Abstract
We present a novel micro-macro hybrid soft-lithography master (MMHSM) fabrication technique where microdevices having both microscale and macroscale features can be replicated with a single soft-lithography step. A poly(methyl methacrylate) (PMMA) master having macroscale structures was first created by a bench-top milling machine. An imprinting master mold having micro-scale structures was then imprinted on the PMMA surface through a hot-embossing process to obtain a PMMA master mold. A poly(dimethylsiloxane) (PDMS) master was then replicated from this PMMA master through a standard soft-lithography process. This process allowed both microscale (height: 3–20 μm, width: 20–500 μm) and macroscale (height: 3.5 mm, width: 1.2–7 mm) structures to co-exist on the PDMS master mold, from which final PDMS devices could be easily stamped out in large quantities. Microfluidic structures requiring macroscale dimensions in height, such as reservoirs or fluidic tubing interconnects, could be directly built into PDMS microfluidic devices without the typically used manual punching process. This significantly reduced alignment errors and time required for such manual fabrication steps. In this paper, we successfully demonstrated the utility of this novel hybrid fabrication method by fabricating a PDMS microfluidic device with 40 built-in fluidic interfaces and a PDMS multi-compartment neuron co-culture platform, where millimeter-scale compartments are connected via arrays of 20 μm wide and 200 μm long microfluidic channels. The resulting structures were characterized for the integrity of the transferred pattern sizes and the surface roughness using scanning electron microscopy and optical profilometry.
Keywords: Soft-lithography, Fluidic interface, PDMS, Cast molding
1 Introduction
Microfluidic devices have emerged as key components in next generation bio/medical applications due to their various advantages over conventionally used devices, such as small sample/reagent volume, faster response/analysis time, and portability (Han et al. 2007; Han and Frazier 2006; Nevill et al. 2007). Many microfluidic systems, including miniaturized biosensors, microfluidic screening systems, and microfluidic cell culture platforms, have been developed for lab-on-a-chip applicaions in silicon, glass and various polymer materials (Cho et al. 2009; Han et al. 2006; Koltay et al. 2004; Vickerman et al. 2008). Although most fluidic components can be seamlessly integrated into a single system using variety of microfabrication techniques, some critical components cannot be easily fabricated using these technologies and require additional fabrication steps or complicated designs. Two such examples are world-to-chip fluidic interfaces and fluidic reservoirs, where the fabrication challenges are primarily due to their millimeter-scale dimensions in heights.
Poly(dimethylsiloxane) (PDMS) is one of the most commonly used materials in bio/medical microdevices because of the easy fabrication process, biocompatibility, and low cost (Jose et al. 2006; Sia and Whitesides 2003; Xia and Whitesides 1998). Fluidic interfaces or reservoirs are typically made in a replicated PDMS device through a manual punching process, using either blunt needles or punch bits (Morales et al. 2008; Park et al. 2006, 2009). The soft material property of PDMS makes it easier to punch holes on these devices compared to other materials such as silicon, glass or other hard polymers. Although this manual process is simple and does not require any sophisticated tools, poor alignment and time consumption make this step a bottleneck when accurate alignment or many fluidic interfaces/reservoirs are needed. This is a critical drawback, especially for screening or cell culture applications where tens or even hundreds of devices are routinely used for each experimental run, as well as for massively parallel fluidic circuit devices where several tens of fluidic interconnects are required (Blazej et al. 2006; Hong and Quake 2003).
