Abstract
AIM: To explore if vascular endothelial growth factor receptor-3 (VEGFR-3) and carcinoembryonic antigen (CEA) can predict overall survival in advanced gastric cancer.
METHODS: VEGFR-3 level was assessed by enzyme-linked immunosorbent assay, and CEA was assessed by chemiluminescence immunoassay in the sera of 81 advanced gastric cancer patients before treatment with oxaliplatin plus 5-fluorouracil and folinic acid.
RESULTS: Median survival time in patients with a low serum VEGFR-3 level was significantly longer than in those with a higher VEGFR-3 level (15.4 mo vs 7.7 mo, P < 0.001). Patients with a low CEA level had a longer survival than those with a higher CEA level (15.8 mo vs 8.6 mo, P < 0.001). Thirty-nine patients with low VEGFR-3 and low CEA levels had a median survival of 19.7 mo (P = 0.0006). The hazard ratio for patients with a high VEGFR-3 level was 2.443 (P = 0.002).
CONCLUSION: High serum VEGFR-3 level is correlated significantly with poor survival. In patients with a high serum level of VEGFR-3, alternative chemotherapy regimens should be considered.
Keywords: Stomach neoplasms, Vascular endothelial growth factor receptor-3, Carcinoembryonic antigen, Oxaliplatin plus 5-fluorouracil and folinic acid protocol, Survival
INTRODUCTION
According to global estimates, gastric cancer is the second most frequent cancer-related cause of death. The incidence of gastric cancer is estimated to be 934 000 cases, with 56% of the new cases occurring in East Asia, 41% in China and 11% in Japan[1]. In 2005, there were approximately 400 000 new cases and 300 000 deaths from gastric cancer in China[2].
Great efforts are being made to develop serological markers that are noninvasive and can easily reflect the dynamic status of the tumor. Among these, carcinoembryonic antigen (CEA) has been used widely as a serological marker in patients with gastrointestinal malignancies[3-5]. CEA was first described by Gold and Freedman in 1965 as an antigen that is expressed by gastrointestinal carcinoma cells, which is secreted in blood or body fluids. Several studies have focused on the utility of CEA and carbohydrate antigen 19-9 (CA19-9) measurement in cancer progression, recurrence, and prognosis in patients with gastric carcinoma[4,6,7].
There has been a dramatic increase in the number of studies of the mechanisms of associated lymphangiogenesis and lymphatic metastasis. It has been recognized that lymphangiogenic growth factors promote the spread of cancer cells to regional lymph nodes[8-10], and one of the most important ones is vascular endothelial growth factor receptors (VEGFR).
Regional lymph node metastasis is an important indicator of tumor aggressiveness, as well as a known prognostic factor[11]. Therefore, it is important to estimate the degree of lymphatic system invasion and lymphangiogenesis in the evaluation of biological tumor aggressiveness and patient outcome. VEGFRs1, 2 and 3 are endothelial-specific receptor tyrosine kinases that are regulated by members of the VEGF family. VEGFR-3 expression has been demonstrated in a variety of human malignancies[12]. The role of the VEGF-C, D and/or VEGFR-3 axis in various types of cancer has been investigated by many research groups[12]. In clinical studies, a negative correlation between VEGF-C, D and/or VEGFR-3 and patient survival time has been reported in non-small cell lung cancer, colorectal carcinoma, endometrial carcinoma, epithelial ovarian carcinoma and primary breast cancer[13-17].
Advanced gastric cancer patients need chemotherapy, but there is currently no established standard regimen; oxaliplatin plus 5-fluorouracil and folinic acid (FOLFOX) is well tolerated. Recently, several phase II studies have yielded a median time to progression (TTP) of 5.4-6.5 mo and a median overall survival (OS) of 9.8-12.6 mo[18-21].
A reliable factor is necessary to predict objectively the effectiveness of some special chemotherapy protocols. Recently, two studies have investigated if gene polymorphisms and mRNA can predict the TTP and OS in advanced gastric cancer treated with FOLFOX[22,23]. For oxaliplatin-associated chemotherapy of advanced gastric cancer, seeking more reliable predictive values for chemotherapy is of great importance in research and clinical settings.
We hypothesized that secreted CEA reflects the tumor burden, and VEGFR-3 is associated with poor survival. The purpose of this study is to find out the prognostic value of serum levels of VEGFR-3 and CEA in gastric cancer patients receiving FOLFOX chemotherapy.
MATERIALS AND METHODS
Patients
Patients with histologically proven locally advanced or metastatic gastric cancer and Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 2 were included in the study. Clinical stage and histological type of gastric cancer were evaluated according to AJCC criteria (the sixth edition). All patients received FOLFOX chemotherapy after resection of primary tumors as follows: oxaliplatin 130 mg/m2 on day 1, plus folinic acid 200 mg/m2 as a 2-h infusion, followed by a 22-h infusion of 5-fluorouracil (5-FU) 450 mg/m2 on days 1-5, every 3 wk. Survival was calculated from the date of diagnosis to the date of last follow-up or death from any cause.
