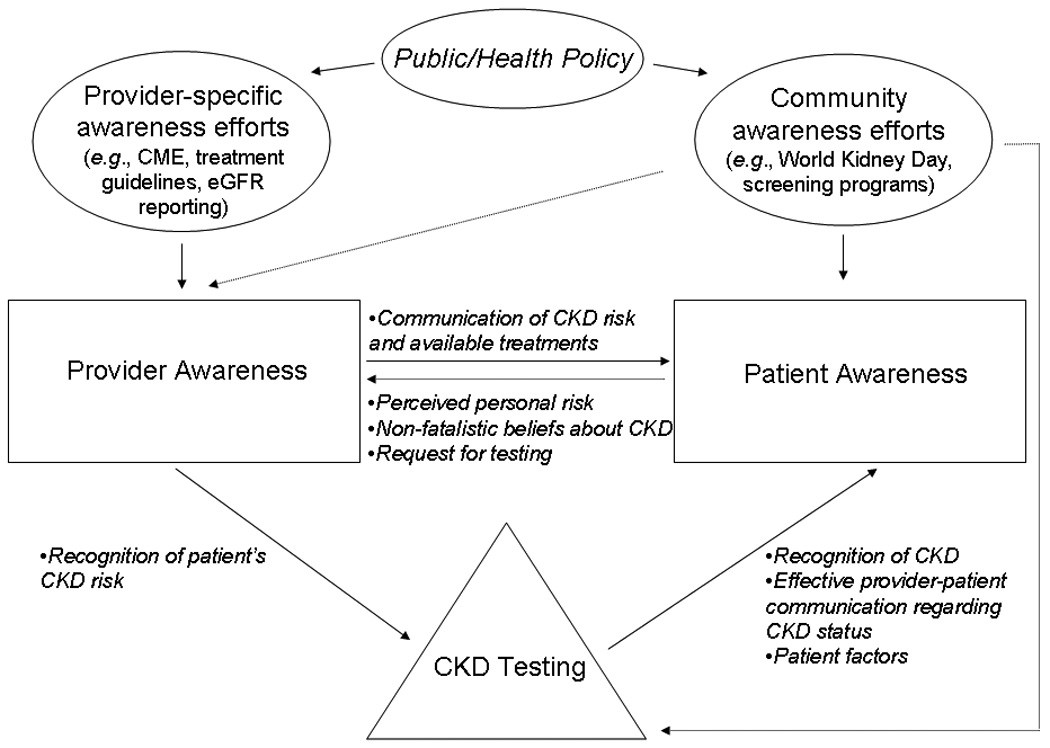
Interaction of provider and patient awareness of CKD, its risk factors, and its consequences. Solid and dotted arrows, purported direct and indirect routes, respectively, of information exchange. CME, continuing medical education.
Patient Awareness of CKD
Patient-level CKD awareness includes both general knowledge of CKD, its risk factors, and consequences and understanding of individual risk and CKD status. Figure 2 shows that awareness of CKD status is dependent on understanding of individual risk, which in turn depends upon general knowledge of CKD. Failure to achieve adequate levels of awareness at each phase likely precludes becoming aware at the next phase; however, the converse, that achieving adequate knowledge will guarantee the next phase of awareness, does not hold. Various psychological and other factors (Figure 2; Table 1) may still prevent the patient from understanding information to which they have been exposed and from acting appropriately upon CKD awareness to improve self-management strategies and, ultimately, clinical outcomes. All these inter-relationships and factors must be considered in measurement and evaluation of patient awareness.
Figure 2.
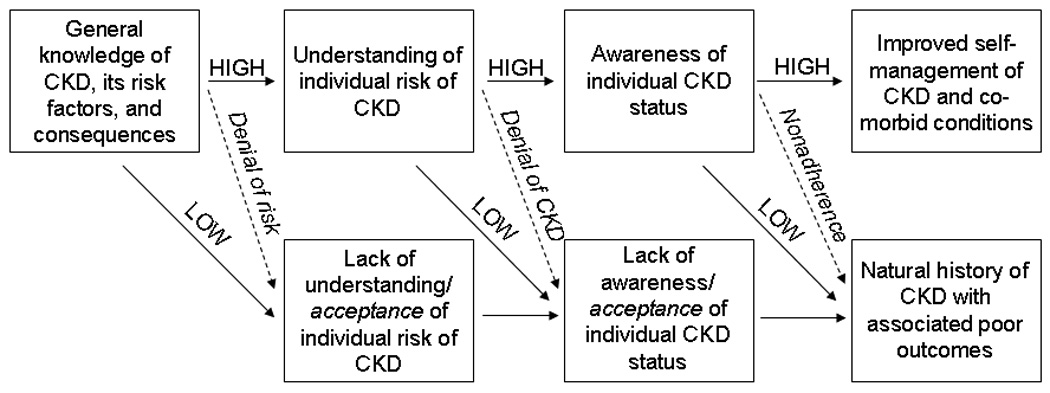
Potential path to awareness and associated outcomes for the CKD patient. Dashed arrows, possible psychosocial effects on awareness, understanding, and outcomes.
Table 1.
