Abstract
Methylphenidate is a psychostimulant widely used in the treatment of attention deficit hyperactivity disorder (ADHD). Here we report a novel paradigm that affords inferences about habituation and attention to a novel stimulus in a familiar environment in a single test session without prior training of the animals. The paradigm was used to assess the effects of methylphenidate (2.5 and 5.0 mg/kg, sc) in young adult, male, Long-Evans rats. Methylphenidate increased locomotor activity during the initial exposure to the test apparatus in a non-dose-related manner. However, upon introduction of a novel spatial stimulus (an alcove) in the familiar environment, methylphenidate-treatment resulted in dose-related increases in distance traveled and inhibition of long dwell times in the alcove, the latter behavior being characteristic of vehicle-treated rats’ response to the alcove condition. These results demonstrate the utility of this paradigm in the elucidation of the behavioral effects of a drug commonly used in the treatment of ADHD. Findings also suggest that species-typical response preferences in rats (e.g., refuge-seeking) may emerge in experimental settings that add spatial novelty to otherwise featureless test enclosures commonly used to assess locomotor activity.
Keywords: spatial stimulus, novelty, attention, actometer, methylphenidate, rat
1. Introduction
Methylphenidate, the most widely used drug in the treatment of attention deficit hyperactivity disorder (ADHD), increases synaptic availability of dopamine by blocking reuptake; in addition, the drug blocks the reuptake of norepinephrine (Kaplan et al., 1998). These effects on brain monoamines appear to ameliorate some of the frontolimbic dysfunction believed to underlie ADHD (Filipek et al., 1997; Lou et al., 1989; Zametkin et al., 1990), and thus improve attention (e.g. time on task) and consequently decrease general activity (e.g., “fidgeting”) in individuals with the disorder.
Although there are several well established tests of attentional function in rodents, such as the sustained attention task (Brockel and Fowler, 1995; Skjoldager and Fowler, 1991), which is analogous to the continuous performance task used in humans (Chee et al., 1989; Kornetsky, 1972), these tests require considerable training of the animals. Accordingly, there is a need for methods that enable evaluation of drug treatments and other manipulations on attention-related behaviors in a single test session without prior training, as well as tests that assess other aspects of attention-related functioning. Based on the observation that individuals with ADHD exhibit higher levels of activity in familiar environments than in novel environments (Sagvolden and Sergeant, 1998; Sleator and Ullmann, 1981), we designed an exploration/habituation/spatial change paradigm that affords inferences about habituation and attention to a novel stimulus in a familiar environment in a single test session that does not require prior training of the animals.
In this report, we present the effects of methylphenidate on the behavior of normal young adult rats in our paradigm, thereby demonstrating the utility of this paradigm to elucidate effects of a drug used in the treatment of ADHD. The results also suggest that species-typical response preferences in rats (in this case, “seeking refuge”) may emerge in experimental settings that add spatial novelty to otherwise featureless test enclosures commonly used to assess locomotor activity.
2. Materials and methods
All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Kansas Medical Center Animal Care and Use Committee.
2.1. Animals and husbandry
Young adult (postnatal day 70–72), male, Long-Evans rats (n = 13 per group; each from a different litter) were bred in-house from breeding stock obtained from Harlan (Indianapolis, IN) as part of a larger diet study. Rats were group-housed (3 per cage) in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle (on at 06:00 h). Except during the behavioral assessment sessions, rats had ad libitum access to chow (AIN-93G; Teklad, Indianapolis, IN) and water. Rats were weighed regularly and were accustomed to handling prior to the commencement of experiments.
2.2. Apparatus
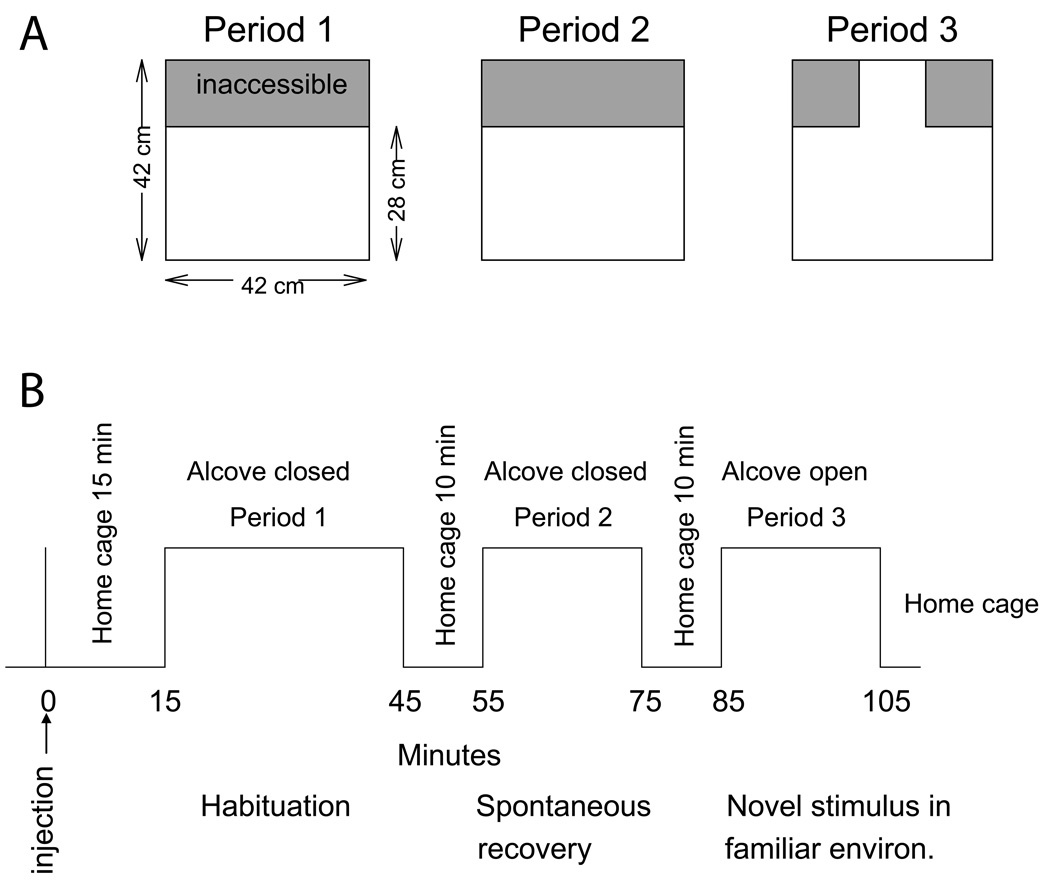
Modified force-plate actometers (Fowler et al., 2001) were used to assess response to environmental spatial change. The modified plexiglass actometer chamber consisted of a “main” compartment (28 × 42 cm) and a small “alcove” (14 × 14 cm) separated from the main compartment area by a removable guillotine-type door (Fig. 1). Spatial resolution of the actometer was less than 2 mm and temporal resolution was 0.02 sec.
