Abstract
A monoclonal antibody (mAb), P4A10, was made to the canine interleukin-2 receptor alpha chain (IL-2Rα; p55; Tac antigen; CD25) to facilitate studies of canine regulatory T-cells (Treg). By non-reduced western blot, P4A10 bound to a 55 kD protein, the expected size of IL-2Rα. In flow cytometry assays, it reacted with a minor population of circulating dog CD3+CD4+ T cells and the majority (>60%) of in vitro PMA-Ionomycin (PMA-IO)-activated canine CD3+ T-cells. P4A10 recognized a hematopoietic cell population enriched for FoxP3+ cells as measured by flow cytometry. The P4A10-selected fractions of T-cells had significantly increased copy numbers of CD25, FoxP3, IL-10, and TGFβ as detected by RT-PCR (reverse transcriptase PCR) compared to the negative fractions. The P4A10-selected cells inhibited 3H (tritiated) thymidine incorporation in a mixed leukocyte reaction (MLR) containing responders of the same origin. P4A10-selected T cells from fresh peripheral blood mononuclear cells had less FoxP3 (p = 0.07) by qRT-PCR (quantitative RT-PCR) and were less suppressive (p=0.01) than in vitro alloantigen-activated Treg. The mAb P4A10 is specific for canine CD25 and can be used to facilitate studies of CD25+FoxP3+ Treg in this clinically relevant large animal model.
Keywords: Monoclonal antibody, CD25, regulatory T cells, dog
1. Introduction
Monoclonal antibodies (mAb) specific to the IL-2Rα protein (CD25) have been useful tools for studies of CD4+CD25+FoxP3+ regulatory T-cells (Treg) in mice (Hori et al, 2003; Sakaguchi et al, 1995), rats (Nolte-'t Hoen et al, 2008), cats (Lankford et al, 2008), pigs (Kaser et al, 2008), monkeys (Gansuvd et al, 2007) and men (Arias et al, 2007; Walker et al, 2003; Walker et al, 2005). Although Tregs have been identified in dogs via intracellular staining of the Treg-associated protein FoxP3 using FJK-16s (Biller et al, 2007), a cross-reactive anti-murine mAb, this technique does not allow for selection of viable cells. Two recent reports (Galkowska et al, 1996; Masuda & Yasuda, 2008) of a cross-reactive human CD25-specific mAb, ACT-1, on activated dog T-cells noted that the binding was of intermediate affinity and ACT-1 was not specifically shown to bind to Treg. ACT-1, however, has remained largely uncharacterized (Schweighoffer et al, 1993). This antibody had not been clustered by an HLDA Workshop, the immunogen consisted of many possible antigens, the binding was of intermediate affinity on dog cells, and the development and characterization were not published. Therefore, to facilitate studies of Treg in the canine model of hematopoietic cell and organ transplantation, we developed and characterized a mAb specific to canine CD25 (P4A10). The mAb P4A10 bound to an approximately 55 kD protein by non-reduced Western blot and in flow cytometry experiments identified a subset of CD3+CD4+ T-cells enriched for FoxP3. Positive selection of cells from canine PBMC which bound the P4A10 mAb resulted in a cell population that was enriched with cDNA for CD25, FoxP3, IL-10 and TGFβ. Additionally, we showed that these P4A10-selected cells had a functional phenotype consistent with Treg as they inhibited the uptake of 3H (tritiated) thymidine in MLR.
2. Methods
2.1. Animals
Dogs were male and female beagle and beagle mix (basenji, golden retriever or mini-mongrel) ranging from 7 to 61 months old (median 16.5 months) and were obtained from the Animal Health Resources at the Fred Hutchinson Cancer Research Center (an AAALAC-certified facility). Adult male mice used for immunization were from Jackson Labs (stock number 00726). All blood draws and antibody production were done with IRO/IACUC approval.
2.2. Cloning
Monoclonal P4A10 was obtained via an adaptation of the protocol described by Oi and Herzenberg (Oi & Herzenberg, 1980). Our method makes use of the Robertsonian (Rb [8.12] 5 Bnr) mouse strain (RBF/DnJ) in conjunction with the FOX-NY myeloma cell line described by Taggart and Samloff (Taggart & Samloff, 1983) and has been published (Wayner & Hoffstrom, 2007). In particular, an enriched, stimulated-T-cell immunogen was prepared with PBMC obtained from heparinized, normal dog blood. The buffy coat from a 1.074 Ficoll density gradient was washed twice with Hank’s to remove platelets, and then monocytes were removed by adherence to plastic with the remaining T-cell-enriched mononuclear cells cultured in complete dog medium (CDM is 45% Waymouth’s, 45% Iscove’s, 10% heat inactivated, pooled, normal fasted male dog sera, and supplemented with sodium pyruvate, glutamine, non-essential amino acids, penicillin and streptomycin; Invitrogen-Gibco) containing 10µg/ml ConA (Sigma-Aldrich). After three days, cells were harvested, washed to remove serum, and a whole, live cellular immunogen was injected i.p. into two mice. The mice were boosted after 6 weeks with freshly prepared cells, and three days later, spleens were removed to obtain splenocytes for hybridoma creation. Resultant antibody-producing hybridomas were screened by flow cytometry of CD3-gated PBMC from freshly drawn dog blood compared to that same dog’s 3-day ConA-activated CD3-gated PBMC. Cells were first incubated in tissue culture supernatant, washed with HH (Hank’s buffered salt solution [Invitrogen] with 2% heat inactivated normal horse serum [Invitrogen] and 0.02% sodium azide [Sigma-Aldrich]), and then incubated with goat-anti-mouse Ig-FITC at 1:500 (Jackson ImmunoResearch) in HH. Cells were washed again and incubated with a biotinylated (BiotinTag; Sigma-Aldrich) CD3 (clone 17.6B3 or 17.6F9 from Peter Moore; UC Davis) in HH followed by another wash step with HH and incubation in HH with 1:100 streptavidin-PE (BD Immunocytometry Systems). After a last wash step in HH, cells were resuspended in HH and acquired by 2-color flow cytometry (FACScan; BD Immunocytometry Systems). Hybridomas producing antibodies that were reactive with most or all activated, but none or few resting, T-cells were cloned by limiting dilution and re-tested.
