Abstract
Human metapneumovirus (hMPV) is a significant cause of respiratory illness in children and adults. Presently, there are no human data regarding the role of antibody for protection against hMPV illness. Therefore, we measured serum and nasal antibody titers against hMPV by EIA and neutralization assay at baseline in hMPV infected adults compared with subjects who remained uninfected. Antibody titers were also compared in patients with mild and severe illness.
Mean serum binding and neutralizing antibody titers of hMPV infected subjects were significantly lower compared to uninfected subjects. Seventy-one percent of subjects with titers ≤10.5 (log 2) were infected compared to 36% with titers >10.5, p = 0.003. There was no difference in the mean acute antibody titers for patients with mild compared to severe illness. Serum antibody may play a role in protection from hMPV infection supporting the development of an hMPV vaccine that stimulates humoral immunity.
Keywords: Human metapneumovirus, Antibody, Immunity
1. Introduction
Human metapneumovirus (hMPV) is an enveloped RNA virus classified in the Paramyxovirus family (Pneumoviridae subfamily) and is closely related to respiratory syncytial virus (RSV). Two major strains, designated A and B each with two subtypes, have been identified by antigenic and genetic analysis [1], [2]. Since its discovery in 2001, infection has been widely reported in infants and young children with an illness similar to RSV and characterized by wheezing and bronchiolitis [3], [4], [5], [6]. Similar to RSV, hMPV infection induces incomplete immunity and re-infections occur throughout life [7], [8]. Illnesses due to hMPV infection resulting in hospitalization and death have been reported in adult populations and the presence of underlying cardiopulmonary conditions and advanced age appear to be risk factors for severe disease [8], [9], [10], [11].
In animal models, infection with hMPV has been shown to be protective from subsequent challenge [12]. In addition, antibody directed against the fusion (F) protein of hMPV exhibits neutralizing activity and has also been shown to be protective in animal models [13], [14]. These data are encouraging that vaccines stimulating humoral immunity might offer benefit in people. At the present time there are no human data regarding the role of serum or mucosal antibody in protection from hMPV infection. Observational studies in children and experimental challenge studies in adults indicate that RSV infection induces antibody responses that offer partial immunity to infection and disease severity [15], [16], [17], [18]. In addition, recent studies suggest that low serum and nasal antibody levels are also risk factors for infection and disease severity in older adults [19], [20]. Although the structural similarities of hMPV and RSV make it reasonable to extrapolate the studies of RSV to hMPV, hMPV-specific human data are desirable. Therefore, we analyzed serum and nasal antibody titers against hMPV in healthy young adults and elderly persons at baseline and in response to natural infection with hMPV.
2. Methods
2.1. Study design
The study encompassed four consecutive winters from 1999 through 2003 in Rochester, New York and involved volunteers who participated in a study of RSV and influenza infections as previously described in detail [21]. Four groups were studied: three prospective cohorts [healthy young adults ages 19–40, community dwelling adults who were either ≥65 years or had chronic cardiopulmonary conditions (high-risk) and residents of a long term care facility (LTCF)], as well as a hospitalized cohort. The prospective cohorts were recruited and enrolled in the late summer-early fall (prior to surveillance) and were followed for a maximum of two consecutive winters. Upon enrollment, demographic and medical history data were recorded and a serum and nasal swab specimen were collected. Serum specimens were collected from all prospective subjects in the fall and spring of each year of participation.
Prospective volunteers notified study personnel of any respiratory symptoms (cough, sore throat, sputum production, nasal congestion, dyspnea, wheezing) or change in baseline respiratory symptoms for high-risk individuals, from November 15 through April 15 each winter. Illness evaluations included a directed respiratory exam, collection of nasal swab and serum specimens. Four to six weeks later a convalescent serum specimen was collected during a follow-up visit.
The hospitalized cohort was recruited from persons with admission diagnoses consistent with an acute cardiopulmonary illness. Eligible subjects included those with admission diagnoses of community or nursing home acquired pneumonia, acute bronchitis, acute exacerbations of COPD or CHF, upper respiratory illness, viral or influenza syndrome, asthma, or respiratory failure. Patients with acute coronary syndrome, myocardial infarction or documented pulmonary embolism were excluded. Acute illness and follow-up evaluations were identical to that used for the prospective cohorts except that hospital records were also reviewed. The University of Rochester Research Subjects Review Board and the Clinical Investigation Committee of Rochester General Hospital approved this study. All subjects or their legal guardians signed informed consent prior to enrollment.