Fig 1. Schematics of the experimental apparatus (top) and test protocol (bottom).
2.3. Procedures
All testing occurred between 10:00 h and 13:00 h and was conducted under dim, indirect lighting conditions. Rats were transported to the testing room in their home cages 1 hr prior to testing. Activity and response to spatial change in the environment were measured during a 3-stage, 90-min session in the modified force-plate actometer chambers (Fig. 1). Rats were injected with methylphenidate (2.5 or 5.0 mg/kg, sc in a volume of 1 ml/kg; Sigma-Aldrich, St. Louis, MO) or saline vehicle (1 ml/kg) and returned to the home cage. These doses were selected because they were reported to induce locomotion, but not stereotypies (Costall and Naylor, 1975; Gupta et al., 1971; Marriott, 1968), findings which we confirmed in a pilot study (data not shown). Fifteen min after injection, each rat was placed in the main compartment (Period 1 - Habituation) with the door to the alcove closed. After 30 min, the rat was removed from the main compartment, placed in the home cage for 10 min, and then returned to the unchanged main compartment (Period 2 – Probe for spontaneous recovery) for 20 min. After Period 2, the rat was again removed from the main compartment to the home cage for 10 min, and then returned to the main compartment with the door to the alcove open (Period 3 - Novel stimulus in familiar environment) for 20 min. Movement of the rat in the main compartment and alcove was automatically quantified by the x–y coordinates and Fz forces derived from the force plate. Analyzing the Fz data (total vertical force) at 50 samples/sec allowed for the quantification of variation in force during all movements (i.e., both locomotor and non-locomotor behaviors).
2.4. Data analysis
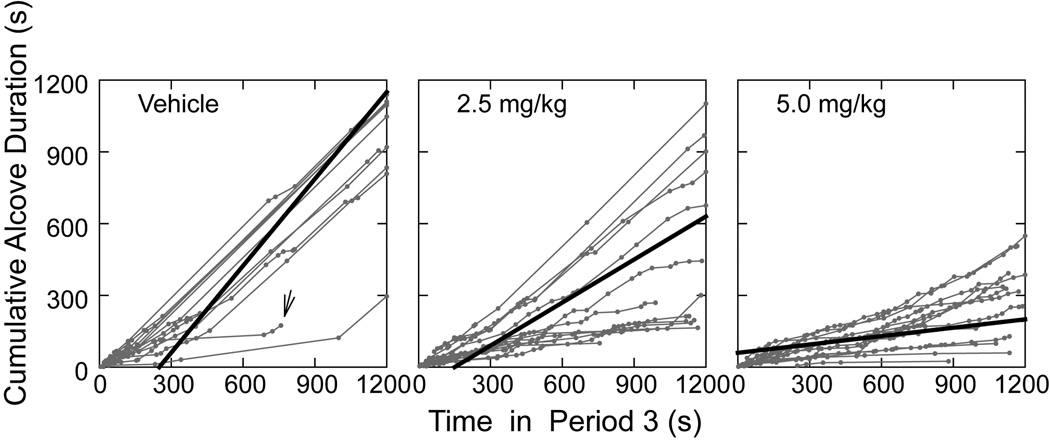
Data are generally presented as group means ± SEM. The dependent variables used to describe behavior were distance traveled, number of low mobility bouts, number of alcove visits, alcove visit durations, and the variation of the vertical or Fz forces during Period 3. An alcove visit was defined as the center of force coordinates of the rats falling within the alcove spatial limits for 0.5 sec or more, followed by center of force coordinates being located outside the alcove space for 0.5 sec or more. Alcove visit duration was the time between the defined entry and exit criteria. The data analysis depicted in Fig. 5 involved the calculation of a separate linear regression coefficient for each of the 39 rats where cumulative alcove visit duration was the dependent variable and elapsed time within Period 3 was the independent (“predictor”) variable. The resulting slopes were subjected to a one-way ANOVA. Group means of these slopes are plotted as the thick lines in Fig. 5. This approach weights each subject equally, whereas a regression for all the aggregated data for the 13 rats in each group does not yield equal weighting. For any of the analyses, when data exhibited evidence of substantially unequal error variances, as is often the case for latency or response duration measures, the data values were subject to log10 transformation before ANOVA procedures were performed. Data were analyzed for statistically significant effects by a variety of ANOVA designs, Tukey’s HSD, paired and non-paired t-tests using SYSTAT v.10.2 (Systat Software, Inc, Chicago, IL). Significance was assumed at P<.05.
Fig. 5. Cumulative alcove occupancy duration as a function of elapsed time in Period 3 for vehicle control and methylphenidate dose groups.
Gray symbols and gray connecting lines show individual rat data. Each individual data point marks the end of a visit or the presence of the rat in the alcove when Period 3 ended. The thick black lines indicate mean lines of best fit reflecting the rate of accumulation of alcove occupancy time during Period 3. The slope for the vehicle group was significantly (P<.01) steeper than the slopes for the two methylphenidate groups. In the plot for vehicle rats, the arrow indicates a rat that was not in the alcove when Period 3 ended, but instead was continuously located a few mm outside the alcove (as determined by inspection of its movement trajectory plot). On the x-axis, the scale markers 300, 600, 900, and 1200 s correspond to the successive 5-min blocks 15, 16, 17, and 18, respectively, of Period 3 in Fig. 2.
3. Results
The first part of this section reports the effects of behavioral manipulations and methylphenidate treatment on distance traveled. The second part of the analysis focuses on additional dependent variables that provide a detailed view of the behavioral responses induced by alcove exposure and how methylphenidate influenced those responses to the novel spatial stimulus.