All patients gave their signed informed consent, and the study was approved by the Institutional Ethics Review Boards.
Quantitative detection of VEGFR-3 and CEA
Sera from 40 healthy volunteers (20 females and 20 males ranging in age from 25 to 60 years) and gastric cancer patients were collected using a serum separator tube and kept frozen at -80°C until assay.
Enzyme-linked immunosorbent assay for serum VEGFR-3 and CEA
Serum was collected before chemotherapy. VEGFR-3 was analyzed with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). An ELISA component kit that measures the extracellular (soluble) domain of VEGFR-3 was employed. Serum VEGFR-3 assays were calibrated against recombinant proteins that consisted of the full-length extracellular domain of the respective receptors. A 96-well microplate was coated with diluted capture antibody, and incubated overnight. After washing, the plate was blocked by adding diluent reagent. Plate preparation was finished. Samples or standards were added, then the plates were washed, detection antibody was added, and washing was repeated. Streptavidin-horseradish peroxidase was added to each well. After washing, substrate solution was added to each well. Finally, stop solution was added to each well. The plate was tapped gently. The optical densities of each well were quantified within 30 min at dual wavelengths of 450 nm corrected to 540 nm using a micro-plate reader.
CEA level of all serum samples was analyzed by chemiluminescence immunoassay (CEA Regent Kit, Abbott Diagnostics). Assays were carried out according to the manufacturer’s instructions using the machine of ARCHITECT i2000 SR.
Statistical analysis
Spearman’s Rho method was used to correlate levels of VEGFR-3 and CEA. The maximal χ2 method of Miller et al[24] and Halpern[25] was adapted to determine which cutoff value can best dichotomize the patients into low- and high-expression CEA and VEGFR-3 subgroups; the Tree method[26] was then applied to optimize these cutoff values. The final cutoff values were confirmed by recursive partitioning and amalgamation using S-Plus software, version 6.1 (Statistical Sciences, Seattle, WA, USA). Cumulative survival rates were determined using the Kaplan-Meier method, and the difference between each group was evaluated by the log-rank method. The cases lost to follow-up were treated as censored data for the analysis of survival rates. A univariate Cox model with overall survival as the dependent variable was constructed and categorized with two factors levels as independent variables, and the factors that were significant in the univariate analysis were included in a multivariate Cox proportional hazards model for survival. Differences were considered significant at P < 0.05. All statistical calculations were performed using the Statistical Package for the Social Sciences, version 13 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
A total of 81 advanced gastric adenocarcinoma patients were included in the study. The median age was 59 years; 55 patients were male and 64 patients had ECOG PS 0-1; and 13 patients had no lymph node involvement. Thirty-eight (46.9%) patients had stage III, and 43 (53.1%) had stage IV disease at the time of diagnosis. Patient characteristics are summarized in Table 1.
Table 1.
Clinical characteristics associated with overall survival of the patients
| Characteristics | n (%) | MST (mo) (95% CI) | P Log-rank test |
| Age (yr) (median: 59, range: 28-73) | |||
| < 59 | 37 (45.7) | 13.900 (8.075-19.725) | 0.610 |
| ≥ 59 | 44 (54.3) | 12.600 (6.595-18.605) | |
| Gender | |||
| Male | 55 (67.9) | 12.600 (7.674-17.526) | 0.384 |
| Female | 26 (32.1) | 15.300 (6.017-24.583) | |
| ECOG | |||
| 0-1 | 64 (79.0) | 14.300 (9.194-19.406) | 0.034 |
| 2 | 17 (21.0) | 9.800 (5.307-14.293) | |
| Lymph node | |||
| Negative | 13 (16.0) | 35.100 (5.695-64.505) | 0.004 |
| Positive | 68 (84.0) | 9.900 (7.480-12.320) | |
| Initial staging | |||
| III | 38 (46.9) | 17.800 (14.187-21.413) | 0.017 |
| IV | 43 (53.1) | 8.600 (6.157-11.043) | |
| Grading | |||
| G2 | 23 (28.4) | 14.500 (2.761-26.239) | 0.584 |
| G3 | 58 (71.6) | 12.600 (7.823-17.377) | |
| Site of tumor | |||
| Proximal stomach | 30 (37.0) | 10.300 (5.283-15.317) | 0.806 |
| Distal stomach | 44 (54.3) | 15.400 (9.380-21.420) | |
| Whole stomach | 7 (8.6) | 10.800 (4.439-17.161) |
MST: Median survival time; ECOG: Eastern Cooperative Oncology Group.
