Abstract
Although the M1 muscarinic receptor is a potential therapeutic target for Alzheimer's disease (AD) based on its wide spread distribution in brain and its association with learning and memory processes, whether its receptor response is altered during the onset of AD remains unclear. A novel [35S]GTPγS binding/immunocapture assay was employed to evaluated changes in M1 receptor function in cortical tissue samples harvested from people who had no cognitive impairment (NCI), mild cognitive impairment (MCI), or AD. M1- function was stable across clinical groups. However, [3H]-oxotremorine-M radioligand binding studies revealed that the concentration of M1 cortical receptors increased significantly between the NCI and AD groups. Although M1 receptor function did not correlate with cognitive function based upon mini-mental status examination (MMSE) or global cognitive score (GCS), functional activity was negatively correlated with the severity of neuropathology determined by Braak staging and NIA-Reagan criteria for AD. Since M1 agonists have the potential to modify the pathologic hallmarks of AD, as well as deficits in cognitive function in animal models of this disease, the present findings provide additional support for targeting the M1 receptor as a potential therapeutic for AD.
Keywords: Bmax, GPCR, functional activity, MCI, muscarinic receptor
Introduction
The cholinergic neurons of the nucleus basalis (NB), which provide the major source of cholinergic innervations to the cerebral cortex and play a key role in memory and attention, are particularly vulnerable in Alzheimer's disease (AD) (Auld, et al., 2002; Mufson, et al., 2003; Mufson and Counts, 2004). Postmortem studies of the AD brain revealed deficits in the cortical presynaptic cholinergic markers choline acetyltransferase (ChAT) activity (Francis, et al., 1999), choline uptake (Nilsson, et al., 1986) and acetylcholine (Ach) release (Rylett, et al., 1983), as well as a loss of cholinergic basal forebrain (CBF) cortical projection neurons (Bowen, et al., 1976; Davies and Maloney, 1976; Whitehouse, et al., 1982). Therefore cholinergic ionotropic (nicotinic) and metabotropic (muscarinic) receptors remain major drug discovery targets for the treatment of AD (Fisher, 2008). In particular, the muscarinic receptor family has shown recent clinical efficacy in improving cognitive deficits associated with AD and schizophrenia (Bodick, et al., 1997; Shekhar, et al., 2008)
There are five members of the muscarinic cholinergic receptor family (M1 –M5), which are widely distributed in the CNS and periphery. Previous studies have shown that the predominantly post-synaptic M1 receptor, which is expressed throughout the cortex and hippocampus (Davies and Verth, 1977) plays a key role in regulating cognition (Anagnostaras, et al., 2002). Short term memory loss as well as attention and executive function deficits associated with AD are thought to be at least in part due to cholinergic deafferentation of basal forebrain input circuits to the hippocampus and cortex and loss of acetylcholine agonism at the post-synaptic M1 receptor. Post-synaptic M1 receptors also play a key role in modulating glutamatergic NMDA receptors which are essential regulators of synaptic action potential propagation (Marino, et al., 1998). Loss of neurotransmitter from pre-synaptic densities usually results in compensatory up-regulation of post-synaptic receptor proteins (Russell, et al., 1986; Benes, et al., 1996). Investigators have measured M1 receptor expression level changes in postmortem brain in normal subjects and AD patients using either radioligand binding techniques or indirectly via quantification of M1 mRNA (Harrison, et al., 1991). Up-regulation of M1 receptor protein density has been observed in AD tissues (Shiozaki, et al., 1999), but the lack of true M1 receptor selectivity of the available radioligands does not provide specific measurement of M1 receptor binding and function. However, M1 selective mRNA in situ hybridization probes show a greater than 2-fold increase in M1 message in postmortem AD brain suggesting that M1 receptor protein is also increased (Harrison, et al., 1991). Measurement of M1 receptor function in human postmortem brain tissue would potentially provide a more direct measure of physiologically relevant M1 activity and signaling potential for therapeutic agonist or positive modulator treatments.
Determining the functional changes in brain M1 receptor activity (Bymaster, et al., 2003; Salah-Uddin, et al., 2008), particularly in people with mild cognitive impairment (MCI), a precursor stage of AD (Petersen, et al., 1999), is of great interest due to its physiologic relevance to cognitive disease processes. Since the M1 receptor belongs to a G-protein coupled receptor (GPCR) superfamily, functional assays measuring the nucleotide exchange of guanosine triphosphate (GTP) for guanosine diphosphate (GDP) bound to the G-protein α-subunits provide a method to evaluate GPCR functional responses in brain membrane preparations under pseudo-equilibrium conditions (DeLapp, et al., 1999). Currently, most muscarinic receptor assays employ antagonist radioligand binding (Ladner, et al., 1995), [35S]GTPγS protein binding to extrapolate total G-protein activation (Gonzalez-Maeso, et al., 2000; Scarr, et al., 2006), or distal signaling in whole cell based assays (Garro, et al., 2001); however, these methods are limited by either their lack of selectivity for the appropriate GPCR-G protein alpha subunit pair or are inappropriate to measure functional responses in postmortem tissue. To overcome these caveats, a more specific and direct measure of GPCR function was developed using a selective G-protein alpha subunit specific antibody capture [35S]GTPγS binding assay (DeLapp, et al., 1999), which uniquely measures muscarinic Gq coupled receptor functional responses in human brain (Salah-Uddin, et al., 2008). As designed, the [35S]GTPγS binding assay predominantly measures M1 receptor functional responses in cortically derived membranes (Bymaster, et al., 2003). This is due to the selectivity of the assay for M1, M3, and M5 Gq-coupled receptors and M1-Gq mediated signaling events that predominate over M3 and M5 receptors in hippocampus and cortex. We applied this assay to investigate whether M1 response remains stable or changes in frontal cortex during the progression of cognitive decline in a carefully annotated human population.