3.1. Distance traveled
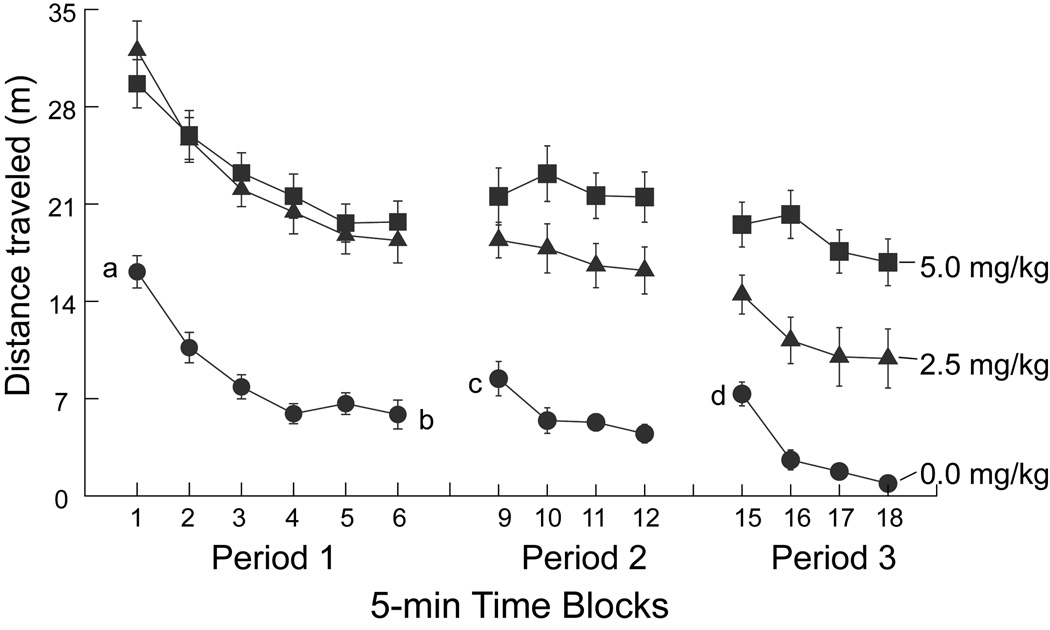
The effects of methylphenidate on distance traveled were assessed across the three observation periods in 5-min time blocks (Fig. 2). During Period 1 (initial habituation), there was a significant main effect of methylphenidate treatment (F2,36=47.761, P<.01), with the 2.5 and 5.0 mg/kg doses of methylphenidate producing similar increases in distance traveled compared to saline (P<.01 for both comparisons); however, the effects of 2.5 and 5.0 mg/kg dose were not statistically different from each other during Period 1. Distance traveled declined significantly across 5-min time blocks, (F5,180=82.812, P<.01), with all groups exhibiting initial high levels of exploratory activity during the first time block and gradually decreasing activity across the next 5 time blocks. In this initial period, there was no interaction between dose and time block.
Fig. 2. Dose-response effects of methylphenidate on distance traveled outside the alcove as a function of 5-min time blocks within Periods 1, 2, and 3.
In Periods 1 and 2 only space outside the alcove was available for occupancy by the rats. Data are presented as the group means ± SEM (n =13 per group). When error bars are not visible, the size of the plot symbol was greater than 2 SEM. Lower case alphabetic characters (a, b, c, d) represent specific means for 5-min blocks that were compared for the control group (0.0 mg/kg).
Although a variety of drugs that increase extracellular striatal dopamine (amphetamine, cocaine, methylphenidate, etc.) can induce a focused stereotypy syndrome that suppresses locomotion, analysis of low-mobility bouts (1 bout is a rat staying within a 4 cm diameter circle for 10 sec continuously) suggested that focused stereotypy did not contribute to the similarity in the effects of the 2.5 and 5.0 mg/kg doses of methylphenidate on distance traveled during Period 1. The number of low-mobility bouts was statistically indistinguishable for these two groups (group means ± SEM were 16.23 ± 2.61 and 16.69 ± 4.65 for 2.5 mg/kg and 5.0 mg/kg groups, respectively). Furthermore, the numbers of low-mobility bouts in both methylphenidate groups were substantially and significantly (P<.01) fewer than the number of low-mobility bouts observed in the vehicle-control group (99.00 ± 7.36) in which low mobility arose from habituation processes and not from drug treatment. Thus, these observations are not consistent with induction of stereotypy at either methylphenidate dose. Accordingly, the possibility of focused stereotypy playing role in the data beyond Period 1 was not further entertained because drug elimination would make the emergence of locomotion-inhibiting focused stereotypy even less probable in Periods 2 and 3 than in Period 1.
To demonstrate that within-period habituation (Leussis and Bolivar, 2006) had occurred as expected for the vehicle-control group, a post hoc paired t-test compared time blocks 1 and 6 in Period 1 (data points labeled a and b in Fig. 2). Confirming this expectation, examination of individual subject data showed that all vehicle-control rats exhibited lower distance traveled at point b than at point a (t12=7.544, P<.01). A decrease in distance traveled across time blocks was also observed for both methylphenidate-treated groups, despite stimulation of locomotor activity by the drug. The difference in initial activity between groups, expressed as the percent decrease in distance traveled between time blocks 1 and 6 in Period 1, indicated that the 5 mg/kg group exhibited less habituation than controls (−35% and −73%, respectively, P<0.01).
During Period 2, there was a significant main effect of methylphenidate dose on distance traveled (F2,36=37.633, P<.01). The decline in distance traveled across time blocks was shallow but significant (F3,108=4.077, P<.01), but time block and dose did not interact significantly. Both methylphenidate groups exhibited significantly more activity than the vehicle group (P<.01 for both comparisons); however, unlike Period 1, the difference between the 2.5 and 5.0 mg/kg groups was significant (P<.05). In addition, re-placement of the rats into the test chamber for Period 2 after a 10-min period in the home cage, resulted in greater locomotor activity in vehicle control rats during the first time block of Period 2 (point c in Fig. 2) compared to the habituated behavior observed for the last time block of Period 1 (point b in Fig. 2) (t12=1.994, P<.05, one tailed directional hypothesis). This comparison was not significant for either of the two methylphenidate dose groups indicating that the drug-related hyperlocomotion interfered with the expression of spontaneous recovery.
During Period 3, when the alcove was available, distance traveled was defined on the basis of movements outside the alcove, as was the case for distance traveled in Periods 1 and 2. Movements inside the alcove were characterized by an analysis of visits to the alcove and variation in force movement as described below (3.2). Given the alcove’s relatively small size (14 cm × 14 cm) and the high resolution of the recording instrument, it was thought that distance traveled inside the alcove would not be a suitable metric for measuring behavior inside the alcove. This choice did not preclude an examination of the effect of alcove availability on distance traveled outside the alcove. The main effect of methylphenidate dose on distance traveled outside the alcove remained significant, (F2,36=31.741, P<.01) in Period 3. The time block effect within Period 3 was also significant (F3,108=29.996, P<.01). However, unlike the case in Periods 1 and 2, the interaction between dose group and time block was significant, (F6,108=3.464, P<.01). Furthermore, the difference in distance traveled outside the alcove between the 2.5 and 5.0 mg/kg groups remained significant (P<.01) and was more pronounced than in Period 2. In addition, in the vehicle group, introduction of the novel spatial stimulus appears to have induced re-exploration of the now familiar main compartment as evidenced by the fact that habituation between Period 2 and Period 3 did not occur [i.e., data point d, which shows the distance traveled only in the main compartment floor area, would be expected to be lower than point c if between-period habituation had occurred, but this was not the case (P>.10 by paired t-test, directional hypothesis)].