Integrated fluidic reservoirs in microfluidic devices need to hold a certain volume of reagents, samples, or buffers. Therefore, they typically require millimeter scale dimensions not only in the planar direction, but also in the vertical direction, which is a challenging size-scale for most microfabrication techniques. In the case of integrated world-to-chip interfaces, millimeter-scale structures have to be embedded to be able to directly integrate tubings that are typically millimeters in sizes. Methods such as attaching tubes or pins on the microstructure patterned master molds to define reservoirs or fluidic interfaces in the replicated PDMS devices have been introduced previously, but the processes were still manual and time consuming (Hong et al. 2008; McDonald et al. 2000). Other inter-connection techniques, such as bonding gasket structures for connecting tubings on the devices as wells as assembling PDMS microfluidic devices on an electric circuit-like substrate with pre-defined fluidic interconnections, have been developed (Brivio et al. 2006; Choi et al. 2001; Quanglio et al. 2008), but these methods are complicated and do not solve the poor alignment issue. Another proposed method involves clamping PDMS devices with custom-designed brackets to fluidic tubes for strong connection and accurate alignment, but it involves complicated preparation steps and still requires the manual punching process (Bhagat et al. 2007; Krulevitch et al. 2002; Zhu et al. 2004).
Here, we present a simple micro-macro hybrid soft-lithography master (MMHSM) fabrication method that enables fabrication of PDMS microfluidic devices having both macroscale (height: 3.5 mm, width: 1.2–7 mm) and microscale (height: 3–20 μm, width: 20–500 μm) structures by a single PDMS soft-lithography process without any manual punching step. The hybrid master mold was first fabricated by defining macroscale reservoirs or fluidic interfaces on a poly(methyl methacrylate) (PMMA) block using a bench-top computer numerical control (CNC) milling machine. Other rapid prototyping equipments such as a stereolithography tool could also be used, however, they are expensive and only a few research labs have access to such equipments. On the other hand, a CNC milling process can be easily done at an extremely low cost in machine shops available in almost all academic institutes. Micrometer-scale structures were fabricated in glass or metal through a glass etching or copper electroplating process, and then hot-embossed against the PMMA block. This process resulted in macroscale and microscale components coinciding within a single PMMA mold. A PDMS replica was then stamped out from the PMMA mold by a soft-lithography process, and was used as the PDMS master mold for the final PDMS device replication. This novel method allowed fabrication of PDMS microfluidic devices with integrated reservoirs or fluidic interfaces, eliminating misalignment issues or design limitations associated with conventionally used manual punching processes. Also, it significantly reduced the fabrication time since all parts of the microdevices, either micro or macro, could be fabricated by a one-step PDMS replication process.
The utility of this novel hybrid fabrication method was demonstrated by making a PDMS microdevice with 40 builtin microfluidic interfaces and two reservoirs as well as a microfluidic multi-compartment neuron co-culture platform with seven macroscale compartments connected via arrays of microfluidic channels. The microdevice with forty integrated fluidic interfaces was an example to show that the process is capable of fabricating large number of integrated fluidic interfaces at once. However, the developed MMHSM technology is not limited to this specific application and can be applied to any microfluidic components that require fluidic interfaces, including pneumatic valve actuation layers or multiplexer devices (Grover et al. 2006; Marcus et al. 2006). Even in those devices where only several fluidic interfaces are needed, most biological experiments using microfluidic devices require large number of devices. The manual punching process will result in inconsistent devices from batch to batch that can significantly impact the performance of the devices, one example being microfluidic gradient generators. The presented MMHSM technology overcomes such shortcomings.
2 Materials and methods
2.1 Design and fabrication
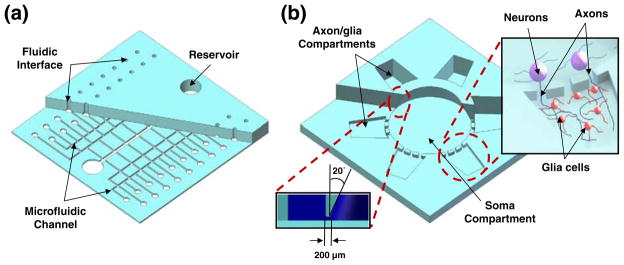
Most microfluidic devices for bio/medical applications are composed of microscale channel/chamber components and macroscale reservoirs or fluidic interfaces. Two different devices with such components have been fabricated to demonstrate the utility of the proposed hybrid fabrication process. The first device, a PDMS microfluidic device having integrated fluidic interfaces and reservoirs, was fabricated to show the validity of the method for a one-step integrated world-to-chip interface fabrication process. The device (50×50×3.5 mm3) was composed of 40 sets of 1.2 mm diameter fluidic interfaces for connection with commonly used 1.58 mm diameter polymer tubings, and two 6 mm diameter reservoirs connected through 500 μm wide and 20 μm high microfluidic channels (Fig. 1(a)).