Association of various established and putative factors with patient awareness and outcomes.
| Measure of awareness |
|||
|---|---|---|---|
| Factor | Increased general knowledge of CKD |
Increased understanding of individual risk of CKD |
Awareness of individual CKD status |
| Demographic | |||
| Increased age | ○21 | ○4, +26,* | |
| Female gender | ○21 | −4 | |
| African-American or other racial minority |
+21 | +4 | |
| Socioeconomic | |||
| Increased income | ○21 | ||
| Higher level of education |
○21 | ○4 | |
| Married status/social support |
|||
| Friends or acquaintances with kidney disease |
|||
| Access-related | |||
| Routine provider | ○4 | ||
| Provider knowledge of CKD |
|||
| Effectiveness of Provider communication |
|||
| Insured | ○4 | ||
| Higher health literacy |
+21 | ||
| Higher health numeracy |
|||
| Cultural/language barriers |
|||
| Exposure to health- related media |
|||
| Clinical | |||
| Family history of CKD |
+/○19,*,** | +19*,** | |
| Diabetes | /○19,*,** | +21,+ 19,*** | 4, ○24 |
| Hypertension | +/○19,*,** | 19*** | +4 |
| Cardiovascular disease |
+25,26 | ||
| Higher CKD stage | +4,22,23,25,26 | ||
| Presence of proteinuria |
+4 | ||
| Presence of CKD symptoms or complications (fatigue/anemia, pain/bone mineral metabolism disorder, etc.) |
|||
| Obesity | ○21 | +4 | |
| Physical activity | ○4,26 | ||
| Number/severity of comorbid conditions |
|||
| Psychological | |||
| Denial | |||
| Worry | |||
| Fear of dialysis/fatalistic attitudes |
|||
| Outcomes | |||
|
Association of type awareness with better self- management or improved outcomes |
+/−21 (blood pressure control), +19 (read information about CKD; spoke to doctor about CKD; got tested for CKD) |
○24*** (target blood glucose) |
|
+, positive effect; −, negative effect; ○, no effect; and blank cell, unknown effect. Effect summarized from peer-reviewed published literature only; superscript numbers represent citations.
African-Americans only.
Family history of CKD, diabetes and hypertension combined as “risk factors.”
Possible positive effect of CKD awareness among males with diabetes only.
Prevalence of patient-level CKD awareness
Measures of patient-level CKD awareness
Patient-level CKD awareness can be measured in multiple, complementary ways. Knowledge of CKD can be ascertained through ability to provide a correct definition for CKD or correctly naming risk factors, symptoms, and diagnostic tests for CKD. Perceived individual risk or susceptibility to CKD can be assessed through binary or graded questionnaire items. Studies of awareness of CKD status require both questionnaire items ascertaining whether the individual is aware that they have CKD and laboratory or diagnostic data to confirm the individual’s CKD status. Such questionnaire items can be difficult to construct and validate, given the variation in medical terminology used to describe CKD (18). For example, in National Health and Nutrition Examination Survey (NHANES) the question “Have you ever been told by a healthcare provider that you have weak or failing kidneys?” is used to assess awareness; while the language of the item is likely easily understood by most survey participants, it is also unlikely that all individuals with albuminuria or slightly reduced kidney function have been told that their kidneys are failing. Additionally, comparison of knowledge, perceived risk, and awareness of CKD status to demographics, socioeconomic, healthcare access, clinical (including presence of CKD and its risk factors), and psychological factors, or association of CKD awareness with outcomes, requires that many elements be included in a single study. Because few studies have been designed to specifically measure CKD awareness, most studies have reported on a portion of possible factors and outcomes, and no studies to date have measured all three phases of awareness simultaneously.
Estimates of patient-level CKD awareness
Knowledge of CKD has been found to be quite low across populations. In a survey of urban African-American adults, <3% named kidney disease as an important health problem, compared to 61% and 55% naming hypertension and diabetes, respectively (19). Additionally, fewer than half of those surveyed could define kidney disease, one-quarter could name a diagnostic test, and 7% knew that protein in the urine was a sign of kidney disease. Only 18%, 14%, 12% and 2% knew that African-American race, diabetes, hypertension and family history of CKD, respectively, were risk factors for the development or progression of CKD (19). In a study of Australian adults with diabetes, knowledge of risk factors was even lower, with only 9% and 3% naming diabetes and hypertension, respectively, as risk factors for CKD (20).
Perceived individual risk of CKD has also been estimated to be quite low. In the urban African-American survey by Waterman et al. (19), only 26% of those with CKD or its risk factors (diabetes, hypertension, or family history of CKD) felt their risk of CKD was “higher than average,” and, perhaps more disturbingly, 52% felt their risk was “lower than average.” In a survey of primary care patients in Maryland at high risk for CKD due to hypertension (21), African-American race, and high rates of uncontrolled blood pressure and diabetes, only 20% felt “very likely” to develop CKD and 33% were “very concerned” about developing CKD, when, in fact, nearly one-third of the surveyed population already had CKD.
