Abstract
Neuronal intermediate filament inclusion disease (NIFID) is an uncommon neurodegenerative condition that typically presents as early-onset, sporadic frontotemporal dementia (FTD), associated with a pyramidal and/or extrapyramidal movement disorder. The neuropathology is characterized by frontotemporal lobar degeneration with neuronal inclusions that are immunoreactive for all class IV intermediate filaments (IF), light, medium and heavy neurofilament subunits and α-internexin. However, not all the inclusions in NIFID are IF-positive and the primary molecular defect remains uncertain. Mutations in the gene encoding the fused in sarcoma (FUS) protein have recently been identified as a cause of familial amyotrophic lateral sclerosis (ALS). Because of the recognized clinical, genetic and pathological overlap between FTD and ALS, we investigated the possible role of FUS in NIFID. We found abnormal intracellular accumulation of FUS to be a consistent feature of our NIFID cases (n = 5). More neuronal inclusions were labeled using FUS immunohistochemistry than for IF. Several types of inclusions were consistently FUS-positive but IF-negative, including neuronal intranuclear inclusions and glial cytoplasmic inclusions. Double-label immunofluorescence confirmed that many cells had only FUS-positive inclusions and that all cells with IF-positive inclusions also contained pathological FUS. No mutations in the FUS gene were identified in a single case with DNA available. These findings suggest that FUS may play an important role in the pathogenesis of NIFID.
Keywords: frontotemporal dementia, frontotemporal lobar degeneration, neuronal intermediate filament disease, fused in liposarcoma, translocated in sarcoma
Introduction
Neuronal intermediate filament inclusion disease (NIFID) is an uncommon neurodegenerative condition [37] that was first recognized as a unique entity, based on the neuropathological finding of neuronal inclusions that are immunoreactive for neurofilament (NF) proteins but negative for tau and α-synuclein [6, 9, 14, 17, 18, 24, 40]. It was initially referred to as neurofilament inclusion (body) disease, however subsequent studies demonstrated the inclusions to be immunoreactive for all class IV intermediate filaments (IF), including α-internexin, as well as light, medium and heavy NF subunits [8, 10, 11].
Twenty cases of NIFID have been published to date [6, 8, 9, 14, 17–19, 24, 27, 33, 40, 41]. Although there is significant variation in the clinical and pathological features [8], enough similarities have emerged to suggest that this represents a distinct entity. The typical presentation is early-onset, sporadic FTD, associated with a pyramidal and/or extrapyramidal movement disorder (Table 1). Additional clinical manifestations have included falls, dystonia, myoclonus, ophthalmoplegia, memory deficits, seizures, eating disorders and psychiatric symptoms. There have been two cases with neurological abnormalities during childhood [24, 33] and, although most cases appear to be sporadic, two had a single relative with dementia or movement disorder [24, 27].
Table 1.
Demographic and clinical features of NIFID cases
| sex | onset (years) | duration (years) | family history | FTD | park | MND | other | |
|---|---|---|---|---|---|---|---|---|
| case 1 | F | 25 | 4 | no | ++ | − | ++ | childhood epilepsy depression poor memory, falls vertical gaze palsy, polyneuropathy dystonia, falls, opsoclonus, mutism |
| case 2 | F | 34 | 7 | no | ++ | − | + | |
| case 3 | M | 58 | 3 | no | ++ | − | − | |
| case 4 | F | 56 | 3 | no | ++ | + | − | |
| case 5 | F | 26* | 4 | no | ++ | − | ++ | |
| mean (N = 5) | 4F:1M | 40 (25–58) | 4 (3–7) | no | 5 (100%) 5 (100%) |
1 (20%) 0 (0%) |
3 (60%) 2 (40%) |
|
| published cases (N = 20) | 1F:1M | 40 (23–70) | 5 (2–13) | no | 19 (95%) 13 (65%) |
15 (75%) 3 (15%) |
16 (80%) 8 (40%) |
FTD, frontotemporal dementia; park, parkinsonism; MND, motor neuron disease.
++ present at onset; +, developed during course of disease.
abnormal development but significant decline beginning at age 26 years.
Statistics in italics for presenting clinical features.
The neuropathological findings in NIFID are also heterogeneous [8]. Chronic degenerative changes may affect a variety of cortical and subcortical regions, with the frontal and temporal lobes and caudate nucleus most consistently and severely involved. Several different types of neuronal cytoplasmic and intranuclear inclusions (NCI and NII, respectively) have been described, that vary in morphology, histochemical staining, immunoreactivity, ultrastructure and anatomical distribution [8, 29, 36]. By definition, these inclusions show no immunoreactivity for tau, α-synuclein or TDP-43 but at least some are immunoreactive for IFs. Antibodies against phosphorylated and phosphorylation independent epitopes of all three NF subunits label some of the inclusions, however α-internexin immunohistochemistry (IHC) tends to be more sensitive [8, 10, 11].
Although the presence of IF-immunoreactive (IF-ir) neuronal inclusions is the defining feature of NIFID, the actual role of IFs in the pathogenesis of this condition remains uncertain. Biochemical studies of post mortem brain tissue have failed to demonstrate any abnormal molecular modification of IFs in NIFID [11, 29] and no pathogenic variants of the corresponding genes have been identified [28]. More importantly, several published reports of NIFID have indicated that only a proportion of the inclusions are IF-ir [6, 18, 24, 36, 41]. The fact that IF IHC may also label the characteristic inclusion bodies of many other common neurodegenerative conditions, in which the primary molecular defect is known to be something other than IFs [1, 10, 23], leaves open the possibility that some other protein may play a more central role in the pathogenesis of NIFID [24, 29, 41].
Recently, two studies have identified mutations in the gene encoding the fused in sarcoma (FUS) (also known as translated in liposarcoma, TLS) protein, as the cause of familial amyotrophic lateral sclerosis (FALS) type 6 [20, 38]. These reports describe the associated pathology as including NCI that are immunoreactive for FUS (FUS-ir) but negative for TDP-43. Because of the recognized clinical, genetic and pathological overlap between ALS and FTD, we speculated that FUS might be the pathological protein in some cases of FTD in which the molecular defect is currently unknown. We recently confirmed this hypothesis in a subgroup of FTD cases that we had previously reported under the name “atypical FTLD-U” (aFTLD-U) [25, 31, 34]. In the present study we extend our investigations of the possible role of FUS in tau/TDP-43-negative FTLD to include NIFID.