2.2. Laboratory diagnostics
2.2.1. RT-PCR
Nasopharyngeal swab specimens were stored at −80 °C and later analyzed for hMPV RNA by real time reverse transcriptase polymerase chain reaction (RT-PCR). Conserved forward and reverse primers and a FAM-labeled probe were selected from hMPV N gene sequences available in Genbank [8].
2.2.2. Serology
2.2.2.1. Serum IgG enzyme immunoassay (EIA)
Serology for hMPV was performed using an EIA in which purified CAN 97-83 strain (a group A virus) was used in the solid phase [8]. Virus was kindly provided by Dr. Guy Boivin (Laval University, Quebec, Canada). Virus was coated onto 96-well EIA plates and serum dilutions (1:1600–1:204,800) incubated overnight. Bound IgG was detected by alkaline phosphatase conjugated goat anti-human IgG, followed by substrate. The serum titer of IgG to hMPV was calculated by comparing the results to standardized control sera. Titers were expressed as the log 2 of the serum dilution in which the optical density is 0.5 and at least five times the background value of wells with no antigen. No reactivity of sera with uninfected LLC-MK2 cells was observed during assay development. Standard and control sera were included in each assay.
2.2.2.2. Nasal IgA enzyme immunoassay (EIA)
IgA to hMPV was measured using methods similar to the serum IgG assay. Four serial 2-fold dilutions (1:8–1:64) of nasal secretions were incubated overnight on antigen coated 96-well plates.
2.2.2.3. Microneutralization assay (MNA)
Nine serial 2-fold dilutions (1:50–1:12,800) of serum in duplicate were made in 50 μl viral growth media (VGM) with porcine trypsin in 96-well microtiter plates. Fifty microliters of virus containing ∼50 pfu of hMPV A strain was added to the serum dilutions and allowed to incubate for 60 min at RT. Positive control wells of virus without sera and negative control wells without virus or sera were included in triplicate on each plate. One hundred microliters of LLC-MK2 cells at a concentration of 3 × 105/ml in VGM with porcine trypsin were added to the serum–virus mixtures and plates incubated at 35 °C in CO2 for 5 days. The plates were fixed with 80% acetone in phosphate buffered saline (PBS) for 15 min at RT, air dried and then blocked for 30 min with PBS with 0.5% gelatin and 2% fetal calf serum (FCS). A monoclonal antibody to the hMPV N protein (produced in our laboratory) was diluted in PBS with 0.5% gelatin/2% FCS/0.5% Tween 20 and incubated at RT for 2 h. Wells were washed and horse radish peroxidase conjugated goat anti-mouse IgG added, followed by 2 h incubation. After washing, O-phenylenediamine dihydrochloride was added and the neutralization titer was defined as the titer of serum that reduced color development by 50% compared to the positive control wells.
2.3. Definition of infection
Symptomatic hMPV infection was defined as a respiratory illness associated with a positive RT-PCR collected at the time of symptoms, or a ≥ 4-fold rise in serum hMPV-specific IgG titer between acute and convalescent serum. Asymptomatic infection was defined as a ≥4-fold rise in hMPV-specific IgG in serum samples bracketing a time frame in which no illnesses were reported.
2.4. Subject groups for comparison
2.4.1. Risk of infection
Subjects with RT-PCR positive hMPV illness in the prospective groups who had baseline serum samples were compared to age and group matched control subjects who had neither hMPV illness diagnosed by serology or RT-PCR, nor asymptomatic seroresponse during the period of study. Subjects with only serological evidence of hMPV infection were not included since seroresponse may be more evident in subjects with low baseline titers and bias these results
2.4.2. Severity of infection
All subjects with symptomatic hMPV infection documented by hMPV RT-PCR and/or a ≥4-fold rise in hMPV-specific IgG were included in the analysis. Subjects who had evidence of other viral infections by RT-PCR, viral culture or serology in addition to hMPV infection were excluded from analysis. Infection was considered severe in subjects who were hospitalized, and mild illness defined as those managed as outpatients.
2.4.3. Response to infection
Subjects with mild hMPV illness documented by RT-PCR or serology were compared to those with severe illness requiring hospitalization. Mixed viral infections were excluded. The effect of age on humoral response to hMPV was evaluated by comparing baseline antibody titers and response to infection in persons <40 years old to those ≥65 years old.
