Abstract
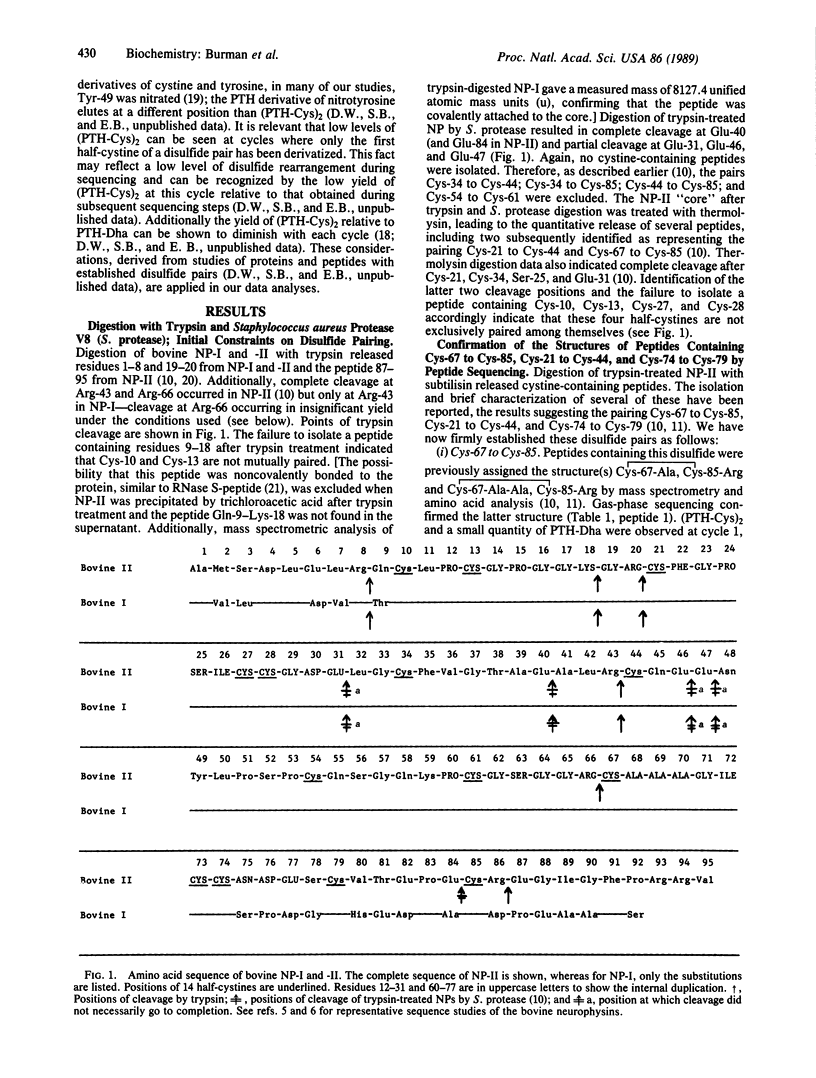
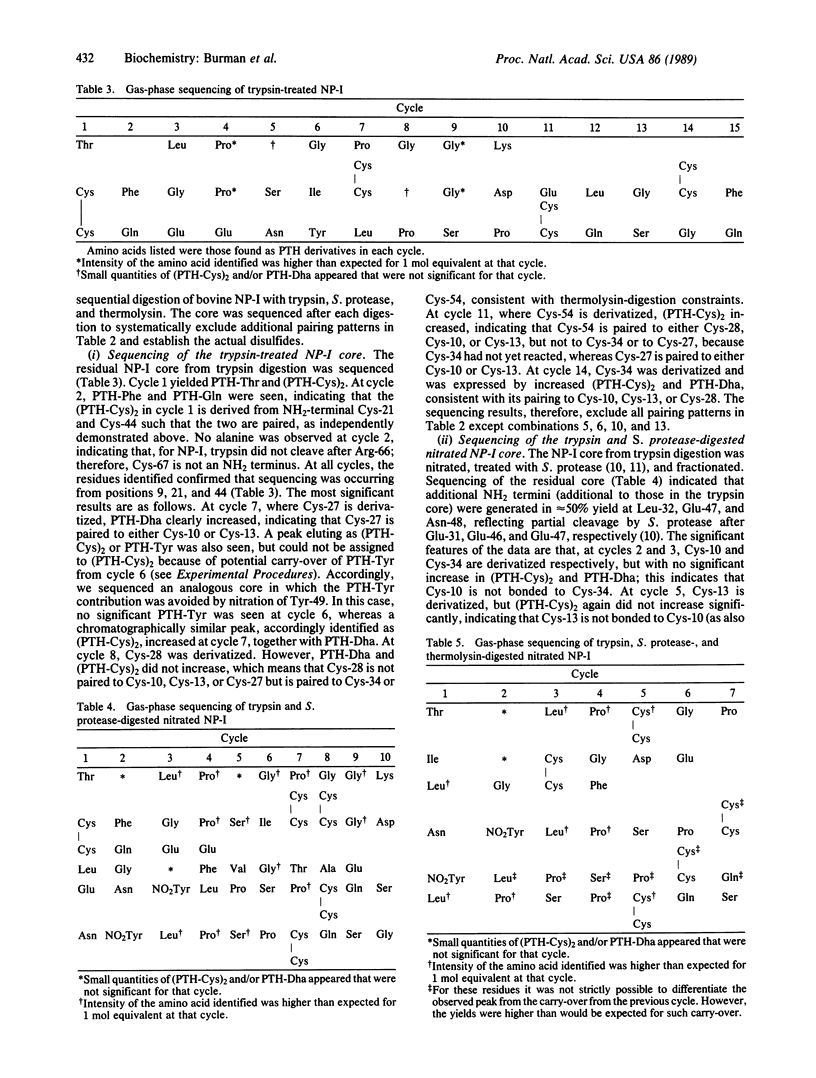
The pairing of the 14 half-cystine residues of bovine neurophysin was established by sequential proteolytic digestion. Purified released peptides and the residual disulfide-linked core were monitored at each step by use of amino acid analysis, gas-phase sequencing, and mass spectrometry. The approach included application of gas-phase sequencing to assign disulfide pairs in peptides containing multiple disulfides. The results demonstrate that neurophysin disulfides are paired in two distinct domains--an NH2 domain (residues 10-54) containing four disulfides and a COOH domain (residues 61-85) containing three disulfides. The specific disulfide bridges are Cys-10 to Cys-54, Cys-13 to Cys-27, Cys-21 to Cys-44, Cys-28 to Cys-34, Cys-61 to Cys-73, Cys-74 to Cys-79, and Cys-67 to Cys-85. The results place the internally duplicated segments of neurophysin (residues 12-31 and 60-77) in separate domains. Disulfide-pairing patterns within each domain are homologous with the exception of the Cys-10 to Cys-54 bond, which is unique to the NH2 domain and which links the two ends of this domain together. The potential role of the Cys-10 to Cys-54 bond in organizing the hormone-binding site is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie D. M., Angal S., Sequeira R. P., Chaiken I. M. Effects of limited tryptic proteolysis of bovine neurophysins on molecular properties of hormone binding, self-association, and antigenicity. Biochemistry. 1982 Dec 7;21(25):6458–6465. doi: 10.1021/bi00268a022. [DOI] [PubMed] [Google Scholar]
- Abercrombie D. M., McCormick W. M., Chaiken I. M. Photoaffinity labeling of the hormone binding site of neurophysin. J Biol Chem. 1982 Mar 10;257(5):2274–2281. [PubMed] [Google Scholar]
- Breslow E., Aanning H. L., Abrash L., Schmir M. Physical and chemical properties of the bovine neurophysins. J Biol Chem. 1971 Sep 10;246(17):5179–5188. [PubMed] [Google Scholar]