Finally, as expected given the low rates of general CKD knowledge and perceived risk among susceptible individuals, most estimates of awareness of disease among those with CKD have been low. In a nationally representative survey of U.S. adults, awareness of disease among those with CKD was <10% overall and <40% even for those with CKD stage 4 (Figure 3); the prevalence of awareness did not show signs of increasing over the period from 1999 to 2006 (4). In a similar survey in Taiwan, reported awareness of CKD, using the same survey item, was 8% and 25% among those with CKD stages 3 and 4, respectively, reporting being told by a provider that they have weak or failing kidneys (22). In the KEEP study, which specifically targeted high-risk U.S. patients for CKD screening, rates of awareness were similarly low, with <10% being aware, even among those with diabetes (23, 24). Reported awareness in cohort studies did not differ dramatically: in a U.S. cohort study in older adults, <10% of individuals with eGFR<60 ml/min/1.73 m2 reported being told that they had kidney disease (25); while in another cohort study of primarily African-Americans, awareness was 16% (26).
Figure 3.
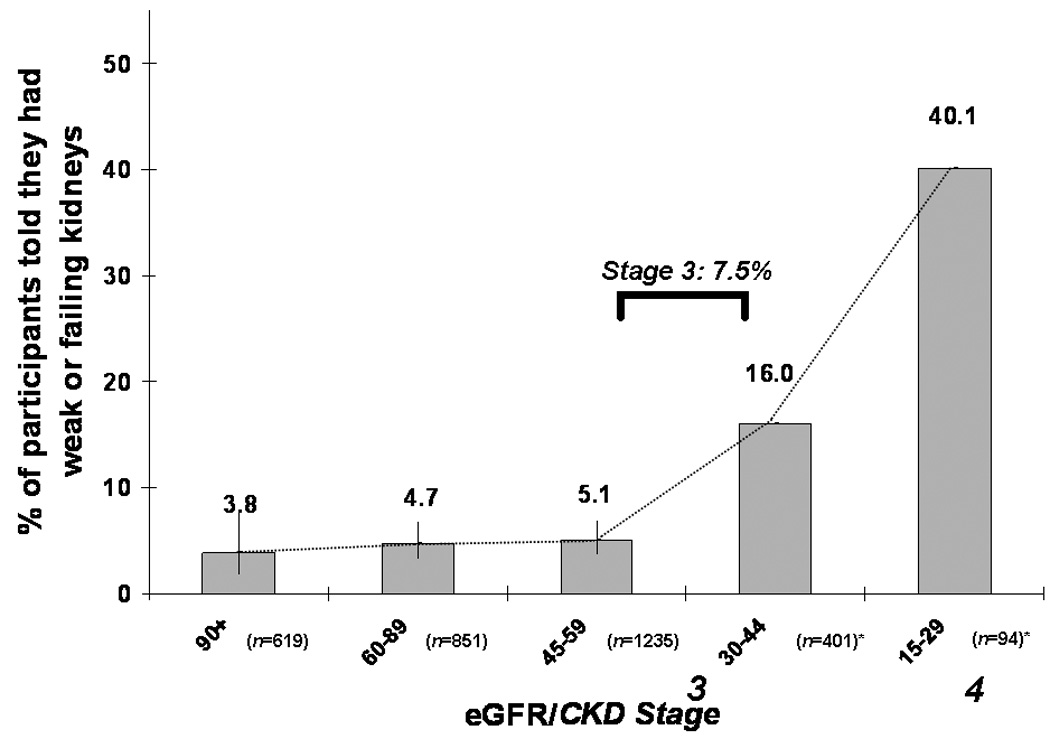
Percentage of U.S. population with albuminuria (single measurement) or CKD stage 3 or 4 who were aware of their disease, by eGFR. Data from the National Health and Nutrition Examination Survey 1999–2006. Bars, 95% CI. Albuminuria defined by albumin:creatinine ratio obtained from spot urine sample, >30 mg/g; GFR, estimated glomerular filtration rate by MDRD Study formula in ml/min/1.73 m2. Modified with permission from Plantinga et al. [4]. *No standard error estimates due to small sample size.
Factors associated with patient-level CKD awareness
Demographic factors
From the limited data available thus far, age of the patient with or at high risk for CKD appears to be unrelated to understanding of personal risk (21) and CKD status (4), although increased age may be associated with greater awareness among African-Americans (Table 1) (26). Understanding of personal risk does not seem to differ by gender (21); however, males with CKD were more likely than females in the United States to be aware of their disease status (12% vs. 5%) (4), likely due to their higher (and more recognizable as abnormal) creatinine levels in the presence of CKD. eGFR reporting by laboratories and refinement of estimating equations (27, 28) may close this gender gap over time. Minority race, and particularly African-American race, is also associated with greater awareness of risk and CKD status (4, 21), perhaps due to greater physician recognition and higher likelihood of having family members or friends with CKD.
Socioeconomic factors
Current literature suggests that income and level of education are unrelated to awareness of personal risk or CKD status, after adjustment for other factors (4, 21), but the association of these factors with general knowledge of CKD has not been examined. Additionally, many other social factors, such as marital status, level of social support, and having friends or acquaintances with CKD (possibly mediated by neighborhood effects), have yet to be examined in relation to awareness of CKD.