Material and methods
Cases
All cases fulfilling current neuropathological diagnostic criteria for NIFID [7] were retrieved from the neurodegenerative disease brain banks at the University of British Columbia, Vancouver, Canada (n = 3) and Ludwig-Maximilians University, Munich, Germany (n = 2). Clinical and pathological details of two of the cases (case 1 and 5, Table 1) have been published previously [24, 33].
For FUS IHC, neurological control cases included FTD with TDP-43 pathology (FTLD-TDP, n = 12, including 2 each of sporadic type 1, sporadic type 2, sporadic type 3, familial with progranulin gene mutations, familial with valosin containing protein gene mutations and familial linked to chromosome 9p), tauopathies (n = 8, including 2 each of Pick’s disease, progressive supranuclear palsy, corticobasal degeneration and argyrophilic grain disease), Alzheimer’s disease (n = 2), Parkinson’s disease combined with dementia with Lewy bodies (n = 2), multiple system atrophy (n = 2), Huntington’s disease (n = 2) and ALS (n = 6, including 2 each of SALS, FALS with SOD1 mutations and FALS with SOD1 mutations excluded). Normal control tissue was from two elderly patients with no history of neurological disease.
FUS antibodies
We tested a number of commercially available anti-FUS antibodies, each of which recognizes a different epitope (Table 2). Immunohistochemistry (IHC) using three of the four antibodies (Bethyl Laboratories A300-302A, Sigma-Aldrich HPA008784 and Santa Cruz Biotechnology sc-47711) demonstrated the normal physiological pattern of staining and also labeled the pathological lesions. The Santa Cruz sc-47711 antibody only worked on frozen sections while the other two showed similar results on sections of formalin fixed, paraffin embedded material. The polyclonal antibody from Sigma-Aldrich was used for all subsequent IHC.
Table 2.
Anti-FUS antibodies tested
| company | product no. | type | epitope (aa 1–526) |
|---|---|---|---|
| Bethyl Laboratories | A300–302A | rabbit polyclonal | N-terminus (aa 1–50) |
| Sigma-Aldrich | HPA008784 | rabbit polyclonal | mid region (aa 86–213) |
| Bethyl Laboratories | A300-292A | rabbit polyclonal | mid region (aa 200–250) |
| Santa Cruz Biotechnology | sc-47711 | mouse monoclonal | C-terminus |
Immunohistochemistry
All IHC was performed on 5 μm thick sections of formalin fixed, paraffin embedded tissue using the Ventana BenchMark® XT automated staining system (Ventana, Tuscon, AZ) and developed with aminoethylcarbizole (AEC). The primary antibodies employed recognized FUS (Sigma-Aldrich anti-FUS; 1:25 – 1:200 with initial overnight incubation at room temperature, following microwave antigen retrieval), ubiquitin (DAKO anti-ubiquitin; 1:500, following microwave antigen retrieval), hyperphosphorylated tau (Innogenetics AT-8; 1:2,000 following microwave antigen retrieval and Sigma TAU-2; 1:1,000 with 3 h initial incubation at room temperature), α-synuclein (Zymed anti-α-synuclein; 1:10,000, following microwave antigen retrieval), Aβ (DAKO anti-beta amyloid; 1:100 with initial incubation for 3 h at room temperature), α-internexin (Zymed anti-alpha-internexin;1:500 with 3 h initial incubation at room temperature, following microwave antigen retrieval), nonphosphorylated neurofilament (NF) (DAKO anti-neurofilament protein; 1:2,000, following protease digestion), phosphorylated neurofilament (pNF) (Sternberger SMI 31; 1:8,000, following protease digestion), p62 (BD Transduction Laboratories p62 Lck ligand; 1:500 following microwave antigen retrieval), TDP-43 (ProteinTech Group anti-TARDBP; 1:1,000 following microwave antigen retrieval) and expanded polyglutamine repeat regions (Chemicon 1C2; 1:1,000, 24 h at room temperature following formic acid pre-treatment).
Based on the amount of normal physiological staining, it was apparent that the anti-FUS sensitivity was greatly influenced by the degree of tissue fixation and that this was only partially reversed by antigen retrieval. Therefore, the dilution of the primary antibody was adjusted in each case (from 1:25 to 1:200) to allow for faint physiological staining that ensured sensitivity (internal positive control) but did not compromise visualization of the pathology.
In cases of NIFID, IHC for ubiquitin, α-internexin and FUS was performed on sections representing a wide range of neuroanatomical regions. For control cases, the region of maximal pathology was evaluated with FUS IHC.
FUS-ir pathology was evaluated using a semiquantitative grading system, similar to that used in several previous studies [24, 25, 34], in which the pathological lesions are scored as none (−), rare (+), occasional (++), common (+++) or numerous (++++). A grading of “rare” indicates that, although present, extensive survey of the tissue section is required for identification. “Occasional” means that the lesions are easy to find but not present in every microscopic field. The pathology is considered “common” when at least one example is present in most high-powered fields. When many lesions are present in every high-powered field, then the lesions are considered to be ”numerous”.
Immunofluorescence
Double-label immunofluorescence was performed on selected regions from NIFID cases using a rabbit polyclonal anti-FUS antibody (Sigma-Aldrich anti-FUS; 1:25) and either a mouse monoclonal anti-ubiquitin antibody (Chemicon 1510; 1:20.000) or a mouse monoclonal α-internexin antibody (Zymed, 1:500). The secondary antibodies were Alexa Fluor 594 conjugated anti-rabbit and Alexa Fluor 488 conjugated anti-mouse (Molecular Probes; 1:500). 4′-6-diamidino-2-phenylindol (DAPI) was used for nuclear counterstaining.