2.3. Western blot
Washed canine PBMC obtained via Ficolling 30 cc of dog blood were resuspended in 50 cc of CDM containing PMA and Ionomycin (IO) (at 100 ng/ml and 1 µg/ml, respectively; Sigma-Aldrich). Cells were cultured overnight in an upright T-75 flask (BD-Falcon) in 5% CO2 at 37°C. Non-adherent cells were decanted and pelleted at 300 rcf for 10 minutes, and then the pellet was resuspended with 50 cc of cold Hank’s balanced salt solution and re-spun. Wash solution was aspirated and the remaining pellet was lysed with 500 µl of M-PER Reagent (Pierce) containing 0.1mg/ml PMSF (Sigma-Aldrich) on ice for 10 minutes. Cells were pelleted at 4°C in a microcentrifuge at 13,000 rpm for 30 minutes, and the supernatant was transferred to a new tube. Protein concentration of the lysate was determined with a BCA Kit (Pierce) and 10 µg/lane was heated for 5 minutes at 95°C in Lane Marker Sample Buffer (Pierce) then used for 10% PAGE (NuPAGE; Invitrogen) with a pre-stained protein marker (BenchMark; Invitrogen). The gel was electroblotted (Sambrook et al, 1989) onto a reinforced nitrocellulose membrane (Hybond-N; Amersham). The blot was blocked for 30 minutes in 5% non-fat dried milk (Carnation) in PBS (Invitrogen) then cut in half. One part was incubated with an IgG1 negative control Mab and the other with P4A10; each antibody was diluted 1:500 (2 µg/ml) in blocking buffer. After washing with 0.1% Tween-20 (Sigma-Aldrich) in PBS (PBST), antibody bound to antigen was detected with a 30-minute incubation with a biotin-conjugated anti-mouse Ig diluted 1:200 in PBST. The blots were washed in PBST, and then a streptavidin-peroxidase complex (ABC Method; Vector Laboratories) was attached for 30 minutes. After a final wash in PBST, visualization was achieved via an enzymatic reaction employing a BCIP/NBT solution (1-Step NBT/BCIP; Pierce) and stopped by dunking the blots in water.
2.4. Flow cytometry
For identifying circulating cells, 100 µl of fresh heparinized dog blood was hemolyzed with 1 ml of ammonium chloride buffer (150 mM NH4Cl, 12 mM NaHCO3, 0.1 mM Na2EDTA; Sigma-Aldrich) in 1.7 ml microcentrifuge tubes for ten minutes and then pelleted at 3,500 rcf for 1 minute in a microcentrifuge. Cell pellets were resuspended in 100 µl HH and probed for cell surface markers with ACT-1-PE (DAKO) and/or canine antibodies to CD3, CD4, CD8 (clones 1E4 and JD3, respectively; Peter Moore at UC Davis),and/or P4A10, then allowed to interact for 20 minutes at room temperature. Antibodies were conjugated with FITC (FHCRC Biologics), PE (Prozyme), Alexa dyes 647 or 488 (Invitrogen), or biotin. Cells probed with a biotinylated antibody were washed with HH and incubated with 1:100 streptavidin PerCP (BD Immuno-cytometry Systems) in HH as a second step reaction. After all antibodies and secondary reagents were attached, cells were washed with 600 µl of HH in a microcentrifuge with 3,500 rcf spins for 1 minute, resuspended in 300 µl of HH, and then transferred into flow cytometry tubes (Falcon) for acquisition of up to 6 parameters. For antibody competitions, PBMC were prepared from 10 ml of heparinized dog blood via a 1.074 Ficoll density gradient and plated in CDM onto a 6-well plate (Falcon) at 5 ml per well and one million cells per ml. Cells were activated by adding PMA-IO and used the next day in flow cytometry antibody competition assays. Cells were harvested into microcentrifuge tubes, washed, and resuspended in 100 µl HH at 1 million per ml. After incubating with antibodies, cells were washed and then transferred to flow cytometry tubes (Falcon) and read in HH on a flow cytometer (FACSCalibur) using the manufacturer’s acquisition and analysis software (CellQuest; BD Immunocytometry Systems). For intracellular staining, a bulk MLR was prepared with PBMC from two dog leukocyte antigen (DLA) mismatched, unrelated dogs. Responders were incubated with gamma-irradiated stimulators for four days, which was previously found to be optimal, (Lesnikova et al, 2006; Lesnikova et al, 2005) and then harvested and surface stained before intracellular staining with cross-species reactive FoxP3 clone 150D (Biolegend) using the manufacturer’s kit and method. Specifically, after surface staining, the cells were pelleted in microcentrifuge tubes and resuspended in 500 µl Fix/Perm solution for 20 minutes. Cells were washed once with 600 µl HH, then again with 500 µl Perm Buffer, and then resuspended in 500 µl Perm Buffer and left at room temperature for 15 minutes. Cells were pelleted, resuspended in 200 µl of Perm Buffer and redistributed as two aliquots of 100 µl. One aliquot was stained with isotype control (IgG1κ-PE or Alexa 647; BD Immunocytometry Systems) and one was stained with FoxP3 (PE or Alexa 647). After 30 minutes at room temperature cells were washed twice with 600 µl HH per wash and resuspended in 500 µl HH for 4-color (6 parameter) flow acquisition (FACSCalibur; BD Immunocytometry Systems).