Materials and Methods
Subjects
Cortical tissue samples were evaluated from 30 individuals who were participants in the Religious Orders Study (ROS), a large longitudinal clinical pathologic study of aging and AD in older Catholic nuns, priests and brothers (Table 1)(Mufson, et al., 2000; Bennett, et al., 2002; DeKosky, et al., 2002). Each participant agreed to an annual detailed clinical evaluation and brain donation at the time of death. For all subjects, cognitive testing scores were available within the last year of life; the average interval from last evaluation to time of death was 15.4 ± 9.8 months, with no significant differences among the three diagnostic groups (p = 0.6). Subjects were clinically categorized as NCI (n = 10, mean age = 82.3 ± 4.5 years, mean MMSE = 28.2 ± 1.0), MCI insufficient to meet criteria for dementia (n = 10, age = 84.1 ± 5.7 years, MMSE = 28.1 ± 1.8), or mild/moderate AD (n = 10, age = 90.2 ± 7.3 years, MMSE = 16.4 ± 5.8). None of the subjections were maintained on anti-cholinergic inhibitors prior to death. The Human Investigation Committee of Rush University Medical Center approved the study.
Table 1.
Clinical, demographic, and neuropathological characteristics by diagnosis category
| Clinical Diagnosis |
Comparison by diagnosis group | |||||
|---|---|---|---|---|---|---|
| NCI (N=10) | MCI (N=10) | AD (N=10) | Total (N=30) | |||
| Age at death (years): | Mean ± SD (Range) | 82.3 ± 4.5 (76–90) | 84.1 ± 5.7 (72–92) | 90.2 ± 7.3 (80–101) | 85.5 ± 6.7 (72–101) | p = 0.05a |
| Number (%) of males: | 5 (50%) | 4 (40%) | 3 (30%) | 12 (40%) | p = 0.9b | |
| Years of education: | Mean ± SD (Range) | 19.4 ± 3.2 (15–25) | 20.0 ± 2.1 (17–24) | 17.9 ± 2.6 (14–22) | 19.1 ± 2.7 (14–25) | p = 0.2a |
| Number (%) with ApoE ε4 allele: | 0 | 0 | 4 (40%) | 4 (13%) | p = 0.5b | |
| MMSE: | Mean ± SD (Range) | 28.2 ± 1.0 (27–30) | 28.1 ± 1.8 (25–30) | 16.4 ± 5.8 (7–24) | 24.2 ± 6.6 (7–30) | p < 0.0001a* |
| Global Cognitive Score (GCS): | Mean ± SD (Range) | 0.71 ± 0.24 (0.37–1.20) | 0.39 ± 0.32 (−0.17, 0.83) | −0.81 ± 0.52 (−1.52, −0.16) | 0.13 ± 0.75 (−1.52, 1.20) | p < 0.0001a* |
| Postmortem interval (hours): | Mean ± SD (Range) | 5.1 ± 3.0 (2.3–12.4) | 4.7 ± 2.4 (2.7–10.0) | 6.1 ± 3.5 (2.7–12.4) | 5.3 ± 2.9 (2.3–12.4) | p = 0.7a |
| Distribution of Braak scores: | I-II | 4 | 5 | 0 | 9 | p = 0.0003a* |
| III-IV | 6 | 5 | 4 | 15 | ||
| V-VI | 0 | 0 | 6 | 6 | ||
| Distribution of NIA Reagan diagnosis (likelihood of AD): | No AD | 0 | 0 | 0 | 0 | p = 0.0005a* |
| Low | 5 | 7 | 0 | 12 | ||
| Intermediate | 5 | 3 | 5 | 13 | ||
| High | 0 | 0 | 5 | 5 | ||
Kruskal-Wallis test.
Fisher's exact test.
Pairwise comparisons with Bonferroni correction showed that there was no significant difference between NCI and MCI, but both were significantly different from AD (p<0.01).
Clinical Evaluation
Details of the ROS clinical evaluation have been published elsewhere (Mufson, et al., 2000; Bennett, et al., 2002; DeKosky, et al., 2002). Briefly, a team of investigators performed a complete annual clinical evaluation that included assessments for stroke (Goldstein and Samsa, 1997) and parkinsonian signs (Bennett, et al., 1997). Trained neuropsychology technicians administered a battery of tests measuring performance in five cognitive domains: orientation, attention, memory, language, and perception (Pittman, et al., 1992). An impaired domain score required impairment on multiple tests within that domain. A board-certified clinical neuropsychologist used these findings to summarize impairment in each cognitive domain as not present, possible, or probable AD. After review of all clinical data from that year and examination of the participant, a board-certified neurologist with expertise in geriatric medicine made a clinical diagnosis. The diagnosis of dementia and AD followed the recommendations of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) (McKhann, et al., 1984). Although there is no consensus criteria for the clinical classification of MCI (Petersen, et al., 1999; Sabbagh, et al., 2006) our MCI cohort was defined as those persons rated as impaired on neuropsychological testing by the neuropsychologist but who were not found to have dementia by the examining neurologist (Mufson, et al., 2000; Bennett, et al., 2002; DeKosky, et al., 2002). These criteria are similar to, or compatible with, those used by others in the field to describe persons who are not cognitively normal but do not meet established criteria for dementia (Rubin, et al., 1989; Flicker, et al., 1991; Petersen, et al., 1999). All members of the ROS population were cognitively assessed using the mini-mental state examination (MMSE) score and a battery of cognitive tests, from which a global cognitive score (GCS) was compiled as a composite z-score indicative of cognitive status (Bennett, et al., 2002). A postmortem interview was conducted with the primary caregiver at the time of death to identify medical conditions that occurred during the interval between the last clinical evaluation and death. Finally, a consensus conference of neurologists and neuropsychologists evaluated all available data and made a summary clinical diagnosis.
Pathological Evaluation and Tissue Preparation
Brains used in this study were processed at autopsy as previously published (Mufson, et al., 2000; Bennett, et al., 2002; DeKosky, et al., 2002). The postmortem interval (PMI) did not differ across groups examined (p = 0.7, Table 1). A neuropathologist conducted a gross examination of brain neuropathology and cases were excluded if they exhibited significant non-AD types of pathologic conditions (e.g., brain tumors, encephalitis, large strokes, and multiple lacunar infarctions). Brains were cut into 1-cm-thick slabs. One hemisphere was immersion-fixed in 4% paraformaldehyde and the opposite hemisphere was snap-frozen in liquid nitrogen. Samples of gray matter from the superior frontal cortex (Brodmann area 10), an area known to contain extensive AD pathology, were dissected based on fiduciary landmarks. All dissections were performed on dry ice to prevent tissue thawing and stored at −80 °C until assayed.