Results
Clinical features
The NIFID cases used in this study had demographic and clinical features, similar to those previously reported for this condition, including early age of onset, short disease duration, absence of family history, initial presentation with FTD [30] and high frequency of pyramidal motor features (Table 1). In two patients, the FTD was manifest as abnormal behaviour, in one as progressive non-fluent aphasia and the other two had a combination of behavioural and non-fluent language dysfunction. The only unusual aspects were the high proportion of female subjects in our group and the relative infrequency of extrapyramidal dysfunction.
Neuropathology (general)
All post mortem brain specimens were small (mean weight = 1050 grams) with symmetric atrophy of the frontal lobes. Chronic degenerative changes of neuronal loss and reactive gliosis were most prominent in the frontal and temporal neocortex, basal ganglia, thalamus, substantia nigra, periaqueductal grey matter, inferior olive and cerebellar dentate nucleus. Decreased myelination of the corticospinal tracts and appreciable loss of lower motor neurons was present in 3/5 cases (cases 1, 2 and 5), however no Bunina bodies were seen.
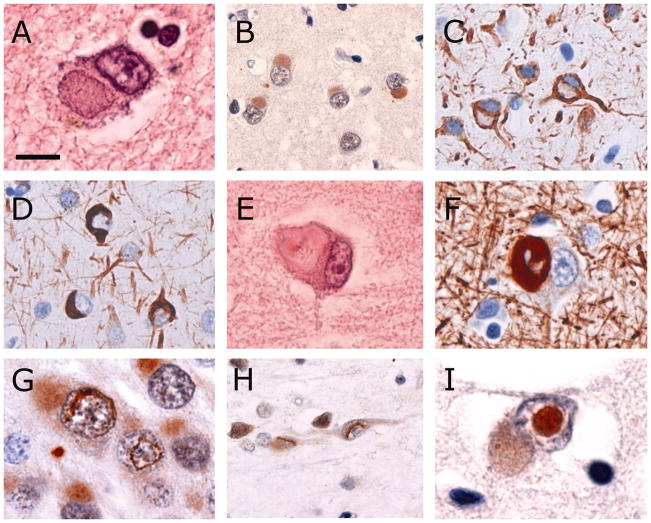
The variety of neuronal inclusions found in our cases was consistent with previous descriptions (Table 3) and, with the exception of round NII (see below), all cases showed the same spectrum of pathological changes. The most common type of NCI was small round, oval or cap-shaped Pick body-like (PBL) inclusions that were well-defined, slightly eosinophilic or basophilic and rarely argyrophilic (Fig. 1a). These were numerous in affected regions of cerebral neocortex, hippocampus and in specific subcortical regions. PBL NCIs showed consistent, but often weak, immunoreactivity for ubiquitin and p62 (Fig. 1b). Immunostaining for IFs was more intense and a greater number were labeled with the α-internexin antibody than for NFs, however, only a small proportion of PBL inclusions demonstrated with hematoxylin and eosin stain or ubiquitin IHC were IF-positive. Interestingly, many neurons that contained an IF-negative PBL NCI showed intense diffuse IF immunoreactivity in the surrounding cytoplasm (Fig. 1c).
Table 3.
Relative frequency and staining intensity of different types of cellular inclusions in NIFID
| HE | ubiquitin | α-internexin | FUS | |
|---|---|---|---|---|
| NCI | ||||
| Pick body-like | ++++ | ++++ weak/moderate |
++ strong |
++++ strong |
| crescents, rings, tangles | + | ++ weak/moderate |
++ strong |
+++ strong |
| hyaline conglomerates | ++ | + weak |
++ strong |
+ strong |
| granular aggregates | − | ++ weak |
− | +++ moderate/strong |
| NII | ||||
| round* | ++ | ++ strong |
− | −/+ moderate/strong |
| filamentous | − | ++ strong |
− | ++ strong |
| GCI | − | − | − | +++ strong |
GCI, glial cytoplasmic inclusion; NCI, neuronal cytoplasmic inclusion; NII, neuronal intranuclear inclusion.
Semiquantitative grading of frequency: −, none; +, rare; ++ occasional; +++, common; ++++, numerous.
Weak, moderate, strong refer to intensity of immunostaining.
round NII were only present in 2/5 cases.
Fig. 1.
Types of neuronal inclusions found in NIFID. Pick body-like inclusions are well-defined, round or oval, slightly eosinophilic or basophilic (a) and show consistent, but often weak, immunoreactivity for ubiquitin (b). They usually do not immunostain for class IV intermediate filaments (IFs), however, many neurons that contain a Pick body-like inclusion show strong, diffuse immunoreactivity for IF in the surrounding cytoplasm (c). Other morphological types of neuronal cytoplasmic inclusions include thin crescents, annular rings and tangle-like inclusions (d). Hyaline conglomerate inclusions appear as irregular, multilobulated masses with a glassy, filamentous appearance and often have a dense, brightly eosinophilic core (e). The filamentous component is strongly immunoreactive for IFs, however the core is often unstained (f). Vermiform neuronal intranuclear inclusions are most frequent in dentate granule cells (g) but also found in pyramidal neurons of the neocortex, hippocampus and some subcortical regions (h). Round, eosinophilic, ubiquitin-immunoreactive neuronal intranuclear inclusions are only present in some cases (i). Neurons with either type of intranuclear inclusion often also contain a Pick body-like cytoplasmic inclusion (g, i). Hematoxylin and eosin (a, e), ubiquitin (b, g–i), α-internexin (c, d) and phosphorylated neurofilament (f) immunohistochemistry. Scale bar = 8 μm (a, e, g, i); 25 μm (b); 20 μm (c, d, h); 15 μm (f).
NCI with other morphologies, including thin crescents, annular rings and tangle-like inclusions were moderately common in the neocortex, hippocampus and striatum. These were not easily appreciated with HE stain and had a similar immunophenotype as the PBL inclusions, being weakly ubiquitin-positive and occasionally showing immunoreactivity for IFs (Fig. 1d).
Hyaline conglomerate (HC) inclusions were much less frequent (rare to occasional) and appeared as irregular, multilobulated masses that often compressed the nucleus (Fig. 1e). They were weakly eosinophilic with a glassy, filamentous appearance and sometimes had a dense, brightly eosinophilic core. Most stained intensely with Bielschowsky silver method. They were most frequent in the neocortex and a number of subcortical regions such as the thalamus, basis pontis and inferior olive. HC inclusions were less often immunoreactive for ubiquitin and most were strongly immunoreactive for IFs (Fig. 1f).