Levels of VEGFR-3 and CEA
Serum VEGFR-3 and CEA levels were detected in all the patients and healthy donors. Serum VEGFR-3 level varied in healthy donors, and the median level was 24.5 ng/mL [range: 0.3-40.3 ng/mL, 95% confidence interval (CI): 19.2-31.7]. In gastric cancer, the median VEGFR-3 level was 41.8 ng/mL (range: 17.2-385.5 ng/mL, 95% CI: 52.5-114.6). A highly significant difference was found in the median VEGFR-3 level between gastric cancer patients (P < 0.01) and healthy donors.
Serum CEA level was variable in healthy donors, and median level was 2.3 ng/mL (range: 0.1-6.2 ng/mL, 95% CI: 1.7-3.9). In gastric cancer, the median CEA level was 13.7 ng/mL (range: 2.2-301.7 ng/mL, 95% CI: 18.3-57.8). A highly significant difference was found in the median CEA level between gastric cancer patients (P < 0.01) and healthy donors.
Using a cutoff value of 70.6, 52 (64.2%) patients had low VEGFR-3 expression levels and 29 (35.8%) patients had higher VEGFR-3 levels. Using a cutoff value of 23.5, 57 (70.4%) patients had low CEA expression levels and 24 (29.6%) had higher levels. There was no significant association between VEGFR-3 and CEA levels (P = -0.136, P = 0.227). No significant association was detected between VEGFR-3 or CEA and clinical parameters.
Survival time
The median survival time for all patients was 13.0 mo (95% CI: 8.820-17.180). A significant association was observed between survival and ECOG PS (P = 0.034), lymph node involvement (P = 0.004) and initial staging (P = 0.017). No other association between clinical characteristics and survival was found (Table 1).
A significant association was also observed between survival and levels of VEGFR-3 (P < 0.05) and CEA (P < 0.05). Median survival for patients with low VEGFR-3 levels was 15.4 mo (95% CI: 11.799-19.001) compared with 7.7 mo (95% CI: 5.691-9.709) for those with higher VEGFR-3 levels (P < 0.001) (Table 2 and Figure 1). Median survival for patients with low CEA levels was 15.8 mo (95% CI: 11.223-20.377) compared with 8.6 mo (95% CI: 7.219-9.981) for those with higher CEA levels (P < 0.001, Table 2).
Table 2.
Serum VEGFR-3 and CEA levels and survival in advanced gastric cancer patients
| Factors | n | MST (mo) (95% CI) | P1 |
| VEGFR-3 (ng/mL) | |||
| Low ≤ 70.6 | 52 | 15.400 (11.799-19.001) | < 0.001 |
| High > 70.6 | 29 | 7.700 (5.691-9.709) | |
| CEA (ng/mL) | |||
| Low ≤ 23.5 | 57 | 15.800 (11.223-20.377) | < 0.001 |
| High > 23.5 | 24 | 8.600 (7.219-9.981) |
Adjusted P-value based on log-rank statistics after 1000 bootstrap simulations. VEGFR-3: Vascular endothelial growth factor receptor-3; CEA: Carcinoembryonic antigen; CI: Confidence interval.
Figure 1.
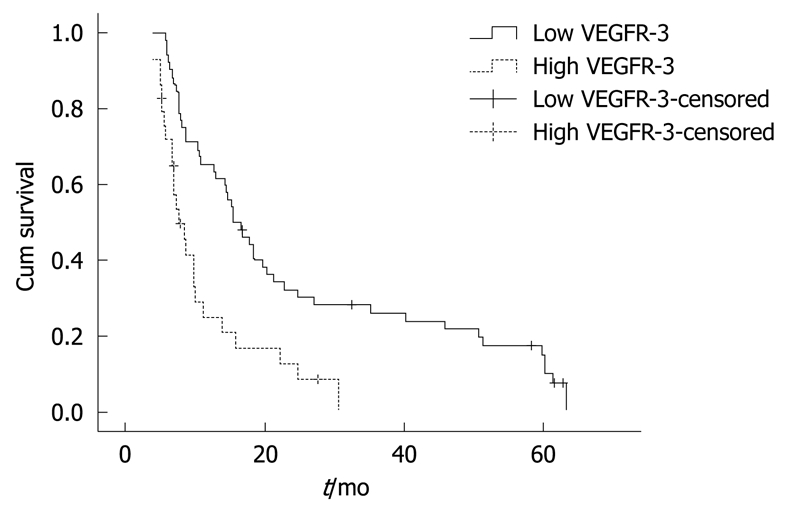
Kaplan-Meier estimates of overall survival by vascular endothelial growth factor receptor-3 (VEGFR-3) levels (n = 81, low VEGFR-3: 52; high VEGFR-3: 29).
Among the 24 patients with high CEA levels, 13 patients with low VEGFR-3 levels had a median survival of 13.0 mo (95% CI: 5.954-20.046) while 11 patients with higher VEGFR-3 levels had a median survival of 7.2 mo (95% CI: 4.622-9.778) (P = 0.003, Figure 2A). Among the 57 patients with low CEA levels, 39 patients with low VEGFR-3 levels had a median survival of 19.7 mo (95% CI: 12.53-26.87) while 18 patients with higher VEGFR-3 levels had a median survival of 9.9 mo (95% CI: 4.651-15.149) (P = 0.006, Figure 2B).