A complete neuropathological analysis was performed on paraffin-embedded sections with special attention to lesions that might contribute to dementia, including brainstem and cortical Lewy bodies, as well as strokes. A pathological diagnosis was made for each case by a neuropathologist blinded to the clinical diagnosis. All cases received Braak scores based upon neurofibrillary tangle pathology (Braak and Braak, 1991) and were assigned a diagnosis based on NIA-Reagan criteria (Table 1) (The National Institute on Aging, 1997).
Membrane Preparation
All procedures were performed at 0–4 °C using pre-chilled reagents as previously described (DeLapp, et al., 1999). Briefly, sucrose buffer (10 mL; 10 mM HEPES, 1 mM EGTA, 1 mM DTT, 10 % sucrose, and 1 tablet/50 mL Complete Protease Inhibitor Cocktail Roche +++1697-498) was added to each tissue sample and homogenized for 15 strokes using a glass homogenizer on ice. The homogenate was centrifuged at 800 × g for 15 min at 4 °C. The supernatant was removed and placed into a new tube and centrifuged at 27,000 × g for 20 min at 4 °C. The pellet was suspended in 15 mL of suspension buffer (10 mM HEPES, 10 mM MgCl2, 1 mM DTT, 1 mM EGTA and 0.5 mM EDTA; pH 7.4) and centrifuged at 27,000 × g for 20 min at 4 °C. The supernatant was removed and pellet was suspended in 1.6 mL suspension buffer. Protein concentration was measured using the Bradford method (Coomassie Plus, Pierce, Rockford, IL) with BSA standards. Samples were aliquoted and stored at −80 °C.
[35S]GTPγS Binding Studies
Frontal cortical membranes were assayed in duplicate using the Gαq/11 [35S]GTPγS immunocapture assay to directly measure the function of predominantly M1 receptors which was previously validated using M1–M5 muscarinic receptor knockout mice (Bymaster, et al., 2003). Briefly, diluted human brain homogenates (100 μL at 5 μg/well, final concentration) were added to a 96-well Costar plates. Oxotremorine-M (Oxo-M; 50 μL) was serial diluted (half-log) starting at 400 μM (4X). Sigma-RBI O100-500MG lot#086K4604 or assay buffer solution (50 μL; 20 mM HEPES, 100 mM NaCl, and 5 mM MgCl2; pH 7.4) was added to each well, mixed, and incubated at room temperature for 15 min. [35S]GTPγS (50 μL; final concentration 500 pM; Amersham, Arlington Heights, IL) was added to each well and mixed. Samples were incubated at room temperature for 60 min. Nonidet P-40 (20 μL of 3% solution; Boehringer Mannheim, Indianapolis, IN) was added and incubated for 5 min at room temperature. Rabbit Polyclonal Antibody: IA purified anti- Gαq/11 (Lot# rgc-Gq-072105-(3,7) (Eli Lilly in-house reagent 1:16.7 diluted with assay buffer for the final 1:200 dilution at 240 μL/well) 20 µL was added and incubated for 60 min at room temperature before adding anti-rabbit SPA beads (50 μL/1.25 mg/well; Amersham; 290 μL/well final incubation volume). The plate was covered with sealing tape and vortexed for 10–15 sec. The plate was incubated at room temperature for 3 h before centrifugation for 10 min at 1000 RPM. [35S] was counted using a Wallac 1450 MicroBeta scintillation counter (Perkin Elmer, Waltham, MA) (1 min/well). The data were analyzed using non-linear regression/one-site sigmoidal dose-response model (GraphPad Prism; La Jolla, CA). Each patient served as its own control for background binding. Using an Excel template, percent stimulation over basal binding levels were calculated. Briefly, duplicate samples were averaged and the number of cpms over background divided by basal counts then multiplied by 100 to yield percent stimulation.
[3H]-Oxotremorine-M Radioligand Binding Studies
Cortical membranes from different patients were incubated with 6 different concentrations of [3H]-oxotremorine-M (PerkinElmer, Boston, MA, NET671) in a receptor binding assay. Incubations were conducted at room temperature for 1 hour in buffer containing 20 mM NaH2PO4 (monobasic), 10 mM MgCl (pH 7.4). Briefly, each sample was tested as a singleton with one row of total binding (100 μL buffer + 50 μL [3H]-oxotremorine-M and 50 μL membranes at 1 μg/μL) and one row to define non-specific binding (50 μL buffer + 50 μL [3H]-oxotremorine-M, 50 μL membranes and 50 μL of atropine for a final concentration of 10 μM). Binding was terminated 60 min later by rapid filtration using a TOMTEC 96-well cell harvester (TOMTEC, Orange, CT) through GF/A filters that had been presoaked with 0.3% polyethyleneimine (Sigma, St Louis) for 2 hours. The filters were washed with 5 mL of ice-cold 50 mM Tris buffer (pH 7.4) and air dried. The dried filters were treated with MeltiLex A melt-on scintillator sheets (Wallac, Gaithersburg, MD), and the radioactivity retained on the filters counted using the Wallac 1205 Betaplate scintillation counter. Raw counts were analyzed using Microsoft Excel to determine the amount of radioligand bound at each concentration. To generate Kd and Bmax values for each patient, samples were tested a total of 2 times and the data analyzed using a non-linear regression/one-site binding hyperbola model (GraphPad Prism; La Jolla, CA).