Healthcare access
Few aspects of healthcare access have been studied in association with CKD awareness. Having a routine provider and health insurance were not associated with awareness of CKD status (4) (Table 1). Health literacy is just beginning to be explored in CKD but does appear to be positively associated with understanding of personal CKD risk (21); health numeracy (29), which describes an individual’s ability to understand numbers as they relate to health (e.g., interpretation of test results, calculation of dosages, and understanding of dietary portions and nutrients), is likely to be quite important in CKD awareness and self-management but remains unexplored. Provider factors, such as provider knowledge of CKD and effectiveness of provider communication, which may depend on both the provider’s skills and other factors like race/ethnicity concordance with the patient (30), have not been studied in regards to CKD awareness. Language and cultural barriers to CKD awareness may also exist. Finally, the effect of exposure to health-related media, through which most awareness campaigns would operate, on patient CKD awareness has not been explored.
Clinical factors
Family history of CKD and having diabetes and hypertension appear to be associated with increased (albeit still unacceptably low) levels of CKD knowledge and understanding of risk (19, 21), and those with diabetes and hypertension were more likely to be aware of their CKD (4) than those without these conditions in a national survey, although the association was not found for diabetes in a screening study targeting high-risk patients (24) (Table 1). Cohort studies have shown that presence of cardiovascular disease also seems to be associated with greater levels of CKD status awareness (25, 26). Regardless of study type, increased severity of CKD appears universally associated with greater awareness of disease status (4, 22, 23, 25, 26), providing some face validity to the measures of awareness used; presence of proteinuria was also associated with greater awareness (4), perhaps due to increased symptoms in these individuals. However, presence of CKD symptoms has not been explored as a predictor of CKD awareness, nor has total number or severity of comorbid conditions. Obesity and physical activity thus far have mostly neutral associations with CKD awareness, after adjustment for other factors (4, 21, 26).
Psychological factors
Currently, issues of denial, worry, and fatalistic attitudes remain unexplored as modifiers or predictors of CKD awareness. Additionally, it is unlikely that these issues comprise a comprehensive listing of all possible psychological factors that could be associated with CKD awareness. However, for awareness campaigns to be effective, such issues must be examined, particularly with regard to identification of patients for whom these factors will prevent action, even with sufficient understanding of their CKD, and strategies for motivation in the face of these obstacles. For example, fatalistic attitudes about CKD may prevent action because of hopelessness stemming from patients’ beliefs that progression to ESRD and dialysis is inevitable; awareness campaigns and provider communication thus should include information not only on the prevalence and consequences of CKD (including the rarity of progressing to ESRD) but also on the treatments available to prevent such consequences.
Outcomes associated with patient-level CKD awareness
Although research among ESRD patients has shown that greater knowledge of their condition leads to better clinical outcomes (31, 32), little data are available on whether awareness of earlier-stage CKD is associated with better clinical outcomes. There is some evidence that those with higher perceived risk for CKD are more likely to seek information, speak to their doctor about CKD, and get tested (19). Interestingly, those with the highest and lowest perceived risk were less likely than those with intermediate perceived risk to have controlled blood pressure (21); for those with the highest perceived risk, psychological factors such as those mentioned above may explain this result. Awareness of CKD status was not generally associated with achieving target blood glucose regardless of diabetes status in the KEEP study (24), but CKD awareness was associated with lower hemoglobin A1C in a group of primary care patients with diabetes and CKD (33). Further research is necessary to determine whether—and for whom—CKD awareness leads to better self-management and better clinical outcomes (particularly, decreased hospitalization, cardiovascular events, and/or mortality). Additionally, the possibly negative consequences of CKD knowledge and awareness, including excess worry about risk and decreased quality of life and depression associated with the diagnosis of chronic disease, should also be explored and weighed against any improvement in clinical outcomes.
Summary of patient-level CKD awareness of CKD
Although current data are sparse and not without limitations, the general consensus is that patient awareness of CKD is unacceptably low. Further exploration of the factors associated with patient-level CKD awareness, behavioral changes as a result of CKD awareness, and the impact of CKD awareness on outcomes is greatly needed to guide awareness efforts. Particularly, the ideal targets (e.g., middle-aged adults with diabetes and/or hypertension) and content (e.g., consistent information on treatment and prevention in addition to risk factors and consequences) for community awareness campaigns should be carefully considered. Also, providers should be armed with not only knowledge and awareness of CKD, as described below, but also with the necessary tools to communicate information effectively and sensitively and to encourage patient self-management.
Healthcare Provider Awareness of CKD
Provider-level CKD awareness includes both general understanding of CKD, its risk factors, and associated complications/consequences and knowledge of patient management strategies to slow CKD progression. As illustrated in Figure 1, inadequate awareness among providers likely precludes patient awareness at both the general and individual levels; and while the consequences of unawareness at this level have not been extensively studied, it seems likely to lead to worse outcomes, including higher patient morbidity and mortality.
Prevalence of provider CKD awareness
Measures of provider CKD awareness
Health-care provider awareness of CKD can be measured in a variety of ways; accurate estimates of prevalence of provider awareness of CKD require an understanding of these different measures, including their strengths and limitations. For example, one proxy for CKD awareness is appropriate CKD identification of patients with renal dysfunction with International Statistical Classification (ICD-9) diagnostic codes. While there are limited data correlating identification of CKD with coding and actual practitioner understanding of chronic kidney disease, ICD-9 codes appear to be a specific, if insensitive, marker at measuring provider awareness of CKD. In a cohort of adult veterans with diabetes and CKD stages 3–5, at least 1 of the 79 ICD-9 codes related to CKD was present in 42.4% of CKD cases identified by laboratory testing and 93.2% of cases with a CKD code did in fact have CKD by laboratory testing (34). Despite these relatively poor performance characteristics, greater CKD documentation with ICD-9 codes has been associated with lower eGFR and a decrease in non-steroidal anti-inflammatory drug prescriptions, lending some validity to use of ICD-9 codes as a measure of physician awareness of CKD (35).