Figure 2.
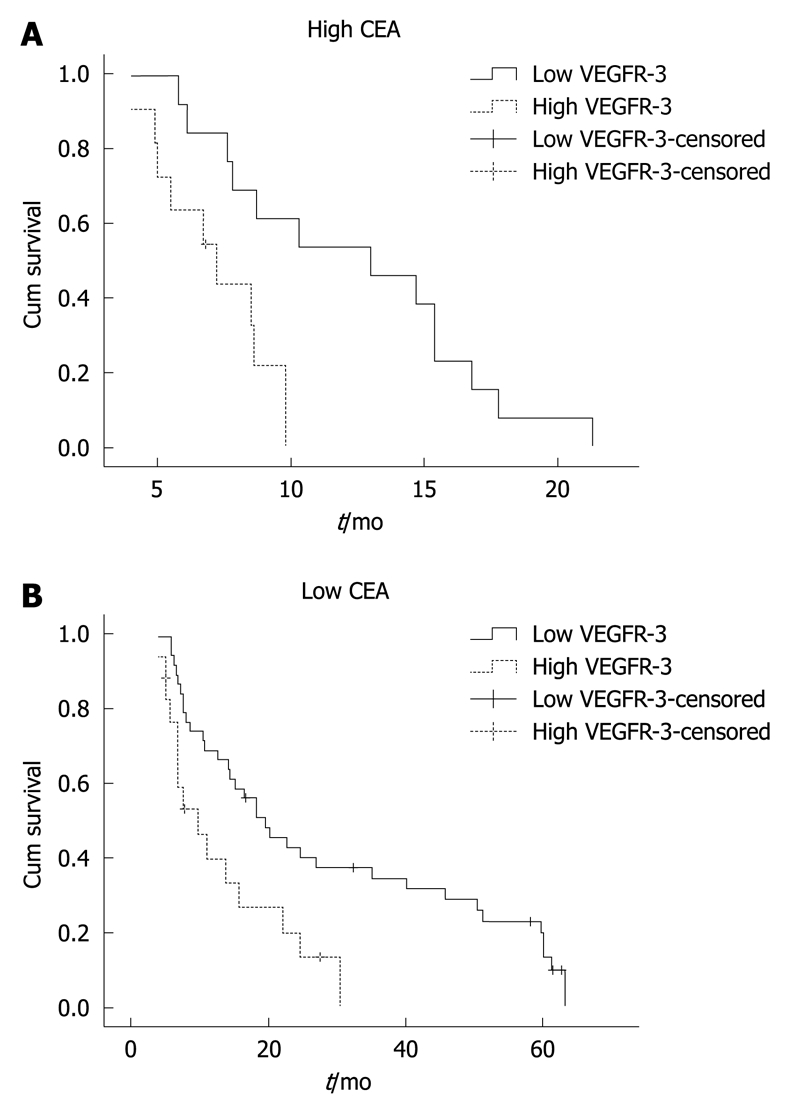
Kaplan-Meier estimates of overall survival according to VEGFR-3 levels in patients. A: Patients with high carcinoembryonic antigen (CEA) levels (n = 24, high CEA and low VEGFR-3: 13, high CEA and high VEGFR-3: 11); B: Patients with low CEA levels (n = 57; low CEA and low VEGFR-3: 39; low CEA and high VEGFR-3: 18).
Multivariate analysis identified VEGFR-3 levels [hazard ratio (HR) = 2.443, P = 0.002], initial staging (HR = 1.844, P = 0.018), and ECOG PS (HR = 2.396, P = 0.011) as independent markers for survival (Table 3), whereas CEA levels (HR = 1.255, 95% CI: 0.721-2.184, P = 0.121) were not an independent marker for survival.
Table 3.
Multivariate analysis of factors associated with overall survival
| Factors | n | Hazard ratio (95% CI) | P |
| ECOG PS | |||
| 0-1 | 64 | 1 (ref.) | |
| 2 | 17 | 2.396 (1.219-4.708) | 0.011 |
| Lymph node | |||
| Negative | 13 | 1 (ref.) | |
| Positive | 68 | 1.846 (0.871-3.912) | 0.110 |
| Initial staging | |||
| III | 38 | 1 (ref.) | |
| IV | 43 | 1.844 (1.109-3.066) | 0.018 |
| VEGFR3 | |||
| Low ≤ 70.6 | 52 | 1 (ref.) | |
| High > 70.6 | 29 | 2.443 (1.374-4.345) | 0.002 |
PS: Performance status.
DISCUSSION
In this study of serum levels of VEGFR-3 and CEA in advanced gastric cancer, multivariate analysis identified VEGFR-3 levels, initial staging, and ECOG PS as independent markers for the survival of the patients who received the FOLFOX regimen. CEA was not an independent marker for survival.