Data Analysis
Both [35S]GTPγS binding and Bmax levels were collected in duplicates. Variance component analysis showed that the within-case variability (relative to between-case variability) was small; therefore the averages of duplicates were used in subsequent analyses. There was one MCI case whose two Bmax values widely differed; this case was excluded in the Bmax analysis. An NCI case showed outlying [35S]GTPγS binding values. Since its duplicate binding values were similar (52.9% and 51.3%), statistical analysis was performed with and without this case without any effect on the statistical outcome. Clinical and neuropathological variables, as well as levels of M1 functional activity were compared across clinical diagnostic groups using the Kruskal-Wallis test or Fisher's exact test; pair-wise comparisons were performed with Bonferroni correction for multiple comparison. In addition, a Jonckheere-Terpstra test (Hollander and Wolfe, 1999) was performed to assess the trend in Bmax levels across diagnostic groups. Associations between M1 functional activity on the one hand, and clinical and neuropathological variables on the other, were assessed by Spearman rank correlation or Wilcoxon rank-sum test. The level of statistical significance was set at 0.05 (two-sided).
Results
Demographics
The NCI, MCI, and AD individuals did not differ in sex, years of education, ApoE ε4 allele status, or postmortem interval (PMI) (see Table 1). Age at death was younger in NCI and MCI than in AD individuals, but the three-group comparison did not reach statistical significance (p=0.0504). The last MMSE score ranged from 7–30. While MMSE was comparable between the NCI and MCI groups, AD cases had significantly lower MMSE scores than either the NCI or MCI subjects (p < 0.01; Table 1). Similarly, the GCS was significantly lower in AD compared to NCI and MCI (p < 0.01; Table 1). Neuropathological evaluation of the three clinical groups by Braak staging and NIA-Reagan criteria of a low, intermediate, or high likelihood of AD also revealed no significant differences between NCI and MCI, but both were significantly different from the AD group (p < 0.01; Table 1).
[35S]GTPγS binding in frontal cortex of NCI, MCI, and AD
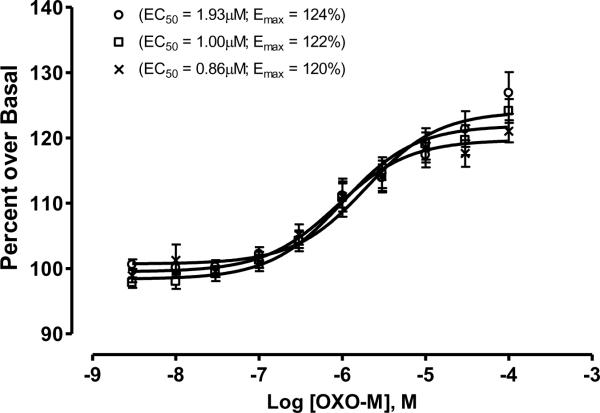
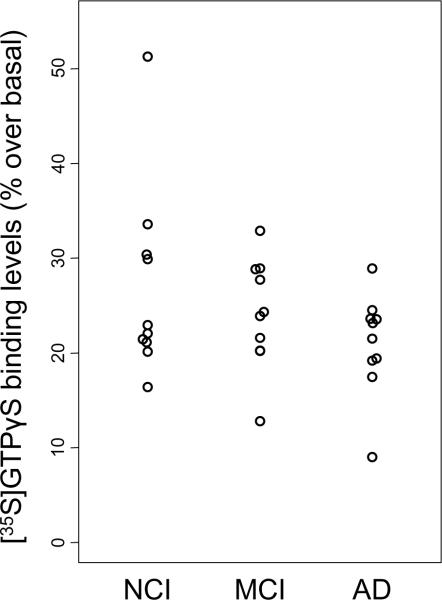
Functional [35S]GTPγS binding was performed in homogenates prepared from cortical tissue samples over a concentration range of 0.001 to 100 μM oxotremorine-M (Fig. 1). The data were normalized as percent stimulation over basal levels in each tissue sample, because of baselines variations. To illustrate maximal efficacy, data were plotted as a scatter plot in the presence of 100 μM oxotremorine-M (Fig. 2 and Table 2). Overall, neither M1 efficacy nor potency differed across the three clinical diagnostic groups tested in this study. Cases with greater years of education tended to have higher functional activity as measured by the [35S]GTPγS assay (Spearman rank correlation, r = 0.44, p = 0.015). However, education level was not associated with clinical diagnosis (Table 1). Interestingly, M1 functional activity determined by GTP function was negatively correlated with both the Braak staging and NIA-Reagan criteria (r = −0.42 and −0.54, p = 0.022 and 0.0021, respectively); cases with more severe neuropathology showed lower M1 functional activity. On the other hand, M1 receptor function did not correlate with cognitive function based upon MMSE or GCS. These results remained essentially unchanged when the outlier in NCI group was excluded.
Figure 1.
Differences in muscarinic receptor function between different patient populations as measured by [35S]GTPγS binding to cortical membranes. Membranes were prepared as described in the Method section from the frontal cortices of the following patients: no cognitive impairment (x), mild cognitive impairment (□), or Alzheimer Disease (○). Approximately 5 μg protein was added to each well then stimulated with various concentration of oxotremorine-m. Percent stimulation was calculated by dividing the number of cpms over basal activity of each sample (wells receiving only buffer, membranes and radioligand). Each point represents 10 patients ran in duplicate and the data analyzed using GraphPad (GraphPad Prism 4.03). Non-linear regression was used to calculate the potency (ED50) and efficacy (Emax) of each patient population.
Figure 2.
Scatter showing levels of specific [35S]GTPγS binding across clinical diagnostic groups in cortical tissue homogenate stimulated with 100 μM Oxotremorine-M. Data were normalized against basal stimulation levels.
Table 2.
Summary of GTP-γ-35S binding levels: % specific binding over basal (averaged across duplicates)
| Clinical Diagnosis |
||||||
|---|---|---|---|---|---|---|
| NCI (N=10) | MCI (N=10) | AD (N=10) | Total (N=30) | P-valuea | ||
| % GTP binding | Mean ± SD (Range) | 26.9 ± 10.1b (16.4–51.3) | 24.1 ± 5.8 (12.8–32.9) | 21.0 ± 5.3 (9.0–28.9) | 24.0 ± 7.5 (9.0–51.3) | 0.4 |
Kruskal-Wallis test.
Case #3 showed exceptionally high binding levels.
