Abstract
In the past three years remarkable discoveries have added three new human polyomaviruses (KIV, WUV and MCV) to a class that previously had two disease-causing members (BKV and JCV). Two monkey polyomaviruses, simian virus 40 (SV40) and B-cell lymphotropic polyomavirus (LPV) are also present in humans. KIV and WUV lack the agnoprotein coding sequence and regulatory microRNA clusters of BKV, JCV and SV40. MCV lacks the agnoprotein sequence but generates microRNAs. KIV, WUV and MCV are all widespread in humans. Although they have distinctive tissue tropisms, they are all likely acquired in childhood. Of these viruses, only MCV has thus far been strongly linked to cancer. Marshalled evidence from diverse sources implicates MCV as an etiological agent of Merkel cell carcinoma. This review compares structural features of the new and previously-known polyomaviruses with the aim of identifying approaches to molecular pathology.
Discovery of three new human polyomaviruses: KIV, WUV and MCV
The past three years have provided witness to a remarkable development in virology. Three new human polyomaviruses (KIV, WUV, and MCV) have been discovered, now bringing the total known number of human polyomaviruses to five members (see Glossary). Beginning 37 years after the discovery of the first polyomaviruses, BKV and JCV, enhanced molecular technology fueled the discovery of the new viruses. The first of these viruses, KIV, was found by randomly amplifying, cloning and sequencing nucleic acids from nasopharyngeal aspirates [1]. Similarly, a random library from nasopharyngeal aspirates of patients with respiratory infections was screened to yield another new polyomavirus, WUV [2]. The latest polyomavirus to be detected, MCV, was sought from Merkel cell carcinoma (MCC) cells because the incidence of MCC is enhanced by immunosupression, suggesting an infectious agent [3]. Immunosuppression is also a factor in polyomavirus associated nephropathy (PVAN) caused by BKV [4,5] and progressive multifocal leukoencephalopathy (PML) caused by JCV [6,7]. In phylogenetic comparisons of several viral proteins, BKV, JCV and simian virus 40 (SV40) are in highly-related classes. However, KIV and WUV lack several features of the earlier-known viruses and MCV is even more distantly related to the known human polyomaviruses. This review focuses on new aspects of the structure of the polyomaviral proteins and microRNAs and integrates these with clinical findings as they relate to classes of these viruses in human disease.
Arrangement of conserved T-antigen-binding elements at origins of replication differs among polyomaviruses
Large T-antigen is a multifunctional protein responsible for both the replication and oncogenic transforming activities of polyomaviruses. Recent reviews thoroughly address the replication initiation, helicase and motor functions of T-antigen [8,9]. It also binds the tumor suppressor proteins Rb and p53, modulating a variety of cell cycle activities critical for tumor formation (see Refs. [10,11] for reviews). Repeats of the DNA element GAGGC are considered large T-antigen binding sites at origins of all previously known polyomaviruses [12-15]. In SV40, the most extensively studied of all polyomaviruses, these sequences are sites for T-antigen assembly, initiating DNA unwinding and subsequent DNA replication. BKV and JCV are nearly identical to SV40 in their arrangement of the origins of replication and the GAGGC elements, or their GCCTC complements (Figure 1). BKV, JCV, and SV40 possess two repeats of GAGGC followed by a palindromic repeat of the GCCTC complements.
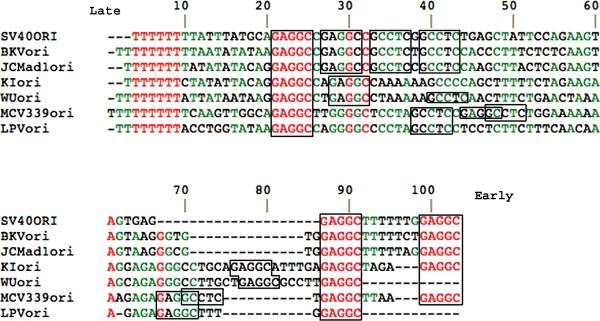
Figure 1. Distinct sequence arrangements in origins of DNA replication of newly discovered human polyomaviruses.
DNA sequences, shown 5’ to 3’ for one strand, were compared for origins of DNA replication of the known human polyomaviruses and for Simian virus 40 (SV40) and Monkey B-cell lymphotropic polyomavirus (LPV) using Multalin [71]. SV40 is included in the figure because of its presence in humans. LPV is included because many humans are reportedly seropositive for it or a homologous virus [65]. Sequences were obtained as described in Box 1. Red lettering denotes 100% identity among all the origins; green lettering denotes >50% identity. Numbering is from the first residue shown for MCV. T-antigen-binding elements, GAGGC or the complement GCCTC, are boxed. MCV is the only virus shown to have the overlapping, palindromic element GAGGCCTC. MCV has two of these elements opposing each other enclosing a highly A-rich segment. These two unusual elements[16] could affect binding of T-antigen hexamers and initiation of DNA replication.
None of the newly-discovered polyomavirus origins have this configuration of T-antigen-binding elements. KIV has no GCCTC complements, while WUV has a single GCCTC complement. Most interestingly, MCV does have two GCCTC elements, but not in a palindromic arrangement. Furthermore, only MCV has two repeats of the overlapping, palindromic element GAGGCCTC (Figure 1), which is an overlapping bidirectional T-antigen binding element. It is not known how T-antigen would bind this element, although it has been reported that MCV T-antigen can bind this element in conjunction with small T-antigen [16]. T-antigen is a 3’ to 5’ helicase [17] that moves processively along the DNA displacing the opposite 5’ to 3’strand in an ATP-dependent fashion. The previously known polyomaviruses replicate their DNA bidirectionally from the origin, requiring processive unwinding by T-antigen in both directions from the origin. When T-antigen is bound to its tetrameric, palindromic elements in SV40, a double-hexameric protein structure can be envisaged [18]. This may be altered in the newly discovered viruses. Two points are evident. First, the GAGGC element is conserved, so that nucleic acid-amino acid interactions are also likely conserved. Second, it is important to determine how T-antigen interacts with the origins of the three new viruses. Five of the 7 shown polyomaviruses possess dual T-antigen binding elements at positions 87 and 99 in Figure 1. These sites may play a role in aspects of replication or transcriptional initiation. SV40, BKV and JCV each have six pentameric T-antigen binding elements. KIV and WUV each have five. B-cell lymphotropic polyomavirus (LPV) has four. MCV, including two palindromic octamers, has eight. Intriguingly, only one of the two MCV palindromic octamers is required for initiation of DNA replication. Mutation analysis indicates that the one beginning at position 44 in Figure 2 is not required whereas the one beginning at position 67 is required [16]. LPV also has a binding element similar to the MCV element at position 67, but LPV has no overlapping, palindromic octamers (Figure 3). Thus, MCV has a replication origin T-antigen loading region distinct from other known human polyomaviruses.