VEGF-C and VEGFR-3 are associated with lymphatic metastasis, mainly via tumor lymphangiogenesis in animal models and human tumors[9,27]. Many previous studies have investigated the association between expression of VEGFR-3 and tumor histology. VEGFR-3 is an independent prognostic factor for survival; the expression of VEGFR-3 is correlated with lymphatic invasion, lymph node metastasis, and poor prognosis for survival[28-30]. In the present study, the median survival time in patients with low serum VEGFR-3 levels was significantly longer than in those with higher VEGFR-3 levels (15.4 mo vs 7.7 mo, P < 0.001). Patients with low VEGFR-3 and CEA levels had a median survival of 19.7 mo (P = 0.0006). The HR for patients with a high VEGFR-3 level was 2.443 (P = 0.002).
Although great efforts have been devoted to improve early detection of gastric cancer, the majority of patients are diagnosed at an advanced stage. The median OS has been shown to be 9.8-12.6 mo after FOLFOX chemotherapy[18-21]. Identification of patients with potentially poor prognosis after FOLFOX chemotherapy would help us to optimize another treatment protocol for patients with advanced gastric cancer.
These findings are similar to those with metastatic malignant melanoma, for which, a high pretreatment serum VEGFR-3 level is correlated significantly with poor prognosis. Patients with a low serum VEGFR-3 level had a higher median disease-free survival than those with a high serum VEGFR-3 level (16.2 mo vs 10.8 mo, χ2 = 3.85, P = 0.022). Median serum VEGFR-3 levels were significantly higher in patients with a high tumor burden than those with a low tumor burden (P = 0.013)[31].
There are few reports on biomarkers with a high and reliable predictive value for chemotherapy. Recently, two studies have investigated predictive factors for FOLFOX chemotherapy in advanced gastric cancer. In one study of genetic polymorphism, the glutathione S-transferase M1 positive genotype showed a significantly longer survival time compared with negative genotype in advanced gastric cancer treated with FOLFOX[22]. In a study of mRNA, the median survival time in patients with low levels of mammalian excision repair via complementing protein ERCC1 was significantly longer than in those with higher levels (15.8 mo vs 6.2 mo, P < 0.0001) in advanced gastric cancer treated with FOLFOX chemotherapy[23].
However, in the present study, the difference in the survival of the patients with a low level and a high level of VEGFR-3 was much more striking (15.4 mo vs 7.7 mo, P < 0.001), with an HR of 2.443. The determination of relative serum VEGFR levels by ELISA and electrochemiluminescence is considered currently to be easier than by immunohistochemistry and quantitative PCR.
It has been shown that VEGFR-3 is expressed not only in lymphatic endothelial cells, but also in tumor cells, and it has been seen in the cytoplasm, along the nuclear and cell membranes, which underlines its potential role in tumor growth[15,32,33]. Despite vast amounts of literature on VEGFR-3 expression in tissues (quantitative PCR and immunohistology)[32,34-37], there are few data about serum levels of VEGFR-3, which is the major axis specific for lymphangiogenesis.
To address the biological and clinical significance of pre-chemotherapeutic serum VEGFR-3 levels in advanced gastric cancer, we compared serum VEGFR-3 levels with clinicopathological parameters. A significant association was observed between survival and ECOG PS, lymph node involvement and initial staging. We found a significant association between the pre-chemotherapeutic serum level of VEGFR-3 and lymph node status. In contrast, there was no correlation between survival and age, sex, tumor grading, or tumor site.
In a recent study of anti-angiogenic treatment, DePrimo et al[38] found that the decrease in the soluble variant of VEGFR-3 could be a marker of sunitinib activity in patients with metastatic renal cell carcinoma. Similar to our results, Rini et al[39] have shown that a high baseline VEGFR-3 level is related to non-response to treatment and shorter progression-free survival in renal cell cancer that is refractory to bevacizumab, when the patients are treated with sunitinib. The presence of high levels of circulating VEGFR-3 in advanced gastric cancer patients might prospectively identify high-risk patients undergoing FOLFOX chemotherapy with a worse prognosis and shorter survival, and special target medicine is needed for therapy.
It is also not known in which way VEGFR-3 contributes to tumor angiogenesis, lymphangiogenesis, tumor progression and metastasis[40,41]. However, our results were from serum, and further investigations are necessary to confirm our observations.
A high CEA level has been shown to be a negative factor for survival in breast, gastric and colorectal cancer[42-47]. In patients with metastatic colorectal cancer receiving cetuximab plus FOLFIRI or FOLFOX-4 chemotherapy, serum CEA could predict progression-free survival time. Survival time in responders assessed by changes in serum CEA was significantly longer than that in non-responders (P = 0.0091)[48].