3. Results
During the study period a total of 257 hMPV infections were identified. Of these, 105 were RT-PCR positive and 142 were identified by serology alone. Sixty-five infections were defined by asymptomatic serologic responses. Of the 192 hMPV infections associated with illnesses, 118 were documented in the hospitalized cohort and 74 occurred in the prospective cohorts; 3 LTCF, 17 young adults, 21 adults ≥65 years and 33 adults with cardiopulmonary disease, of whom 6 were hospitalized. Forty-two illnesses were associated with mixed viral infections and 150 were due to hMPV alone. Of the 118 hospitalized subjects, 91(77%) had hMPV identified as a single pathogen. Underlying medical conditions in this group were common with 45%, 66% and 26% having chronic cardiac, pulmonary conditions and diabetes mellitus, respectively. Eighteen percent received oral corticosteroids chronically and 26% utilized home oxygen therapy.
Baseline hMPV-specific serum and nasal antibody levels were lower among subjects who became infected compared to those who remained free of hMPV infection (Table 1 ). The mean serum binding and neutralizing antibody titers of infected subjects were significantly lower compared to non-infected subjects although the magnitude of the difference in titers was modest. The relationship between antibody and infection can be seen when cases and controls are combined and divided according to baseline serum neutralizing titer. The percentage of infected subjects increased with decreasing quartiles of serum neutralizing titers (Fig. 1 ) and 27 of 38 (71%) subjects with neutralizing titers ≤10.5 (log 2) were infected compared to 14 of 39 (36%) subjects with titers >10.5, p = 0.003.
Table 1.
Baseline antibody titers of hMPV infected subjects compared to uninfected controls.
| hMPV infected (N = 41) | No hMPV (N = 36) | p-Value | |
|---|---|---|---|
| Nasal IgA | 3.93 ± 1.22 | 4.46 ± 1.41 | 0.08 |
| Serum IgG | 11.93 ± 1.25 | 12.86 ± 1.23 | 0.001 |
| Serum MNA | 10.38 ± 0.83 | 10.88 ± 0.88 | 0.02 |
Fig. 1.
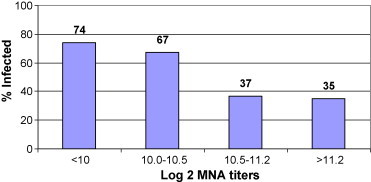
The serum neutralizing antibody values of 41 hMPV infected (RT-PCR + only) and 36 age matched uninfected controls were combined and ranked according to antibody titer into quartiles. The percentage of infected subject is shown by the bar for each quartile.
Despite these findings, low acute antibody titers of hMPV were not associated with severe disease. Mean acute serum EIA or neutralizing antibody titers were similar for patients with mild disease and more severe illness requiring hospitalization (Table 2 ). In fact, mean acute titers were slightly higher in the hospitalized patients compared to the outpatient group. Of note, the mean days of symptoms prior to evaluation was 3.2 ± 1.7 vs. 4.8 ± 4.1, p = 0.002 in the outpatient and hospitalized cohorts, respectively. Severe illness was associated with a trend toward a more vigorous antibody response as evidenced by higher convalescent titers in the hospitalized group compared to the outpatients.
Table 2.
Response to infection.
| N | Mild | N | Severe | p-Value | |
|---|---|---|---|---|---|
| EIA-acute | 57 | 12.03 ± 1.36 | 93 | 12.27 ± 1.95 | 0.42 |
| EIA convalescent | 56 | 14.93 ± 2.11 | 68 | 16.23 ± 1.93 | 0.0005 |
| EIA rise | 56 | 2.88 ± 2.23 | 68 | 4.07 ± 2.15 | 0.003 |
| MNA-acute | 57 | 10.67 ± 0.87 | 92 | 10.83 ± 1.13 | 0.36 |
| MNA convalescent | 55 | 12.57 ± 1.92 | 21 | 13.20 ± 1.60 | 0.19 |
| MNA rise | 55 | 1.89 ± 1.95 | 18 | 2.69 ± 1.90 | 0.13 |
Finally, we examined the effect of age on humoral responses to hMPV infection, since we had previously noted older persons had more vigorous responses to RSV infection than young healthy adults [22]. The mean ages of the young and older adults in the outpatient cohorts who were infected with hMPV were 34 ± 5 years and 72 ± 6 years, respectively. Young subjects had lower serum antibody titers as measured EIA but similar neutralizing titers at baseline (Table 3 ). However, older adults with mild hMPV had a significantly greater response in binding antibody and a trend toward greater neutralizing antibody responses compared to young adults with mild hMPV illness.
Table 3.