2.5. Quantitative reverse-transcribed (qRT) PCR
Primers were designed for qRT-PCR of canine FoxP3 from the human and murine FoxP3 consensus sequences and real time qRT-PCR was performed for canine FoxP3 as previously published (Nash et al, 2009) http://www3.interscience.wiley.com/journal/122410096/suppinfo). Primers, probes and amplification criteria for canine G3PDH (control), TGFβ and IL10 messages were from (Peeters et al, 2006). Quantitative RT-PCR was performed on cDNA from CD3+ T cells using Platinum qPCR Supermix-UDG with Rox kits (Invitrogen, Carlsbad, CA) in a real-time ABI 7900 thermocycler (Applied Biosystems, Foster City, CA). The conditions included two holds at 50°C and 95° for 2 minutes each, 45 cycles of 15 seconds at 95°C, and 1 minute at 60°C during which the fluorescence data was collected. Absolute copy numbers were calculated based on FoxP3, IL-10 or TGFβ standard curves, and samples were normalized to a second standard curve for the housekeeping gene G3PDH. Each sample was run in duplicate alongside negative controls to exclude genomic contamination.
2.6. Semi-quantitative reverse-transcribed (RT) PCR
CD25 primers were designed from the canine genome database and contained the following primer set: CD25 Forward 5′-CGCCGCCCATGGACGAAGTT-3′ and CD25 Reverse 5′-GACGCAGCTCGCCACTGCTA-3′. RT-PCR for CD25 was done on the same cDNA stocks from the four dogs that had been used to qRT-PCR for FoxP3, TGFβ and IL10. Amplification was done for 45 cycles at 58°C for 1 minute, 72°C for 1 minute and 94°C for 1 minute. Products were separated on a 1% agarose gel and stained with ethidium bromide.
2.7. Immunomagnetic selection
Cells were depleted of CD8 and positively selected with P4A10 from freshly prepared PBMC and from day 4 MLR containing irradiated DLA mismatched stimulators. CD8 antibody JD3 was added at 1 µg/100 million cells/ml, and P4A10 was added at 0.4 µg/100 million cells/ml in HH. Both antibodies were allowed to incubate for 20 minutes at 4°C on a rocker. After incubation, unbound antibody was washed away with HH and rat-anti-mouse IgG2a/2b beads were added at 500 µl/500 million cells/ml in HH and allowed to attach at 4°C for 20 minutes. Preps were then sorted on Deplete program on an AutoMACS (Miltenyi) and the negative fractions kept for P4A10 selection. Negative fractions were washed with HH and resuspended at 500 million cells/ml in HH and then anti-mouse-IgG1 magnetic beads (Miltenyi) were added at 500µl/500 million cells/ml in HH and rocked at 4°C for 20 minutes. Immunomagentically labeled cells were positively selected with program PoselD on an AutoMACS. Selected cells were counted, washed and resuspended at 1 million per ml in CDM for functional analysis and flow phenotyping QC.
2.8. Functional analysis
The suppressive ability of P4A10-selected T cells was measured by 3H thymidine incorporation in one-way MLR with responders from the same dog as the P4A10-selected T cells were obtained. PBMC collected in heparin from two DLA mismatched dogs were prepared as stimulators from 1.074 Ficoll density gradients. Cells were adjusted to 1 million per ml in CDM, and the stimulators were γ-irradiated with 25 Gray (GammaCell 3000 Elite; Atomic Energy of Canada) to prevent their proliferation. MLRs were plated in triplicates onto a 96-well round bottom plates (Falcon) with 100 µl each to achieve 100,000 responders and stimulators per well in a final volume of 200 µl. P4A10-selected T cells were then placed into the wells at increasing titers from 1:200 to 1:5 (P4A10-selected T cell:Responder) and the plate incubated at 37°C and 5% CO2 for 6 days. On day 6, 1 µCi of 3H thymidine (Perkin-Elmer) was added to each well, and cells were incubated for an additional 16 hours. MLRs were harvested onto a porous matrix (Perkin-Elmer), then dried and overlaid with MicroScint-20 scintillation fluid (Perkin-Elmer) for detection on a TopCount NXT automated plate reader (Perkin-Elmer). Suppression of allogeneic proliferation was detected as a decrease in 3H thymidine signal compared to MLR without added P4A10-selected T cells.
3. Results
3.1. P4A10 binds to an activation antigen
P4A10 was isotyped as IgG1. P4A10 reacted with 4.9 ± 2.1% (n=8) of CD3-gated cells in hemolyzed whole blood from normal dogs (data not shown), and activation of resting PBMC with PMA-IO resulted in the expression of the P4A10-related antigen on the majority (>60%) of lymphocytes within 24 hours (Figure 1a).