Excluding this case: Mean ± SD = 24.2 ± 5.7, Range = (16.4–33.6), P-value = 0.5.
[3H]-Oxotremorine-M radioligand binding in frontal cortex in NCI, MCI, and AD
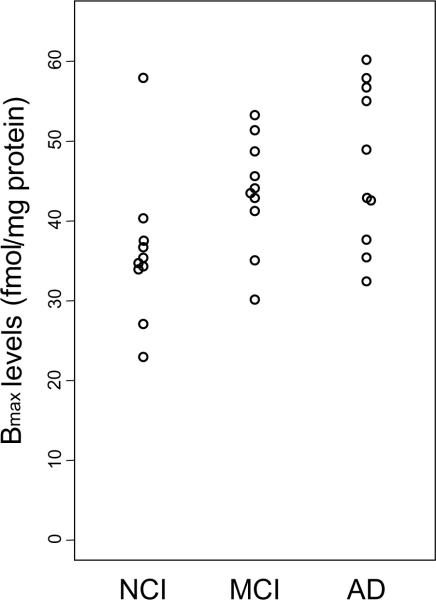
Changes in the relative concentrations of cortical muscarinic receptors across clinical populations were determined using concentrations of [3H]-Oxo-M that spanned the Kd and resulted in full saturation of the specific binding. Non-specific binding was defined as binding in the presence of a saturating concentration of atropine. The total receptor expression (Bmax) showed an increasing trend from NCI to MCI to AD (p = 0.008, Jonckheere-Terpstra test) with their respective mean ± SD = 36.1 ± 9.2, 42.5 ± 6.5, and 47.0 ± 10.1 fmol/mg protein (Fig. 3 and Table 3). Bmax for the AD group was significantly higher than NCI (p = 0.023, or p = 0.0055 after exclusion of the outlier in NCI), indicating a higher muscarinic receptor concentration. The correlation between Bmax and cognitive function did not reach statistical significance (r = −0.32 for MMSE and r = −0.33 for GCS, p = 0.09 for both). We also did not find an association between Bmax and the clinical and neuropathological variables examined (data not shown).
Figure 3.
Scatter plot showing differences in Bmax between human cortical membranes labeled with [3H]-Oxotremorine-M. The total concentration of receptor sites bound to [3H]-Oxotremorine-M at infinitely high concentration of agonist (Bmax) showed a significant increasing trend across the three groups (p = 0.008).
Table 3.
Summary of Bmax levels (averaged across duplicates)
| Clinical Diagnosis |
||||||
|---|---|---|---|---|---|---|
| NCI (N=10) | MCIb (N=9) | AD (N=10) | Total (N=29) | P-valuea | ||
| Bmax levels | Mean ± SD (Range) | 36.1 ± 9.2 (22.9–57.9) | 42.5 ± 6.5 (30.1–51.4) | 47.0 ± 10.1 (32.4–60.2) | 41.8 ± 9.7 (22.9–60.2) | 0.034 |
Kruskal-Wallis test; pair-wise comparison with Bonferroni correction showed NCI < AD.
Case #3 was not included in the statistical evaluation due widely different duplicate values
Discussion
Deficits in pre-synaptic cholinergic function in AD are well characterized and contribute significantly to the pathologic process of this disease. In fact, the pathology of cholinergic basal forebrain neurons is one of the best predictors of memory impairment in AD (Terry, et al., 1991; Samuel, et al., 1994). In the present study, we used a novel [35S]GTPγS binding/immunocapture method (DeLapp, et al., 1999; Bymaster, et al., 2003) to measure the functional activity of the M1 receptor during the progression of AD. It was previously shown using M1 (−/−) deficient mice that Gq-coupled PI hydrolysis was essentially lost in hippocampus and cortex, but not in the other two Gq-coupled muscarinic receptor subtypes (M3 and M5) (Bymaster, et al., 2003). We found that M1 functional activity was preserved; however, the amount of high-affinity M1 cortical receptors increased significantly between the NCI and AD groups. The present analysis was performed using tissue from a cohort of retired clergy who died with a clinical diagnosis based on an extensive series of cognitive tests obtained within 12 months proximate to death (Bennett, et al., 2002) and were not receiving cholinesterase inhibitors. This approach was not confounded by selection bias, which occur when subjects are categorized and subsequently chosen based on neuropathological criteria (DeKosky, et al., 2002). Although previous studies indicated that the subjects analyzed were diagnosed clinically and confirmed histopathologically (Araujo, et al., 1988; Aubert, et al., 1992), there remain differences in the stages of AD examined between the present and these early investigations. Most studies evaluated end stage AD cases, whereas in the present study the level of dementia was mainly mild to moderate AD. Although M1 receptor function did not correlate with cognitive function based upon MMSE or GCS, functional activity was negatively correlated with the severity of neuropathology determined by Braak staging and NIA-Reagan criteria for AD. In the present study, postmortem neuropathological evaluation revealed that 50% of the NCI and 30% of the MCI cases meet the NIA-Reagan criteria of an intermediate likelihood of AD. The discrepancy between clinical and neuropathological diagnosis in people without dementia has been reported previously in studies using ROS cases (Mufson, et al., 1997; Bennett, et al., 2002) as well as other cohorts (Mufson, et al., 1997; Bennett, et al., 2002; Price, et al., 2009). Recently, a multi-center study of non-demented aged people revealed that of the 97 cases examined, 41% were classified with a CERAD criteria of possible, probable, or definite AD whereas only 19% were classified with a NIA-Reagan criteria of intermediate to high AD (Price, et al., 2009). Together these findings suggest that there is a disconnect between current neuropathological and clinical criteria indicative of the onset of AD. Moreover, classic AD neuropathology by itself may not be a necessary precondition for the initiation of changes in brain chemistry including the response of the cholinergic receptor system during the early stages of the disease.