Unlike ICD-9 codes, other metrics of provider awareness of CKD may provide insight into provider understanding of renal physiology, CKD risk factors, clinical presentation and complications, correct CKD management, and knowledge regarding appropriate timing of nephrology referral. Questionnaire data can reveal level of provider awareness of CKD as a public health problem and may identify barriers to improved CKD knowledge and care. However, questionnaires are prone to volunteer bias and thus may not accurately reflect the current state of provider awareness of CKD. Physician problem lists with associated plans can provide more in-depth information about provider understanding of CKD and management, but examining such problem lists cannot be performed efficiently on a large scale.
Estimates of provider awareness of CKD
Estimates of primary care provider (PCP) recognition of kidney disease remain alarmingly low. Reported physician documentation of CKD with ICD-9 codes in a large managed care cohort with greater than 10,000 individuals with CKD stages 3–5, was only 14.4% (35). PCP awareness was significantly associated with patient gender and age, lower eGFR and at least one of the following comorbid conditions: hypertension, diabetes, congestive heart failure and peripheral vascular disease. eGFR was the strongest predictor of CKD identification, but proper identification was documented in merely 6%, 14%, 31% and 50% of patients with eGFR 50–59, 40–49, 30–30 and 20–29 ml/min/1.73 m2, respectively (35). This low prevalence of CKD documentation was similar within a Veterans Administration (VA) Hospital system in Michigan (CDC CKD Surveillance Project, unpublished report) and in a geriatric Caucasian population in the Mid-South VA Healthcare network (36).
While ascertainment of physician awareness of chronic diseases with ICD-9 coding has its aforementioned limitations, these data are consistent with other studies that examined physician knowledge directly with questionnaires and clinical vignettes. In one study using several clinical scenarios to ascertain knowledge surrounding basic CKD awareness and management; 40% of U.S. family physicians failed to recognize progressive CKD in a patient with a serum creatinine of 2.0 mg/dl and gross proteinuria (37). Agrawal et. al. (38) demonstrated similar rates of awareness among internal medicine residents (physicians-in-training) across the United States. Again using a clinical vignette describing a patient with persistent proteinuria and eGFR of 76 ml/min/1.73 m2, only 54% of responders correctly identified proteinuria as a marker of chronic kidney disease. However, 65% of residents correctly identified CKD stage 3 with an eGFR range of 30–59 ml/min/1.73 m2 and nearly 87% and 73% noted that eGFR and a random urine albumin:creatinine ratio should be used to evaluate a patient at high risk of CKD (38).
Examination of medical records to ascertain PCP recognition of CKD has yielded similar results. In one academic outpatient family medicine practice (39), only 13.9% physicians documented awareness of CKD stage 3, defined by any written evidence of CKD recognition in the medical chart, including CKD in the problem list, ordering of diagnostic investigations for renal impairment, or referral to a nephrologist. It is important to note that CKD recognition increased dramatically after an educational curriculum, to 85.1%, suggesting that provider identification of CKD could be improved through targeted interventions (39).
Estimates of provider awareness of CKD risk factors
General awareness of CKD risk factors appears more widespread than knowledge of CKD itself. Surveys demonstrate high levels of knowledge of diabetes and hypertension as risk factors, with 99% of family practice and internal medicine trainees identifying them as strong risk factors in web-based questionnaires (38, 40). Minority status, age, and family history of CKD were less well-recognized as risk factors. A survey of PCPs practicing in predominantly African-American communities also revealed that a third of respondents (34.4%) did not consider family history of kidney disease to be associated with increased risk of CKD and 22% did not consider African American race to be a risk factor for development of CKD (41).
Estimates of provider awareness of CKD guidelines
Several studies indicate that only 22–30% of family practice physicians and general internists are aware of the existence of National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines regarding management of patients with CKD; in contrast, approximately 80% of the same physicians report that the American Diabetes Association (ADA) and Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC) guidelines influence CKD care (37, 41). These data help explain the differing rates of knowledge vis-à-vis CKD risk factors detailed above; the ADA and JNC guidelines both emphasize hypertension and diabetes as significant CKD risks, but both fail to describe race and family history of CKD as additional risk factors, which are included in the KDOQI guidelines.
Factors associated with provider CKD awareness
Demographic factors
Current data demonstrate that family physicians with more than 10 years in clinical practice are less likely to recognize CKD and less likely to recommend patient referral to nephrologists for advanced care (Table 2) (37). Other studies that indicate more recently trained physicians have greater awareness of KDOQI guidelines and risk factors for development of CKD further corroborate this finding (38, 40). However, no association has been found between physician race/ethnicity and gender and differing rates of CKD awareness and nephrology referrals (37, 38)
Table 2.