Fig. 1.
Schematic illustrations of PDMS microdevices fabricated by the MMHSM fabrication process. (a) A PDMS microfluidic device with 40 integrated fluidic interfaces and two reservoirs. (b) A PDMS multi-compartment microfluidic neuron co-culture platform. (Inset 1: Illustration showing the isolation of axonal layer inside the axon/glia compartment from neuronal soma and dendrites by the microfluidic channels, Inset 2: Cross-sectional view showing truncated cone shaped soma compartment)
The second device demonstrated was a 3.5 mm thick PDMS multi-compartment neuron co-culture platform composed of one 7 mm diameter circular soma compartment (approximately 90 μl) and six square-shaped satellite axon/glia compartments (approximately 25 μl) that were 200 μm apart from the soma compartment (Fig. 1(b)). These compartments were connected via arrays of micro-fluidic channels that were 20 μm wide and 3 μm in height. Punching 3.5 mm deep millimeter-scale reservoirs that are only 200 μm apart was not achievable through the conventionally used manual punching process. This microfluidic neuron co-culture platform was designed to isolate axonal layers from its neuronal soma and dendrites. Shallow micro-fluidic channel arrays confine neuronal soma only inside the soma compartment while allowing axons to grow into the neighboring axon/glia compartments (Fig. 1(b)—upper right inset). Such devices can be used to conduct localized neuron and glia interaction studies in a controlled in vivo like environment (Park et al. 2009; Taylor et al. 2005).
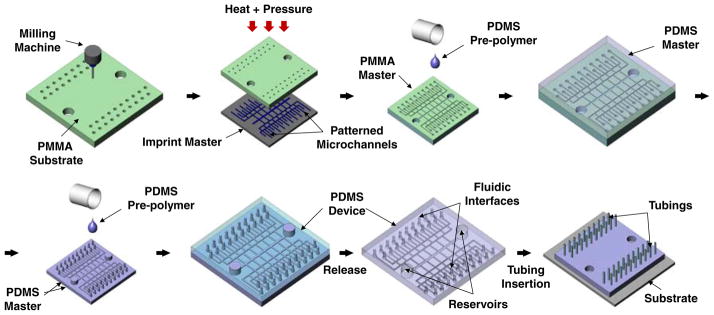
Macroscale structures were first cut into a 76.2 mm× 76.2 mm PMMA block (McMaster-Carr, Atlanta, GA) using a bench-top CNC milling machine (MDX 40, Roland, Irvine, CA). The PMMA block was then sonicated in isopropyl alcohol (IPA) (Sigma Aldrich, St. Louis, MO) for 10 min and dried with N2 gas to remove residual debris from the milling process. The hot-embossing master for fluidic interface application was fabricated by electroplating copper on a 3-inch diameter silicon wafer. Photoresist patterns with width of 500 μm (thickness: 50 μm) were created on a Cu seed layer deposited silicon wafer with a negative photoresist (NR4-8000P, Futurrex, Inc., Franklin, NJ) using standard photolithography. Electroplating was performed at a current density of 10 mA/cm2 for 120 min, followed by removal of the photoresist in acetone. This resulted in an imprint master with 500 μm wide and 20 μm high ridge structures. The imprint master was then aligned and hot-embossed against a PMMA block having CNC machined interfaces and reservoirs to transfer the micro-channel patterns. The hot-embossing process was conducted at 115°C with 1,082 kPa of pressure for 5 min using a temperature controlled hydraulic press (Specac Ltd., London, UK). A PDMS master was then replicated from this PMMA master by pouring PDMS pre-polymer on the master (10:1 mixture, Sylgard® 184, Dow Corning, Inc., Midland, MI), followed by curing at 85°C for 60 min. The final PDMS device with integrated fluidic interfaces and reservoirs was replicated from the PDMS master by a single soft-lithography process, same as described above, and assembled on a cleaned glass substrate after oxygen plasma treatment (Plasma cleaner, Harrick Plasma, Ithaca, NY). Finally, 1.58 mm diameter Teflon FEP tubings (Upchurch Scientific, Inc., Oak Harbor, WA) were inserted directly into fluidic interfaces on the PDMS device. Figure 2 shows the overall MMHSM fabrication process.