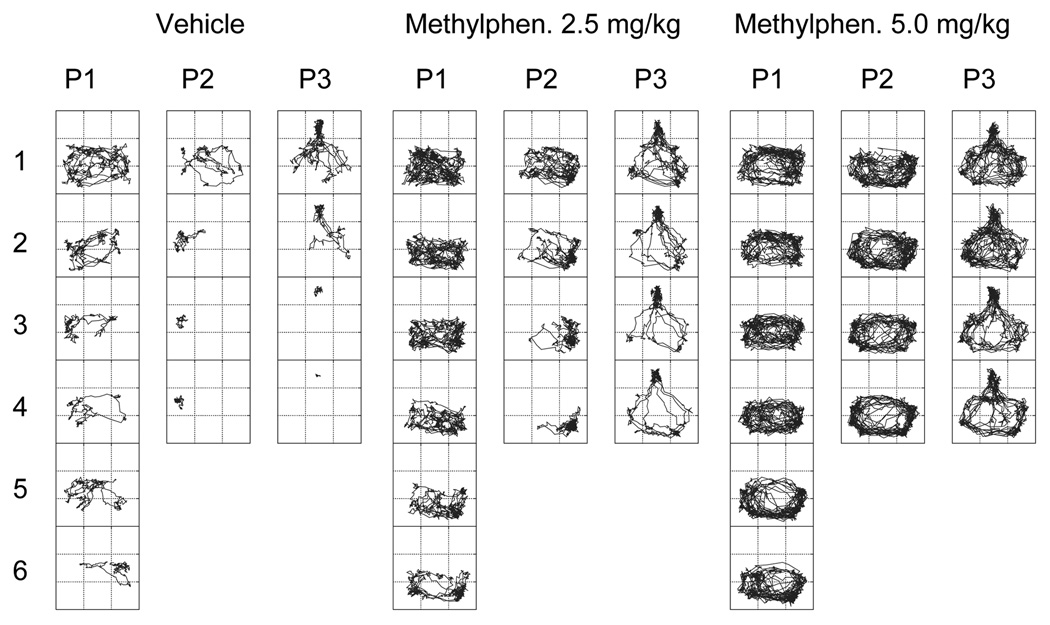
Tracings of movement trajectories within the actometer across the three periods are shown in Fig. 3 for one representative rat from each of the three methylphenidate dose groups. These patterns of movement are in accord with the statistical analyses based on distance traveled. However, these tracings of the rats’ use of space show that the distance traveled measure, as a behavioral descriptor, neglects important information about a rats’ response to methylphenidate. For example, Fig. 3 shows that the vehicle rat spent the entire 4th 5-min time block of Period 3 inside the alcove, while the two methylphenidate rats shared time between the alcove and the space outside the alcove during the corresponding time block.
Fig. 3. Movement trajectories of individual rats shown in 5-min blocks within each of three successive periods.
Representative movement trajectories are presented for three different rats from each of the treatment groups. Numbers 1 through 6 along the left side of the figure represent successive 5-min time blocks. Note that the back 1/3 of the chamber was inaccessible in Periods 1 and 2 (see Fig. 1). Inaccessible regions can be discerned by noting that no tracings are present in those regions (back third of the chamber in Periods 1 and 2, and left and right back corners in Period 3).
3.2. Alcove derived measures of behavior
3.2.1. Number and duration of alcove visits
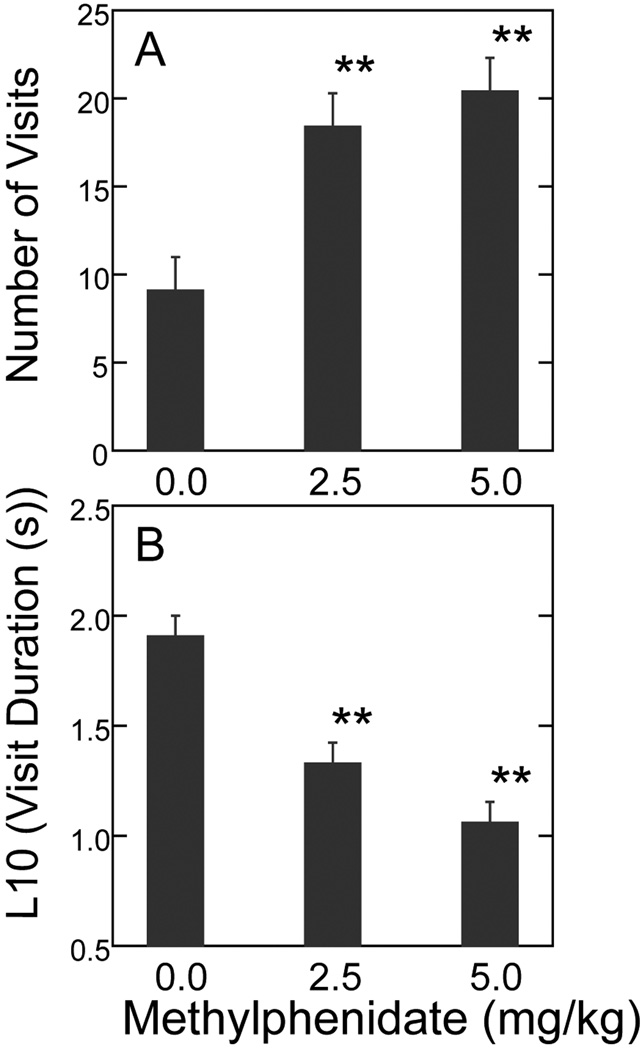
Data in Fig. 4 (Panel A) show that the number of alcove visits was significantly higher for methylphenidate-treated rats than for the vehicle controls (F2,36=12.000, P<.01); however, post hoc analysis did not detect any difference between the two doses of methylphenidate. Panel B in Fig. 4 shows that the methylphenidate-treated rats displayed mean visit durations that were significantly shorter than those of the controls (F2,36=7.521, P<.01). Again, post hoc comparisons for visit duration did not detect a reliable difference between the two dose groups.
Fig. 4. Number of alcove visits (Panel A) and mean alcove visit duration (Panel B) for Period 3 as a function of methylphenidate dose.
Data are presented as the group means ± SEM (n = 13 per group). ** P<.01 vs. vehicle control by Tukey’s post hoc test.