In the current investigation, we measured total high affinity muscarinic receptor density using the full agonist radioligand, 3H-oxotremorine-M which revealed a significant increase in the Bmax between the NCI and AD subjects indicating a rise in cortical muscarinic receptor concentration late in the disease process. By contrast other studies reported that cortical Bmax levels remained unchanged or decreased between control and AD cases (Smith, et al., 1987; Araujo, et al., 1988; Flynn, et al., 1991; Aubert, et al., 1992; Ladner, et al., 1995). The inconsistencies between the present and previous findings may be related to methodological and/or patient population differences. For example, previous studies used a different biochemical approach to assessed global M1 binding sites (Araujo et al., 1988) and different clinical and neuropathological criteria (Araujo et al., 1988; Aubert et al., 1992). Variability in methodology and patient populations reported in the literature are similar to inconsistencies found between muscarinic neuroimaging studies evaluating both muscarinic function and density in AD (Holman, et al., 1985; Weinberger, et al., 1991; Brown, et al., 2003; Kemp, et al., 2003; Pakrasi, et al., 2007). Moreover, interpretation derived from previous postmortem tissue studies are tempered by the fact that many of the procedures used have not been validated for their ability to selectively detect M1 receptors and may include measurement of additional muscarinic receptor subtypes. Previous investigations determined muscarinic density and affinity state using the modestly selective M1/M4 preferring antagonist, [3H]pirenzepine, in the presence and absence of non-hydrolyzable guanine nucleotide to quantify high vs. low affinity binding states. Furthermore, Ladner and Lee (1999) reported that loss of high affinity binding was agonist dependent and that oxotremorine-M affinity was unchanged between control and AD case using antagonist labeling techniques. The current study evaluated total muscarinic receptor high affinity agonist binding sites using [3H]oxotremorine-M. While [3H]oxotremorine-M is not a muscarinic receptor subtype selective radioligand, the agonist binding state is more likely relevant to the functional state of the receptor and served as a control for differences observed in the direct functional measure. In addition, we also observed an order of magnitude difference in the range of the NCI muscarinic receptor concentrations compared to earlier investigations which ranged from 300 to 500 fmol/mg protein in control frontal cortex (Araujo, et al., 1988; Aubert, et al., 1992) further reflecting the ratio of total, using [3H]pirenzepine vs. high affinity muscarinic receptor density. Moreover, prefrontal cortical brain areas are enriched in M1 and M4 receptor subtypes over M2, M3, and M5 receptors (Buckley, et al., 1988; Pearce and Potter, 1991).
Muscarinic cholinergic receptors activate G proteins of the Gi/o and Gq/11 subtypes that modulate the activity of second messengers and ion channels involved in learning and memory processes. Previous studies suggest that M2 muscarinic receptor coupling via Gi/o is preserved in AD brains (Cowburn, et al., 1992), but M1 receptor coupling to Gq/11 is reduced (Ladner, et al., 1995). Although the mechanisms contributing to a reduced coupling in AD remain unknown, it may relate to decreased levels of Gq/11 (Kelly, et al., 2005). In fact, Kelly et al demonstrated a significant relationship between the levels of cognition and synaptic plasma membrane Gq/11. The impact of chronic M1 agonist treatments such as AF102B (Nitsch, et al., 2000) and talsaclidine (Hock, et al., 2003) on AD has not been well-examined. Both drugs significantly decreased CSF Aβ in AD patients (Nitsch, et al., 2000; Hock, et al., 2003); however the clinical significance of these findings remains to be established. Preclinical studies showed that treatment with M1 agonist resulted in decreased CSF Aβ that was paralleled by decreased levels of cortical soluble Aβ (Beach, et al., 2001).
In the current investigation, there was a non- statically significant trend towards a loss of functional binding using the Gαq/11 [35S]GTPγS immunocapture assay to directly measure the function of predominantly M1 receptors. Perhaps, this is due to the small sample size examined in the present study or other factors. Even so, if there is a loss of Gαq/11 in AD, its impact was not significant enough to dramatically change muscarinic cholinergic receptor activation in the current study. Others have employed similar techniques to ensure that only M1-dependent activation was recorded in human postmortem tissues. In this regard, N-ethylmaleimide (NEM) was used to irreversibly uncouple cholinergic receptors from Gαi/o proteins (Salah-Uddin, et al., 2008). Under these conditions, Gαi/o-[35S]GTPγS binding that may have occurred under normal conditions was eliminated by the act of decoupling, thus resulting in the capture and quantification of [35S]GTPγS bound to the Gαq/11 subunit. However, it is not clear why NEM would be selective for Gi/o over Gq. In addition, these authors used MT-7, a putative selective M1 receptor toxin (Adem and Karlsson, 1997) to show that the M1 receptor accounted for more than 90 % of Oxo-M-stimulated Gαq/11-[35S]GTPγS binding (Salah-Uddin, et al., 2008). It is unlikely that selective M1 agonists or modulators will restore muscarinic signaling in AD if most M1 receptors are uncoupled and unresponsive to agonist binding (Ladner, et al., 1995). Conversely, it is possible that sufficient receptor reserve allows for stable M1 receptor function in the absence of a full complement of receptors.
To our knowledge, this is the first investigation exploring M1 function during the progression of AD using the [35S]GTPγS immunocapture assay showing that MCI cases were not significantly different compared to NCI or AD in either function. Our [35S]GTPγS binding experiments demonstrated that receptor function remains stable in AD compared with NCI despite the significant increase in the density of high affinity muscarinic receptors using 3H-oxotremorine-M. Although the mechanism(s) underlying the increase in M1 receptor concentration in the AD frontal cortex remains to be clarified, and it is possible that the higher M1 Bmax response found in AD may be due to relative brain shrinkage or loss of non-M1 bearing cells in the cortex; an up-regulation in response to afferent denervation is well established. Moreover, the observations that M1 level and function are preserved in MCI and that the M1 Bmax is increased in AD, support the observation that select components of the cortical cholinergic system are differentially affected in AD (Davies, 1999; Gilmor, et al., 1999; DeKosky, et al., 2002).