Figure 1.
a) Activation of canine PBMC with PMA-IO results in the appearance of P4A10-antigen on the majority of cells within 24 hours. All samples were stimulated at time zero in individual wells then harvested, stained and fixed for later acquisition. PBMC were gated by FSC and SSC to eliminate debris and back-gated in unstained FL-3 to eliminate auto-fluorescence. Percentages are above that of cells incubated in medium only, and data are representative of three separate experiments. P4A10 reacted with 4.9 ± 2.1% (n=8) of CD3-gated cells in hemolyzed whole blood from normal dogs (data not shown).
b) P4A10 recognizes a unique doublet at approximately 55 kD by western blot. Overnight PMA-IO-activated dog PBMC lysate was probed with either mouse IgG1 (left lane) or P4A10 (center lane). A non-specific common band is seen at approximately 116 kD in both blot strips.
3.2. P4A10 recognizes a unique doublet at approximately 55 kD by Western blot
Since P4A10 was elicited against whole activated cells and then screened against 3-day ConA-activated vs. freshly isolated PBMC containing non-activated canine T-cells, the antigen it recognized was unknown. To help identify the cognate antigen, a lysate from activated canine PBMC was probed with P4A10 using a non-reducing Western blot technique. This revealed a unique doublet with a molecular weight of 55 kD (Figure 1b).
3.3. P4A10 identifies cells expressing FOXP3 by flow cytometry
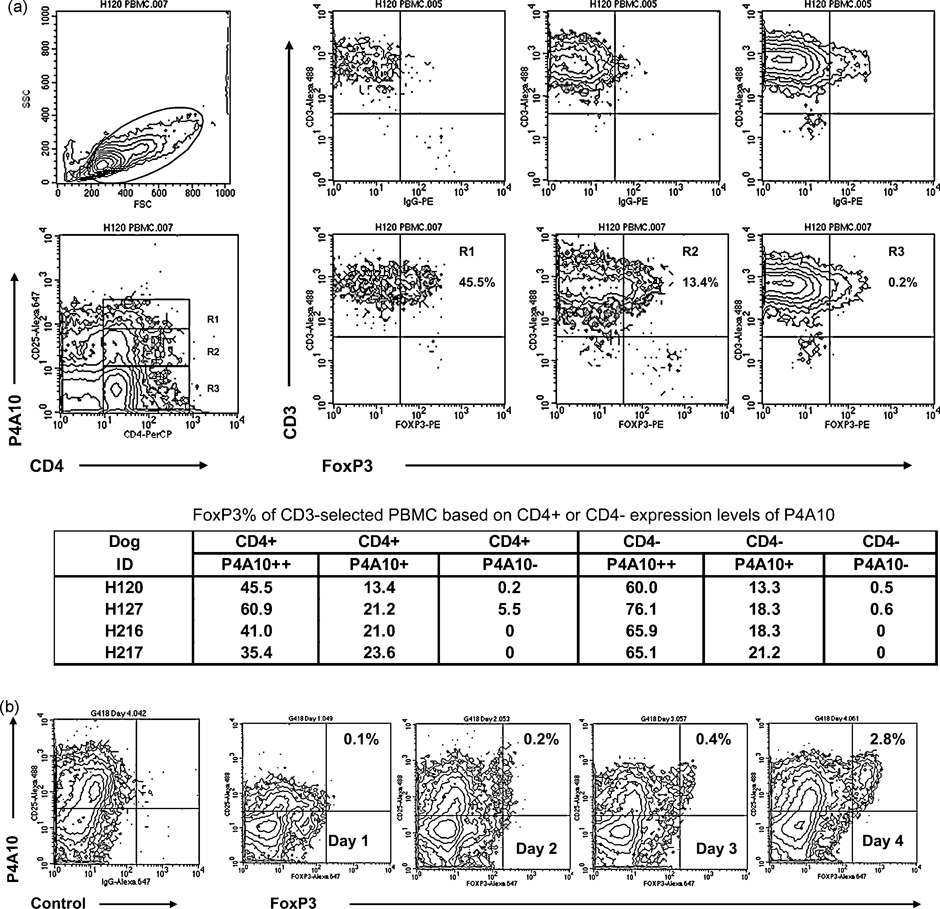
PBMC from two dogs were immunomagnetically selected with CD3 and surface stained with CD3, CD4 and P4A10 followed by intracellular staining with FoxP3. Total CD4+P4A10+ content was 6.9% and 7.5% with higher density P4A10 cells also staining for FoxP3 (Figure 2a). In a separate experiment, alloantigen activation via MLR resulted in increased expression of both FoxP3 and P4A10 with over 99% of FoxP3 bright cells also positive for P4A10 at day 4 (Figure 2b).
