Abstract
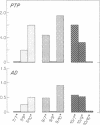
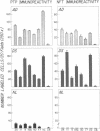
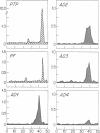
We identified by a monoclonal antibody-based immunoradiometric assay high concentrations of an exocrine pancreatic protein called pancreatic thread protein (PTP) in several areas of Alzheimer disease (AD) brain. The saline-extractable soluble immunoreactivity shares at least three epitopes in common with the native pancreatic form of the protein; the Mr varies from approximately 17,000 to 20,000. Quantitative measurements of PTP immunoreactivity in various regions of several AD brains revealed levels varying from 12 to 295 ng/g of tissue (mean, 116 ng/g) compared with 1-11 ng/g of tissue (mean, 5 ng/g) found in comparable areas of control brains. Immunocytochemistry performed with the anti-PTP monoclonal antibodies demonstrate PTP immunoreactivity within large pyramidal neurons--many of which contain neurofibrillary tangles in both AD and Down syndrome. Less accumulation was observed in astrocytes, and some PTP immunoreactivity was found extracellularly. The highest number of labeled cells in AD and Down syndrome was seen in the hippocampal formation. Fewer positive-staining cells were noted in normal and disease control brains. We conclude, therefore, that an exocrine pancreatic protein is present in the central nervous system of normal individuals at low levels; in AD brain concentrations of this protein are much higher.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Bellet D. H., Ozturk M., Bidart J. M., Bohuon C. J., Wands J. R. Sensitive and specific assay for human chorionic gonadotropin (hCG) based on anti-peptide and anti-hCG monoclonal antibodies: construction and clinical implications. J Clin Endocrinol Metab. 1986 Dec;63(6):1319–1327. doi: 10.1210/jcem-63-6-1319. [DOI] [PubMed] [Google Scholar]
- De Caro A. M., Bonicel J. J., Rouimi P., De Caro J. D., Sarles H., Rovery M. Complete amino acid sequence of an immunoreactive form of human pancreatic stone protein isolated from pancreatic juice. Eur J Biochem. 1987 Oct 1;168(1):201–207. doi: 10.1111/j.1432-1033.1987.tb13405.x. [DOI] [PubMed] [Google Scholar]
- De Caro A., Lohse J., Sarles H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1176–1182. doi: 10.1016/s0006-291x(79)80031-5. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gross J., Brauer A. W., Bringhurst R. F., Corbett C., Margolies M. N. An unusual bovine pancreatic protein exhibiting pH-dependent globule-fibril transformation and unique amino acid sequence. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5627–5631. doi: 10.1073/pnas.82.17.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Carlson R. I., Brauer A. W., Margolies M. N., Warshaw A. L., Wands J. R. Isolation, characterization, and distribution of an unusual pancreatic human secretory protein. J Clin Invest. 1985 Dec;76(6):2115–2126. doi: 10.1172/JCI112216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. N Engl J Med. 1986 Apr 10;314(15):964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manetto V., Perry G., Tabaton M., Mulvihill P., Fried V. A., Smith H. T., Gambetti P., Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Ozturk M., Bellet D., Manil L., Hennen G., Frydman R., Wands J. Physiological studies of human chorionic gonadotropin (hCG), alpha hCG, and beta hCG as measured by specific monoclonal immunoradiometric assays. Endocrinology. 1987 Feb;120(2):549–558. doi: 10.1210/endo-120-2-549. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Altered structural proteins in plaques and tangles: what do they tell us about the biology of Alzheimer's disease? Neurobiol Aging. 1986 Nov-Dec;7(6):425–432. doi: 10.1016/0197-4580(86)90055-2. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Bell D. S., Podlisny M. B., Price D. L., Cork L. C. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer's disease. Science. 1987 Feb 20;235(4791):873–877. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- Stout D. L., Becker F. F. Fluorometric quantitation of single-stranded DNA: a method applicable to the technique of alkaline elution. Anal Biochem. 1982 Dec;127(2):302–307. doi: 10.1016/0003-2697(82)90177-4. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Van Broeckhoven C., Genthe A. M., Vandenberghe A., Horsthemke B., Backhovens H., Raeymaekers P., Van Hul W., Wehnert A., Gheuens J., Cras P. Failure of familial Alzheimer's disease to segregate with the A4-amyloid gene in several European families. Nature. 1987 Sep 10;329(6135):153–155. doi: 10.1038/329153a0. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]