Abstract
Objective
The authors evaluated the accuracies and ease of use of several commonly used microanastomosis training models (synthetic tube, chicken wing, and living rat model).
Methods
A survey was conducted among neurosurgeons and neurosurgery residents at a workshop held in 2009 at the authors' institute. Questions addressed model accuracy (similarity to real vessels and actual procedures) and practicality (availability of materials and ease of application in daily practice). Answers to each question were rated using a 5-point scale. Participants were also asked what types of training methods they would chose to improve their skills and to introduce the topic to other neurosurgeons or neurosurgery residents.
Results
Of the 24 participants, 20 (83.3%) responded to the survey. The living rat model was favored for model accuracy (p < 0.001; synthetic tube -0.95 ± 0.686, chicken wing, 0.15 ± 0.587, and rat, 1.75 ± 0.444) and the chicken wing model for practicality (p < 0.001; synthetic tube -1.55 ± 0.605, chicken wing, 1.80 ± 0.523, and rat, 1.30 ± 0.923). All (100%) chose the living rat model for improving their skills, and for introducing the subject to other neurosurgeons or neurosurgery residents, the chicken wing and living rat models were selected by 18 (90%) and 20 (100%), respectively.
Conclusion
Of 3 methods examined, the chicken wing model was found to be the most practical, but the living rat model was found to represent reality the best. We recommend the chicken wing model to train surgeons who have mastered basic techniques, and the living rat model for experienced surgeons to maintain skill levels.
Keywords: Cerebral revascularization, Microsurgery, Training
INTRODUCTION
Microneurovascular anastomosis is useful technique in a number of neurovascular diseases. However, adequate practice and experience are necessary to achieve good results after bypass surgery, and regular practice is required to improve or maintain surgical skills. Accordingly, various microanastomosis training models have been introduced, for example, the suturing of surgical gloves or sponges, and the use of synthetic tube, chicken wing, or living rat models3-9).
We have conducted a "Micro-Anastomosis Hand-on Workshop" annually from 2007 for Korean neurosurgeons and neurosurgery residents at our institute. By surveying participants at the 2009 workshop, we sought to identify the most effective microanastomosis training model with respect to their abilities to develop and maintain surgical skills.
MATERIALS AND METHODS
Training methods
At the 2009 workshop, we introduced three microanastomosis training models, namely, the synthetic tube, the chicken wing, and the living rat model. After lectures on basic suture methods and suture practice with sponge, participants practiced with the three models over a 2 day period.
The synthetic tube model
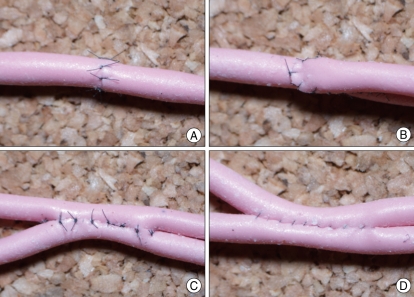
We provided 1 and 2 mm diameter synthetic tubes obtained from "European Cerebral Revascularization and Endovascular Stroke Treatment Course (Inselspital Bern, University of Bern, Switzerland)". This tube is softer than other types of synthetic tube, which makes it more convenient for manipulation and suturing. Using these tubes, the participants performed end-to-end, end-to-side, and side-to-side anastomoses using 10-0 nylon under a microscope (Fig. 1).
Fig. 1.
Microanastomosis training with synthetic tube. Various types of anastomoses including end-to-end anastomosis (A), end-to-side anastomosis (B), and side-to-side anastomosis [interrupted (C) and continuous (D) sutures] can be trained with synthetic tube.
The chicken wing model
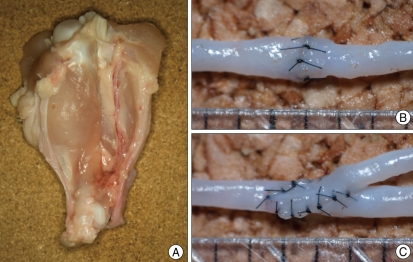
To obtain a large caliber brachial artery, we purchased refrigerated chickens from a farm. The skin was dissected from the shoulder to the wing tip on the ventral side of the wing, and the brachial artery was identified between the biceps brachii and the triceps brachii, along the shaft of the humerus (Fig. 2). The artery harvested from wings was -1 mm in diameter. Under the microscope, participants practiced end-to-end, end-to-side, and side-to-side anastomoses using 10-0 nylon. When anastomosis was completed, patencies were evaluated by injecting saline into vessels using a 24-gauge needle.
Fig. 2.
Microanastomosis training with chicken wing vessels. The brachial artery is harvested between the biceps and triceps brachii (A). Trainee performs end-to-end anastomosis (B) and end-to-side anastomosis (C) with the brachial artery.