Figure 2.
a) P4A10Hi T cells in blood are highly enriched for cells expressing FoxP3. PBMC were obtained from fresh blood and immunomagnetically selected with CD3. T cells were not activated before selection. The P4A10-selected cells were then surface stained with CD3, CD4 and P4A10 prior to intracellular staining with FoxP3 clone 150D or isotype control. P4A10Hi T cells were highly enriched for FoxP3 compared to P4A10intermediate or P4A10Lo. This is consistent with observations in humans that CD4+CD25Hi T cells are enriched with cells expressing FoxP3. However, there were 3.4 +/− 2.2% (n=4) CD4−CD25+ cells also found in fresh CD3-selected PBMC. Flow phenotyping data from four dogs obtained in two experiments (presented in tabular form) shows that the CD4− cells were also more likely to be FoxP3 positive if they were also expressing the P4A10 antigen. Cells in Figure 2a were gated on CD4+ or CD4− and FoxP3 percentage as a function of P4A10 expression levels (R1, R2 or R3) is above that of the respective isotype control for FoxP3.
b) Emergence of FoxP3Hi cells during alloantigen activation. Reciprocal bulk MLRs were set up between two DLA-mismatched unrelated dogs and followed for four days. Cells were collected daily, stained for P4A10 then fixed for subsequent intracellular FoxP3 staining with clone 150D in a single acquisition. Cells were gated by FSC and SSC to include cells and blasts and also to back-gate out debris, then quadrants were set to isolate the Day 4 FoxP3 plus P4A10 double positive population vs. the Day 4 isotype control. Over 99% of FoxP3Hi cells were also positive for P4A10 in each of the two MLRs at Day 4 (data shown from one of two dogs).
3.4. P4A10-selection of activated T cells are markedly enriched for FoxP3 expression
Eight immunomagnetic selections with P4A10 were done on freshly prepared PBMC or 4-day alloantigen-stimulated PBMC from four normal dogs, and then cDNA was prepared and probed for CD25 and FOXP3. RT-PCR of CD25 showed an increased signal in the P4A10+ sorts of both fresh and stimulated PBMC as compared to the respective P4A10− sorts (Figure 3a). By qRT PCR, the P4A10+ cells had increased copy numbers of FoxP3, TGFβ and IL10 compared to the P4A10− cells from fresh PBMC (1429 ± 349 vs. 52 ± 48; p < 0.0001, 821 ± 230 vs. 100 ± 109; p = 0.029 and 93 ± 66 vs. 0 ± 0; p = 0.001 respectively; Figure 3b). P4A10-selected cells from alloantigen-activated cultures had a significantly increased copy number of FoxP3 compared to the P4A10− fractions, however no statistical differences in the TGF or IL-10 copy number were observed between the two fractions of activated T cells possibly due to large variabilities in the measurements (FoxP3 14413 ± 11795 vs. 4 ± 7, p = 0.05; TGFβ 15144 ± 19640 vs. 8 ± 16, p = 0.17; IL-10 182 ± 232 vs. 0 ± 0, p = 0.26). Activated P4A10-selected T cells tended to contain 1 log more FoxP3 copy number than those selected from fresh PBMC (14413 ± 11795 vs. 1429 ± 349 copies, p = 0.07; Figure 3c).
Figure 3.
a) P4A10+ cell fractions are positive for CD25 by PCR. Fresh PBMC from four different dogs were immunomagnetically depleted of CD8 then positively sorted with P4A10 into positive and negative fractions. Resultant sorts were enriched 60-fold in CD3+CD25+CD4+ cells compared to CD3+CD25+CD8+ cells. Copy DNA was prepared and amplified with primers specific for canine CD25 (and canine G3PDH, data not shown) and run out on an agarose gel then stained with ethidium bromide. There was only visible signal in the positive fractions: (left-to-right) MW marker, respective negative and positive P4A10 fractions for dogs G401, G819, G653 and G368. b) Expression of FoxP3, IL-10 and TGFβ in P4A10 positive and negative cell fractions before and after activation in MLR. Copy number (Y-axis), as compared to the signal from 100,000 copies of G3PDH in a titration curve established by qPCR, of the P4A10+ fractions of the same fresh sorts from Figure 3a) had significantly increased copy numbers of FoxP3 (p<0.0001), IL-10 (p=0.001) and TGFβ (p=0.029) over the respective P4A10− fractions. T-cells from these same four dogs that had been activated in a mixed leukocyte reaction (MLR) and then P4A10-selected, not only contained more FoxP3 message than the negative activated fractions (p=0.05), but tended to have more FoxP3 than the fresh (p=0.07) P4A10+ fractions when normalized to 100,000 copies of housekeeping gene G3PDH from activated vs. fresh cell sorts. Bars represent normalized copy number means ± 1 SD.
c) P4A10+ T cells inhibit tritiated thymidine uptake in MLR. Functional analysis of P4A10-selected T cells showed that they markedly inhibited uptake of tritiated thymidine and that MLR-activated P4A10+ cells inhibited uptake almost twice as much as fresh non-activated P4A10+ cells (p=0.01 for both 1:10 and 1:20). Bars represent means ± 1 SD.
3.5. P4A10-selected T cells are inhibitory consistent with a Treg phenotype
As Treg had been demonstrated to inhibit 3H thymidine incorporation in MLR, P4A10-selected cells from the experiment above were titered into primary MLRs containing responders of the same origin as the P4A10-selected cells. Inhibition of 3H thymidine incorporation was observed with P4A10-selected cells from both sources; however, more suppression was seen with the activated P4A10-selected cells than with those selected from fresh PBMC (Figure 3c). At a ratio of 1:10 (Treg:responder), alloantigen-stimulated P4A10-selected cells inhibited 3H thymidine incorporation in MLR an average of 72.8 ± 5.5% compared to an average of 44.3 ± 8.3% suppression observed when adding cells selected from fresh PBMC (p=0.01). At a ratio of 1:20, primed cells suppressed the MLRs 81.8 ± 6.2% vs. 39.5 ± 11.2% for unprimed cells (p=0.01).