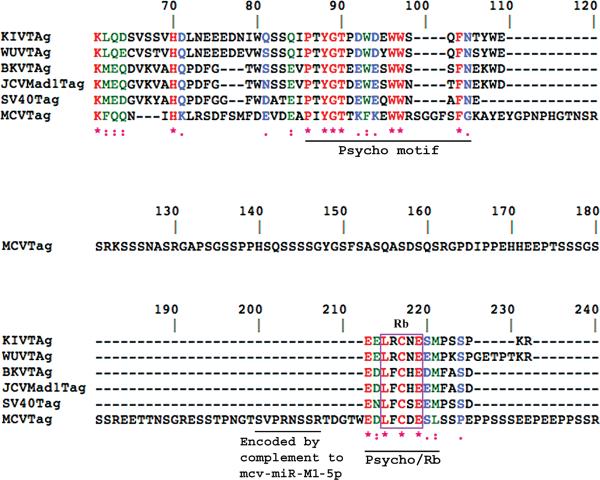
Figure 2. Protein sequence alignment of Rb-binding regions of large T-antigens of human polyomaviruses.
The alignment employed Clustal W [72], which weighs conservative substitutions in aas. Numbering begins based on the SV40 T-antigen sequence. 100% identities are in red lettering and denoted below by “*”. Green lettering and a “:” denote highly conserved aa's. Blue lettering and “.” denote a less conservative aligned aa. The motif LXCXE (boxed) is essential in T-antigens for binding the retinoblastoma tumor suppressor protein, Rb. The Psycho motif is conserved among T-antigens and influences Rb activities. Note that only in MCV is this motif interrupted by a sequence extending from aa 106 to aa 212. The central line of sequence from 121 to 180 is shown for MCV alone because none of the other T-antigens have any sequence in this region. This interrupting sequence could represent an intron, but there are no stop codons preventing its translation. It contains a sequence, SVPRNSSR, encoded by a perfect complement to the miRNA mcv-miR-M1-5p (Figure 3). This sequence in MCV almost immediately precedes the Rb-binding LFCDE motif. LPV is not included in Figure 2 because, except for certain of the important motifs, it is not overtly homologous to several of the other T-antigens.
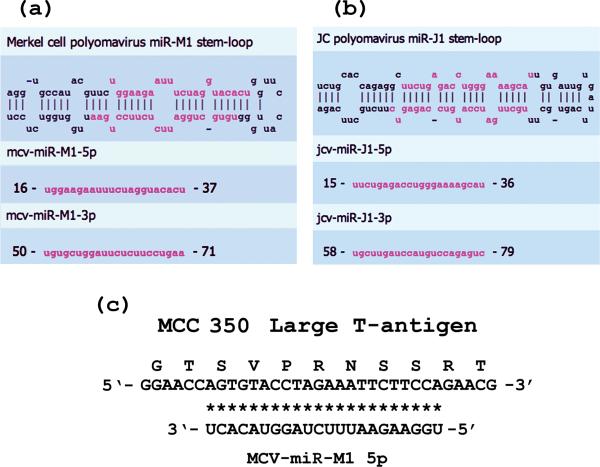
Figure 3. MiRNAs and pre-miRNAs generated by MCV and JCV.
MiRNAs for MCV [37] and JCV [36] have recently been reported. Alignment of stem-and-loop structures for an MCV pre-miRNA (a) and a JCV pre-miRNA (b) are shown and have been adapted from the mirbase website (www.mirbase.org). Sequences of the 22 nt miRNAs are shown in pink lettering. A perfect match between mcv-miR-M1-5p and a DNA sequence encoding a segment of MCV large T-antigen is shown in (c). As shown in Figure 2, this sequence is N-terminal to the Rb-binding motif of MCV T-antigen.
Large T-antigen proteins distinguish MCV from other human polyomaviruses
The T-antigen proteins of the three previously known polyomaviruses are highly conserved and T-antigens from SV40 and BKV are >70% identical in amino acid (aa) content (Box 1). BKV and JCV T-antigens possess an indentity of 83%. Despite the high degree of conservation between BKV, JCV and SV40, clearly the non-identical aas are crucial. T-antigens of SV40 and JCV will support replication of polyomavirus DNA in murine cells while BKV T-antigen will not [19]. The three newly discovered viruses are different. The large T-antigens of JCV and WUV are only 49% identical to each other. Furthermore, the MCV large T-antigen is distinct among those of the human polyomaviruses. All of the other T-antigens are only about 30% identical to that of MCV, and this is largely due to conservation among a few critical elements.
LPV T-antigen domains are not strongly homologous to those of any of the human polyomaviruses, including MCV. However, overall the LPV T-antigen is slightly more homologous to those of KIV, WUV and JCV than is that of MCV although the identities are <45% in all cases.
The first 100 aa of all the T-antigens are well conserved and include the J domain. This domain is similar to the DnaJ chaperone protein of Escherichia coli and interacts with the ATPase domain of the mammalian HSP70 chaperone [20-22]. The J domain includes the sequence HPDKGG, which is precisely conserved in T-antigens of all five human polyomaviruses and SV40. The T-antigen Rb-binding domain contains a Psycho domain that modulates Rb activities [23,24] and the box LXCXE, which is important for binding of T-antigen to a pocket region of the Rb protein [25]. The Psycho domain of the MCV T-antigen is interrupted by 106 aas containing 40 S or T residues (Figure 2). This S-rich “spacer” separates the LFCDE motif from the rest of the domain, in contrast to all of the other T-antigens. The effect of this separation on Rb binding and Rb functional inactivation by MCV T-antigen remains to be determined. SV40 and MCV have been linked to naturally-occurring neoplasias in primates. It is thus of interest that T-antigens of these viruses differ markedly in their Rb-binding configurations. LPV T-antigen is not included in Figure 2. It is considerably shorter than the other T-antigens, and it lacks a Psycho domain. It does have the HPDKGG J-element and an LFCSE Rb-binding element.