Factors associated with provider awareness of CKD and CKD risk factors.
| Measure of awareness |
||
|---|---|---|
| Factor | Increased identification of CKD |
Increased understanding of CKD risk factors |
| Demographic37,38,40 | ||
| <10 years of clinical practice |
+ | + |
| Age Female gender | ○ | |
| African-American or other racial minority |
○ | |
| Practice Characteristics37,40 | ||
| <50% practicing clinical medicine |
+ | |
| Size of practice | ○ | |
| Practice setting (academic center vs community hospital vs private practice) |
○ | |
| Geographic location % of patient panel with CKD, as definted by laboratory data |
||
| Urban practice | ○ | |
| Specialty37,40 | ||
| Family Pracitioner | − | − |
| Internist | + | + |
| Nurse Practitioner/Physician Assistant |
||
| Residency Training Characteristics38 | ||
| US training program | ○ | |
| University vs. community program |
○ | |
| Program with an in- house nephrology fellowship program |
||
| Participation in an inpatient nephrology elective rotation |
||
| Participation in a CKD clinic |
||
| Presence of an Educational curriculum on nephrology for residents |
||
+, positive effect; −, negative effect; ○, no effect. and blank cell, unknown effect. Effect summarized from peer-reviewed published literature only; superscript numbers represent citations.
Practice characteristics
Somewhat surprisingly, PCPs who spend less than 50% of their time practicing clinical medicine are more likely to correctly diagnose CKD stages 3–4, though the difference was non-significant in a multivariable-adjusted analysis (Table 2). These physicians are also more likely to recommend nephrology referral. Size of practice, practice setting (community hospital vs. academic center vs. private), and census region (urban vs. rural) were not associated with differing rates of CKD identification or nephrology referral (37).
A difference has been noted in CKD awareness and referral rates between general internists and family practitioners (77% vs. 64% and 98% vs. 69%, respectively) (37). Perhaps family practitioners minimize the number of specialty referrals compared to their general internist colleagues because of decreased informal access to medical specialists and a greater desire to maintain continuity of care. However, it is noteworthy that differences in referral pattern are strongly related to differences in awareness of clinical guidelines and confidence in the effectiveness of care (37) suggesting a lack of knowledge surrounding CKD management by many family practitioners. Interestingly, online surveys administered to both family medicine and internal medicine trainees reveal similar rates of knowledge surrounding CKD risk factors and complications and anti-hypertensive management in CKD patients (40). Practice patterns among the two groups of physicians are thus more similar among trainees, suggesting that the gap in CKD awareness and management may decline over time.
Of note, CKD awareness among mid-level providers such as nurse practitioners and physician assistants has not been studied. With the PCP workforce shrinking in the United States, the dissemination of primary care will rely more heavily on these providers. More extensive evaluation of their knowledge and practice patterns is thus warranted.
Consequences associated with provider CKD awareness
There are little data that demonstrate improved patient outcomes with greater provider awareness of CKD. However, in one prospective cohort study, an association between nephrology referral and greater preservation of renal function in diabetic patients with early CKD was noted; the benefit was largely mediated by better blood pressure control and more extensive use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (42). The importance of CKD awareness at earlier stages of disease for implementation of evidence-based medication regimens cannot be underestimated. Additional studies examining the effect of increased provider awareness on patient awareness of CKD and patient behavioral changes are key. In addition, the effects of earlier CKD diagnosis on subsequent progression of kidney dysfunction and cardiovascular outcomes are essential to document and understand. Potential negative outcomes associated with increased provider awareness of CKD cannot be neglected. Earlier detection of CKD may become an unnecessarily large financial burden on an already over-extended healthcare system if no positive outcomes are delineated, and the numbers of nephrologists in the United States may be insufficient to handle referrals of all patients with early-stage CKD. Furthermore, increased provider awareness of CKD may lead to overuse of PCP time, relegating other important chronic diseases to the background of medical care.
Summary of provider awareness of CKD
Early referral of patients to nephrologists is optimal, but given the relatively small number of practicing nephrologists nationwide, nephrologists cannot exclusively manage all patients with CKD. The burden of CKD management thus falls largely on PCPs. Although current data are limited, the general consensus is that awareness of CKD by all types of PCPs is unacceptably low and knowledge of CKD management is particularly poor among family practitioners. Further exploration of the factors associated with decreased CKD knowledge and the impact of CKD awareness on individual patient outcomes and public health outcomes is necessary to guide awareness efforts moving forward. In particular, next steps should focus on evaluation of CKD awareness and management among mid-level providers and increasing CKD knowledge and awareness of KDOQI guidelines among family practitioners. In addition, educational efforts aimed at improving CKD awareness in training programs should be considered, even though this may be a challenging undertaking, given the requirements and restrictions placed upon U.S. training programs.
