Abstract
We recently experienced a case of synovial sarcoma in the posterior neck, which involved adjacent bony structures. Synovial sarcoma is rare, malignant soft tissue tumor that occur predominantly in the lower extremities. Wide surgical excision with involved tissue is the treatment of first choice, because most synovial sarcomas reveal aggressive features. We removed the tumor with involved bony structures and patient was given postoperative radiation therapy. Despite these treatment options, the patient died 1 year after surgery. We report this case with a review of the literature.
Keywords: Synovial sarcoma, Posterior neck, Bony involvement
INTRODUCTION
Synovial sarcoma is an uncommon neoplasm that has been reported to account for 5% to 10% of all soft tissue sarcomas9,12). The tumors are mainly found in para-articular regions of the lower extremities, and only 3% to 10% of all synovial sarcomas arise from the head and neck9,11,16). Among these, only a few cases have been reported to originate in the posterior neck. Synovial sarcoma is usually seen in young adults (20-30 years' group), and the disease is more common in men than women with a 3 : 2 ratio9,11). Histopathologically, synovial sarcoma shows a biphasic pattern consisting of both an epithelial round cell component and a fibrous spindle cell component11). However, some tumors show a monophasic pattern, as well as myxoid, calcifying, and bone forming forms. Due to such a varity of histopathological findings, synovial sarcomas of the head and neck are confused with other mesenchymal and non-mesenchymal tumors15). In addition, immunohistochemical and cytogenetic examinations are important for diagnosis.
Total surgical excision with a wide margin is a widely used treatment for synovial sarcoma, although the optimal treatment strategy has not yet been established. If risk for locoregional recurrence is high, postoperative radiation therapy is recommended14). In this article, we report on a case of posterior neck synovial sarcoma with a large size (> 5 cm) and adjacent bony involvement, with a review of the literature.
CASE REPORT
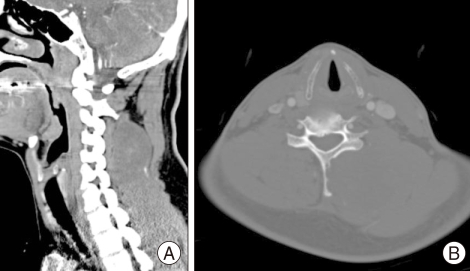
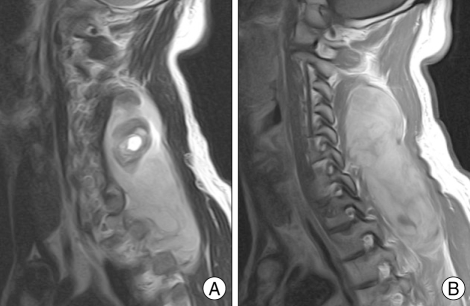
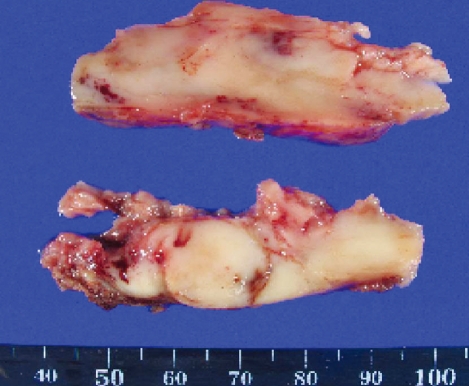
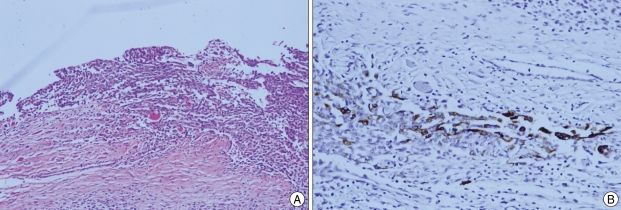
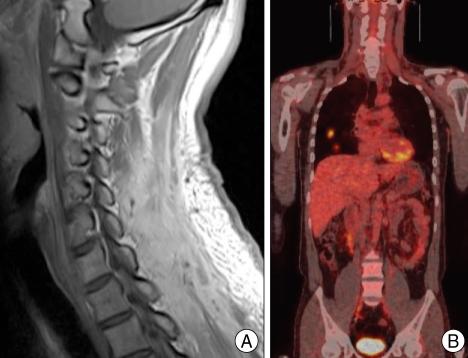
A 39-year-old man visited our hospital complaining of posterior neck pain and left shoulder pain for six months. He had no other symptoms or past medical history. Neck computed tomography (CT) was performed first and it revealed a 5.5 × 4 × 12 cm sized non-calcified and slightly hypodense soft tissue mass in the paraspinal muscle from C3 to T3, with destructive changes of the C4-5 spinous process and lamina (Fig. 1). Cervical magnetic resonance imaging (MRI) showed mixed signal intensity in the lesion with a central cystic component (Fig. 2A). The lesion was heterogenous, and enhanced by gadolinium (Fig. 2B). Considering the CT and MRI findings, we determined that the lesion might be a malignant tumor originated in the paraspinal muscle of the posterior neck. We