The p53- and DNA-binding sites of T-antigens comprise multiple overlapping elements. Major p53-binding elements are located in aa position 123 to 215, corresponding to SV40 numbering, and DNA binding elements (not shown) are located in positions 123 to 246, depending on various reports. This region is moderately conserved, with specific motifs identical in all of the T-antigens. Moreover, the p53 binding regions of BKV and JCV are 90% identical in sequence (Box 1). BKV, JCV and SV40 p53/DNA-binding regions are 54-56% identical to that of MCV. KIV and WUV are most different from MCV in their p53 binding regions, at 42% and 43%, respectively.
All of the T-antigens, including that of LPV, possess highly-conserved Walker A and B boxes [26] in their helicase domains. The BKV, JCV and SV40 helicase domains are all at least 90% identical in sequence (Box 1). T-antigen helicase domains from all the human polyomaviruses range from 60% to 84% in identity. Furthermore, the Walker A box, an ATP-binding motif, is present as KGPI(N/D)SGKT in every example. KIV and WUV are 78% identical to each other in the helicase region, and are thus almost as closely related in this region as are BKV and JCV (84%).
It is clear that the polyomaviral large T-antigens differ widely amongst each other, with MCV most distinct. It will be valuable to relate disease capacity to the ability of T-antigens to bind p53 and DNA.
Distinguishing classes of polyomaviruses by VP1 capsid protein sequences and agnoprotein
BKV, JCV and SV40 capsids each contain 360 molecules of the VP1 protein organized as pentamers around a central VP2 or VP3 protein. Through binding to sialic acid residues on the cell surface, VP1 plays an essential role in entry of all polyomaviruses into cells. It has now been found that the MCV VP1 protein binds to sialic acids on ganglioside GT1b [27]. Gangliosides are glycolipids with a ceramide moiety spanning the cell membrane. It has been reported that certain amino acids on the surface of JCV VP1 show accelerated mutation in viral sequences isolated from PML patients but not from control subjects [28]. It has also recently been demonstrated that certain JCV VP1 loop mutations are associated with a favorable prognosis for PML [29]. VP1 sequences separate the polyomaviruses into three classes: BKV-JCV-SV40, KIV-WUV and MCV (Box 2). The three new viruses are much less closely related to each other regarding VP1 than are the older known viruses.
It should also be noted that none of the three newly discovered polyomaviruses possesses a distinct open reading frame corresponding to the agnoprotein, a small auxiliary protein that facilitates the effects of the larger five polyomavirus-encoded proteins (see Ref. [30]). This lack of agnoprotein indicates differences between the previously known polyomaviruses and those newly discovered in regulation of viral maturation and oncogenic transformation.
Polyomavirus microRNAs: a new level of regulation
MicroRNAs (miRNAs) have been implicated as posttranscriptional regulators of gene expression, particularly at junctions of pathways involved in development, cell cycle control and oncogenesis [31,32]. Recent reviews address the topic that many viruses generate miRNAs [33-35]. Classically, miRNAs are small RNA molecules generated by cleavage of longer stem-and-loop structures transcribed by cellular RNA polymerase II followed by unwinding to produce 22 nt single-stranded RNAs. Either strand thus produced can function as an miRNA. miRNAs function by pairing with complementary sequences in cellular or viral mRNAs leading to impaired translation of the mRNA or cleavage of the hybrid product. Generally, cleavage occurs only when there is a perfect complement of the 22 nt, and most such reactions have been described for viral miRNA. Sequences of SV40, BKV and JCV miRNAs are not homologous. Despite this, these miRNAs conserve function regarding directing cleavage of the large T-antigen [36].
Thus far no miRNA sequences have been reported for KIV or WUV. However, the MCV genome generates an miRNA [37]. The stem-and-loop structures generated by MCV as pre-miRNAs are not homologous to those generated by JCV. The MCV-mir-M1 5p miRNA has 100% complementarity to a sequence from the large T-antigen mRNA of MCV strain MCC 350 (Figure 3c) [37]. Note that this complement is in the sequence of the mRNA immediately preceding that which would encode the Rb-binding region (Figure 2 and Figure 3). The consequences of this tantalizing miRNA-binding location are not known. The miRNA shown in Figure 3a markedly downregulates early MCV transcripts late in infection [37].
Tissue tropism and acquisition of human polyomaviruses
Although KIV and WUV [38-40] as well as MCV [41-43] have been found in respiratory tract samples, percentages of patients with any given infection were low, and there is no firm evidence at this time for an etiological role of any of the newly-discovered polyomaviruses in respiratory disease [43,44]. All of the human polyomaviruses are detected globally and are most likely acquired in early childhood [7]. Nonetheless, there are important distinctions regarding routes of acquisition and tissue tropism. KIV and WUV are detected in pediatric respiratory samples while BKV and JCV are much less common there [38]. In contrast, BKV and JCV are found in blood and urine and persist in urothelial tissue [4,45], while KIV and WUV were not found in blood and urine [38]. An oral-fecal route of transmission of MCV [46] has been proposed, and MCV may persist in tonsils [42]. Reports now indicate a widespread non-tumoral tissue distribution of MCV. Recently MCV DNA has been detected in anogenital and oral samples (31%) and eyebrow hair (50%) of HIV-positive men and in forehead swabs (62%) of healthy individuals [47]. It may thus be considered that MCV transmission could be effected through skin contact or saliva.