Influence of Current Community Efforts and Public Policy on CKD Awareness
As shown in Figure 1, public policy can affect both patient and provider awareness. World Kidney Day (10, 11; www.worldkidneyday.org) is an annual event that began in 2006 and is jointly sponsored by the NKF and International Society of Nephrology, “to raise awareness of the importance of our kidneys to our overall health and to reduce the frequency and impact of kidney disease and its associated health problems worldwide.” The World Kidney Day effort aims to raise awareness in the community by offering free screening and by targeting various media outlets with education about CKD, its risk factors, and consequences. The KEEP study (12, 13) is another effort of the NKF that targets the community by offering free screening for any individual at risk (aged 18 years or older, with hypertension, diabetes, and/or family history of CKD). KEEP has screened >130,000 individuals in cities thoughout the United States since its inception in 1997. Both of these NKF efforts have increased general knowledge of CKD and awareness of disease status among patients. Greater awareness of disease status at earlier stages would allow patients to take full advantage of The Medicare Improvement for Patients and Providers Act of 2008, which mandates six educational sessions for individuals with CKD stage 4 to prepare for ESRD and make informed choices regarding treatment options, which would in turn increase knowledge and awareness in those with severe CKD. Finally, provider awareness could be directly affected by health system policy that incentivizes attainment of CKD-related clinical performance guidelines (7–9) or continuing education related to CKD.
Conclusions
Levels of awareness and knowledge among patients and providers must be improved to prevent CKD, its progression, and its many consequences. More research is needed to guide implementation of awareness campaigns, including determining appropriate content and target populations, but many challenges exist. Changing definitions of CKD and eGFR equations, and subsequent prevalence estimates, could confuse providers and patients alike (27, 43). Many factors, such as patient health literacy/numeracy and psychological factors and patient-provider communication, which likely play a large part in modifying CKD awareness and resulting preventive behaviors, are difficult to measure. Additionally, no single dataset or study population will be sufficient to study all predictors and outcomes of CKD awareness, and outcome studies require substantial resources. Despite these challenges, further studies are essential to fully understand the factors and consequences associated with CKD awareness. However, because such studies will take time, efforts to increase awareness through dissemination of basic CKD information, may need to be implemented simultaneously with these studies, given the high prevalence of CKD and its risk factors.
Acknowledgements
Dr. Powe is partially supported by grant K24DK02643 from the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. Dr. Powe is Principal Investigator and Ms. Plantinga is a Co-Investigator in the Centers for Disease Control and Prevention Surveillance Project. Report contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None of the authors have any conflicts of interest to report.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50(2):169–180. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER, 3rd, Saran R, Messer KL, Levey AS, Powe NR. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med. 2008;168(20):2268–2275. doi: 10.1001/archinte.168.20.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickolas TL, Frisch GD, Opotowsky AR, Arons R, Radhakrishnan J. Awareness of kidney disease in the US population: findings from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2000. Am J Kidney Dis. 2004;44(2):185–197. doi: 10.1053/j.ajkd.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16(1):180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 7.American DA. Standards of Medical Care in Diabetes-2009. Diabetes Care. 2009;32:S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: common, harmful and treatable--World Kidney Day 2007. Am J Nephrol. 2007;27(1):108–112. doi: 10.1159/000099801. [DOI] [PubMed] [Google Scholar]
- 11.Chin HJ, Ahn JM, Na KY, Chae DW, Lee TW, Heo NJ, Kim S. The effect of the World Kidney Day campaign on the awareness of chronic kidney disease and the status of risk factors for cardiovascular disease and renal progression. Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp512. in press. [DOI] [PubMed] [Google Scholar]
- 12.Bakris G, Collins AJ. Executive summary: Kidney Early Evaluation Program (KEEP) 2007 Annual Data Report. Am J Kidney Dis. 2008;51(4):S1–S2. doi: 10.1053/j.ajkd.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Vassalotti JA, Li S, Chen SC, Collins AJ. Screening populations at increased risk of CKD: the Kidney Early Evaluation Program (KEEP) and the public health problem. Am J Kidney Dis. 2009;53(3 Suppl 3):S107–S114. doi: 10.1053/j.ajkd.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 14.Lin CL, Chuang FR, Wu CF, Yang CT. Early referral as an independent predictor of clinical outcome in end-stage renal disease on hemodialysis and continuous ambulatory peritoneal dialysis. Ren Fail. 2004;26(0886-022; 5):531–537. doi: 10.1081/jdi-200031733. [DOI] [PubMed] [Google Scholar]
- 15.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137(6):479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 16.Roderick P, Jones C, Drey N, Blakeley S, Webster P, Goddard J, Garland S, Bourton L, Mason J, Tomson C. Late referral for end-stage renal disease: a region-wide survey in the south west of England. Nephrol Dial Transplant. 2002;17(7):1252–1259. doi: 10.1093/ndt/17.7.1252. [DOI] [PubMed] [Google Scholar]