Two types of NII were identified. In all cases, ubiquitin IHC demonstrated single straight, curved or twisted, thick filamentous (vermiform) NII that were most numerous in the dentate granule cells but also found in pyramidal neurons of the neocortex, hippocampus and some subcortical regions (Figs. 1g, h). These were not visible with HE stain or with IHC for IFs or p62. In addition, large, round, brightly eosinophilic NII were present in only 2/5 cases (Fig. 1i), a similar frequency as has been described in previous NIFID series [8]. These were most numerous in the neocortex and rare in the hippocampus and subcortical regions. They showed intense immunoreactive for ubiquitin and p62 but not for IFs. Neither type of NII was immunoreactive with the 1C2 antibody. Neurons with either type of NII often also contained a PBL NCI (Figs. 1g, i).
No significant pathology was demonstrated with IHC for Aβ, tau, α-synuclein or TDP-43.
FUS immunohistochemistry
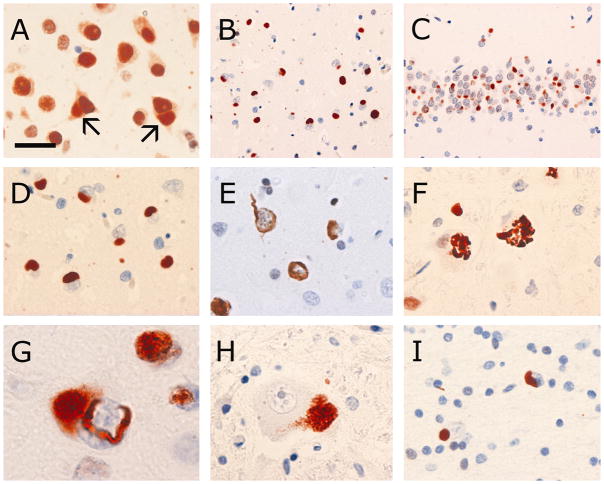
The normal physiological staining pattern of FUS was equally well demonstrated in normal controls, neurological controls and NIFID cases. This consisted of strong immunoreactivity of neuronal nuclei, weaker but consistent staining of neuronal cytoplasm and more variable reactivity of glial nuclei (Fig. 2a). Both the nuclear and cytoplasmic staining was generally diffuse but with occasional small granular structures. With one exception, none of the controls showed any abnormal FUS immunoreactivity. Specifically, FUS IHC did not label senile plaques, neurofibrillary tangles, dystrophic neurites, Lewy bodies, Lewy neurites, Pick bodies, ballooned neurons, neuronal inclusions in ALS or FTLD with TDP pathology or glial inclusions in tauopathies or MSA. The exception was HD in which the characteristic small round NII were strongly and uniformly FUS-ir, a finding that has been reported previously [13].
Fig. 2.
FUS immunohistochemistry in NIFID. The normal physiological staining pattern of FUS is demonstrated with higher concentrations of primary antibody and consists of strong immunoreactivity of neuronal nuclei, weaker but consistent staining of neuronal cytoplasm and more variable reactivity of glial nuclei (a). Neurons with FUS-immunoreactive (FUS-ir) inclusions (arrows) still retain some normal physiological FUS staining (a). Abundant FUS-ir pathology is present in all affected brain regions, including the neocortex (b) and hippocampus (c). FUS-ir Pick body-like inclusions (a –d), crescentic, annular and tangle-like neuronal cytoplasmic inclusions (e) are numerous. FUS immunohistochemistry occasionally labels structures recognizable as hyaline conglomerate inclusions (f, inferior olive). Many neurons with a FUS-ir vermiform intranuclear inclusion also contain a Pick body-like cytoplasmic inclusion (g). Aggregates of coarse cytoplasmic granules are common in many subcortical regions, including lower motor neurons of the spinal cord (h). Glial cytoplasmic inclusions are common in the cerebral white matter (i). FUS immunohistochemistry with primary antibody from Sigma-Aldrich (HPA008784 vs. epitope aa 86–213) (a–i) or Bethyl Laboratories (A300–302A vs. epitope aa 1–50) (c, insert). Scale bar = 25 μm (a, e); 60 μm (b, c); 30 μm (d); 20 μm (f, h, i); 8 μm (g).
In cases of NIFID, FUS IHC labeled NCI and NII of similar morphology, number and anatomical distribution as were demonstrated with ubiquitin IHC (Figs. 2a–g). However, the FUS immunoreactivity was much more intense (Table 3) and in some anatomical regions, more FUS-positive inclusions were present (Table 4).
Table 4.
Semiquantitative grading of immunoreactive pathology in different anatomical regions
| anatomical region | ubiquitin | α-internexin | FUS |
|---|---|---|---|
| frontal cortex | ++++ | +++ | ++++ |
| hippocampus - dentate | ++++ | ++ | ++++ |
| hippocampus - pyramidal | +++ | + | ++++ |
| entorhinal cortex | +++ | ++ | ++++ |
| striatum | ++ | +++ | +++ |
| globus pallidus | + | + | ++ |
| thalamus | ++ | ++ | +++ |
| substantia nigra | ++ | + | ++++ |
| periaqueductal grey | +++ | ++ | ++++ |
| pontine nuclei | ++ | ++ | +++ |
| inferior olive | ++ | ++ | +++ |
| cranial nerve XII | ++ | + | ++ |
| spinal cord - ventral grey | ++ | + | +++ |
| cerebellum - cortex | − | − | − |
| cerebellum - dentate | + | + | +++ |
Semiquantitative grading: −, none; +, rare; ++ occasional; +++, common; ++++, numerous.
Scores represent mean values for all types of inclusions in all cases (n = 5).