3.6. P4A10 competes with human mAb ACT-1 on activated dog T-cells
Since ACT-1 was reported as CD25-specific and had been reported to stain canine ConA- and PHA-activated lymphocytes (Galkowska et al, 1996; Masuda & Yasuda, 2008), co-staining experiments with P4A10 and ACT-1 were conducted. As seen in Figure 4a, when added together at appropriate concentrations, P4A10-FITC and mAb ACT-1-PE co-stained PMA-IO-activated canine PBMCs. In order to achieve equivalent co-staining with ACT-1, a 1/400 concentrate of P4A10 was required, and less than twice the concentration of P4A10 as ACT-1 could completely reduce the fluorescent signal of ACT-1-PE to background. In this competitive flow cytometry assay, we were unable to eliminate the fluorescent signal of P4A10-FITC using mAb ACT-1 conjugated to PE. To determine if competition existed for the same epitope, unconjugated P4A10 was added after binding ACT-1-PE to PMA-IO-activated dog T-cells. Figure 4b shows low concentrations of P4A10 successfully competing with PE-conjugated antibody ACT-1.
Figure 4.
a) P4A10 and ACT-1 recognize the same T cell subset. P4A10 co-staining with cross-reactive human CD25-specific clone ACT-1 on PMA-IO activated canine PBMC. P4A10-FITC (vertical axis) at 0.01 µg/ml and ACT-1-PE (horizontal axis) at 4 µg/ml were added individually (left and middle panels, respectively) or together (right panel). Data are representative of five experiments.
b) Unconjugated P4A10 competes with the PE-conjugated ACT-1 for cell surface CD25 on PMA-Ionomycin-activated dog T-cells. ACT-1-PE (X-axis) at 1.6 µg/ml and CD3-FITC (Y-axis) were added first in all samples and then unconjugated P4A10 was added at 0.5 and 1 µg/ml in the middle and right panels, respectively. The data shown represent one of three dogs from a single experiment.
4. Discussion
A mAb specific for canine CD25 was developed for use in pre-clinical experiments. The antigen to which the mAb binds had a molecular weight of 55kD, the reported molecular weight of the IL2Rα protein (CD25) (Leonard et al, 1982). The doublet observed in the Western may represent both soluble and membrane-bound IL2Rα (Morris & Waldmann, 2000), differential glycosylation of the protein (Leonard et al, 1982), or perhaps an alternatively spliced isoform (Leonard et al, 1982). The characterization of antibody P4A10 was also done in part by comparing it to the human CD25 mAb (ACT-1) even though ACT-1 had not been well-characterized. In combination with other data that we obtained on the phenotype and function of T-cells selected by P4A10, we concluded that P4A10 is specific for canine CD25.
We have observed that only a small percentage of CD3+ T cells in the peripheral blood of a normal dog are CD25+ and potentially have a Treg phenotype. Most Tregs in normal dogs are found in the lymph nodes and spleen (Nash et al, 2009; Biller et al, 2007). After activation of canine T-cells from the peripheral blood in MLR, we confirmed that there was a marked increase in the percentage of CD25+ T cells (Masuda & Yasuda, 2008). Activation of T-cells in MLR also resulted in a 10-fold increase of FoxP3 and TGFβ copy number but not IL-10. T-cells expressing IL-10 (Tr1) have been described as having significant regulatory functions and appear to be distinct from FoxP3+ regulatory cells that constitutively express CD25 (Battaglia et al, 2006; Roncarolo & Gregori, 2008). The lack of increased expression of IL-10 in canine CD25+ T cells activated in MLR would suggest that a canine equivalent of Tr1 did not contribute to the increased regulatory activity we observed in the functional analysis.
CD8-depleted, CD25+-selected canine T-cells have in vitro regulatory activity. Activation of the T-cells in MLR not only increased levels of FoxP3 copy number in CD3+ T cells but also made these cells more suppressive when added back to a MLR (Walker et al, 2003; Walker et al, 2005). The ability to CD8-deplete and CD25-select to obtain an enriched cell population of Treg will facilitate studies of ex vivo expanded Treg in a transplantation model. While P4A10 was being developed, our group also did studies in which ACT-1 was successfully used to phenotype canine T-cells by flow cytometry as well as for immunomagnetic selection of T-cells including Treg after activation in MLR for ex vivo Treg expansion with canine artificial APC (Lesnikova et al, 2006; Lesnikova et al, 2005). We are continuing these studies of the canine Treg with the P4A10 mAb.
P4A10 could also be potentially used as an immunomodulatory agent for treatment of dogs with autoimmune or non-infectious inflammatory diseases or in an experimental model of hematopoietic cell or solid organ transplantation. Two agents, basiliximab and daclizumab, are specific for human CD25 and are used for prevention of acute rejection after solid organ transplantation in humans without increasing the risk for opportunistic infections (Vincenti et al, 1998; Nashan et al, 1997). Efficacy has also been demonstrated or suggested for some non-infectious inflammatory disorders such as asthma, multiple sclerosis or uveitis (Busse et al, 2008; Rose et al, 2007; Yeh et al, 2008; Bielekova et al, 2004). The mechanism of action may involve inhibition of proinflammatory cytokines by the IL-2R blockade of activated T-cells. IL-2R blockade with daclizumab was not effective treatment for ulcerative colitis (Van Assche et al, 2006). Elimination of Treg by these agents may potentially compromise the overall effectiveness of this treatment for non-infectious inflammatory diseases. However, even where this was observed, inflammation resulting from the primary disease process may still be substantially reduced (Oh et al, 2009). It has also been proposed that CD25-specific mAb could be successfully used as an anti-tumor agent for those hematopoietic malignancies expressing CD25 (Waldmann, 2007).