MCV as an etiological agent of MCC
High frequency of MCV in MCC
All polyomaviruses can transform cells and the name polyoma is derived from this property. Both of the previously-known human polyomaviruses and SV40 are associated with human tumors, although their identification as etiological agents remains uncertain [10,45,48,49]. It is unknown whether polyomaviruses KIV and WUV can transform cultured cells, and they have not been associated with any neoplasias. Among the new polyomaviruses, however, MCV is an exception. It was initially reported that 8 of 10 samples of MCC were positive for MCV versus 5 of 59 MCV-positive control samples from various tissues and 4 of 25 control skin samples [3]. MCV was discovered by using a digital transcriptome subtraction procedure. Why was a new virus specifically sought in MCC, a rare skin cancer affecting about 1 in 105 people? The reason was that MCC primarily affects elderly and immunosuppressed individuals, including AIDS patients. There is a 13-fold increase in MCC incidence in AIDS versus the general population [50]. This epidemiological profile is characteristic of an infectious agent. This initial finding of MCV in 80% of MCC cases was rapidly confirmed by others [46,51-54]. In a recent extensive study, Sihto et al. analyzed 114 MCC samples from Finland from 1979 through 2004 and found 79.8% positive for MCV using quantitative PCR and DNA sequencing [55].
The remarkable consensus of positive findings is not without its complexities. Notably, nearly 20% of MCC cases have no detectable MCV. Does this mean that these cases are not associated with MCV? Bhatia et al. have reported 74% of MCC cases positive for MCV, but many of these had <1 virus copy per 300 cells [56]. They suggested that an infected cell could contribute to transformation of neighboring uninfected cells by paracrine mechanisms. Alternatively, MCV may not contribute at all to oncogenesis. The results of Bhatia and colleagues were contradicted by Houben et al. [57]. They reported that 86% of MCC samples are positive for MCV and that the minimal copy number per cell is approximately 1. Moreover, their data show that in MCV-positive tumors, 100% of tumor cells express large T-antigen, as determined by immunohistochemistry [57]. In contrast, MCV-negative MCC tumors did not stain positively for T-antigen. Considering all evidence, we are left to conclude that a major subset of MCC tumors is associated with the presence of MCV.
Clonal chromosomal integration of MCV and T-antigen mutations in MCC
Association of any virus with a neoplasia, including MCV, is insufficient evidence for etiology [48]. However, there is evidence accumulating via numerous avenues, presenting a powerful inductive argument for an etiology of MCV in MCC. The initial report of MCV noted that in 75% of MCV-positive MCC samples MCV was present as a chromosomally-integrated copy [3]. The gene for large T-antigen was fused to that of a cellular receptor tyrosine phosphatase at chromosome band 3p14.2. Chromosomal integration is a characteristic of viruses causing cancer in humans, including what may currently be regarded as a solid paradigm for human cancer viruses, human papilloma virus (HPV) [58]. Integration may be oncogenic by disrupting viral genes, by creating virus-cell fusion proteins or by disregulating expression of cellular oncogenes or tumor suppressor genes.
Perhaps the most significant aspect of this MCV integration in MCC was that it was clonal [3]. Thus each aliquot analyzed from the MCC tumor from a given patient had the virus integrated in the same human genomic site. Tellingly, in each different individual with an integrated MCV virus, the virus was integrated in a different genomic site [3]. Clonal integration of MCV in MCC has been corroborated by a separate group, including use of fluorescence in situ hybridization (FISH) [54]. This is essential, but not sufficient, evidence for etiology. It means that there was clonal expansion of a single cell in which the virus was specifically integrated. That is, the virus was integrated in the cell before the cell clonally expanded. That means that MCV was present in the original MCC tumor cell prior to or during oncogenic transformation. It must be noted that in the 25% of MCC samples without an integrated MCV the virus was present episomally. At this time the number of cases analyzed is too small to make an airtight case for clonal integration of MCV in MCC. Nonetheless, the evidence presented is compelling and adds to a growing case for etiology of MCV in MCC.
Chromosomally-integrated MCV in MCC possesses mutated or truncated large T-antigen, inactivating the helicase activity of the encoded protein [54,59]. This is characteristic of oncogenically transforming viruses [58]. The virus is prevented from replicating and causing death of the host cell, contributing to oncogenic transformation. Seven of 8 truncating mutations were for premature stop codons [59] and all of the premature stops were C-terminal to the Rb minimal binding site. Three were near the C-terminus of the DNA-binding region, which also includes p53 binding sequences. Thus, while Rb binding would remain intact in certain mutated T-antigens, p53 binding would be questionable in more than half of them. All observed mutations disrupted the T-antigen helicase domain. The premature stop codons would all eliminate this domain, thus eliminating the ability of MCV to replicate while potentially allowing it to interact with Rb and, possibly, p53. The MCC T-antigen mutations are called signature mutations [59,60] because they have not been detected in MCV present outside of MCC tumors.
Clinical factors associated with MCV in MCC
MCC regularly presents as a red skin lesion of nonremarkable appearance although it may grow rapidly. After resection and treatment, it frequently reappears within two years, metastasizing to many organs including the lung and liver [50]. Ultraviolet (UV) radiation, e.g., sun exposure, is a pathogenic factor in MCC. A 100-fold increase in occurrence of MCC has been reported in psoriasis patients treated with UVA and methoxsalen [61]. Immunosuppression [62] and, in particular AIDS [63], increase the incidence of MCC.
In a study of more than 100 MCC cases in Finland, with nearly 80% MCV-positive, MCV presence was less frequent in patients who had regional lymph node involvement at diagnosis. Patients with MCV-positive tumors had a better than 3-fold 5-year survival rate than did those with MCV-negative tumors [55]. These findings indicate that MCV presence in MCC tumors affects clinical outcome.
Conclusions and future implications
There is little doubt that the discovery of three new polyomaviruses will trigger an influx of research into several primary areas. There will be a major effort to find out what as yet unknown diseases are associated with each of them. Integration of structural and clinical features indicates that, like their previously known counterparts, they will be disease agents. The new polyomaviruses are not as closely structurally related as BKV, JCV and SV40, yet they share structural features known to be involved in disease by their well-known relatives. For example, all share an Rb-binding motif that is conserved through evolution and disease-associated mutations.