Fig. 2.
Fabrication and assembly steps to create a PDMS microdevice having two integrated macroscale reservoirs and 40 fluidic interfaces
For the multi-compartment neuron co-culture platform application, a wet-etched glass slide was used as the embossing mold. Microridge patterns were created on a cleaned glass substrate with a positive photoresist (S1818™, Micro-Chem Corp., Newton, MA) followed by wet etching of the glass substrate in buffered oxide etch (BOE) (J. T. Baker, Phillipsburg, NJ) at room temperature for 5–7 min to obtain 20 μm wide and 3 μm tall glass ridge structures. The photoresist layer that served as the etch mask was then removed in acetone.
2.2 Scanning electron microscopy
PMMA masters, PDMS masters, and PDMS devices fabricated by the MMHSM process were sputter coated with 100Å of Au/Pd in argon plasma. Images were acquired at 12 kV acceleration using a scanning electron microscope (SEM) (JEOL 6400, JEOL Ltd., Tokyo, Japan).
2.3 Surface profilometry
Surface roughness and average sizes of the microchannel arrays on the PMMA masters, PDMS masters, and PDMS devices were analyzed using an optical surface profilometer (Veeco NT9100, Veeco, Plainview, NY) to look for any pattern distortion or significant shrinkage that might have occurred during the fabrication process.
3 Results and discussions
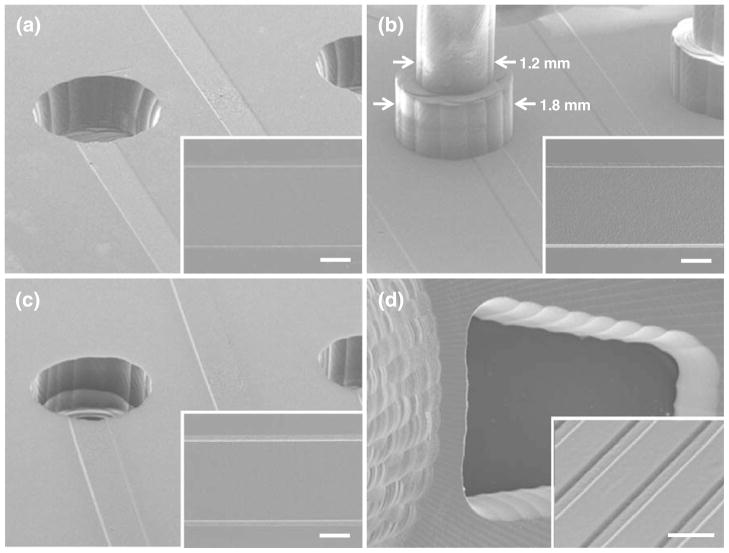
Figure 3(a–c) shows SEM images of the PMMA master, PDMS master, and PDMS device for the 40 fluidic interfaces integrated microdevice fabricated by the MMHSM process. Figure 3(d) shows the PDMS multi-compartment neuron co-culture device showing millimeter-scale compartments connected via arrays of 3 μm high microchannels. It can be seen that microscale channels and macroscale fluidic interfaces were successfully transferred to the final PDMS device.
Fig. 3.