In conclusion, it is well established that Ach plays a necessary role in the learning and memory. For instance, animals that have specific cholinergic lesions replaced by modified graphs can restore learning and memory deficits (Nilsson et al, 1992). In addition, clinical data reveal that the use of cholineresterase inhibitors significantly improves cognitive measures, yet the overall clinical benefits are limited (Davis, et al., 1992; Farlow, et al., 1992). Our findings are particularly timely given recent in vivo and in vitro evidence that M1 agonists have the potential to modify the pathologic hallmarks of AD as well as deficits in cognitive function in transgenic animal models of this disease (Nitsch, et al., 1992; Genis, et al., 1999; Beach, et al., 2001; Caccamo, et al., 2006; Fisher, 2008). Further research will be required in order to determine whether therapeutics selective for the M1 receptor might be viable pharmacological targets for the treatment of AD.
Acknowledgments
This work was supported in part by TGA000257, PO1AG14449, and P50AG10161 We are indebted to the altruism and support of the hundreds of nuns, priests and brothers participating in the Religious Orders Study. A list of participating groups can be found at the website: http://www.rush.edu/rumc/page-R12394.html. We also thank all the members of the Rush ADC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures CCF, DAS, and YT are employees of Eli Lilly & Company. EJM consults and has stock in Ceregene.
References
- Adem A, Karlsson E. Muscarinic receptor subtype selective toxins. Life Sci. 1997;60:1069–1076. doi: 10.1016/s0024-3205(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2002;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer's disease. J. Neurochem. 1988;50:1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Aubert I, Araujo DM, Cecyre D, Robitaille Y, Gauthier S, Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J. Neurochem. 1992;58:529–541. doi: 10.1111/j.1471-4159.1992.tb09752.x. [DOI] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Beach TG, Walker DG, Potter PE, Sue LI, Fisher A. Reduction of cerebrospinal fluid amyloid beta after systemic administration of M1 muscarinic agonists. Brain Res. 2001;905:220–223. doi: 10.1016/s0006-8993(01)02484-2. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses' ratings of parkinsonian signs with a modified Unified Parkinson's Disease Rating Scale. Neurology. 1997;49:1580–1587. doi: 10.1212/wnl.49.6.1580. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown D, Chisholm JA, Owens J, Pimlott S, Patterson J, Wyper D. Acetylcholine muscarinic receptors and response to anti-cholinesterase therapy in patients with Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:296–300. doi: 10.1007/s00259-002-1028-6. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J. Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur. J. Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Cowburn RF, O'Neill C, Ravid R, Winblad B, Fowler CJ. Preservation of Gi-protein inhibited adenylyl cyclase activity in the brains of patients with Alzheimer's disease. Neurosci. Lett. 1992;141:16–20. doi: 10.1016/0304-3940(92)90324-z. [DOI] [PubMed] [Google Scholar]
- Davies P. Challenging the cholinergic hypothesis in Alzheimer disease. J. Am. Med. Assoc. 1999;281:1433–1434. doi: 10.1001/jama.281.15.1433. [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Davies P, Verth AH. Regional distribution of muscarinic acetylcholine receptor in normal and Alzheimer's-type dementia brains. Brain Res. 1977;138:385–392. doi: 10.1016/0006-8993(77)90758-2. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- DeLapp NW, McKinzie JH, Sawyer BD, Vandergriff A, Falcone J, McClure D, Felder CC. Determination of [35S]guanosine-5'-O-(3-thio)triphosphate binding mediated by cholinergic muscarinic receptors in membranes from Chinese hamster ovary cells and rat striatum using an anti-G protein scintillation proximity assay. J. Pharmacol. Exp. Ther. 1999;289:946–955. [PubMed] [Google Scholar]
- Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer's disease. The Tacrine Study Group. J. Am. Med. Assoc. 1992;268:2523–2529. [PubMed] [Google Scholar]
- Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer's disease. Neurotherapeutics. 2008;5:433–442. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- Flynn DD, Weinstein DA, Mash DC. Loss of high-affinity agonist binding to M1 muscarinic receptors in Alzheimer's disease: implications for the failure of cholinergic replacement therapies. Ann. Neurol. 1991;29:256–262. doi: 10.1002/ana.410290305. see comment. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatr. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro MA, Lopez de Jesus M, Ruiz de Azua I, Callado LF, Meana JJ, Salles J. Regulation of phospholipase Cbeta activity by muscarinic acetylcholine and 5-HT(2) receptors in crude and synaptosomal membranes from human cerebral cortex. Neuropharmacology. 2001;40:686–695. doi: 10.1016/s0028-3908(00)00206-9. [DOI] [PubMed] [Google Scholar]
- Genis I, Fisher A, Michaelson DM. Site-specific dephosphorylation of tau of apolipoprotein E-deficient and control mice by M1 muscarinic agonist treatment. J. Neurochem. 1999;72:206–213. doi: 10.1046/j.1471-4159.1999.0720206.x. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer's disease. J. Comp. Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke. 1997;28:307–310. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Rodriguez-Puertas R, Gabilondo AM, Meana JJ. Characterization of receptor-mediated [35S]GTPgammaS binding to cortical membranes from postmortem human brain. Eur. J. Clin. Pharmacol. 2000;390:25–36. doi: 10.1016/s0014-2999(99)00827-4. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Barton AJ, Najlerahim A, McDonald B, Pearson RC. Increased muscarinic receptor messenger RNA in Alzheimer's disease temporal cortex demonstrated by in situ hybridization histochemistry. Brain Res. Mol. Brain Res. 1991;9:15–21. doi: 10.1016/0169-328x(91)90125-h. [DOI] [PubMed] [Google Scholar]
- Hock C, Maddalena A, Raschig A, Muller-Spahn F, Eschweiler G, Hager K, Heuser I, Hampel H, Muller-Thomsen T, Oertel W, Wienrich M, Signorell A, Gonzalez-Agosti C, Nitsch RM. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer's disease. Amyloid. 2003;10:1–6. doi: 10.3109/13506120308995249. [DOI] [PubMed] [Google Scholar]