- Breslow E. Optical activity of bovine neurophysins and their peptide complexes in the near ultraviolet. Proc Natl Acad Sci U S A. 1970 Oct;67(2):493–500. doi: 10.1073/pnas.67.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow E., Pagnozzi M., Co R. T. Chemical modification or excision of neurophysin arginine-8 is associated with loss of peptide-binding ability. Biochem Biophys Res Commun. 1982 May 14;106(1):194–201. doi: 10.1016/0006-291x(82)92077-0. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Burman S., Breslow E., Chait B. T., Chaudhary T. Application of high-performance liquid chromatography in neurophysin disulfide assignment. J Chromatogr. 1988 Jun 29;443:285–298. doi: 10.1016/s0021-9673(00)94800-3. [DOI] [PubMed] [Google Scholar]
- Burman S., Breslow E., Chait B. T., Chaudhary T. Partial assignment of disulfide pairs in neurophysins. Biochem Biophys Res Commun. 1987 Oct 29;148(2):827–833. doi: 10.1016/0006-291x(87)90950-8. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M., Kotelchuck D., Walter R., Breslow E. Evolution of neurophysin proteins: the partial sequence of bovine neurophysin-I (vasopressin-oxytocin-carrier proteins-automated amino-acid-sequence analysis-homology-protein evolution). Proc Natl Acad Sci U S A. 1972 Feb;69(2):431–434. doi: 10.1073/pnas.69.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait B. T., Field F. H. A rapid, sensitive mass spectrometric method for investigating microscale chemical reactions of surface adsorbed peptides and proteins. Biochem Biophys Res Commun. 1986 Jan 14;134(1):420–426. doi: 10.1016/0006-291x(86)90580-2. [DOI] [PubMed] [Google Scholar]
- Chauvet M. T., Chauvet J., Acher R. Guinea pig MSEL-neurophysin. Sequence comparison of eight mammalian MSEL-neurophysins. Int J Pept Protein Res. 1987 Nov;30(5):676–682. doi: 10.1111/j.1399-3011.1987.tb03379.x. [DOI] [PubMed] [Google Scholar]