The living rat model
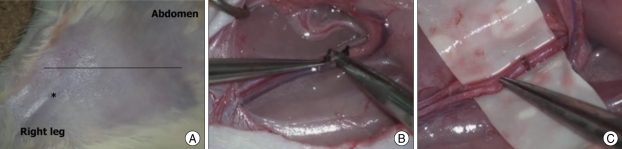
Sprague Dawley rats weighing 300 g were used, and the National Institutes of Health guidelines for the care and use of laboratory animals were adhered to throughout. All procedures were performed under general anesthesia provided by an intraperitoneal injection of Zoletil 30 mg/kg and Xylazine 10 mg/kg. Anesthesia was maintained using half the induction doses. Hairs at skin incisions were removed beforehand using a commercially available depilatory. In the femoral region (Fig. 3), a skin incision was made across the inguinal fold to visualize the common femoral artery and vein. Connective and fat tissue surrounding the vessels were then dissected carefully. The inferior epigastric artery and vein were then ligated with 4-0 black silk and cut with a microscissors. The dissection was then continued to the superficial femoral artery and vein, and the femoral artery was isolated from the vein. The exposed femoral artery measured about 1 mm in diameter.
Fig. 3.
Microanastomosis training using the living rat model in the right femoral region. Skin incision is made crossing the inguinal fold after hair removal. The superficial femoral artery (*) is observed under skin (A). The inferior epigastric artery and vein are ligated with 4-0 black silk and cut using microscissor (B). After the common femoral artery is cut, end-to-end anastomosis is done with 10-0 nylon (C).
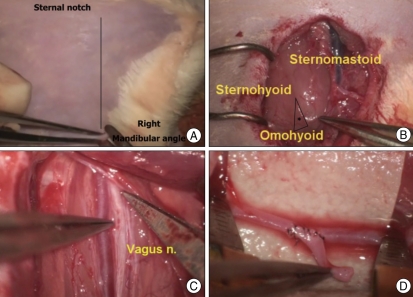
In the cervical region (Fig. 4), a vertical incision was made from the mandibular angle to the sternal notch. After dissecting subcutaneous tissue, the jugular vein was identified, and the triangle limited by the sternomastoid, sternohyoid, and omohyoid muscles was located in the medial region of the jugular vein. The common carotid artery and the vagus nerve are located in this triangle. The common carotid artery was separated from adjacent structures after sharp dissection of connective tissues. Isolated common carotid artery also measured -1 mm in diameter. The participants performed various types of anastomoses using these arteries.
Fig. 4.
Microanastomosis training using the living rat model in the right cervical region. Skin is incised with scissor after hair removal (A). The triangle (*) limited by the sternomastoid, sternohyoid, and omohyoid muscles is located medial to the jugular vein (B). The common carotid artery and vagus nerve are exposed deep in this triangle (C). Trainee performs end-to-side anastomosis with an arterial graft (D).
Evaluation of the accuracies and practicalities of the three anastomosis training methods
We carried out a survey of workshop participants by electronic mail (E-mail). Twenty-four neurosurgeons and neurosurgery residents participated in the workshop and 20 (83.3%) responded to the survey. Survey questions addressed model accuracy (similarity to real vessels and actual procedures) and practicality (material procurement and ease of use). Answers to the survey question scored using a 5-point scale (worst -2, poor -1, average 0, good 1, or excellent 2). We asked participants which training method they would chose to improve their skills and to introduce the topic to other neurosurgeons or neurosurgery residents. In addition, participants were asked to submit their personal opinions. Statistical analysis was conducted using SPSS version 17 (SPSS, Chicago, IL, USA) and the average scores for method accuracy and practicality were compared using the analysis of variance test. Statistical significance was accepted for p-values of < 0.05.
RESULTS
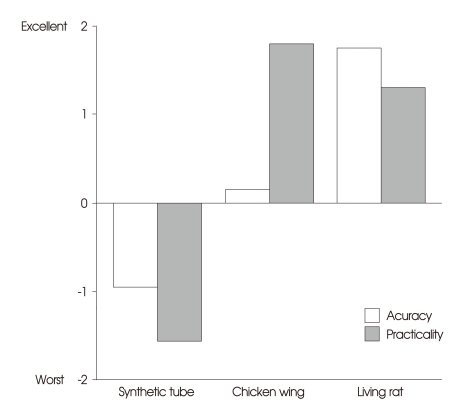
The participants responded that the living rat model was the most accurate (p < 0.001; synthetic tube -0.95 ± 0.686, chicken wing, 0.15 ± 0.587, and living rat, 1.75 ± 0.444) (Fig. 5). In terms of practicality, chicken wing model was favored (p < 0.001; synthetic tube -1.55 ± 0.605, chicken wing, 1.80 ± 0.523, and rat, 1.30 ± 0.923) (Fig. 5).
Fig. 5.
The accuracies and practicalities of the three training methods. Accuracy is defined as similarity with real vessels and procedures, and is found to be best in the living rat model. However, the chicken wing model is found to be a more practical option in terms of procurement and freedom from regulatory restrictions.