In all anatomical regions of all cases, more pathology was demonstrated with IHC for FUS than with any of the IF antibodies (Table 4). There were many more FUS-ir PBL, crescentic, annular and tangle-like NCI than were seen with IHC for NF proteins or α-internexin. The only type of NCI that was better demonstrated with IF IHC were the HCs. Although FUS IHC occasionally labeled complex NCI with HC morphology (Fig. 2f), more often, neuronal populations known to harbour HCs showed only small round dots of FUS immunoreactivity. FUS IHC also demonstrated a number of types of inclusions that were not seen at all with IHC for IFs. These included the vermiform NII, aggregates of coarse cytoplasmic granules in some neuronal populations and glial cytoplasmic inclusions. The focal aggregates of FUS-ir coarse granules were easily distinguished from non-immunoreactive lipofuscin and were most common in the brainstem, cerebellar dentate nucleus and lower motor neurons of the spinal cord (Fig. 2h). Glial cytoplasmic inclusions were common in the cerebral white matter and included small round bodies adjacent to the nucleus and small tangle-like inclusions (Fig. 2i). Based on the size and shape of the adjacent nuclei, these inclusions appeared to be in both oligodendrocytes and astrocytes.
Although the variation in staining intensity prevented quantitation, it was evident that neurons harbouring FUS-ir inclusions (either NCI or NII) often still retained at least some of their normal physiological FUS staining (Fig. 2a). Most of the IHC was performed using a single antibody that recognizes a mid-region epitope of FUS (Sigma-Aldrich HPA008784, against epitope aa 86–213), however, we confirmed that the inclusions were also immunoreactive with antibodies against the C- and N-terminus (Table 2, Fig. 2c).
Double-label immunofluorescence
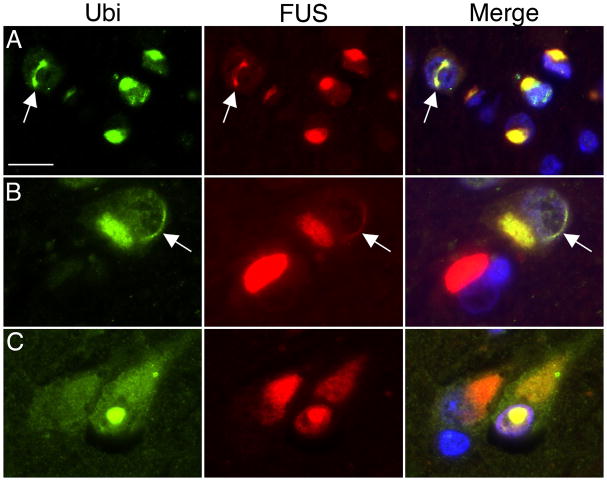
Double-labeling immunofluorescence with a combination of ubiquitin and FUS antibodies confirmed that all the ubiquitin-ir inclusions were also FUS-positive (Fig. 3). Moreover, there were significant numbers of NCI that only labeled for FUS and not for ubiquitin (Fig. 3b). The vermiform NIIs showed consistent co-localization of both ubiquitin and FUS (Fig. 3).
Fig. 3.
Double-label immunofluorescence for ubiquitin (green) and FUS (red) in NIFID. Merged images show cell nuclei stained with DAPI (blue). Ubiquitin-positive neuronal cytoplasmic inclusions (NCIs) and vermiform neuronal intranuclear inclusions (NIIs, arrows) always show strong labeling for FUS (a, b). Occasionally, FUS-positive NCIs do not label for ubiquitin (red NCI in merged image, b). A round NII showing colocalization of ubiquitin and FUS. Scale bar = 15 μm (a, b); 10 μm (c).
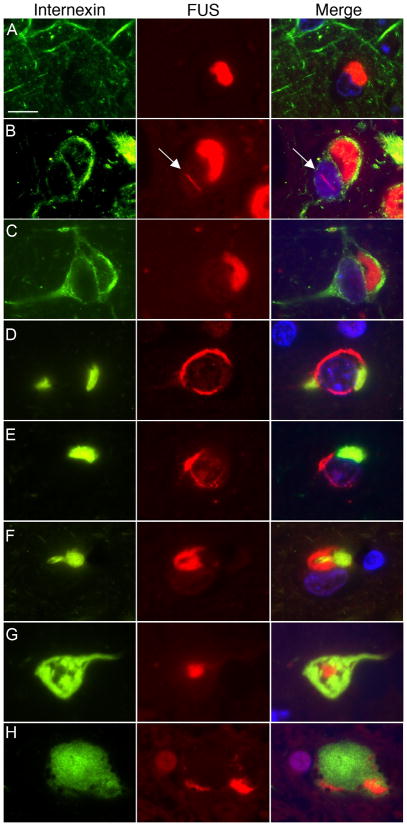
Double-labeling for FUS and α-internexin confirmed the IHC results and was particularly helpful in clarifying the relationship between the two proteins in the different types of inclusion. First, many neurons contained NCIs that were only immunoreactive for FUS (Fig. 4a–c). Some of these cells with FUS-only NCI showed intense diffuse α-internexin immunoreactivity in the surrounding cytoplasm (Fig. 4b, c). Vermiform NII were only FUS-positive and never labeled for α-internexin (Fig. 4b). Secondly, a significant proportion of neurons contained inclusions immunoreactive for both markers. Notably, all of the neurons with compact α-internexin-positive deposits also contained FUS-ir inclusions. Importantly however, FUS and α-internexin clearly labeled discrete inclusions or different regions of an inclusion in these cells and there was only marginal overlap between the proteins (Figs. 4d–h). HC inclusions were composed mainly of α-internexin but always had at least a small FUS-ir component, either as an intensely stained central dot (Fig. 4g), or at the periphery of inclusion (Fig. 4h).
Fig. 4.
Double-label immunofluorescence for α-internexin (green) and FUS (red) in NIFID. Merged images show cell nuclei stained with DAPI (blue). Many neuronal cytoplasmic inclusions (NCIs) only label for FUS (a–c). A subset of neurons with NCI that are only FUS-positive show strong diffuse cytoplasmic staining for α-internexin (b, c). Vermiform neuronal intranuclear inclusions (arrow) only label for FUS (b). Neurons with compact α-internexin-positive inclusions always show additional FUS pathology (d–h). However, note that each marker labels separate components of the inclusions, with only marginal overlap. Hyaline conglomerate inclusions (g, h) are composed mainly of α-internexin but always have at least a small FUS-immunoreactive component, either as a central dot (g), or at the periphery of inclusion (h). Scale bar = 10 μm.