3.2.2. Visit duration as a function of time
The data presented so far suggest that vehicle-treated rats spent more time in the alcove than in the main compartment compared to methylphenidate-treated rats, but these data are not directly informative on the patterning of visits across Period 3. To elucidate how visit durations changed as a function of the passage of time in Period 3, we plotted cumulative alcove visit durations as a function of elapsed time in Period 3 (Fig. 5, gray points and gray lines connecting the points for each rat). This is analogous to an operant response cumulative record, except that the y-axis is not cumulative number of visits. Instead the ordinate is cumulative visit duration. For each rat, a linear regression was performed to compute the slope of the linear relation between accumulating alcove dwell time and elapsed time within Period 3. The steeper the slope, the higher the rate of accumulation of time in the alcove. Plots of the records for each subject give an indication of the number of visits (each data point marks the end of an alcove visit). The regression coefficients (13 coefficients for each dose group) were subjected to a one-way AVONA, which indicated a significant drug treatment effect, F(2,36)=14.193, P<.01. Post hoc analyses showed that the methylphenidate groups had slopes significantly lower than the control group (P<.01, both comparisons), but the two drug groups were not significantly different from each other. Therefore, with this analysis we were able to show that alcove occupancy grew much faster with time for the vehicle group than for the drug groups
3.2.3. Latency to enter the alcove and early visit durations
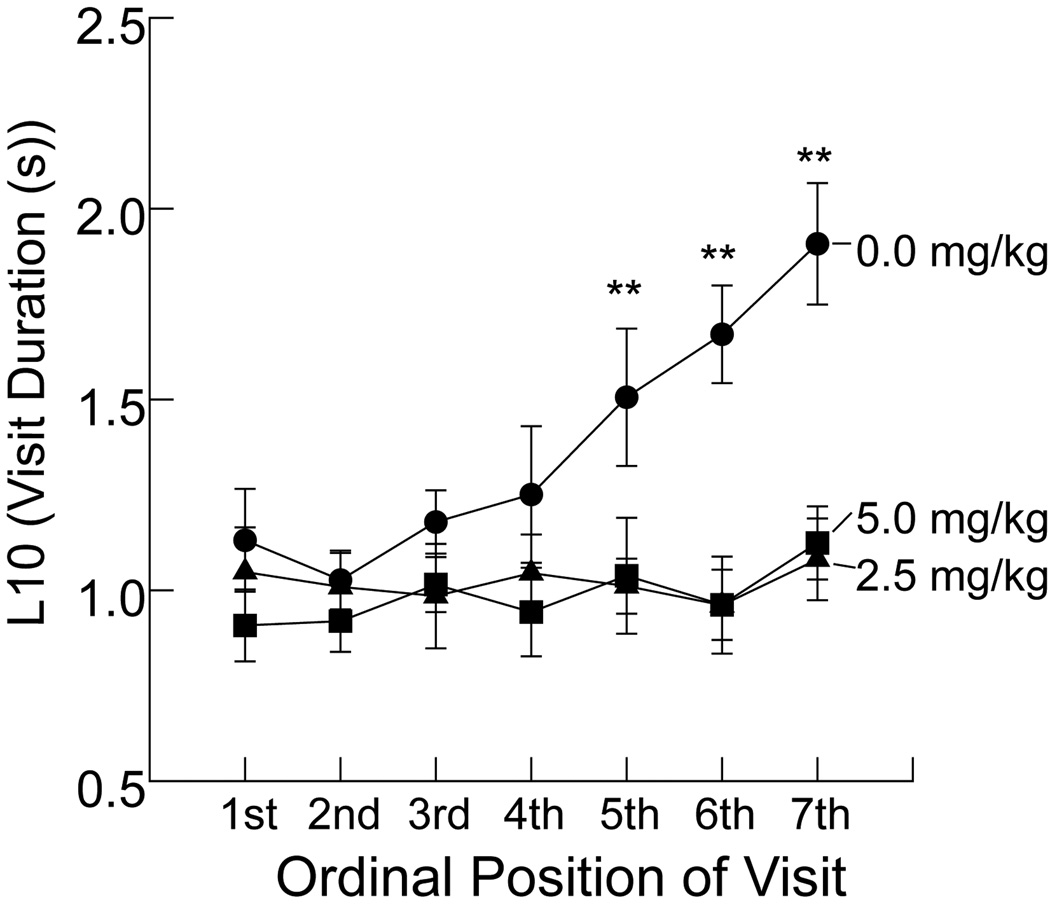
Although the analysis represented in Fig. 5 showed that alcove visit time grew much faster for rats in the vehicle group compared to the methylphenidate group, it does not provide detail about the first few visits. Accordingly, this subset of the data was examined with additional analyses. By one-way ANOVA, latency to enter the alcove during the initial time block of Period 3 did not differ significantly among the three groups. The group means for the control, 2.5 mg/kg, and 5.0 mg/kg groups were (in s) 5.1 (±1.5), 11.2 (±3.0), and 13.7 (±4.0), respectively. Discovery of the alcove was approximately equally quick by the 3 groups (but with a trend for methylphenidate to delay entry into the alcove). Fig. 6 provides group averages for the first seven alcove visits in Period 3. Seven was chosen because it was a practical limit beyond which there was a large amount of missing data for the vehicle group which overall had a mean number of seven visits (see Fig. 4, panel A). A two-way repeated measures ANOVA (with group means imputed for a total of 5 cells in the vehicle group for the 6th and 7th visits) indicated significant drug treatment, ordinal position, and interaction effects with test statistics of F(2, 36)=10.449, P<.01; F(6, 211)= 4.712, P<.01; and F(6,211)=2.665, P<.01, respectively. Post hoc analysis (t-test, vehicle versus the two drug groups combined) showed that the vehicle group first significantly diverged from the methylphenidate groups by the 5th visit. (t37=2.802, P<.01). Thus, the beginning of the 5th alcove visit can be seen as the time in Period 3 when the vehicle-treated rats began to show a significant preference for occupying the alcove over the main compartment; the geometric mean (i.e., antilog of the mean Log10 times) marking the beginning of the 5th visit was 198.2 s (±42.0). Taken together, these analyses of the early alcove visits suggest that on the average there were no significant methylphenidate effects until after the 4th visit, and this was about the time that the vehicle rats began to express long-duration visits.
Fig. 6. Group average alcove visit duration as a function of ordinal position of visits in Period 3.
Note that the log10 scale value of 1.0 on the ordinate indicates 10 s and 2.0 represents 100 s. Data are presented as group means ± SEM. ** P<.01 vs. vehicle control by Tukey’s post hoc test.
3.2.4. Force variability in the alcove
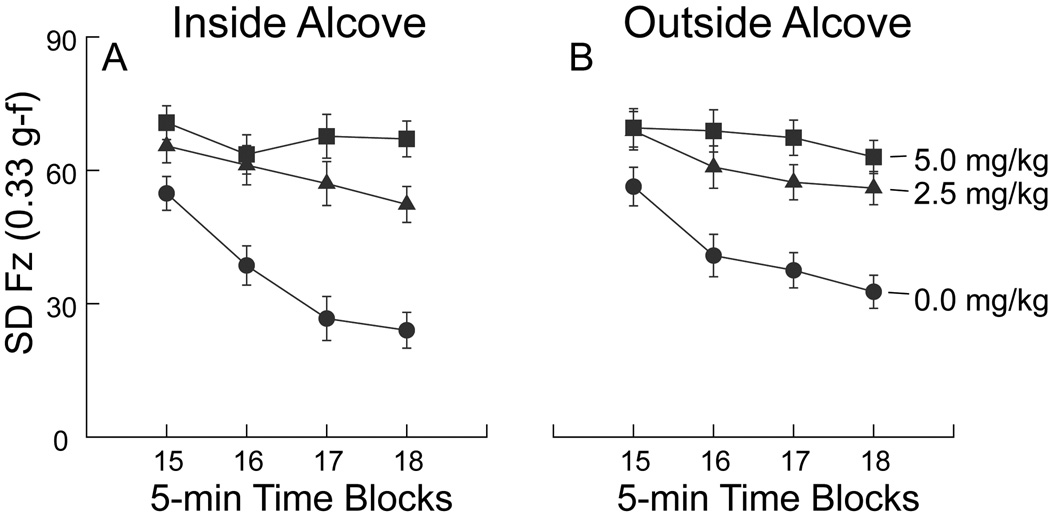
With the force-plate method of measuring a specimen’s location (x–y coordinate at each instant), the force information and the position information are independent mathematically. This is why the same force plate actometer can be used to track the movements of a rat or a mouse (Fowler et al., 2001). Analysis of the force data provides information about reactive force production during movement. Such force measures can be used to operationalize concepts such as strength or relaxation of movements even if the specimen is “staying in one place”. Accordingly, the standard deviation of force variation in Period 3 was used as a dependent variable to examine further the impact on behavior of the alcove and methylphenidate manipulations. For data collected while rats were inside the alcove (Fig. 7, Panel A), ANOVA detected significant effects of methylphenidate dose (F2,36=21.234, P<.01), time block (F3,108=17.137, P<.01), and an interaction of time block-by-treatment (F6,108=5.497, P<.01). Outside the alcove (Fig. 7, Panel B), the methylphenidate dose effect was significant (F2,36=15.599, P<.01), as was the time block effect (F3,108=12.720, P<.01). However, the interaction effect was not significant. Post hoc analyses did not detect differences between the 2.5 and 5.0 mg/kg doses either inside or outside the alcove. Post hoc analyses further indicated that the SD of Fz was significantly higher for both drug groups than the vehicle-control group both inside and outside the alcove. These data showed that methylphenidate treatment led to higher reactive force levels than those exhibited by the vehicle-control group and that reactive forces can be used to chart the course of habituation to a novel environmental event when levels of locomotion are already relatively low. The sample raw force data presented in Fig. 8 are generally in accord with the analyses based on the group data.