Competition between P4A10 and ACT-1 for binding a shared epitope on canine CD25 was observed on canine leukocytes, but CD25-specific antibodies recognizing similar epitopes and even of the same isotype (ACT-1 and P4A10 are both mouse IgG1) may have different biological effects (Wijdenes et al, 1992). An important factor in the effectiveness of antibody immunotherapies is that the antibody bioactivity may be decreased in the settings where the target antigen exists in a soluble form (Hagg & Junghans, 1998). The human IL2 α receptor exists as a 45 kDa soluble form (sIL2Rα) and is present in serum during allograft rejection (Morris & Waldmann, 2000). Elevated levels of sIL2Rα are also biomarkers of tumor load that can be targeted with anti-CD25 antibody therapy (Bien & Balcerska, 2008). Therefore, a high affinity antibody would be needed to overcome this barrier. The humanized CD25 antibody RA8 was being selected for further studies in part because of its low dissociation constant (Arias et al, 2007). P4A10 is potentially such an antibody as its affinity was seen to be substantially greater than that of ACT-1.
In summary, using mitogen-activated dog T-cells as an immunogen, we have developed a mouse-derived mAb (P4A10) that recognizes canine CD25. P4A10 can be efficiently used for the identification and selection of CD25+ canine Treg. Selection and evaluation of functional canine Treg is an important step for the understanding of their biological properties in disease or in the canine model of allogeneic hematopoietic cell or solid organ transplantation.
Acknowledgments
We thank G.K. Venkataraman for his analytical expertise. We wish to thank the AHR staff for great care of the dogs and in particular Alix Joslyn, and Michele Spector, DVM. We also thank Helen Crawford, Bonnie Larson, Karen Carbonneau and Sue Carbonneau for their help in preparing the manuscript and for their administrative support.
Supported in part by NIH grants AI69879, DK42716 and CA15704 from the NIH, DHHS, Bethesda, MD.
Non-Standard Abbreviations
- CDM
Complete Dog Medium
- DLA
Dog Leukocyte Antigen
- 3H
Tritiated
- HH
2% Horse Serum in Hank’s Balanced Salt Solution
- IO
Ionomycin
- qRT-PCR
quantitative Reverse Transcriptase-PCR
- RT-PCR
Reverse Transcriptase-PCR
- sIL2Rα
soluble IL2Rα
- Treg
regulatory T-cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement:
The authors of this report have no financial or personal relationships with other people or organizations that could inappropriately influence (bias) this work. The work was funded through the National Institutes of Health and the authors were solely responsible for the study design, the collection, analysis and interpretation of data as well as writing the manuscript.
References
- Arias RS, Flanagan ML, Miller KD, Nien YC, Hu P, Gray D, Khawli LA, Epstein AL. RA8, a human anti-CD25 antibody against human Treg cells. Hybridoma. 2007;26:119–130. doi: 10.1089/hyb.2006.0041. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application (Review) Seminars in Immunology. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Richert N, Howard T, Blevins G, Markovic-Plese S, McCartin J, Frank JA, Wurfel J, Ohayon J, Waldmann TA, McFarland HF, Martin R. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta [erratum appears in Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17565] Proceedings of the National Academy of Sciences. 2004;101:8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien E, Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review (Review) Biomarkers. 2008;13:1–26. doi: 10.1080/13547500701674063. [DOI] [PubMed] [Google Scholar]
- Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Veterinary Immunology and Immunopathology. 2007;116:69–78. doi: 10.1016/j.vetimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, Vexler V, Shames RS. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine. 2008;178:1002–1008. doi: 10.1164/rccm.200708-1200OC. [DOI] [PubMed] [Google Scholar]
- Galkowska H, Waldemar LO, Wojewodzka U. Reactivity of antibodies directed against human antigens with surface markers on canine leukocytes. Veterinary Immunology and Immunopathology. 1996;53:329–334. doi: 10.1016/S0165-2427(96)05604-8. [DOI] [PubMed] [Google Scholar]
- Gansuvd B, Asiedu CK, Goodwin J, Jargal U, Deckard LA, Andrades P, Guarcello V, Thomas JM. Expansion of CD4+CD25+ suppressive regulatory T cells from rhesus macaque peripheral blood by FN18/antihuman CD28-coated Dynal beads. Human Immunology. 2007;68:478–490. doi: 10.1016/j.humimm.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg DS, Junghans RP. Measurement and biological correlates of antibody bioactivity during antibody immunotherapies. Journal of Immunological Methods. 1998;219:7–21. doi: 10.1016/s0022-1759(98)00096-9. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Kaser T, Gerner W, Hammer SE, Patzl M, Saalmuller A. Phenotypic and functional characterisation of porcine CD4(+)CD25(high) regulatory T cells. Veterinary Immunology and Immunopathology. 2008;122:153–158. doi: 10.1016/j.vetimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Lankford S, Petty C, LaVoy A, Reckling S, Tompkins W, Dean GA. Cloning of feline FOXP3 and detection of expression in CD4+CD25+ regulatory T cells. Veterinary Immunology and Immunopathology. 2008;122:159–166. doi: 10.1016/j.vetimm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WJ, Depper JM, Uchiyama T, Smith KA, Waldmann TA, Greene WC. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982;300:267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lesnikova M, Nikitine A, Mason N, Nash RA, Georges GE. Ex vivo expanded T regulatory (Treg) cells block conversion of mixed chimeras to complete donor chimerism. Blood. 2006;108(Part 2):382b. #5168.(Abstract) [Google Scholar]