could remove the tumor completely as en bloc through left paraspinal approach. On operative findings, the lesion was well capsulated, soft, and gray in color. The upper part of the tumor consisted of old hematoma (Fig. 3). C4-5 spinous process and lamina were resected together, because they appeared to be destructive lesions due to tumor invasion. However, there was no adhesion between main mass lesion and spinous process. Histologically, the tumor was composed mainly of epithelial cells and spindle cells (Fig. 4A). Immunohistochemical staining for cytokeratin-7 showed positive findings (Fig. 4B). Neural markers showed negative findings. Based on these results, the tumor was diagnosed as a biphasic synovial sarcoma. Biopsy from surgical margin showed positive finding to tumor. Postoperative investigations of metastasis were done. Whole body positron emission tomography (PET) CT and bone scan showed no increased uptake in lesions. No abnormal metastatic lesions were seen on the chest and abdominal CT. The patient was referred to a radiation oncologist for postoperative radiation therapy. Intensity modulated radiation therapy (IMRT) was performed with total 7,000 cGy. A follow up MRI six month later showed no recurrent lesions on the previous operation site (Fig. 5A). However, PET CT showed a well defined multiple hypermetabolic nodule at both lung fields, representative of metastatic lesions (Fig. 5B). We planed adjuvant systemic chemotherapy, however the patient refused. The patient died due to metastatic complications 1 year after the surgery.
Fig. 1.
A : Non-enhanced neck computed tomography (CT) scan shows a mass lesion with mixed density in the left paraspinal area. B : Neck CT scan in bone setting showed destruction of the spinous process and lamina of cervical vertebra.
Fig. 2.
A : Cervical T2-weighted sagittal magnetic resonance (MR) image reveals mixed signal (mainly high signal intensity) in the lesion with an internal cystic component. B : Cervical T1-weighted sagittal enhanced MR image showing the heterogenous enhanced, well demarcated mass lesion in the posterior neck.
Fig. 3.
In gross finding, the removed mass is well capsulated, soft, gray colored, and low vascular lesions. The upper part of the tumor consists of old hematoma.
Fig. 4.
A : Microscopically, the tumor consists of spindle cell and epithelial cell components. B : On immunohistochemistry, the tumor isfocally stained by CK-7.
Fig. 5.
A : Six months after surgery, a follow up MR image shows no recurrent lesion on the previous operation site. B : Follow up positron emission tomography (PET) scan reveals multiple lung metastasis.
DISCUSSION
Synovial sarcoma can occur at all ages, but most frequently affects young adults and adolescents9). The most common locations in the head and neck regions are the upper aerodigestive tract, parapharyngeal spaces, and joints between the cervical vertebrae8,15). The tumor has also occurred in various other anatomic sites, including the neck, trunk, thymus, kidney, bone, skin, nervous system, liver, pleura, ovary, and mediastinum10-12,17).