Discussion
The neuropathology associated with clinical FTD is heterogeneous, with the common feature being relatively selective degeneration of the frontal and temporal lobes (frontotemporal lobar degeneration, FTLD) [7, 35]. As with many other neurodegenerative conditions, the pathology of most cases of FTLD also includes the presence of abnormal intracellular protein aggregates. In recent years it has become popular to classify the FTLDs based on the molecular defect that is presumed to be pathogenic or most characteristic [7, 26]. The majority of cases are associated with the abnormal accumulation of either tau protein (FTLD-tau) or TDP-43 (FTLD-TDP) [26]. However, there remain a number of uncommon FTLD subtypes in which there is no evidence of pathological tau or TDP-43 and in which the nature of the molecular defect is unknown; these include dementia lacking distinctive histopathology (DLDH, now designated FTLD-ni for “no inclusions”), basophilic inclusion body disease (BIBD) and FTLD with protein inclusions that are only detectable with IHC for proteins of the ubiquitin proteasome system (FTLD-UPS). The pathology of NIFID is now designated as FTLD-IF because IF-immunoreactive NCIs are the most characteristic feature, however, the role of IFs in the pathogenesis remains uncertain.
Recently, mutations in the FUS gene, on chromosome 16, have been identified as a cause of familial ALS (FALS) [20, 38]. In the two initial studies, a total of 14 different mutations were reported in 26 unrelated families, representing 4% of FALS in these combined series. The clinical phenotype was classical ALS, with no associated cognitive dysfunction. Post-mortem pathology was described in four patients and included FUS-ir NCIs in lower motor neurons, in the absence of TDP-43 pathology [38].
FUS is a ubiquitously expressed protein [2, 3] that binds to RNA [12, 42] and DNA [31] and is involved in diverse cellular processes including cell proliferation [5], DNA repair [4], transcription regulation, RNA splicing [39] and the transport of RNA between intracellular compartments [42]. In most cell types, FUS is present in both the nucleus and cytoplasm, however in neurons there is proportionally more in the nucleus and expression in glia is exclusively nuclear [3]. FUS may be involved in neuronal plasticity and the maintenance of dendritic integrity by transporting mRNA, including those encoding actin-related proteins, to dendritic spines for local translation in response to synapticstimulation [15, 16]. In contrast, FUS deficient neurons show decreased spine arborization and morphology [15]. Chromosomal translocation of the 5′ portion of FUS results in several fusion oncogenes that are each associated with specific types of human cancer, including myxoid liposarcoma, Ewing’s sarcoma and acute myeloid leukemia [22].
Because of the recognized clinical, pathological and genetic overlap between ALS and FTD, and the high degree of functional homology between FUS and another ALS/FTD associated protein (TDP-43) [21], we hypothesized that FUS might also be the pathological protein in some cases of tau/TDP-43-negative FTLD. In a recent study [31], we evaluated FUS in subgroup of FTD cases that we had previously reported under the designation “atypical FTLD-U” (aFTLD-U), because they have an unusual clinical phenotype and neuropathology characterized by inclusions that are only immunoreactive for ubiquitin and other proteins of the UPS, such as p62 (FTLD-UPS) [25, 34]. In that study, we found that all the ubiquitin/p62-ir neuronal inclusions in aFTLD-U cases were also immunoreactive for FUS [31]. FUS IHC also demonstrated previously unrecognized inclusions in glial cells. The pathological changes were labeled with multiple antibodies that recognize different epitopes across the entire FUS protein. Immunoblot analysis confirmed increased amounts of insoluble FUS in post-mortem aFTLD-U brain tissue from these cases. All cases were sporadic and molecular genetic analysis did not identify any mutations in the FUS gene or abnormal levels of FUS mRNA expression. The specificity of this finding was confirmed by the absence of FUS-ir pathology in neurological control cases with FTLD-tau and FTLD-TDP, as well as other common neurodegenerative conditions. We interpreted these findings as indicating that FUS is the pathological protein in aFTLD-U and that the associated pathology should therefore be reclassified as FTLD-FUS.
In the present study, we performed a similar evaluation of the possible role of FUS protein in NIFID. Although this condition is defined by the presence of NCI that are immunoreactive for IF proteins, it is questionable whether or not IF proteins play a direct role in the pathogenesis [24, 29, 41]. In several previous reports of NIFID there is a clear indication that only a proportion of the inclusions stain for IFs [6, 18, 24, 36, 41]. Moreover, this finding is not disease specific since NF proteins have been demonstrated as a minor component of the characteristic neuronal inclusions in a number of neurodegenerative conditions (including Alzheimer’s Disease, Parkinson’s disease, dementia with Lewy bodies, Pick’s disease, progressive supranuclear palsy and ALS), where they are not believed to represent the primary molecular defect [1, 23]. Less information is available for α-internexin, however one study demonstrated immunoreactivity in a proportion of neurofibrillary tangles, Lewy bodies and NCI in FTLD-TDP [10]. These findings, together with the lack of evidence for any molecular genetic or biochemical alteration of IFs in NIFID [11, 28, 29] suggests that some other protein may be more central to the pathogenesis.
The findings of this study suggest that FUS may be involved in the pathogenesis of NIFID and is more likely to be the major pathological protein than IFs. IHC showed that, in all cases, FUS-ir inclusions were more numerous than those labeled for IFs. There were several specific types of FUS-ir inclusions that never labeled for IFs (i.e. vermiform NII, granular aggregates in neurons and glial cytoplasmic inclusions) (Table 3) and some anatomical regions had abundant FUS-ir pathology but little or no IF pathology (Table 4). Importantly, double-label immunofluorescence demonstrated that all cells with IF-ir NCI also had focal accumulation of FUS. Finally, the major types of FUS-ir inclusions in NIFID are very similar in morphology and anatomical distribution to those previously described in our aFTLD-U cases which lack any IF pathology [31]. In particular, the vermiform NII, that were present in all our NIFID cases, and which have been noted in some previous reports [8, 18], appear to be a specific feature of cases with FUS-ir pathology [25, 31, 34].