- Lesnikova M, Nikitine A, Pogosov L, Mason N, Nash RA, Georges GE. Ex vivo expansion of canine CD4+CD25+ regulatory T cells (Treg) with artificial antigen presenting cells (aAPC) Blood. 2005;106(Part 1):867a. #3102.(Abstract) [Google Scholar]
- Masuda K, Yasuda N. The antibody against human CD25, ACT-1, recognizes canine T-lymphocytes in the G2/M and G0/G1 phases of the cell cycle during proliferation. Journal of Veterinary Medical Science. 2008;70:1285–1287. doi: 10.1292/jvms.70.1285. [DOI] [PubMed] [Google Scholar]
- Morris JC, Waldmann TA. Advances in interleukin 2 receptor targeted treatment (Review) Annals of the Rheumatic Diseases. 2000;59 Suppl. 1:i109–i114. doi: 10.1136/ard.59.suppl_1.i109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash RA, Yunusov M, Abrams K, Hwang B, Castilla-Llorente C, Chen P, Farivar AS, Georges GE, Hackman RC, Lamm WJE, Lesnikova M, Ochs HD, Randolph-Habecker J, Ziegler SF, Storb R, Storer B, Madtes DK, Glenny R, Mulligan MS. Immunomodulatory effects of mixed hematopoietic chimerism: immune tolerance in canine model of lung transplantation. American Journal of Transplantation. 2009;9:1037–1047. doi: 10.1111/j.1600-6143.2009.02619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients [erratum appears in Lancet 1997 Nov 15;350(9089):1484] Lancet. 1997;350:1193–1198. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- Nolte-'t Hoen EN, Boot EP, Wagenaar-Hilbers JP, van Bilsen JH, Arkesteijn GJ, Storm G, Everse LA, van Eden W, Wauben MH. Identification and monitoring of effector and regulatory T cells during experimental arthritis based on differential expression of CD25 and CD134. Journal of Leukocyte Biology. 2008;83:112–121. doi: 10.1189/jlb.0607436. [DOI] [PubMed] [Google Scholar]
- Oh U, Blevins G, Griffith C, Richert N, Maric D, Lee CR, McFarland H, Jacobson S. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Archives of Neurology. 2009;66:471–479. doi: 10.1001/archneurol.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi VT, Herzenberg LA. Immunoglobulin-producing hybrid cell lines. In: Mishell BB, Shiigi SM, editors. Selected Methods in Cellular Immunology. San Francisco, CA: W.H. Freeman; 1980. p. 351. [Google Scholar]
- Peeters D, Peters IR, Clercx C, Day MJ. Quantification of mRNA encoding cytokines and chemokines in nasal biopsies from dogs with sino-nasal aspergillosis. Veterinary Microbiology. 2006;114:318–326. doi: 10.1016/j.vetmic.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? (Review) European Journal of Immunology. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of Immunology. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, Lazarovits AI, Buck D, Shaw S. Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with Hallmarks of gut-trophism. Journal of Immunology. 1993;151:717–729. [PubMed] [Google Scholar]
- Taggart RT, Samloff IM. Stable antibody-producing murine hybridomas. Science. 1983;219:1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Sandborn WJ, Feagan BG, Salzberg BA, Silvers D, Monroe PS, Pandak WM, Anderson FH, Valentine JF, Wild GE, Geenen DJ, Sprague R, Targan SR, Rutgeerts P, Vexler V, Young D, Shames RS. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor (CD25), for the treatment of moderately to severely active ulcerative colitis: a randomised, double blind, placebo controlled, dose ranging trial. Gut. 2006;55:1568–1574. doi: 10.1136/gut.2005.089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, Backman L, Burdick J. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. New England Journal of Medicine. 1998;338:161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma (Review) Oncogene. 2007;26:3699–3703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25− cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. Journal of Clinical Investigation. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner EA, Hoffstrom BG. Development of monoclonal antibodies to integrin receptors (Review) Methods in Enzymology. 2007;426:117–153. doi: 10.1016/S0076-6879(07)26007-0. [DOI] [PubMed] [Google Scholar]
- Wijdenes J, Roy C, Morel-Fourrier B, Racadot E. Monoclonal antibodies in human organ transplantation and auto-immune diseases (Review) Therapie. 1992;47:283–287. [PubMed] [Google Scholar]
- Yeh S, Wroblewski K, Buggage R, Li Z, Kurup SK, Sen HN, Dahr S, Sran P, Reed GF, Robinson R, Ragheb JA, Waldmann TA, Nussenblatt RB. High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, non-infectious uveitis. Journal of Autoimmunity. 2008;31:91–97. doi: 10.1016/j.jaut.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]