It is likely that MCV is the etiological agent of at least a subset of MCC. Evidence thus far parallels that for viruses generally accepted as playing a causal role in cancer, such as HPV in cervical carcinoma [64]. The evidence enumerated also forms an interlocking set with other data to offer further support for a causal role of MCV. For example, the fact that MCC is a rare tumor can in part be explained by observations that MCV is not only integrated but also mutated, thus requiring at least two statistically-rare events. The increased MCC incidence in AIDS suggests that the immune system has some control over either the viral infection or the cancer. This could explain the better prognosis for MCV-positive MCC patients. How, given the evidence at hand, does one address the issue that there are two subsets of MCC, one associated with MCV and one that is not? One explanation could be that MCV, once present, caused genetic changes in Merkel cells that induced the cells to clonally expand in an oncogenic state without continued presence of the virus. This is the “hit and run” hypothesis, much disdained by many virologists, but which cannot be completely discounted here. Because the MCV T-antigen can interact with Rb, it can potentially cause genetic changes that would persist after the virus is gone. Another explanation is that mechanisms contributing to the same oncogenic state in MCC, including inactivating Rb tumor suppression, can be achieved by means other than MCV. This is a solid hyptothesis, but it appeals for an explanation of why Merkel cells are peculiarly susceptible to such means. In any case, an etiological role of MCV in a major subset of MCC should stimulate research on understanding and treating not only MCV, but polyomavirus infections in general.
Does MCV also cause any other prevalent cancers? The role of MCV in MCC will trigger a search for additional oncogenic human polyomaviruses. One such candidate has already been menitioned here—LPV. While many people are reportedly seropositive for LPV [65,66], it is conceivable that the human virus is distinct from the LPV characterized in monkeys. It is doubtful that the full array of polyomaviruses existing in humans has been identified.
In which tissues do each of the polyomaviruses persist? BKV, JCV and SV40 can each persist in urothelial tissues, but the new viruses may not. It is not fully known where even the older viruses reside to evade the immune system during disease. As is MCV, BKV has also been detected in saliva [67]. This raises new considerations. These two apparently widespread viruses may be, at least at certain times, in the same place at the same time. Is coinfection possible? Does horizontal gene transfer or recombination contribute to the evolution of polyomaviruses? Can one polyomavirus influence infection by or transmission of another? Answers to these questions will aid in understanding the disease capabilities of this class of viruses. The polyomaviruses usually persist asymptomatically. Are they reactivated to cause disease, or are they freed by immunosuppression alone? It is unlikely that immunosuppression alone can trigger emergence of all polyomaviral diseases. PML is more prevalent in AIDS than in immunosuppressed transplant patients, and a direct effect of HIV upon JCV infection in the brain has been characterized [68,69]. Immunosuppression clearly plays a role, and an activation mechanism is suggested by the observation that certain types of immunosuppression, e.g., treatment with natalizumab, are more effective than others at triggering PML [70]. To what, if any, activating signals do the new polyomaviruses respond?
The issue of polyomaviral miRNAs must also be soundly addressed. BKV, JCV and SV40 each generate miRNAs which autoregulate early protein expression. MCV generates a functionally-similar miRNA. Each of these viruses is implicated in either cell transformation or cancer etiology. The new viruses KIV and WUV do not generate similar miRNAs. Are they capable of oncogenic transformation? Do the viral miRNAs contribute to transforming capacity?
The discovery of three new viruses within a given family, after more than 50 years of intensive research on this family, is a dramatic development. It is clear from these discoveries that accelerating technology will soon expand the range of diseases caused in humans by polyomaviruses.
Box 1. Individual comparisons of critical functional domains of polyomavirus T-antigens.
The viral protein sequences compared in Table I, and used throughout all figures and tables in this review, were all from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/). The viruses and their accession numbers (parentheses) are as follows: SV40 (JO2400.1), BKV (VO1108), JCV Mad1 (JO2226), KIV Stockholm 60 (EF127906), WUV (EF444549), MCV 350 (EU375803), and LPV, monkey B-cell lymphotropic virus (M30540).
Alignments were done using Multalin [71], which optimizes for identities. Phylogenetic trees were not used because they are variable depending on the algorithm used. The T-antigen column compares the entire protein for each pair of viruses. The helicase column compares a 45 aa segment that includes Walker A and B boxes. The p53/DNA-binding column compares a 93 aa segment with multiple regions reportedly involved in p53 and DNA-binding.
Note that overall the MCV T-antigen (T-antigen column) is only approximately 30% homologous to all the other polyomaviruses, whereas KIV vs. WUV is 70% homologous and JCV vs. BKV is 83% homologous. KIV and WUV are least homologous to MCV in the p53/DNA-binding region (42% and 43%, respectively). Thus, in important aspects, among the three new viruses, KIV and WUV are very different from MCV.
LPV is slightly more homologous to the other new viruses and to JCV in the T-antigen column, but LPV has much lower homologies to these other polyomaviruses in the p53/DNA-binding region. There is no evidence from these comparisons that LPV is closely related to MCV.
Box 2. VP1 protein sequences distinguish classes of polyomaviruses.
Given the different disease states associated with polyomaviruses and their vastly different tissue and cell tropisms, one might expect this to be reflected in the protein sequences of VP1 (Table I).
A comparison of VP1 sequences is revealing, distinguishing more prominently among classes of polyomaviruses than among individual viruses. The alignment of aa sequences employed ClustalW [72], which weighs conserved aas. The previously known and closely related viruses BKV and JCV share 78.2% identity. Many strains of these viruses have been sequenced, and GenBank accession numbers are given in Box 1. This high degree of identity is even more surprising given that many of the differences are highly conservative substitutions of aas. There are a few short motifs that differ between these sequences, and clearly these are important since BKV and JCV VP1 proteins bind different sialic acid-containing receptors. JCV has a tropism for glial cells while BKV does not. Even greater identity, 81.3%, is shared between BKV and SV40. Perhaps the homologies among these three VP1 sequences distinguish a class of polyomaviruses that can be harbored in urothelial tissues. KIV and WUV present a different pattern of homologies. KIV and JCV VP1 proteins share only 27.5% aa identities. This relatively weak homology is not reflected in phylogenetic analyses that place KIV and WUV in a broad branch of primate polyomaviruses much closer to JCV than to MCV. Similarly to KIV and JCV, KIV and MCV VP1s share only 26.4% identity. In contrast, KIV and WUV VP1s are 65.5% identical, a relatively high degree, although not as high as BKV, JCV and SV40. MCV is frequently phylogenetically compared to LPV, although these viruses differ significantly in functional motifs of proteins. Their VP1 aas are 52% identical, and so MCV is more closely related to LPV than to KIV or JCV regarding VP1. LPV DNA sequences have recently been reported in peripheral blood of patients with leukoencephalopathies [73]. Thus the VP1 protein homologies distinguish three classes of polyomaviruses found in humans: BKV-JCV-SV40, KI-WU and MCV, each of which are very similar among class members, but each of which differs greatly from members of other classes. This VP1 homology comparison may reflect differences in tissue tropisms among these different classes of polyomaviruses.