Fig. 7. Variation in vertical force as a function of 5-min block and location within the force plate actometer during Period 3.
Data are presented as group means ± SEM (n = 13 per group). “SD” in the ordinate label is an abbreviation for “standard deviation.” Sample raw data for Fz time series are shown in Fig. 8.
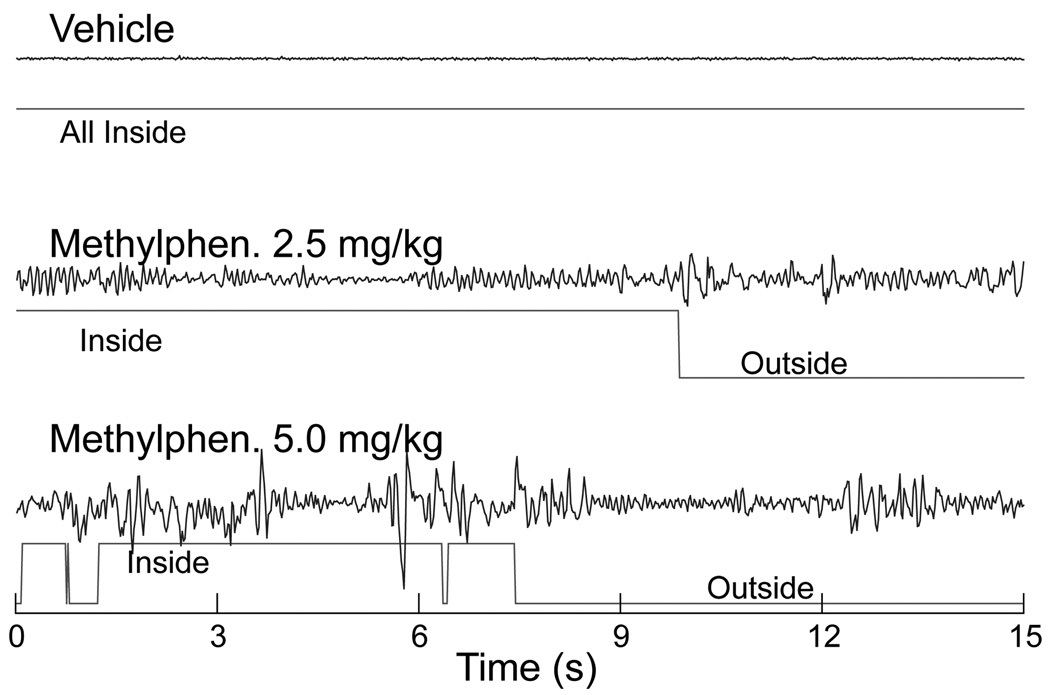
Fig. 8. Representative time series of vertical force variation of Fz over the first 15 s of the 4th time block of Period 3.
Data are from the same three rats whose data are shown in Fig. 3. Force data from the load plate were collected at 50 samples/sec. Inside (event line “up”) and outside (event line “down”) refer to spatial categories in the actometer (inside or outside of the alcove) where the rat was located when these data were gathered. Raw data such as these served as the basis for the quantitative assessment of force output depicted in Fig. 7
4. Discussion
The novel behavioral paradigm described here enables the assessment of exploratory behavior, habituation, and response to a spatial change in a familiar environment in a single test session and requires no prior training of the animals. The behavioral changes occurring during habituation of the exploratory response represents an elementary form of behavioral plasticity involving learning, memory, arousal level, and attention (Berlyne, 1969; Leussis and Bolivar, 2006), processes highly relevant for assessing the effects of psychoactive drugs, particularly psychostimulants. In addition, the test enables assessment of within-period habituation, which involves 1) learning in the form of gaining familiarity with a novel environment; 2) adaptation in the form of between-period habituation, which involves memory of the prior period (Leussis and Bolivar, 2006); and 3) response to spatial change.
Methylphenidate increases synaptic availability of dopamine and norepinephrine by blocking reuptake (Kaplan et al., 1998). In rats, methylphenidate has a proportionately larger effect on increasing extracellular norepinephrine in the dorsal hippocampus compared to the elevation of extracellular dopamine produced in the nucleus accumbens (Kuczenski and Segal, 2001, 2002). In agreement with previous studies in rodents (Costall and Naylor, 1975; Gupta et al., 1971; Marriott, 1968), the present findings indicate that, at the doses tested, methylphenidate increased locomotion, but did not induce stereotypy. Overall, the effects of methylphenidate were more dose-related later, compared to earlier, in the in the 90-min assessment procedure. In addition, methylphenidate significantly increased the amplitude of reactive force variation during movements.