Response to infection in non-hospitalized young and older adults.
| N | Young | N | Old | p-Value | |
|---|---|---|---|---|---|
| EIA-acute | 17 | 11.40 ± 0.96 | 48 | 12.25 ± 1.29 | 0.002 |
| EIA convalescent | 15 | 13.80 ± 1.71 | 48 | 15.80 ± 1.49 | 0.0001 |
| EIA rise | 15 | 2.43 ± 1.15 | 48 | 3.55 ± 1.63 | 0.02 |
| MNA-acute | 17 | 10.67 ± 0.98 | 50 | 10.67 ± 1.35 | 1.0 |
| MNA convalescent | 15 | 11.89 ± 1.97 | 50 | 12.82 ± 2.06 | 0.13 |
| MNA rise | 15 | 1.23 ± 1.33 | 50 | 2.30 ± 2.22 | 0.08 |
4. Discussion
Since its discovery less than a decade ago, hMPV has been established as a significant respiratory pathogen in adults and children [23]. Thus, an effective vaccine to prevent infection and serious illness would be highly desirable. Our study is the first report data in humans linking risk of infection with low serum hMPV binding and neutralizing antibody. These results are very similar to the relationship of serum and nasal antibody to risk of infection we previously observed with RSV in the same adult cohort [19], [20]. Although the absolute differences in mean antibody titers of infected compared to non-infected subjects were quite modest, virus exposure of uninfected controls cannot be guaranteed in studies of natural infection. Therefore, true differences in pre-infection immunity would be more readily identified using experimental challenge studies. Nevertheless, we believe our data provide support for the development of hMPV vaccine candidates that stimulate humoral immunity.
Unlike our previous study of risk factors for severe disease in adults with RSV, we did not find an association of low serum neutralizing antibody levels and risk of hospitalization [20]. The acute blood samples were drawn significantly later in illness in the hospitalized subjects compared to the outpatients, and this discrepancy could have obscured true baseline levels in the more severely ill group. However, the same issue was noted in our previous study of RSV infected patients in whom a relationship of low antibody status and risk of hospitalization could be demonstrated. It is possible that hMPV re-infection elicits a more brisk amnestic immune response than RSV, although given the close genetic relationship of these viruses this seems unlikely. Alternatively, hMPV related hospitalizations might be driven more by underlying diseases of the host rather than inadequate immunity to the pathogen. Several studies have suggested that illness due to RSV is more severe than hMPV in young children and underlying medical conditions and age appear to be risk factors for severe illness in adults [24], [25]. Yet, we observed similar rates of underlying cardiopulmonary diseases, diabetes and functional impairment to our previous cohort of patients hospitalized with RSV infection [21]. Thus, the link between low serum antibody and severe RSV disease and lack of a similar association with hMPV remains unexplained.
Interestingly, we found that older adults exhibited a more robust antibody response as measured by EIA or neutralization assay compared to young adults infected with hMPV illnesses of similar severity. Pre-infection hMPV antibody levels were higher in older subjects, suggesting that aging per se does not result in a defect in humoral immunity. The more exuberant antibody response observed in older adults may be due to immune dysregulation associated with poor viral clearance [26]. We observed a similar finding in a previous study of young and older adults infected with RSV [22]. While both viruses are members of the pneumovirus subfamily, these data suggest this observation represents a more general phenomenon associated with aging rather than a viral specific immune response. To fully explore this possibility, the immune responses to other unrelated respiratory viruses such as coronaviruses or adenoviruses should be evaluated.
Our study has several limitations which include the relatively small sample size, especially young adults with symptomatic illness. In addition, hMPV diagnosis was not group A and group B specific and EIA and neutralizing assays utilized group A virus. It is possible that different or more dramatic changes might have been detected if group specific antibody levels were evaluated separately in group A and group B specific infections. However, we have previously demonstrated nearly identical serologic response by EIA using the either CAN 97-83 (group A) and CAN 98-75 (group B) strains of hMPV as antigen regardless of the infecting viral strain [8]. In addition, we have recently found that serum neutralizing antibody titers to group A and group B viruses are highly correlated (R = 0.87) [27]. Minimal differences in neutralizing activity directed against group A and group B viruses is not surprising since the conserved F protein has been found to be highly immunogenic and protective, whereas the antigenically more diverse G protein is not [28]. Lastly, by using purified whole virus in our EIA, we are not able to detect F or G antibody specific immune responses.
In summary, mean serum antibody levels are significantly lower in adults who subsequently become infected with hMPV compared to those who remain infection free. These data suggest that serum antibody plays a protective role in hMPV infection and support development of an hMPV vaccine.
Footnotes
Funding support: This work was supported by grants (AI055861 and AI045465) from the National Institutes of Allergy Immunology and Infectious Diseases.