Synovial sarcomas are very aggressive tumors. The reported 5-year survival rate for patients with synovial sarcoma is approximately 40% to 602,11). If the tumor is located in the skull base or paraspinal region, tumor size was more than 5 cm, or the tumor infiltrated adjacent structures, it has the worse prognosis8). On the other hand, patients with calcification or synovial sarcoma of the upper aerodigestive tract had an excellent prognosis8,11).
Radiologic diagnosis of synovial sarcoma is difficult. On CT findings, the tumor was shown to be slightly hypodense, well demarcated, with homogenous enhancement16). However, the tumor may be seen sometimes with heterogenous enhancement. Therefore, these results may be difficult to distinguish from benign tumors or other malignant tumors. Calcifications are seen in < 30% of all synovial sarcomas16). MRI found that a head and neck synovial sarcoma on T1-weighed images is shown most frequently isointense to gray matter and has a signal intensity similar to that of glandular or fat tissue on T2-weighed images9). Well-demarcated, yet heterogeneous mucosal or nonmucosal lesions, containing septations, hemorrhage, internal cystic formations, calcifications, or multilocularity, can also be seen in synovial sarcoma9). However, there is no definitive characteristic finding in terms of density and intensity, then radiologic diagnosis is uncertain16).
Histopathological examination and immunohistochemical staining are very important to the diagnosis of synovial sarcoma due to radiologic uncertainty. Histopathologically, synovial sarcoma shows a biphasic pattern consisting of both epithelial and spindle cells. Some tumors show monophasic patterns consisting of only 1 component11,15). Clinical features were different between biphasic and monophasic tumors. Monophasic tumors tended to present at sizes of < 5 cm, while biphasic tumors tend to present at sizes of > 5 cm. Biphasic tumors were more likely tended to infiltrate bone. However, no differences were found in demographic characteristics and history of prior radiation exposure8,14).
Immunohistochemical staining has also been a useful diagnostic tool, because synovial sarcoma can be found in unexpected locations and morphology can vary. Most synovial sarcomas are immunoreactive for cytokeratin, epithelial membrane antigen, and bcl-2. Tumors may express S100 protein, and CD99. However, immunoreactivity for CD34 is not expressed6,15).
Cytologic study is helpful for the diagnosis of synovial sarcoma. Nienty percent of synovial sarcomas have an identifiable translocation between chromosomes 18 and X that result in the fusion of the SYT gene on chromosome 18 and the SSX-1 or SSX-2 gene on chromosome X8,13,15). Tumors with the SYT-SSX2 gene fusion transcript type showed higher survival rates and better metastasis-free survival rates than the tumor with the SYT-SSX1 gene fusion transcript type. Therefore, cytologic study can be a prognostic factor as well as definitive a diagnostic tool13,15).
The first optimal treatment strategy for synovial sarcoma is surgical excision with negative margins7,18). Additional radiation therapy in the management of synovial sarcoma has been shown to improve local control as compared with surgery alone1,4). However, there was no significant difference in disease free survival or local control between preoperative and postoperative radiation therapy4). Patients treated with radiation therapy after surgical excision had higher survival and lower recurrence rates than those treated with chemotherapy after surgical excision8). The role of chemotherapy has not yet been established, although intensive systemic chemotherapy with cisplatin, adriamycion, ifoafamide, and other chemotherapeutic agents was performed in many cases15). Recently, among patients with synovial sarcoma undergoing resection with curative intent, anthracyclineifosfamide (AI) chemotherapy was associated with improved survival in a retrospective analysis5). Canter et al.3) suggested that a nomogram based on preoperative variables may improve decision-making with regards to selecting patients most likely to benefit from neoadjuvant/adjuvant chemotherapy. Therefore, we think systemic chemotherapy will play an important role in the treatment for control of distant metastasis of synovial sarcomas.
In our case, there were many poor prognostic factors. These were the tumor larger than 5 cm, paraspinal location, internal hemorrhage, and adjacent bony destruction with invasion. However, we performed only surgical resection and postoperative radiation therapy. Eventually, the patient died 1 year after the surgery due to distant pulmonary metastasis. Therefore, we think that if immediately adjuvant chemotherapy was planed and performed, the prognosis might have been the better.