All of the 20 responders chose the living rat model as a training method for improving their skills, although 4 responded that the chicken wing model was a suitable additional training method. In terms of introducing microanastomosis to other neurosurgeons and neurosurgery residents, the chicken wing and rat models were selected by 18 (90%) and 20 (100%), respectively.
DISCUSSION
Microneurovascular bypass is very useful surgical technique, but since the International Cooperative Study of Extracranial/Intracranial Arterial Anastomosis was published in 198510), the number of microsurgical cerebral revascularizations performed has decreased1,2). Today, young neurosurgeons and neurosurgery residents have little opportunity to experience revascularization surgery. Furthermore, although a surgeon can perform bypass surgery, it is difficult to maintain and improve skill levels, without routine clinical experience. Accordingly, the best way to maintain surgical skill level is to practice regularly.
Many reports have described microanastomosis methods3-9). Of them, our results demonstrate that the living rat model is the most accurate model and that the chicken wing model is the most practical. These reasons for results are relatively easy to understand. Synthetic tube is difficult to obtain in Korea, and is expensive. Furthermore, it is difficult to manipulate synthetic tubes and suture them because they are harder than actual vessels. However, the rat model is a practice with real vessels of living animal, which are only slightly thicker than the human cortical arteries. Thus, the living rat model provides an experience that closely resembles actual anastomosis, and allows trainees to improve microsurgical and microinstrumental skills confidently through dissection of the cervical and femoral regions in rat. However, training with living rats must be performed in an animal laboratory, and rats are not readily available in some institutes. In contrast, although the chicken wing model is not a practice with living animal, it is cheap, readily available, and allows the training to be performed anywhere.
We believe that the training models utilized in the workshop should be used after trainees have mastered vessel manipulation and suturing with a fine needle. Therefore, we consider that when young neurosurgeons and neurosurgery residents are introduced to anastomosis techniques for the first time, that they learn suturing and manipulation techniques using sponges or synthetic tubes. Based on our results, we recommend that regular chicken wing training is the most practical option to improve the skills of surgeons that have mastered basic techniques, and that the living rat model can be used to maintain the skills of experienced surgeons.
CONCLUSION
Of various the microanastomosis training methods examined, the chicken wing model was found to be the most practical and the living rat model the most accurate. Therefore, we recommend that the chicken wing model can be used to train surgeons who have mastered the basic techniques and that the living rat model can be used to maintain the skills of experienced surgeons.
Acknowledgements
This study was supported by a grant from the Korean Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (grant no : A06-0171-B51004-06N1-00040B).
References
- 1.Ausman JI, Diaz FG. Critique of the extracranial-intracranial bypass study. Surg Neurol. 1986;26:218–221. doi: 10.1016/0090-3019(86)90152-7. [DOI] [PubMed] [Google Scholar]
- 2.EC/IC Bypass Study Group. Failure of extracranialintracranial arterial bypass to reduce the risk of ischemic stroke Results of an international randomized trial. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 3.Grober ED, Hamstra SJ, Wanzel KR, Reznick RK, Matsumoto ED, Sidhu RS, et al. The educational impact of bench model fidelity on the acquisition of technical skill : the use of clinically relevant outcome measures. Ann Surg. 2004;240:374–381. doi: 10.1097/01.sla.0000133346.07434.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hino A. Training in microvascular surgery using a chicken wing artery. Neurosurgery. 2003;52:1495–1497. doi: 10.1227/01.neu.0000065174.83840.62. discussion 1497-1498. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Tsutsumi K, Adachi S, Tanaka S, Saito K, Kunii N. Effectiveness of suturing training with 10-0 nylon under fixed and maximum magnification (x 20) using desk type microscope. Surg Neurol. 2006;66:183–187. doi: 10.1016/j.surneu.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 6.Kanazawa R, Teramoto A. The realization of preferable operative working space through the microsurgical training with rats-the importance of the process. Surg Neurol. 2009;71:380–387. doi: 10.1016/j.surneu.2007.09.039. discussion 387. [DOI] [PubMed] [Google Scholar]
- 7.Lausada NR, Escudero E, Lamonega R, Dreizzen E, Raimondi JC. Use of cryopreserved rat arteries for microsurgical training. Microsurgery. 2005;25:500–501. doi: 10.1002/micr.20153. [DOI] [PubMed] [Google Scholar]
- 8.Olabe J, Olabe J. Microsurgical training on an in vitro chicken wing infusion model. Surg Neurol. 2009;72:695–699. doi: 10.1016/j.surneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Peled IJ, Kaplan HY, Wexler MR. Microsilicone anastomoses. Ann Plast Surg. 1983;10:331–332. doi: 10.1097/00000637-198304000-00015. [DOI] [PubMed] [Google Scholar]
- 10.EC/IC Bypass Study Group. The International Cooperative Study of Extracranial/Intracranial Arterial Anastomosis (EC/IC Bypass Study) : methodology and entry characteristics. Stroke. 1985;16:397–406. doi: 10.1161/01.str.16.3.397. [DOI] [PubMed] [Google Scholar]