The results of our double-label immunofluorescence were particularly intriguing and offer an explanation for the heterogenous nature of the inclusions found in NIFID, by demonstrating that each type of inclusion may have multiple elements with differing biochemical composition. PBLs seem to be composed almost exclusively of FUS with varying degrees of ubiquitination. The major component of most HCs is bundles of IFs that always surround or are adjacent to small FUS aggregates. Crescentic, annular and tangle-like NCIs each have discrete regions composed of FUS and IFs. NII seem to be mostly FUS with a high degree of ubiquitination. These findings are reminiscent of previous studies that have described the ultrastructure of different types of inclusions in NIFID as having variable composition of different types of filaments and granular material in various arrangements [6, 8, 9, 18, 24, 29, 36]. Moreover, two studies that employed immuno electron microscopy (EM) found that IFs often localized to different regions of an inclusion than were labeled for ubiquitin or p62 [29, 36]. Final resolution of this issue should now be possible by performing immuno EM using a combination of FUS and IF antibodies.
Finally, the relative distribution of FUS and IF demonstrated with double-labeling also suggests a temporal sequence for the formation of some NCI. The presence of intense, diffuse cytoplasmic staining for IF in some neurons with FUS-ir NCI is similar to what has been reported in previous IHC studies [24, 36, 41] and suggests that an initial defect in FUS activity (either loss of some normal function or toxic effect of abnormal FUS aggregates) may lead to a secondary abnormality in the expression, processing or intracellular trafficking of IFs. This seems quite plausible, given the normal role of FUS in such processes as transcription regulation [39] and the transport of mRNA encoding cytoskeletal proteins from the soma into cell processes [16]. Alternatively, the strong IF-immunoreactivity may simply represent concentration of the normal cellular allotment of IF as the FUS-ir NCI compresses the cytoplasm. Regardless of the mechanism, the higher concentration of IFs in these cells could predispose to the formation of abnormal focal aggregates of IF proteins that might be geographically distinct from the pre-existing FUS inclusion. Our finding that when compact accumulations of FUS and IF occur in the same cell, they do not co-localize but form discrete inclusions, is consistent with this model, rather than normal physiological IF simply becoming secondarily entrapped within abnormal FUS aggregates. Future in vitro studies of the effect of aberrant FUS activity (such as expression of pathogenic FUS mutations) on IFs, should help to clarify the temporal and spatial relationship between FUS and IF pathology.
In summary, we have demonstrated abundant abnormal accumulation of FUS to be a consistent feature of NIFID. The semiquantitative IHC results and the findings with double-label immunofluorescence suggest that FUS accumulation occurs earlier and is therefore more likely to play a central role in the disease pathogenesis than the abnormal accumulation of IFs, which may be a secondary phenomenon. According to the recent consensus recommendations for FTLD nomenclature, the neuropathology of NIFID should therefore be revised from the current designation of FTLD-IF to FTLD-FUS (NIFID) [26]. However, whether or not FUS truly represents the primary pathological protein defect in NIFID will require more detailed molecular studies and genetic analysis of additional cases. Further studies are also needed to determine the full spectrum of neurodegenerative conditions characterized by pathological FUS and the clinical and pathological relationships between these conditions should be reevaluated. Although all of the cases of FTLD-FUS we have identified to date (both aFTLD-U and NIFID) have been sporadic, mutations in FUS should obviously be considered in any case of familial disease with FUS pathology. Finally, the identification of FTLD-FUS as a new molecular category provides further evidence that ALS and FTD are closely related neurodegenerative conditions.
Acknowledgments
We thank Margaret Luk, Mareike Schroff and Mirjam Lutz for their excellent technical assistance. This work was supported by grants from Canadian Institutes of Health Research (grant number 74580, IM); the Pacific Alzheimer Research Foundation (IM); the Deutsche Forschungsgemeinschaft (SFB 596, MN); the Stavros-Niarchos Foundation (MN); the Synapsis Foundation (MN); and the German Brain Bank “BrainNet” (HK).
Contributor Information
Manuela Neumann, Institute of Neuropathology, University Hospital of Zürich, Zurich, Switzerland.
Sigrun Roeber, Center for Neuropathology and Prion Research, Ludwig-Maximilians University, Munich, Germany.
Hans A. Kretzschmar, Center for Neuropathology and Prion Research, Ludwig-Maximilians University, Munich, Germany
Rosa Rademakers, Department of Neuroscience, Mayo Clinic College of Medicine, Jacksonville, FL, USA.
Matt Baker, Department of Neuroscience, Mayo Clinic College of Medicine, Jacksonville, FL, USA.