Table I.
Amino acid comparisons of critical domains of different human polyomaviruses, SV40 and LPV
| Comparison | T-antigen | Helicase | P53/DNA-binding |
|---|---|---|---|
| JCV vs. BKV | 83% aa identity | 84% | 90% |
| JCV vs. MCV | 31% | 64% | 56% |
| BKV vs. MCV | 31% | 64% | 54% |
| SV40 vs. BKV | 73% | 84% | 85% |
| SV40 vs. MCV | 29% | 67% | 56% |
| KIV vs. MCV | 30% | 73% | 42% |
| KIV vs. WUV | 70% | 78% | 68% |
| WUV vs. MCV | 31% | 71% | 43% |
| WUV vs. JCV | 49% | 82% | 54% |
| LPV vs. JCV | 38% | 56% | 42% |
| LPV vs. KIV | 42% | 60% | 36% |
| LPV vs. WUV | 41% | 64% | 38% |
| LPV vs. MCV | 39% | 62% | 51% |
Table I.
Amino acid (aa) comparisons of VP1 proteins of different polyomaviruses
| Comparison | VP1 |
|---|---|
| SV40 vs. BKV | 81% aa identity |
| BKV vs. JCV | 78% |
| KIV vs. WUV | 66% |
| KIV vs. JCV | 28% |
| KIV vs. MCV | 26% |
| JCV vs. MCV | 42% |
| LPV vs. MCV | 52% |
Acknowledgements
I thank Drs. Ann E. Campbell and Dianne C. Daniel for proofreading and insightful comments on this manuscript. Dr. Aurora Esquela-Kerscher provided helpful discussion regarding microRNAs.
Glossary
- Polyomavirus
polyomaviruses are small, capsid viruses containing approximately 5 kbp of double-stranded, circular DNA. They all contain a non-coding transcriptional control region that regulates both early and late genes, in opposite directions, and which includes the origin of DNA replication. The early genes are small and large T-antigens, which are the oncogenically-transforming proteins and which initiate viral DNA replication. The late genes are VP1, VP2 and VP3, which make up the capsid. Because polyomaviruses are so small, they rely heavily on cellular proteins for both DNA replication and gene transcription. All polyomaviruses are so named because they can transform many types of animal cells in culture
- BKV, JCV and SV40
these are viruses previously known to be harbored in humans. BK virus (BKV) and JC virus (JCV) are polyomaviruses that primarily infect humans, in which they cause debilitating diseases. BKV and JCV acronyms are derived from initials of the patients who bore them. Simian virus 40 (SV40) is a virus that causes kidney tumors in monkeys. SV40 was inadvertently injected into million of humans, where it persists, as a contaminant of early polio virus vaccines
- KIV, WUV, and MCV
these are the newly discovered human polyomaviruses. KIV and WUV are derived from the institutions at which they were discovered, Karolinska Institutet and Washington University, respectively. The acronym MCV is derived from the tumor cell type, Merkel cell carcinoma, from which the virus was isolated
- LPV
this is monkey B-cell lymphotropic polyomavirus. The monkey virus was characterized many years ago, as was the presence of antibodies to it in human sera. In that regard, the virus can be regarded as previously known. It is not known at this time whether a virus recently detected in human blood is identical to LPV or is an undiscovered LPV-like virus not identical to monkey LPV. If it is not identical to monkey LPV, then it can be regarded as another new human polyomavirus
- Transcriptional control region
this DNA region is centered about the T-antigen-binding sites in the origin of viral DNA replication. It contains promoter and enhancer elements for early and late gene transcription. These are oriented for regulation of cellular RNA polymerase II and control both early and late gene transcription
- Origin of replication
initial DNA unwinding originates here allowing the cellular DNA polymerase apparatus to enter and proceed. The viruses, however, rely on their own initiation protein, T-antigen, to bind specific sites and unwind the origin. T-antigen also serves as the replicative helicase, unwining DNA at replication forks
- Helicase domains
these are protein sequence motifs common to DNA and RNA processively unwinding enzymes. There are several of these motifs common to AAA+ proteins, which are ATPase motor proteins, and they all include what have been defined as Walker A and B boxes. These are ATP and MG++-binding motifs
- PML (progressive multifocal leukoencephalopathy)
a brain infection of oligodendroglial cells caused by JCV in immunocompromised patients and patients infected with AIDS
- PVAN (polyomavirus associated nephropathy)
a disease caused by polyomavirus responsible for a high percentage of kidney transplant rejections. BKV is the primary cause of this infection, brought about by immunosuppression, although common immunohistochemical tests for T-antigen do not distinguish between BKV and JCV, both of which persist in the urothelial system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan TF, Borden EC, McBain JA, Padgett BL, Walker DL. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980;92:373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- 5.Trofe J, Hirsch HH, Ramos E. Polyomavirus-associated nephropathy: update of clinical management in kidney transplant patients. Transpl Infect Dis. 2006;8:76–85. doi: 10.1111/j.1399-3062.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Berger JR, Major EO. Progressive multifocal leukoencephalopathy. Semin Neurol. 1999;19:193–200. doi: 10.1055/s-2008-1040837. [DOI] [PubMed] [Google Scholar]