In another study, a preoperative CEA level was an independent prognostic factor in patients with node-positive Dukes’ C colorectal cancer treated with 5-FU-based adjuvant chemotherapy[49]. However, the role of CEA in predicting chemosensitivity remains controversial. In a prospective study, by multivariate analysis, serum CA19-9 level (P < 0.001) was found to be an independent prognostic factor, whereas pretreatment serum CEA level was not considered to be a significant prognostic indicator in patients with metastatic colorectal cancer treated with 5-FU-based chemotherapy[50].
In the present study, patients with lower CEA levels had a longer survival than those with higher CEA levels, especially in patients with higher VEGFR-3 levels. VEGFR-3 over-expression increases proliferation of MCF7 cells, but proliferation of BT474 cells is reduced drastically when endogenous VEGFR-3 is down-regulated[51]. Producing a plausible explanation of why a meaningful number of patients with low VEGFR-3 and CEA levels had the longest survival is one of the aims of the present study.
Irinotecan and taxane-based regimens have been used in the treatment of advanced gastric cancer patients, with a similar survival to those attained with FOLFOX[52-54]. Irinotecan or taxane-based regimens could be the better alternative for patients with high VEGFR-3 levels. A randomized customized trial is warranted in this setting.
Targeting the VEGF-C/VEGFR-3 axis may be therapeutically significant for certain types of tumors. Thus, the continued discovery and characterization of factors that regulate VEGF-C or VEGFR-3 are essential for developing new therapies that limit the spread of cancer. In a recent study of gastric tumors, the target drug Ki23057 inhibited the phosphorylation of VEGFR-3 in lymphatic endothelial cells. The degree of lymphatic invasion and lymphangiogenesis was significantly (P < 0.05) lower in the gastric tumors treated by Ki23057[55]. Therefore, the target medicine is required to be developed in the future. In particular, new drugs that block the VEGFC/VEGFR-3 signaling pathway may provide useful anticancer therapeutics by mechanisms other than the blockage of lymphangiogenesis.
In conclusion, the data from our study indicate that serum VEGFR-3 level is the most significant prognostic indicator of patients with advanced gastric cancer. It is recommended that stratification for further clinical trials of patients with advanced gastric cancer should be carried out according to serum VEGFR-3 levels. Combined analysis of the VEGF-C/VEGF-D/VEGFR-3 system might be useful for identifying patients with an unfavorable clinical outcome, thereby helping refine therapeutic decisions in gastric cancer.
In our study, we only detected the levels of VEGFR-3 and CEA before chemotherapy. Changes in these levels after chemotherapy, especially with regard to differences between the responding and non-responding groups, need further researches.
COMMENTS
Background
Carcinoembryonic antigen (CEA) has been used widely as a serological marker in patients with gastrointestinal malignancies. Several reports have focused on the utility of CEA measurements in cancer progression, recurrence, and prognosis in patients with gastric carcinoma. Vascular endothelial growth factor receptor-3 (VEGFR-3) expression has been demonstrated in a variety of human malignancies. CEA reflects the tumor burden, and VEGFR-3 is associated with tumor progression. For oxaliplatin-associated chemotherapy of advanced gastric cancer, seeking a more reliable predictive marker for chemotherapy has been of great interest in research and clinical settings.
Research frontiers
In this study, serum VEGFR-3 and CEA levels were assessed in advanced gastric cancer. The authors observed that VEGFR-3 and CEA could help predict survival in advanced gastric cancer patients treated with oxaliplatin plus 5-fluorouracil and folinic acid (FOLFOX).
Innovations and breakthroughs
High serum VEGFR-3 levels are correlated significantly with poorer survival. In patients with a higher serum VEGFR-3 level, alternative chemotherapy regimens should be considered.
Applications
The results of this study will help predict survival in advanced gastric cancer patients treated with FOLFOX chemotherapy.
Peer review
In this paper, the authors analyzed the clinicopathological parameters, serum CEA, serum VEGFR-3 levels and survival of advanced gastric cancer patients treated with FOLFOX. Based on the results of the study, the authors concluded that a high serum VEGFR-3 level is significantly correlated to poorer survival of the patients.