Ian R. A. Mackenzie, Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada
References
- 1.Al Chalabi A, Miller CC. Neurofilaments and neurological disease. Bioessays. 2003;25:346–355. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- 2.Aman P, Panagopoulos I, Lassen C, et al. Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS. Genomics. 1996;37:1–8. doi: 10.1006/geno.1996.0513. [DOI] [PubMed] [Google Scholar]
- 3.Andersson MK, Stahlberg A, Arvidsson Y, et al. The multifunctional FUS, EWS, and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 2008;9:37. doi: 10.1186/1471-2121-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baechtold H, Kuroda M, Sok J, Ron D, Lopez BS, Akhmedov AT. Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLD/FUS and is able to promote D-loop formation. J Biol Chem. 1999;274:34337–34342. doi: 10.1074/jbc.274.48.34337. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand P, Akhmedov AT, Delacote F, Durrbach A, Lopez BS. Human POMp75 is identified as the pro-oncoprotein TLS/FUS: both POMp75 and POMp100 DNA homologous pairing activities are associated with cell proliferation. Oncogene. 1999;18:4515–4521. doi: 10.1038/sj.onc.1203048. [DOI] [PubMed] [Google Scholar]
- 6.Bigio EH, Lipton AM, White CL, Dickson DW, Hirano A. Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol. 2003;29:239–253. doi: 10.1046/j.1365-2990.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- 7.Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns NJ, Grossman M, Arnold SE, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–1384. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairns NJ, Perry RH, Jaros E, et al. Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neuroscience Letters. 2003;341:177–180. doi: 10.1016/s0304-3940(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 10.Cairns NJ, Uryu K, Bigio EH, et al. α-Internexin aggregates are abundant in neuronal intermediate filament inclusion disease (NIFID) but rare in other neurodegenerative diseases. Acta Neuropathol (Berl) 2004;108:213–223. doi: 10.1007/s00401-004-0882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns NJ, Zhukareva V, Uryu K, et al. α-internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol. 2004;164:2153–2161. doi: 10.1016/s0002-9440(10)63773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA- binding protein in human myxoid liposarcoma. Nature. 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 13.Doi H, Okamura K, Bauer PO, et al. RNA-binding protein TLS is a major nuclear aggregate-interacting protein in Huntingtin exon 1 with expanded polyglutamine-expressing cells. J Biol Chem. 2008;283:6489–6500. doi: 10.1074/jbc.M705306200. [DOI] [PubMed] [Google Scholar]
- 14.Duyckaerts C, Mokhtari K, Fontaine B, et al. Maladie de Pick généralisée: une dézmence mal nommée caractérisée par des inclusions neurofilamentaires. Rev Neurol. 2003;159:219. [Google Scholar]
- 15.Fujii R, Okabe S, Urushido T, et al. The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol. 2005;15:587–593. doi: 10.1016/j.cub.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 16.Fujii R, Takumi T. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J Cell Sci. 2005;118:5755–5765. doi: 10.1242/jcs.02692. [DOI] [PubMed] [Google Scholar]
- 17.Gearing M, Castellano AA, Hunter SB, et al. Unusual neuropathological findings in a case of primary lateral sclerosis. J Neuropathol Exp Neurol. 2003;62:555. [Google Scholar]
- 18.Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- 19.Josephs KA, Uchikado H, McComb RD, et al. Extending the clinicopathological spectrum of neurofilament inclusion disease. Acta Neuropathol. 2005;109:427–432. doi: 10.1007/s00401-004-0974-4. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski TJ, Bosco DA, LeClerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 21.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law WJ, Cann KL, Hicks GG. TLS, EWS, and TAF15: a model for transcriptional integration of gene expression. Brief Funct Genomic Proteomic. 2006;5:8–14. doi: 10.1093/bfgp/ell015. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Xie F, Siedlak SL, et al. Neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci. 2004;61:3057–3075. doi: 10.1007/s00018-004-4268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie IR, Feldman H. Neurofilament inclusion body disease with early onset frontotemporal dementia and primary lateral sclerosis. Clin Neuropathol. 2004;23:183–193. [PubMed] [Google Scholar]
- 25.Mackenzie IRA, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131:1282–1293. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina-Porcel L, Llado A, Rey MJ, et al. Clinical and pathological heterogeneity of neuronal intermediate filament inclusion disease. Arch Neurol. 2008;65:272–275. doi: 10.1001/archneurol.2007.37. [DOI] [PubMed] [Google Scholar]
- 28.Momeni P, Cairns NJ, Perry RH, et al. Mutation analysis of patients with neuronal intermediate filament inclusion disease (NIFID) Neurobiol Aging. 2006;27:778.e1–6. doi: 10.1016/j.neurobiolaging.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Mosaheb S, Thorpe JR, Hashemzadeh-Bonehi L, Bigio EH, Gearing M, Cairns NJ. Neuronal intranuclear inclusions are ultrastructurally and immunologically distinct from cytoplasmic inclusions of neuronal intermediate filament inclusion disease. Acta Neuropathol. 2005;110:360–368. doi: 10.1007/s00401-005-1057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 31.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA. A New Subtype of Frontotemporal lobar degeneration with FUS pathology. Brain. doi: 10.1093/brain/awp214. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrotti D, Bonatti S, Trotta R, et al. TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis. EMBO J. 1998;17:4442–4455. doi: 10.1093/emboj/17.15.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roeber S, Bazner H, Hennerici M, Porstmann R, Kretzschmar HA. Neurodegeneration with features of NIFID and ALS--extended clinical and neuropathological spectrum. Brain Pathol. 2006;16:228–234. doi: 10.1111/j.1750-3639.2006.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roeber S, Mackenzie IR, Kretzschmar HA, Neumann M. TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol. 2008;116:147–157. doi: 10.1007/s00401-008-0395-x. [DOI] [PubMed] [Google Scholar]
- 35.Trojanowski JQ, Dickson D. Update on the neuropathological diagnosis of frontotemporal dementia. J Neuropathol Exp Neurol. 2001;60:1123–1126. doi: 10.1093/jnen/60.12.1123. [DOI] [PubMed] [Google Scholar]
- 36.Uchikado H, Li A, Lin WL, Dickson DW. Heterogeneous inclusions in neurofilament inclusion disease. Neuropathol. 2006;26:417–421. doi: 10.1111/j.1440-1789.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 37.Uchikado H, Shaw G, Wang DS, Dickson DW. Screening for neurofilament inclusion disease using alpha-internexin immunohistochemistry. Neurology. 2005;64:1658–1659. doi: 10.1212/01.WNL.0000160328.17975.9C. [DOI] [PubMed] [Google Scholar]
- 38.Vance C, Rogelj B, Hortobagyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, Embree LJ, Tsai S, Hickstein DD. Oncoprotein TLS interacts with serine-arginine proteins involved in RNA splicing. J Biol Chem. 1998;273:27761–27764. doi: 10.1074/jbc.273.43.27761. [DOI] [PubMed] [Google Scholar]
- 40.Yokoo H, Oyama T, Hirato J, Sasaki A, Nakazato Y. A case of Pick’s disease with unusual neuronal inclusions. Acta Neuropathol. 1994;88:267–272. doi: 10.1007/BF00293404. [DOI] [PubMed] [Google Scholar]
- 41.Yokota O, Tsuchiya K, Terada S, et al. Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol. 2008;115:561–575. doi: 10.1007/s00401-007-0329-z. [DOI] [PubMed] [Google Scholar]
- 42.Zinszner H, Sok J, Immanuel D, Ron D. TLD (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci. 1997;110:1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]