SEM images of (a) PMMA master, (b) PDMS master, and (c) PDMS device showing microchannels connected to millimeter-scale fluidic interfaces (Insets: Enlarged view of 500 μm wide channels). Scale bars, 200 μm. (d) Bottom-side of the PDMS multi-compartment neuron co-culture device showing arrays of 3 μm deep and 20 μm wide microfluidic channels connecting the soma compartment and the axon compartment. Scale bar: 50 μm
The PDMS master was easily released from the PMMA master after the soft-lithography process without any surface treatment of the PMMA master. However, the final PDMS devices firmly adhered to the PDMS master after the curing process and could not be peeled off without damaging the microstructures when no coating was used. In order to facilitate the release process, the PDMS master was vapor coated with (tridecafluoro-1,1,2,2-tetrahydrooctyl) trichlorosilane (United Chemical Technologies, Inc., Bristol, PA) for 10 min and rinsed with IPA to remove excessive coating residues. Chemical treatment of the PDMS master solved the adhesion issue, but the high aspect ratio of the PDMS walls separating reservoirs and fluidic tubing interfaces still posed a challenge, and careful attention had to be paid when designing the reservoirs or fluidic interfaces on the PMMA master. When reservoirs or fluidic interfaces were too close to each other compared to the final thickness of the PDMS device, for example when the aspect ratio of the PDMS wall was 17.5: 1, the wall part of the PDMS was torn during the PDMS device replication process. For the multi-compartment neuron co-culture platform, the soma compartment and the axon/glia compartments were only 200 μm apart and it was challenging to replicate a 3.5 mm thick PDMS device from its master without damaging the device. Therefore, the design of the soma compartment was modified from a cylinder shape into a truncated cone shape with sidewalls tilted 20° toward the center (Fig. 1(b) lower left inset). This modified structure not only strengthened the PDMS walls separating the compartments, but also facilitated the release of the device. Using this method, no damage to the final PDMS devices was observed. In case of the PDMS device with integrated fluidic interfaces, the highest aspect ratio of the PDMS wall separating two macroscale structures was 1.06 : 1, so no damage was observed during the fabrication process. However, the bottom part of the 1.2 mm diameter pillars was enlarged to 1.8 mm to make it more mechanically robust (Fig. 3(b)).
Fluidic interfaces and reservoirs on the PMMA master were engraved using a 0.8 mm square end mill (Roland, Irvine, CA). Only the area where macroscale structures, such as the fluidic interfaces or reservoirs, exist was engraved with the CNC milling machine. The rest of the PMMA surface was left intact to preserve the smooth and polished surface to obtain PDMS devices with smooth surfaces. Average surface roughness of the PMMA master and the imprint master before the hot-embossing process was 9.89±0.88 nm and 14.14± 1.06 nm respectively. After the hot-embossing process, surface roughness of the PMMA master changed to 14.19± 2.44 nm, showing that no significant changes had been made to the surface roughness during the hot-embossing process other than due to the imprint master surface roughness. This result matches well with recent results reported by Desai et al. that multiple self-replications, making a new master from a master fabricated by a soft-lithography process, have little effect on the surface roughness and dimensions of the final replica (Desai et al. 2009). SEM images of the sample surfaces showed smooth surface profiles (Fig. 3), and the final PDMS devices did not show any fluidic seal failure between the device and polystyrene cell culture substrates even for reversible PDMS bonding. This was confirmed by assembling a PDMS multi-compartment neuron co-culture device on a poly-d-lysine (PDL) coated polystyrene culture plate after treating it with oxygen plasma followed by 30 min sterilization in 70% ethanol. The fluidic seal was tightly maintained through 4 weeks of neuron culture without any leakage. The imprint master used in this paper was fabricated by electroplating copper on a silicon wafer or by wet etching a glass slide, but any imprint master can be used. For example, a glass or silicon imprint master fabricated by a deep reactive ion etching (DRIE) process could also be used.
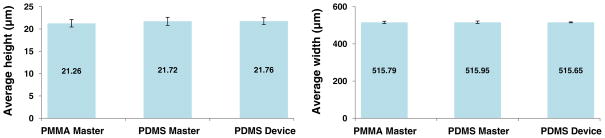
In order to confirm that the original geometry of the pattern was not distorted during the MMHSM fabrication process, average width/depth of microchannels from the PMMA master, the PDMS master, and the PDMS device were compared using SEM images and optical surface profile measurements (Fig. 4). The average width and depth of channels changed from 515.8±5.9 μm (PMMA master, n=10) to 515.7±2.7 μm (PDMS device, n=4) and from 21.3±0.8 μm (PMMA master, n=8) to 21.7±0.8 μm (PDMS device, n=6), respectively. The dimension changes were less than 1.88%, showing that almost no changes had been made during the replication process and that this process is very reliable.