Footnotes
Peer reviewer: Masahiro Iizuka, MD, PhD, Director, Akita Health Care Center, Akita Red Cross Hospital, 3-4-23, Nakadori, Akita, 010-0001, Japan
S- Editor Wang JL L- Editor Ma JY E- Editor Zheng XM
References
- 1.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–424. doi: 10.1136/pgmj.2004.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001;22:318–322. doi: 10.1159/000050633. [DOI] [PubMed] [Google Scholar]
- 4.Mihmanli M, Dilege E, Demir U, Coskun H, Eroglu T, Uysalol MD. The use of tumor markers as predictors of prognosis in gastric cancer. Hepatogastroenterology. 2004;51:1544–1547. [PubMed] [Google Scholar]
- 5.Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000;16:105–110. doi: 10.1155/2000/595492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SR, Jang JS, Lee JH, Roh MH, Kim MC, Lee WS, Qureshi W. Role of serum tumor markers in monitoring for recurrence of gastric cancer following radical gastrectomy. Dig Dis Sci. 2006;51:2081–2086. doi: 10.1007/s10620-006-9166-5. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, Kodaira S, Okajima K, Nakazato H. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003;6:142–145. doi: 10.1007/s10120-003-0240-9. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 10.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 11.Adachi Y, Shiraishi N, Suematsu T, Shiromizu A, Yamaguchi K, Kitano S. Most important lymph node information in gastric cancer: multivariate prognostic study. Ann Surg Oncol. 2000;7:503–507. doi: 10.1007/s10434-000-0503-1. [DOI] [PubMed] [Google Scholar]
- 12.Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, Chou CH, Jeng YM, Wang MY, Chang KJ, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457–464. doi: 10.1002/cncr.11073. [DOI] [PubMed] [Google Scholar]
- 14.Mylona E, Alexandrou P, Mpakali A, Giannopoulou I, Liapis G, Markaki S, Keramopoulos A, Nakopoulou L. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol. 2007;33:294–300. doi: 10.1016/j.ejso.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 15.White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, Murray JC. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669–1675. [PubMed] [Google Scholar]
- 16.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H, et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9:1361–1369. [PubMed] [Google Scholar]
- 17.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Umemoto M, Sakamoto T, Sato S, et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003;88:237–244. doi: 10.1038/sj.bjc.6600701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keam B, Im SA, Han SW, Ham HS, Kim MA, Oh DY, Lee SH, Kim JH, Kim DW, Kim TY, et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer. 2008;8:148. doi: 10.1186/1471-2407-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZF, Guo QS, Zhang XQ, Yang XG, Guan F, Fu Z, Wang MY. Biweekly oxaliplatin in combination with continuous infusional 5-fluorouracil and leucovorin (modified FOLFOX-4 regimen) as first-line chemotherapy for elderly patients with advanced gastric cancer. Am J Clin Oncol. 2008;31:259–263. doi: 10.1097/COC.0b013e31815d43ee. [DOI] [PubMed] [Google Scholar]
- 20.Luo HY, Xu RH, Zhang L, Li YH, Shi YX, Lin TY, Han B, Wang F, Qiu MZ, He YJ, et al. A pilot study of oxaliplatin, fluorouracil and folinic acid (FOLFOX-6) as first-line chemotherapy in advanced or recurrent gastric cancer. Chemotherapy. 2008;54:228–235. doi: 10.1159/000140467. [DOI] [PubMed] [Google Scholar]
- 21.Zhao JG, Qiu F, Xiong JP, Zhang L, Xiang XJ, Yu F, Yan J, Zhan ZY, Feng M. A phase II study of modified FOLFOX as first-line chemotherapy in elderly patients with advanced gastric cancer. Anticancer Drugs. 2009;20:281–286. doi: 10.1097/CAD.0b013e328324bbc1. [DOI] [PubMed] [Google Scholar]
- 22.Seo BG, Kwon HC, Oh SY, Lee S, Kim SG, Kim SH, Han H, Kim HJ. Comprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI. Oncol Rep. 2009;22:127–136. [PubMed] [Google Scholar]
- 23.Wei J, Zou Z, Qian X, Ding Y, Xie L, Sanchez JJ, Zhao Y, Feng J, Ling Y, Liu Y, et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br J Cancer. 2008;98:1398–1402. doi: 10.1038/sj.bjc.6604317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller R, Siegmund D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–1016. [Google Scholar]
- 25.Halpern J. Maximally selected chi-square statistics for small samples. Biometrics. 1982;38:1017–1023. [Google Scholar]
- 26.LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics. 1992;48:411–425. [PubMed] [Google Scholar]
- 27.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 28.Choi JH, Oh YH, Park YW, Baik HK, Lee YY, Kim IS. Correlation of vascular endothelial growth factor-D expression and VEGFR-3-positive vessel density with lymph node metastasis in gastric carcinoma. J Korean Med Sci. 2008;23:592–597. doi: 10.3346/jkms.2008.23.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jüttner S, Wissmann C, Jöns T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Höcker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 30.Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer. 2005;115:388–392. doi: 10.1002/ijc.20859. [DOI] [PubMed] [Google Scholar]