- 17.Jungers P, Massy ZA, Nguyen-Khoa T, Choukroun G, Robino C, Fakhouri F, Touam M, Nguyen AT, Grunfeld JP. Longer duration of predialysis nephrological care is associated with improved long-term survival of dialysis patients. Nephrol Dial Transplant. 2001;16(12):2357–2364. doi: 10.1093/ndt/16.12.2357. [DOI] [PubMed] [Google Scholar]
- 18.Charles RF, Powe NR, Jaar BG, Troll MU, Parekh RS, Boulware LE. Clinical testing patterns and cost implications of variation in the evaluation of CKD among US physicians. Am J Kidney Dis. 2009;54(2):227–237. doi: 10.1053/j.ajkd.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterman AD, Browne T, Waterman BM, Gladstone EH, Hostetter T. Attitudes and behaviors of African Americans regarding early detection of kidney disease. Am J Kidney Dis. 2008;51(4):554–562. doi: 10.1053/j.ajkd.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 20.White SL, Polkinghorne KR, Cass A, Shaw J, Atkins RC, Chadban SJ. Limited knowledge of kidney disease in a survey of AusDiab study participants. Med J Aust. 2008;188(4):204–208. doi: 10.5694/j.1326-5377.2008.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 21.Boulware LE, Carson KA, Troll MU, Powe NR, Cooper LA. Perceived Susceptibility to Chronic Kidney Disease among High-risk Patients Seen in Primary Care Practices. J Gen Intern Med. 2009;24(10):1123–1129. doi: 10.1007/s11606-009-1086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, Horng SS, Chang YK, Yang WC. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis. 2006;48(5):727–738. doi: 10.1053/j.ajkd.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Saab G, Whaley-Connell AT, McCullough PA, Bakris GL. CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;52(2):382–383. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Whaley-Connell A, Sowers JR, McCullough PA, Roberts T, McFarlane SI, Chen SC, Li S, Wang C, Collins AJ, Bakris GL. KEEP Investigators: Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) Am J Kidney Dis. 2009;53(4 Suppl 4):S11–S21. doi: 10.1053/j.ajkd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 25.McClellan WM, Newsome BB, McClure LA, Cushman M, Howard G, Audhya P, Abramson JL, Warnock DG. Chronic kidney disease is often unrecognized among patients with coronary heart disease: The REGARDS Cohort Study. Am J Nephrol. 2009;29(1):10–17. doi: 10.1159/000148645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flessner MF, Wyatt SB, Akylbekova EL, Coady S, Fulop T, Lee F, Taylor HA, Crook E. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53(2):238–247. doi: 10.1053/j.ajkd.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. A. nn Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 29.Huizinga MM, Elasy TA, Wallston KA, Cavanaugh K, Davis D, Gregory RP, Fuchs LS, Malone R, Cherrington A, Dewalt DA, Buse J, Pignone M, Rothman RL. Development and validation of the Diabetes Numeracy Test (DNT) BMC Health Serv Res. 2008;8:96. doi: 10.1186/1472-6963-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907–915. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 31.Cavanaugh KL, Merkin SS, Plantinga LC, Fink NE, Sadler JH, Powe NR. Accuracy of patients' reports of comorbid disease and their association with mortality in ESRD. Am J Kidney Dis. 2008;52(1):118–127. doi: 10.1053/j.ajkd.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grubbs V, Gregorich SE, Perez-Stable EJ, Hsu CY. Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol. 2009;4(1):195–200. doi: 10.2215/CJN.03290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanaugh KL, Wright J, wallston ke, Elasy TA, Ikizler TA, Rothman RL. Effect of CKD awareness on diabetes control and self-care is modified by health literacy [abstract] J Am Soc Nephrol. 2006;20 421A. [Google Scholar]
- 34.Kern EF, Maney M, Miller DR, Tseng CL, Tiwari A, Rajan M, Aron D, Pogach L. Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res. 2006;41(2):564–580. doi: 10.1111/j.1475-6773.2005.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guessous I, McClellan W, Vupputuri S, Wasse H. Low documentation of chronic kidney disease among high-risk patients in a managed care population: a retrospective cohort study. BMC Nephrol. 2009;10:25. doi: 10.1186/1471-2369-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouseph R, Hendricks P, Hollon JA, Bhimani BD, Lederer ED. Under-recognition of chronic kidney disease in elderly outpatients. Clin Nephrol. 2007;68(6):373–378. doi: 10.5414/cnp68373. [DOI] [PubMed] [Google Scholar]
- 37.Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48(2):192–204. doi: 10.1053/j.ajkd.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal V, Ghosh AK, Barnes MA, McCullough PA. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol. 2009;4(2):323–328. doi: 10.2215/CJN.03510708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbari A, Swedko PJ, Clark HD, Hogg W, Lemelin J, Magner P, Moore L, Ooi D. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med. 2004;164(16):1788–1792. doi: 10.1001/archinte.164.16.1788. [DOI] [PubMed] [Google Scholar]
- 40.Lenz O, Fornoni A. Chronic kidney disease care delivered by US family medicine and internal medicine trainees: results from an online survey. BMC Med. 2006;4:30. doi: 10.1186/1741-7015-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47(1):72–77. doi: 10.1053/j.ajkd.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Ramirez HR, Jalomo-Martinez B, Cortes-Sanabria L, Rojas-Campos E, Barragan G, Alfaro G, Cueto-Manzano AM. Renal function preservation in type 2 diabetes mellitus patients with early nephropathy: a comparative prospective cohort study between primary health care doctors and a nephrologist. Am J Kidney Dis. 2006;47(1):78–87. doi: 10.1053/j.ajkd.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Melamed ML, Bauer C, Hostetter TH. eGFR: is it ready for early identification of CKD? Clin J Am Soc Nephrol. 2008;3(5):1569–1572. doi: 10.2215/CJN.02370508. [DOI] [PMC free article] [PubMed] [Google Scholar]