CONCLUSION
Synovial sarcoma is an aggressive tumor, and survival rates are associated with tumor location, size, histologic differentiation and adjacent bony extension. Considering our case, we suggest that synovial sarcoma with known poor prognostic factors should be treated earlier with multimodality approaches, including surgery, radiation therapy, and chemotherapy, with frequent follow up examinations. Finally, the tumor should be included in the differential diagnosis of posterior neck soft tissue.
References
- 1.Alektiar KM, Leung D, Zelefsky MJ, Brennan MF. Adjuvant radiation for stage II-B soft tissue sarcoma of the extremity. J Clin Oncol. 2002;20:1643–1650. doi: 10.1200/JCO.2002.20.6.1643. [DOI] [PubMed] [Google Scholar]
- 2.Bergh P, Meis-Kindblom JM, Gherlinzoni F, Berlin O, Bacchini P, Bertoni F, et al. Synovial sarcoma : identification of low and high risk groups. Cancer. 1999;85:2596–2607. doi: 10.1002/(sici)1097-0142(19990615)85:12<2596::aid-cncr16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14:8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis AM, O'Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Eilber FC, Brennan MF, Eilber FR, Eckardt JJ, Grobmyer SR, Riedel E, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246:105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998;2:401–421. doi: 10.1016/s1092-9134(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 7.Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities : a series of patients treated at a single institution. J Clin Oncol. 2005;23:96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 8.Harb WJ, Luna MA, Patel SR, Ballo MT, Roberts DB, Sturgis EM. Survival in patients with synovial sarcoma of the head and neck : association with tumor location, size, and extension. Head Neck. 2007;29:731–740. doi: 10.1002/hed.20564. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch RJ, Yousem DM, Loevner LA, Montone KT, Chalian AA, Hayden RE, et al. Synovial sarcomas of the head and neck: MR findings. AJR Am J Roentgenol. 1997;169:1185–1188. doi: 10.2214/ajr.169.4.9308488. [DOI] [PubMed] [Google Scholar]
- 10.Holla P, Hafez GR, Slukvin I, Kalayoglu M. Synovial sarcoma, a primary liver tumor--a case report. Pathol Res Pract. 2006;202:385–387. doi: 10.1016/j.prp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Ishiki H, Miyajima C, Nakao K, Asakage T, Sugasawa M, Motoi T. Synovial sarcoma of the head and neck : rare case of cervical metastasis. Head Neck. 2009;31:131–135. doi: 10.1002/hed.20856. [DOI] [PubMed] [Google Scholar]
- 12.Jang KS, Min KW, Jang SH, Paik SS, Tae K, Jang SJ, et al. Primary synovial sarcoma of the thyroid gland. J Korean Med Sci. 2007;22(Suppl):S154–S158. doi: 10.3346/jkms.2007.22.S.S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153–160. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Shin E. Treatment outcomes for patients with synovial sarcoma of the head and neck. Expert Rev Anticancer Ther. 2008;8:371–373. doi: 10.1586/14737140.8.3.371. [DOI] [PubMed] [Google Scholar]
- 15.Ochi N, Uozumi M, Doi K, Tanimoto H, Ohbayashi C, Inagaki H, et al. Synovial sarcoma of the neck. ORL J Otorhinolaryngol Relat Spec. 2004;66:42–45. doi: 10.1159/000077233. [DOI] [PubMed] [Google Scholar]
- 16.Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck : CT and MR imaging findings of eight patients. AJNR Am J Neuroradiol. 2001;22:851–857. [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CJ, Ferrier AJ, Russell P, Danieletto S. Primary synovial sarcoma of the ovary : first reported case. Pathology. 2005;37:385–387. doi: 10.1080/00313020500254339. [DOI] [PubMed] [Google Scholar]
- 18.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy : an analysis of 1225 patients. Cancer. 2003;97:2530–2543. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]