References
- 1.Biacchesi S., Skiadopoulos M.H., Boivin G., Hanson C.T., Murphy B.R., Collins P.L. Genetic diversity between human metapneumovirus subgroups. Virology. 2003;315:1–9. doi: 10.1016/s0042-6822(03)00528-2. [DOI] [PubMed] [Google Scholar]
- 2.van den Hoogen B.G., Besterbroer T.M., Osterhaus A.D., Fouchier R.A. Analysis of the genomic sequence of a human metapneumovirus. Virology. 2002;295:119–132. doi: 10.1006/viro.2001.1355. [DOI] [PubMed] [Google Scholar]
- 3.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn J.S. Human metapneumovirus: a newly emerging respiratory pathogen. Curr Opin Infect Dis. 2003;16:255–258. doi: 10.1097/00001432-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Williams J.V., Wang C.K., Yang C.F., Tollefson S.J., House F.S., Heck J.M. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins J.A., Erdman D.D., Weinberg G.A., Edwards K., Hall C.B., Walker F.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin G., De Serres G., Hamelin M.E., Cote S., Argouin M., Tremblay G. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44:1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 8.Walsh E.E., Peterson D.R., Falsey A.R. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–2496. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinello R.A., Esper F., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53:248–254. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boivin G., Abed Y., Pelletier G., Ruel L., Moisan D., Cote S. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Human metapneumovirus pneumonia in adults: results of a prospective study. Clin Infect Dis. 2008;46:571–574. doi: 10.1086/526776. [DOI] [PubMed] [Google Scholar]
- 12.van den Hoogen B.G., Herfst S., de Graaf M., Sprong L., van Lavieren R., van Amerongen G. Experimental infection of macaques with human metapneumovirus induces transient protective immunity. J Gen Virol. 2007;88:1251–1259. doi: 10.1099/vir.0.82663-0. [DOI] [PubMed] [Google Scholar]
- 13.Herfst S., de Graaf M., Schrauwen E.J., Ulbrandt N.D., Barnes A.S., Senthil K. Immunization of Syrian golden hamsters with F subunit vaccine of human metapneumovirus induces protection against challenge with homologous or heterologous strains. J Gen Virol. 2007;88:2702–2709. doi: 10.1099/vir.0.83084-0. [DOI] [PubMed] [Google Scholar]
- 14.Williams J.V., Chen Z., Cseke G., Wright D.W., Keefer C.J., Tollefson S.J. A recombinant human monoclonal antibody to human metapneumovirus fusion protein that neutralizes virus in vitro and is effective therapeutically in vivo. J Virol. 2007;81:8315–8324. doi: 10.1128/JVI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glezen W.P., Paredes A., Allison J.E., Taber L.H., Frank A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–715. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 16.Henderson F.W., Collier A.M., Clyde W.A., Denny F.W. Respiratory syncytial-virus infections, reinfections and immunity. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 17.Lee F.E., Walsh E.E., Falsey A.R., Betts R.F., Treanor J.J. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res. 2004;63:191–196. doi: 10.1016/j.antiviral.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Hall C.B., Walsh E.E., Long C.E., Schnabel K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 19.Walsh E.E., Falsey A.R. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. doi: 10.1086/421524. [DOI] [PubMed] [Google Scholar]
- 20.Walsh E.E., Peterson D.R., Falsey A.R. Risk factors for severe respiratory syncytial virus infection in elderly adults. J Infect Dis. 2004;189:233–238. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 21.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 22.Walsh E.E., Falsey A.R. Age related differences in humoral immune response to respiratory syncytial virus infection in adults. J Med Virol. 2004;73:295–299. doi: 10.1002/jmv.20090. [DOI] [PubMed] [Google Scholar]
- 23.Kahn J.S. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boivin G., De Sarres G., Cote S., Gilca R., Abed Y., Rochette L. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viazov S., Ratjen F., Scheidhauer R., Fiedler M., Roggendorf M. High prevalence of human metapneumovirus infection in young children and genetic heterogeneity of the viral isolates. J Clin Microbiol. 2003;41:3043–3045. doi: 10.1128/JCM.41.7.3043-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller R.A. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 27.Falsey A.R., Formica M.A., Walsh E.E. Microneutralization assay for the measurement of neutralizing antibodies to human metapneumovirus. J Clin Virol. 2009;46:314–317. doi: 10.1016/j.jcv.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skiadopoulos M.H., Biacchesi S., Buchholz U.J., Riggs J.M., Surman S.R., Amaro-Carambot E. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78:6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