- Hollander M, Wolfe D. Nonparametric Statistical Methods. Wiley; New York: 1999. [Google Scholar]
- Holman BL, Gibson RE, Hill TC, Eckelman WC, Albert M, Reba RC. Muscarinic acetylcholine receptors in Alzheimer's disease. In vivo imaging with iodine 123-labeled 3-quinuclidinyl-4-iodobenzilate and emission tomography. J. Am. Med. Assoc. 1985;254:3063–3066. doi: 10.1001/jama.254.21.3063. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Storie K, Skamra C, Bienias J, Beck T, Bennett DA. Relationship between Alzheimer's disease clinical stage and Gq/11 in subcellular fractions of frontal cortex. J. Neural Transm. 2005;112:1049–1056. doi: 10.1007/s00702-004-0243-7. [DOI] [PubMed] [Google Scholar]
- Kemp PM, Holmes C, Hoffmann S, Wilkinson S, Zivanovic M, Thom J, Bolt L, Fleming J, Wilkinson DG. A randomised placebo controlled study to assess the effects of cholinergic treatment on muscarinic receptors in Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:1567–1570. doi: 10.1136/jnnp.74.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner CJ, Celesia GG, Magnuson DJ, Lee JM. Regional alterations in M1 muscarinic receptor-G protein coupling in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 1995;54:783–789. doi: 10.1097/00005072-199511000-00005. [DOI] [PubMed] [Google Scholar]
- Ladner CJ, Lee JM. Reduced high-affinity agonist binding at the M-1 muscarinic receptor in Alzheimer's disease brain: Differential sensitivity to agonists and divalent cations. Exp. Neurol. 1999;158:451–458. doi: 10.1006/exnr.1999.7116. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc. Natl. Acad. Sci. U S A. 1998;95:11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer's disease. Exp. Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, Saragovi HU, Kordower JH. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer's disease. J. Comp. Neurol. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Nordberg A, Hardy J, Wester P, Winblad B. Physostigmine restores 3H-acetylcholine efflux from Alzheimer brain slices to normal level. J. Neural Transm. 1986;67:275–285. doi: 10.1007/BF01243353. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer's disease. Ann. Neurol. 2000;48:913–918. [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Pakrasi S, Colloby SJ, Firbank MJ, Perry EK, Wyper DJ, Owens J, McKeith IG, Williams ED, O'Brien JT. Muscarinic acetylcholine receptor status in Alzheimer's disease assessed using (R, R) 123I-QNB SPECT. J. Neurol. 2007;254:907–913. doi: 10.1007/s00415-006-0473-8. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Potter LT. Coupling of m1 muscarinic receptors to G protein in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1991;5:163–172. doi: 10.1097/00002093-199100530-00002. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pittman J, Andrews H, Tatemichi T, Link B, Struening E, Stern Y, Mayeux R. Diagnosis of dementia in a heterogeneous population. A comparison of paradigm-based diagnosis and physician's diagnosis. Arch. Neurol. 1992;49:461–467. doi: 10.1001/archneur.1992.00530290043010. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr., Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin EH, Morris JC, Grant EA, Vendegna T. Very mild senile dementia of the Alzheimer type. I. Clinical assessment. Arch. Neurol. 1989;46:379–382. doi: 10.1001/archneur.1989.00520400033016. [DOI] [PubMed] [Google Scholar]
- Russell RW, Ehlert FJ, Hwa JJ. Relation between behaviorally augmented tolerance and upregulation of muscarinic receptors in the CNS: effects of chronic administration of chronic administration of scopolamine. Psychopharmacology (Berl) 1986;88:33–39. doi: 10.1007/BF00310509. [DOI] [PubMed] [Google Scholar]
- Rylett RJ, Ball MJ, Colhoun EH. Evidence for high affinity choline transport in synaptosomes prepared from hippocampus and neocortex of patients with Alzheimer's disease. Brain Res. 1983;289:169–175. doi: 10.1016/0006-8993(83)90017-3. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Shah F, Reid RT, Sue L, Connor DJ, Peterson LK, Beach TG. Pathologic and nicotinic receptor binding differences between mild cognitive impairment, Alzheimer disease, and normal aging. Arch. Neurol. 2006;63:1771–1776. doi: 10.1001/archneur.63.12.1771. [DOI] [PubMed] [Google Scholar]
- Salah-Uddin H, Thomas DR, Davies CH, Hagan JJ, Wood MD, Watson JM, Challiss RA. Pharmacological assessment of m1 muscarinic acetylcholine receptor gq/11 protein coupling in membranes prepared from postmortem human brain tissue. J. Pharmacol. Exp. Ther. 2008;325:869–874. doi: 10.1124/jpet.108.137968. [DOI] [PubMed] [Google Scholar]
- Samuel W, Terry RD, DeTeresa R, Butters N, Masliah E. Clinical correlates of cortical and nucleus basalis pathology in Alzheimer dementia. Arch. Neurol. 1994;51:772–778. doi: 10.1001/archneur.1994.00540200048015. [DOI] [PubMed] [Google Scholar]
- Scarr E, Keriakous D, Crossland N, Dean B. No change in cortical muscarinic M2, M3 receptors or [35S]GTPgammaS binding in schizophrenia. Life Sci. 2006;78:1231–1237. doi: 10.1016/j.lfs.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Iseki E, Uchiyama H, Watanabe Y, Haga T, Kameyama K, Ikeda T, Yamamoto T, Kosaka K. Alterations of muscarinic acetylcholine receptor subtypes in diffuse lewy body disease: relation to Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr. 1999;67:209–213. doi: 10.1136/jnnp.67.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Perry EK, Perry RH, Fairbairn AF, Birdsall NJ. Guanine nucleotide modulation of muscarinic cholinergic receptor binding in postmortem human brain--a preliminary study in Alzheimer's disease. Neurosci. Lett. 1987;82:227–232. doi: 10.1016/0304-3940(87)90135-2. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- The National Institute on Aging. R.I.W.G.o.D.C.f.t.N.A.o.A.s.D. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol. Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- Weinberger DR, Gibson R, Coppola R, Jones DW, Molchan S, Sunderland T, Berman KF, Reba RC. The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia. A controlled study with 123IQNB and single photon emission computed tomography. Arch. Neurol. 1991;48:169–176. doi: 10.1001/archneur.1991.00530140061018. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]