Although the force-plate technology records the force variations that accompany grooming and wall rearing, two behaviors often targeted in locomotor activity studies of CNS stimulants (e.g., Wooters and Bardo, 2009), quantifying these specific behaviors was not attempted in this project. Nevertheless, by looking at relevant literature, it may be informative to consider whether or not the methylphenidate-related results for the SD of Fz data may have been influenced by grooming and rearing. In the dose range used here, it is unlikely that the observed methylphenidate-related increase in force variability (Fig. 7) was a reflection of increases in either grooming or wall rearing. After a single methylphenidate dose of 5.6 mg/kg, sc., Wooters and Bardo (2009) reported an insignificant increase in rears and a non-significant decrease in amount of grooming (data derived from video-taped observations on male Sprague-Dawley rats from the same supplier we used). Preliminary data from our laboratory (Fowler et al., 2009) showed that 2.5 mg/kg d-amphetamine sulfate significantly increased amplitude of force changes during runs covering 45 cm in 1.5 s in a 0.8 × 0.8 m force-plate actometer. Given that both amphetamine and methylphenidate block norepinephrine transporters as well as dopamine transporters, and given that norepinephrine increases spinal motoneuron excitability (White et al., 1991), we suggest that, in part, the methylphenidate-elevated force variability shown in Fig. 7 was a result of the drug’s induction of greater locomotion than that seen in controls combined with more forceful limb movements during locomotion. The increased force variation during locomotion induced by methylphenidate may also partly be the result of increased brain dopamine since increased force production has been shown to be associated with activation of dopamine-rich brain loci (e.g., ventral striatum; Schmidt et al., 2009). The foregoing points plus the relatively low rates of methylphenidate-related occurrence of grooming [about 1.4 instances in 3 min (Wooters and Bardo, 2009)] and rearing [7.0 instances in 3 min (Wooters and Bardo, 2009)], along with brief durations of these behaviors in the undrugged state [grooming: 4–6 s/episode (Aldridge and Berridge, 1998); wall rearing: 1–5s/rear (unpublished observations)], suggest that these behaviors did not substantially contribute to the methylphenidate-induced elevation of SD of Fz (Fig. 7). Instead, the amount and intensity of locomotion probably underlie methylphenidate’s effect on SD of Fz in this setting.
Even though overall activity level was higher in the methylphenidate-treated rats, within-period habituation to the novel test chamber environment (Period 1) occurred in both control and methylphenidate-treated rats as indicated by the parallel activity curves across Period 1 time blocks; However, methylphenidate-treated rats habituated less than controls relative to initial activity. The decreased within-period habituation with methylphenidate was similar to that reported in apomorphine-treated rats (Carlsson, 1972) and likely reflects the increased stimulation of D1-like and D2-like dopamine receptors produced by both drugs.
The introduction of spatial change in the environment (i.e., the novel alcove stimulus) in Period 3 produced the greatest differences between the methylphenidate-treated and vehicle-control rats, and also showed the greatest dose-related effects of methylphenidate. In controls, the introduction of the novel alcove in Period 3 led to initially increased locomotor activity and progressively increasing alcove occupancy times. Accordingly, for vehicle-control rats, the introduction of the novel stimulus of the alcove was a “preferred” space as indicated by their long dwell times there compared to the main compartment of the chamber that they had already explored. Preferential exploration of novel stimuli by normal control rats has also been reported in a number of studies using various paradigms such as introduction of a novel object, etc. (e.g., Buhot and Naili, 1995; Dai and Carey, 1994; Oitzl et al., 1994). Furthermore, in our paradigm, exposure of the control group to the alcove prompted re-exploration of the outer, main compartment of the chamber.
Methylphenidate altered the rats’ responses to the presence of the alcove. In the initial time block of Period 3, both methylphenidate dose groups exhibited a small decrease in outside-the-alcove distance traveled compared to the last time block of Period 2 (see Fig. 2). In contrast, the vehicle group exhibited a significant increase in outside-the-alcove distance traveled for the same time blocks (block 12 versus block 15). These results differ from previous reports that the locomotor effects of cocaine or amphetamine, psychostimulants with mechanisms of action similar to methylphenidate’s, were potentiated in a “completely” novel environment compared to a familiar one (Carey and Damianopoulos, 1996; Kyatkin, 1992; Uslaner et al., 2001); however, these earlier studies compared exposures to drugs in novel environments relative to the home cage, and thus possibly assessed different processes than those produced by the introduction of the open alcove in the test apparatus used here. Methylphenidate did not significantly impair rats’ latency to enter the alcove for the first time, nor did the drug differentially affect the duration of the first few visits compared to the vehicle control condition. However, after about 3 min in Period 3, the methylphenidate-treated rats made many more alcove visits that were of shorter duration than those exhibited by the rats that received vehicle injections. When in the alcove, methylphenidate-treated rats moved more and more forcefully than controls. These data show that methylphenidate did not impair initial sampling of or attention to the alcove space, but the drug markedly interfered with the expression of quiet resting and lack of locomotion that was characteristic of the rats in the control group.
The relatively quick adoption of the alcove by the vehicle-control rats suggests that the alcove may have provided a species-relevant stimulus of biological importance. Work by several investigators (e.g., Townsend, 1997; Whishaw et al., 2006) has shown that “shelters” or “refuges” placed within the boundaries of larger “open fields” are readily occupied by rats. The smaller enclosures within the larger spaces had dimensions similar to those of the alcove used here; thus, each space (“shelter”, “refuge”, alcove) was small enough to allow the rat to cutaneously and/or vibrissally sense multiple walls concurrently. Townsend (1997) interpreted the rats’ preference for shelters in terms of anxiety reduction, whereas Whishaw et al. (2006) suggested that occupancy of a small enclosed space (“refuge”) located within a larger space (a soccer field!) reflected a rat’s innate tendency to seek spaces that optimize bodily security (e.g., to increase probability of avoidance of, or escape from, predators). This work with shelters provides a plausible explanation for the fact that the alcove produced a spatial preference and a rapid exploration/habituation response to the alcove in the vehicle-control rats. Although, this interpretation makes sense for the vehicle-control group, it is difficult to reconcile this idea with the disruptive effect of methylphenidate treatment on alcove behavior; that is, methylphenidate treatment interfered with the normal expression of the adaptive preference for a shelter or refuge in an open field. It has been suggested that amphetamine and cocaine, drugs with brain/behavioral effects similar to methylphenidate, increase fear and anxiety in rats (e.g., Blanchard and Blanchard, 1999; Markham et al., 2006). If optimization of security and reduction of fear/anxiety are reflexively intertwined processes in a rat (i.e., the evocation of shelter seeking is also accompanied by fear and anxiety), then methylphenidate treatment would be expected to increase, not decrease, alcove occupancy because drug-induced increased fear/anxiety would make the alcove (“shelter”, “refuge”) a more preferred location than it would be in the non-drugged state. An alternative interpretation of the disruptive effect of methylphenidate on alcove behavior in Period 3 is that methylphenidate induced (reflexively-elicited) compulsive locomotion or inability to stay in one place for more than 10 to 20 sec. The locomotion of methylphenidate-treated rats can be aptly described as “reflexively-evoked” because (1) the locomotion overrode the expression of a biologically important response and (2) the locomotion bouts and alcove visits were relatively constant across the 20 min of Period 3 (e.g., data for the 5.0 mg/kg dose in Fig. 5). While these interpretations may appear contradictory to the therapeutic effects of methylphenidate in ADHD patients, the drug appears to produce “paradoxical” effects in ADHD patients as suggested by the report that dopamine transporter function appears to be altered in ADHD patients (Mazei-Robison et al., 2008); thus, the effects in normal rats or humans may differ from effects in individuals with ADHD. Furthermore, laboratory assay procedures for describing the behavioral effects of drugs in animals need not have face validity in order to have predictive validity for a particular disorder (Geyer and Markou, 1995).