- Chauvet M. T., Codogno P., Chauvet J., Acher R. Comparison between MSEL- and VLDV-neurophysins. Complete amino acid sequences of porcine and bovine VLDV-neurophysins. FEBS Lett. 1979 Feb 1;98(1):37–40. doi: 10.1016/0014-5793(79)80146-5. [DOI] [PubMed] [Google Scholar]
- Cohen P., Nicolas P., Camier M. Biochemical aspects of neurosecretion: neurophysin--neurohypophyseal hormone complexes. Curr Top Cell Regul. 1979;15:263–318. doi: 10.1016/b978-0-12-152815-7.50011-9. [DOI] [PubMed] [Google Scholar]
- Drenth J. The structure of neurophysin. J Biol Chem. 1981 Mar 25;256(6):2601–2602. [PubMed] [Google Scholar]
- Furth A. J., Hope D. B. Studies on the chemical modification of the tyrosine residue in bovine neurophysin-II. Biochem J. 1970 Feb;116(4):545–553. doi: 10.1042/bj1160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Judd A. K., Schoolnik G. K. Importance of disulfide bridges in the structure and activity of Escherichia coli enterotoxin ST1b. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8907–8911. doi: 10.1073/pnas.84.24.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Titani K., Walsh K. A. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987 Dec 15;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- Menendez-Botet C. J., Breslow E. Chemical and physical properties of the disulfides of bovine neurophysin-II. Biochemistry. 1975 Aug 26;14(17):3825–3835. doi: 10.1021/bi00688a015. [DOI] [PubMed] [Google Scholar]
- Peyton D., Sardana V., Breslow E. Application of peptide-mediated ring current shifts to the study of neurophysin-peptide interactions: a partial model of the neurophysin-peptide complex. Biochemistry. 1987 Mar 24;26(6):1518–1525. doi: 10.1021/bi00380a004. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Schlesinger D. H., Frangione B., Walter R. Covalent structure of bovine neurophysin-II: localization of the disulfide bonds. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3350–3354. doi: 10.1073/pnas.69.11.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]