Fig. 4.
Average height and width of the microchannels in the PMMA master, PDMS master and final PDMS device fabricated through the MMHSM process
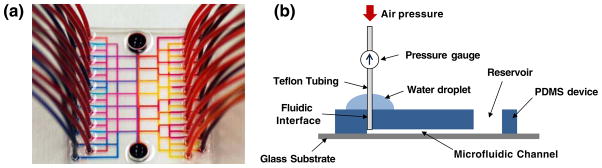
Finally, 40 Teflon FEP tubings (Ø 1.58 mm) were inserted into the PDMS device with integrated fluidic interfaces (Fig. 5(a)). Fluids were introduced through the 40 fluidic inlets and were successfully collected at two reservoirs without any leakage at the interface. To further investigate the robustness of the fluidic interface, a pressure threshold experiment was conducted. Schematic illustration of the experimental setup is shown in Fig. 5(b). A PDMS device with the integrated fluidic interfaces was bonded on a glass substrate after oxygen plasma treatment, followed by Teflon tubing insertion. Water droplets were applied around the inserted tubings to observe leakage while applying pressure to the fluidic interface. The fluidic interface did not show any leakage for applied pressure of up to 345 kPa. Although some fluidic connections using clamps provide stronger pressure tolerance (Bhagat et al. 2007), most bio/medical microfluidic experiments do not require high pressure. The pressure tolerance of 345 kPa obtained through our fluidic interface is comparable to other widely used press-fit type fluidic interfaces (Christensen et al. 2005; Thorsen et al. 2002). The MMHSM fabrication process presented in this paper is not limited to fluidic interfaces or reservoirs, and can be widely used for a broad range of applications requiring both micro and macro scale structures in a single system.
Fig. 5.
(a) A photographic image of a 3.5 mm thick PDMS microfluidic device (50×50 mm2) with 40 Teflon tubings connected to the integrated fluidic interfaces. Four different color dyes were used for visualization. (b) Schematic illustration showing the pressure threshold experimental setup
4 Conclusions
We have developed a novel fabrication technique where PDMS microdevices with micro and macro scale structures can be fabricated by a single step soft-lithography process. The processing step is simple and significantly reduces the fabrication time. Also, it eliminates misalignment or fluidic interface integration issues associated with the widely used manual PDMS punching process, hence enabling high-throughput microdevice fabrication with good batch-to-batch quality control. Forty fluidic interfaces showing no leakage were successfully integrated into a PDMS micro-fluidic system. Millimeter-scale compartments only 200 μm apart were also successfully fabricated and replicated using this process. We believe that this simple MMHSM fabrication technique can be widely used to mass fabricate various high-throughput bio/medical PDMS microdevices at low cost and reduced time.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Mental Health (NIH/NIMH) grant #1R21MH085267.
Contributor Information
Jaewon Park, Email: arum.han@ece.tamu.edu, Department of Electrical and Computer Engineering, College of Engineering, Texas A&M University, College Station, TX 77843-3128, USA.
Jianrong Li, Department of Veterinary Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX 77843, USA.
Arum Han, Department of Electrical and Computer Engineering, College of Engineering, Texas A&M University, College Station, TX 77843-3128, USA. Department of Biomedical Engineering, College of Engineering, Texas A&M University, College Station, TX 77843, USA.