- 7.Eash S, Manley K, Gasparovic M, Querbes W, Atwood WJ. The human polyomaviruses. Cell Mol Life Sci. 2006;63:865–876. doi: 10.1007/s00018-005-5454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enemark EJ, Joshua-Tor L. On helicases and other motor proteins. Curr Opin Struct Biol. 2008;18:243–257. doi: 10.1016/j.sbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanning E, Zhao K. SV40 DNA replication: from the A gene to a nanomachine. Virology. 2009;384:352–359. doi: 10.1016/j.virol.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng J, DeCaprio JA, Fluck MM, Schaffhausen BS. Cellular transformation by Simian Virus 40 and Murine Polyoma Virus T antigens. Semin Cancer Biol. 2009;19:218–228. doi: 10.1016/j.semcancer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeCaprio JA. How the Rb tumor suppressor structure and function was revealed by the study of Adenovirus and SV40. Virology. 2009;384:274–284. doi: 10.1016/j.virol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Frisque RJ. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983;46:170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomerantz BJ, Hassell JA. Polyomavirus and simian virus 40 large T antigens bind to common DNA sequences. J Virol. 1984;49:925–937. doi: 10.1128/jvi.49.3.925-937.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shortle DR, Margolskee RF, Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979;76:6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978;13:165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- 16.Kwun HJ, Guastafierro A, Shuda M, Meinke G, Bohm A, Moore PS, Chang Y. The minimum replication origin of merkel cell polyomavirus has a unique large T-antigen loading architecture and requires small T-antigen expression for optimal replication. J Virol. 2009;83:12118–12128. doi: 10.1128/JVI.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz GS, Dean FB, Hurwitz J, Matson SW. The unwinding of duplex regions in DNA by the simian virus 40 large tumor antigen-associated DNA helicase activity. J Biol Chem. 1988;263:383–392. [PubMed] [Google Scholar]
- 18.Mastrangelo IA, Hough PV, Wall JS, Dodson M, Dean FB, Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989;338:658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- 19.Mahon C, Liang B, Tikhanovich I, Abend JR, Imperiale MJ, Nasheuer HP, Folk WR. Restriction of human polyomavirus BK virus DNA replication in murine cells and extracts. J Virol. 2009;83:5708–5717. doi: 10.1128/JVI.00300-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley WL, Georgopoulos C. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc Natl Acad Sci U S A. 1997;94:3679–3684. doi: 10.1073/pnas.94.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawai ET, Butel JS. Association of a cellular heat shock protein with simian virus 40 large T antigen in transformed cells. J Virol. 1989;63:3961–3973. doi: 10.1128/jvi.63.9.3961-3973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright CM, Seguin SP, Fewell SW, Zhang H, Ishwad C, Vats A, Lingwood CA, Wipf P, Fanning E, Pipas JM, et al. Inhibition of Simian Virus 40 replication by targeting the molecular chaperone function and ATPase activity of T antigen. Virus Res. 2009;141:71–80. doi: 10.1016/j.virusres.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson EM, Chen PL, Krachmarov CP, Barr SM, Kanovsky M, Ma ZW, Lee WH. Association of human Pur alpha with the retinoblastoma protein, Rb, regulates binding to the single-stranded DNA Pur alpha recognition element. J Biol Chem. 1995;270:24352–24360. doi: 10.1074/jbc.270.41.24352. [DOI] [PubMed] [Google Scholar]
- 24.White MK, Johnson EM, Khalili K. Multiple roles for Puralpha in cellular and viral regulation. Cell Cycle. 2009;8:1–7. doi: 10.4161/cc.8.3.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radulescu RT. The ‘LXCXE’ hydropathic superfamily of ligands for retinoblastoma protein: a proposal. Med Hypotheses. 1995;44:28–31. doi: 10.1016/0306-9877(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 26.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson KD, Garcea RL, Tsai B. Ganglioside GT1b is a putative host cell receptor for the Merkel cell polyomavirus. J Virol. 2009 doi: 10.1128/JVI.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunyaev SR, Lugovskoy A, Simon K, Gorelik L. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML). PLoS Genet. 2009;5:e1000368. doi: 10.1371/journal.pgen.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delbue S, Branchetti E, Bertolacci S, Tavazzi E, Marchioni E, Maserati R, Minnucci G, Tremolada S, Vago G, Ferrante P. JC virus VP1 loop-specific polymorphisms are associated with favorable prognosis for progressive multifocal leukoencephalopathy. J Neurovirol. 2009;15:51–56. doi: 10.1080/13550280802425467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalili K, White MK, Sawa H, Nagashima K, Safak M. The agnoprotein of polyomaviruses: a multifunctional auxiliary protein. J Cell Physiol. 2005;204:1–7. doi: 10.1002/jcp.20266. [DOI] [PubMed] [Google Scholar]
- 31.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 32.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 33.Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe. 2008;3:375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan CS. New roles for large and small viral RNAs in evading host defences. Nat Rev Genet. 2008;9:503–507. doi: 10.1038/nrg2349. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–686. doi: 10.1038/nature03576. [DOI] [PubMed] [Google Scholar]
- 36.Seo GJ, Fink LH, O'Hara B, Atwood WJ, Sullivan CS. Evolutionarily conserved function of a viral microRNA. J Virol. 2008;82:9823–9828. doi: 10.1128/JVI.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo GJ, Chen CJ, Sullivan CS. Merkel cell polyomavirus encodes a microRNA with the ability to autoregulate viral gene expression. Virology. 2009;383:183–187. doi: 10.1016/j.virol.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Bialasiewicz S, Whiley DM, Lambert SB, Nissen MD, Sloots TP. Detection of BK, JC, WU, or KI polyomaviruses in faecal, urine, blood, cerebrospinal fluid and respiratory samples. J Clin Virol. 2009;45:249–254. doi: 10.1016/j.jcv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Le BM, Demertzis LM, Wu G, Tibbets RJ, Buller R, Arens MQ, Gaynor AM, Storch GA, Wang D. Clinical and epidemiologic characterization of WU polyomavirus infection, St. Louis, Missouri. Emerg Infect Dis. 2007;13:1936–1938. doi: 10.3201/eid1312.070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payungporn S, Chieochansin T, Thongmee C, Panjaworayan N, Samransamruajkit R, Theamboolers A, Poovorawan Y. Detection and discrimination of WU/KI polyomaviruses by real-time PCR with melting curve analysis. J Virol Methods. 2008;153:70–73. doi: 10.1016/j.jviromet.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Bialasiewicz S, Lambert SB, Whiley DM, Nissen MD, Sloots TP. Merkel cell polyomavirus DNA in respiratory specimens from children and adults. Emerg Infect Dis. 2009;15:492–494. doi: 10.3201/eid1503.