In conclusion, the present findings demonstrate the utility of this behavioral paradigm for assessing exploratory activity, within-period habituation, and response to a spatial modification of a test environment in a single test session and without prior training. More specifically, the effects of methylphenidate on alcove-related behavior highlights the complex interplay among novelty, habituation, and drug-elicited behaviors in rats. These findings also indicate that species-typical response preferences in rats, such as refuge-seeking, may be discerned in test paradigms that introduce spatial novelty to otherwise featureless test enclosures commonly used to assess locomotor activity. In the future, it will be interesting to determine the effects of other psychostimulants and other classes of psychoactive drugs in this paradigm and to further assess the biological relevance of the “refuge-like” properties of the alcove.
Acknowledgements
The authors thank Marlies K. Ozias, Paul F. Davis, and Guillermo Lona for expert technical assistance. Supported by NIH MH071599 (BL), P30 HD02528 (BL, SCF), and P20 RR016475 from the INBRE Program of the National Center for Research Resources (BL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a "natural action" approach to movement sequence. J Neurosci. 1998;18:2777–2787. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyne DE. Arousal, reward and learning. Ann N Y Acad Sci. 1969;159:1059–1570. doi: 10.1111/j.1749-6632.1969.tb12997.x. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Brockel B, Fowler S. Effects of chronic haloperidol on reaction time and errors in a sustained attention task: Partial reversal by anticholinergics and by amphetamine. The Journal of Pharmacology and Experimental Theraputics. 1995;275:1090–1098. [PubMed] [Google Scholar]
- Buhot MC, Naili S. Changes in exploratory activity following stimulation of hippocampal 5-HT1A and 5-HT1B receptors in the rat. Hippocampus. 1995;5:198–208. doi: 10.1002/hipo.450050306. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN. Cocaine conditioning and sensitization: The habituation factor. Pharmacol Biochem Behav. 1996;84:128–133. doi: 10.1016/j.pbb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Carlsson SG. Effects of apomorphine on exploration. Physiol Behav. 1972;9:127–129. doi: 10.1016/0031-9384(72)90282-x. [DOI] [PubMed] [Google Scholar]
- Chee P, Gordon L, Schachar R, Lindsay P, Wachsmuth R. Effects of event rate and display time on sustained attention in hyperactive, normal, and control children. Journal of Abnormal Child Psychology. 1989;17:371–391. doi: 10.1007/BF00915033. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Actions of dopaminergic agonists on motor function. Adv Neurol. 1975;9:285–297. [PubMed] [Google Scholar]
- Dai H, Carey RJ. A new method to quantify behavioral attention to a stimulus object in a modified open-field. J Neurosci Methods. 1994;53:29–34. doi: 10.1016/0165-0270(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Voronstova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J. Neurosci. Meth. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Voronstova E, Mahoney LP. Chicago, IL: Society for Neuroscience; 2009. Actometer size interacts significantly with the effects of amphetamine on rats’ locomotor behavior: Analyses based on distance traveled and gait dynamics. 2009 Abstract Viewer/Itinerary Planner Program No. 843.7. [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: FE Bloom, DJ Kufer., editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 787–798. [Google Scholar]
- Gupta BD, Dandiya PC, Gupta ML. A psycho-pharmacological analysis of behaviour in rats. Jpn J Pharmacol. 1971;21:293–298. doi: 10.1254/jjp.21.293. [DOI] [PubMed] [Google Scholar]
- Kaplan DM, M.A. G, Reiss AL. Attention deficit and development disorders. In: SJ Enna, JT Coyle., editors. Pharmacological Management of Neurological and Psychiatric Disorders. New York: McGraw-Hill; 1998. pp. 137–176. [Google Scholar]
- Kornetsky C. The use of a simple test of attention as a measure of drug effects in schizophrenic patients. Psychopharmacologia. 1972;24:99–106. doi: 10.1007/BF00402907. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- Kyatkin EA. State-dependent peculiarities of cocaine-induced behavioral sensitization and their possible reasons. Int. J. Neuropharmacol. 1992;67:93–105. doi: 10.3109/00207459208994776. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Lou H, Henricksen L, Bruhn P, Borner H, Nielson J. Striatal dysfunction in attention deficit hyperkinetic disorder. Arch. Neurol. 1989;46:48–52. doi: 10.1001/archneur.1989.00520370050018. [DOI] [PubMed] [Google Scholar]
- Markham CM, Yang M, Blanchard RJ, Blanchard DC. Effects of D-amphetamine on defensive behaviors related to fear and anxiety. Pharmacol Biochem Behav. 2006;83:490–499. doi: 10.1016/j.pbb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Marriott AS. The effects of amphetamine, caffeine and methylphenidate on the locomotor activity of rats in an unfamiliar environment. Int J Neuropharmacol. 1968;7:487–491. doi: 10.1016/0028-3908(68)90059-2. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci. 2008;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, de Kloet ER. The effect of corticosterone on reactivity to spatial novelty is mediated by central mineralocorticosteroid receptors. Eur J Neurosci. 1994;6:1072–1079. doi: 10.1111/j.1460-9568.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder--from brain dysfunctions to behaviour. Behav Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- Skjoldager P, Fowler SC. Scopolamine attenuates the motor disruptions but not the attentional disturbances induced by haloperidol in a sustained attention task in the rat. Psychopharmacology (Berl) 1991;105:93–100. doi: 10.1007/BF02316869. [DOI] [PubMed] [Google Scholar]
- Sleator EK, Ullmann RK. Can the physician diagnose hyperactivity in the office? Pediatrics. 1981;67:13–17. [PubMed] [Google Scholar]
- Schmidt L, Cléry-Melin ML, Lafargue G, Valabrègue R, Fossati P, Dubois B, Pessiglione M. Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J Neurosci. 2009 Jul 29;29(30):9450–9457. doi: 10.1523/JNEUROSCI.1951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend P. Use of in-cage shelters by laboratory rats. Animal Welfare. 1997;6:95–103. [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gharbawie OA, Clark BJ, Lehmann H. The exploratory behavior of rats in an open environment optimizes security. Behav Brain Res. 2006;171:230–239. doi: 10.1016/j.bbr.2006.03.037. [DOI] [PubMed] [Google Scholar]
- White SR, Fung SJ, Barnes CD. Norepinephrine effects on spinal motoneurons. Prog Brain Res. 1991;88:343–350. doi: 10.1016/s0079-6123(08)63821-2. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. Nicotinic receptors differentially modulate the induction and expression of behavioral sensitization to methylphenidate in rats. Psychopharmacology (Berl) 2009;204:551–562. doi: 10.1007/s00213-009-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zametkin AJ, Nordahi TE, Gross M, King C, Semple WE, Rumsey J, Hamburger S, Cohen RM. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N. Engl. J. Med. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]