References
- Bhagat AAS, Jothimuthu P, Pais A, Papautsky I. J Micromech Microeng. 2007;17:42. [Google Scholar]
- Blazej RG, Kumaresan P, Mathies RA. PNAS. 2006;103:7240. doi: 10.1073/pnas.0602476103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivio M, Verboom W, Reinhoudt DN. Lab Chip. 2006;6:329. doi: 10.1039/b510856j. [DOI] [PubMed] [Google Scholar]
- Cho Y, Kim HS, Frazier AB, Chen Z, Shin DM, Han A. J Microelectromech Syst. 2009;18:808. [Google Scholar]
- Choi JW, Oh KW, Han A, Okulan N, Wijayawardhana CA, Lannes C, Bhansali S, Schlueter KT, Heineman WR, Halsall HB, Nevin JH, Helmicki AJ, Henderson HT, Ahn CH. Biomed Microdevices. 2001;3:191. [Google Scholar]
- Christensen A, Chang-Yen D, Gale B. J Micromech Microeng. 2005;15:928. [Google Scholar]
- Desai SP, Freeman DM, Voldman J. Lab Chip. 2009;9:1631. doi: 10.1039/b822081f. [DOI] [PubMed] [Google Scholar]
- Grover WH, Ivester RHC, Jensen EC, Mathies RA. Lab Chip. 2006;6:623. doi: 10.1039/b518362f. [DOI] [PubMed] [Google Scholar]
- Han A, Frazier AB. Lab Chip. 2006;6:1412. doi: 10.1039/b608930e. [DOI] [PubMed] [Google Scholar]
- Han KH, Han A, Frazier AB. Biosens Bioelectron. 2006;21:1907. doi: 10.1016/j.bios.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Han A, Yang L, Frazier AB. Clin Cancer Res. 2007;13:139. doi: 10.1158/1078-0432.CCR-06-1346. [DOI] [PubMed] [Google Scholar]
- Hong JW, Quake SR. Nat Biotechnol. 2003;21:1179. doi: 10.1038/nbt871. [DOI] [PubMed] [Google Scholar]
- Hong C, Bao D, Thomas MS, Clift JM, Vullev VI. Langmuir. 2008;24:8439. doi: 10.1021/la801752k. [DOI] [PubMed] [Google Scholar]
- Jose NM, Prado LASDA, Schiavon MA, Redondo SUA, Yoshida IVP. J Polym Sci B Polym Phys. 2006;45:299. [Google Scholar]
- Koltay P, Steger R, Bohl B, Zengerle R. Sens Actuators A. 2004;116:483. [Google Scholar]
- Krulevitch P, Benett W, Hamilton J, Maghribi M, Rose K. Biomed Microdevices. 2002;4:301. [Google Scholar]
- Marcus J, Anderson W, Quake S. Anal Chem. 2006;78:3084. doi: 10.1021/ac0519460. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJA, Whitesides GM. Electrophoresis. 2000;21:27. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Morales R, Riss M, Wang L, Gavin R, Rio JAD, Alcubilla R, Claverol-Tinture E. Lab Chip. 2008;8:1896. doi: 10.1039/b802165a. [DOI] [PubMed] [Google Scholar]
- Nevill J, Cooper R, Dueck M, Breslauer D, Lee L. Lab Chip. 2007;7:1689. doi: 10.1039/b711874k. [DOI] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor A, Rhee SW, Jeon NL. Nat Protoc. 2006;1:2128. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- Park J, Koito H, Li J, Han A. Biomed Microdevices. 2009;11:1145. doi: 10.1007/s10544-009-9331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanglio M, Canavese G, Giuri E, Marasso SL, Perrone D, Cocuzza M, Pirri CF. J Micromech Microeng. 2008;18:055012. [Google Scholar]
- Sia SK, Whitesides GM. Electrophoresis. 2003;24:3563. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- Taylor A, Blurton-Jones M, Rhee SW, Cribbs D, Cotman C, Jeon NL. Nat Methods. 2005;2:599. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen T, Maerki SJ, Quake SR. Science. 2002;298:580. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- Vickerman V, Blundo J, Chung S, Kamm R. Lab Chip. 2008;8:1468. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Whitesides G. Angew Chem. 1998;37:550. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhang Q, Feng H, Ang S, Chau FS, Liu WT. Lab Chip. 2004;4:337. doi: 10.1039/b401834f. [DOI] [PubMed] [Google Scholar]