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kantola K, Sadeghi M, Lahtinen A, Koskenvuo M, Aaltonen LM, Mottonen M, Rahiala J, Saarinen-Pihkala U, Riikonen P, Jartti T, et al. Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J Clin Virol. 2009;45:292–295. doi: 10.1016/j.jcv.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loyo M, Guerrero-Preston R, Brait M, Hoque M, Chuang A, Kim M, Sharma R, Liegeois N, Koch W, Califano J, et al. Quantitative detection of merkel cell virus in human tissues and possible mode of transmission. Int J Cancer. 2009 doi: 10.1002/ijc.24737. 10.1002/ijc.24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norja P, Ubillos I, Templeton K, Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40:307–311. doi: 10.1016/j.jcv.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinreb DB, Desman GT, Amolat-Apiado MJ, Burstein DE, Godbold JH, Jr., Johnson EM. Polyoma virus infection is a prominent risk factor for bladder carcinoma in immunocompetent individuals. Diagn Cytopathol. 2006;34:201–203. doi: 10.1002/dc.20429. [DOI] [PubMed] [Google Scholar]
- 46.Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, Arora R, Hanson NC, Tassello JA, Frosina D, et al. Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009;33:1378–1385. doi: 10.1097/PAS.0b013e3181aa30a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland U, Mauch C, Kreuter A, Krieg T, Pfister H. Merkel cell polyomavirus DNA in persons without merkel cell carcinoma. Emerg Infect Dis. 2009;15:1496–1498. doi: 10.3201/eid1509.081575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, Roizman B. Infectious agents and cancer: criteria for a causal relation. Semin Cancer Biol. 2004;14:453–471. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 49.White MK, Gordon J, Reiss K, Del Valle L, Croul S, Giordano A, Darbinyan A, Khalili K. Human polyomaviruses and brain tumors. Brain Res Brain Res Rev. 2005;50:69–85. doi: 10.1016/j.brainresrev.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Zhan FQ, Packianathan VS, Zeitouni NC. Merkel cell carcinoma: a review of current advances. J Natl Compr Canc Netw. 2009;7:333–339. doi: 10.6004/jnccn.2009.0025. [DOI] [PubMed] [Google Scholar]
- 51.Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516–521. doi: 10.1038/modpathol.2009.3. [DOI] [PubMed] [Google Scholar]
- 52.Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, Zur Hausen A. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68:5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 53.Paulson KG, Lemos BD, Feng B, Jaimes N, Penas PF, Bi X, Maher E, Cohen L, Leonard JH, Granter SR, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547–1555. doi: 10.1038/jid.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sastre-Garau X, Peter M, Avril MF, Laude H, Couturier J, Rozenberg F, Almeida A, Boitier F, Carlotti A, Couturaud B, et al. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J Pathol. 2009;218:48–56. doi: 10.1002/path.2532. [DOI] [PubMed] [Google Scholar]
- 55.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938–945. doi: 10.1093/jnci/djp139. [DOI] [PubMed] [Google Scholar]
- 56.Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW. Merkel cell carcinoma subgroups by merkel cell polyomavirus DNA relative abundance and oncogene expression. Int J Cancer. 2009 doi: 10.1002/ijc.24676. 10.1002/ijc.24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houben R, Schrama D, Alb M, Pfohler C, Trefzer U, Ugurel S, Becker JC. Comparable expression and phosphorylation of the retinoblastoma protein in merkel cell polyoma-virus positive and negative merkel cell carcinoma. Int J Cancer. 2009 doi: 10.1002/ijc.24790. 10.1002/ijc.24790. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 59.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105:16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.zur Hausen H. A specific signature of Merkel cell polyomavirus persistence in human cancer cells. Proc Natl Acad Sci U S A. 2008;105:16063–16064. doi: 10.1073/pnas.0808973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8:153–158. [PubMed] [Google Scholar]
- 62.Buell JF, Trofe J, Hanaway MJ, Beebe TM, Gross TG, Alloway RR, First MR, Woodle ES. Immunosuppression and Merkel cell cancer. Transplant Proc. 2002;34:1780–1781. doi: 10.1016/s0041-1345(02)03065-8. [DOI] [PubMed] [Google Scholar]
- 63.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 64.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 65.Brade L, Muller-Lantzsch N, zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6:301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- 66.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeffers LK, Madden V, Webster-Cyriaque J. BK virus has tropism for human salivary gland cells in vitro: implications for transmission. Virology. 2009;394:183–193. doi: 10.1016/j.virol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Krachmarov CP, Chepenik LG, Barr-Vagell S, Khalili K, Johnson EM. Activation of the JC virus Tat-responsive transcriptional control element by association of the Tat protein of human immunodeficiency virus 1 with cellular protein Pur alpha. Proc Natl Acad Sci U S A. 1996;93:14112–14117. doi: 10.1073/pnas.93.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stettner MR, Nance JA, Wright CA, Kinoshita Y, Kim WK, Morgello S, Rappaport J, Khalili K, Gordon J, Johnson EM. SMAD proteins of oligodendroglial cells regulate transcription of JC virus early and late genes coordinately with the Tat protein of human immunodeficiency virus type 1. J Gen Virol. 2009;90:2005–2014. doi: 10.1099/vir.0.011072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 71.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delbue S, Tremolada S, Branchetti E, Elia F, Gualco E, Marchioni E, Maserati R, Ferrante P. First identification and molecular characterization of lymphotropic polyomavirus in peripheral blood from patients with leukoencephalopathies. J Clin Microbiol. 2008;46:2461–2462. doi: 10.1128/JCM.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]