- 31.Mouawad R, Spano JP, Comperat E, Capron F, Khayat D. Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: correlation with clinical parameters and outcome. Eur J Cancer. 2009;45:1407–1414. doi: 10.1016/j.ejca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Jenny B, Harrison JA, Baetens D, Tille JC, Burkhardt K, Mottaz H, Kiss JZ, Dietrich PY, De Tribolet N, Pizzolato GP, et al. Expression and localization of VEGF-C and VEGFR-3 in glioblastomas and haemangioblastomas. J Pathol. 2006;209:34–43. doi: 10.1002/path.1943. [DOI] [PubMed] [Google Scholar]
- 33.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 34.Bando H, Brokelmann M, Toi M, Alitalo K, Sleeman JP, Sipos B, Gröne HJ, Weich HA. Immunodetection and quantification of vascular endothelial growth factor receptor-3 in human malignant tumor tissues. Int J Cancer. 2004;111:184–191. doi: 10.1002/ijc.20211. [DOI] [PubMed] [Google Scholar]
- 35.Leclers D, Durand K, Cook-Moreau J, Rabinovitch-Chable H, Sturtz FG, Rigaud M. VEGFR-3, VEGF-C and VEGF-D mRNA quantification by RT-PCR in different human cell types. Anticancer Res. 2006;26:1885–1891. [PubMed] [Google Scholar]
- 36.Li R, Younes M, Wheeler TM, Scardino P, Ohori M, Frolov A, Ayala G. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate. 2004;58:193–199. doi: 10.1002/pros.10321. [DOI] [PubMed] [Google Scholar]
- 37.Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201:93–99. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 38.DePrimo SE, Bello C. Surrogate biomarkers in evaluating response to anti-angiogenic agents: focus on sunitinib. Ann Oncol. 2007;18 Suppl 10:x11–x19. doi: 10.1093/annonc/mdm409. [DOI] [PubMed] [Google Scholar]
- 39.Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon CS, DePrimo SE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 40.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 41.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 42.Kochi M, Fujii M, Kanamori N, Kaiga T, Kawakami T, Aizaki K, Kasahara M, Mochizuki F, Kasakura Y, Yamagata M. Evaluation of serum CEA and CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer. 2000;3:177–186. doi: 10.1007/pl00011715. [DOI] [PubMed] [Google Scholar]
- 43.Ogata Y, Murakami H, Sasatomi T, Ishibashi N, Mori S, Ushijima M, Akagi Y, Shirouzu K. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. J Surg Oncol. 2009;99:65–70. doi: 10.1002/jso.21161. [DOI] [PubMed] [Google Scholar]
- 44.Park BW, Oh JW, Kim JH, Park SH, Kim KS, Kim JH, Lee KS. Preoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomes. Ann Oncol. 2008;19:675–681. doi: 10.1093/annonc/mdm538. [DOI] [PubMed] [Google Scholar]
- 45.Park JW, Lim SB, Kim DY, Jung KH, Hong YS, Chang HJ, Choi HS, Jeong SY. Carcinoembryonic antigen as a predictor of pathologic response and a prognostic factor in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and surgery. Int J Radiat Oncol Biol Phys. 2009;74:810–817. doi: 10.1016/j.ijrobp.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 46.Perez RO, São Julião GP, Habr-Gama A, Kiss D, Proscurshim I, Campos FG, Gama-Rodrigues JJ, Cecconello I. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum. 2009;52:1137–1143. doi: 10.1007/DCR.0b013e31819ef76b. [DOI] [PubMed] [Google Scholar]
- 47.Uehara M, Kinoshita T, Hojo T, Akashi-Tanaka S, Iwamoto E, Fukutomi T. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 2008;13:447–451. doi: 10.1007/s10147-008-0773-3. [DOI] [PubMed] [Google Scholar]
- 48.Tsai HL, Chang YT, Chu KS, Chen CF, Yeh YS, Ma CJ, Wu DC, Kuo CH, Chan HM, Sheen MC, et al. Carcinoembryonic antigen in monitoring of response to cetuximab plus FOLFIRI or FOLFOX-4 in patients with metastatic colorectal cancer. Int J Biol Markers. 2008;23:244–248. doi: 10.1177/172460080802300408. [DOI] [PubMed] [Google Scholar]
- 49.Wang WS, Chen PM, Chiou TJ, Liu JH, Fan FS, Lin TC, Jiang JK, Yang SH, Yen CC, Wang HS, et al. Factors predictive of survival in patients with node-positive colorectal cancer in Taiwan. Hepatogastroenterology. 2000;47:1590–1594. [PubMed] [Google Scholar]
- 50.Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS, et al. CA19-9 as the most significant prognostic indicator of metastatic colorectal cancer. Hepatogastroenterology. 2002;49:160–164. [PubMed] [Google Scholar]
- 51.Kurenova EV, Hunt DL, He D, Fu AD, Massoll NA, Golubovskaya VM, Garces CA, Cance WG. Vascular endothelial growth factor receptor-3 promotes breast cancer cell proliferation, motility and survival in vitro and tumor formation in vivo. Cell Cycle. 2009;8:2266–2280. doi: 10.4161/cc.8.14.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moehler M, Eimermacher A, Siebler J, Höhler T, Wein A, Menges M, Flieger D, Junginger T, Geer T, Gracien E, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer. 2005;92:2122–2128. doi: 10.1038/sj.bjc.6602649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh SC, Sur HY, Sung HJ, Choi IK, Park SS, Seo JH, Jeen YT, Chun HJ, Shin SW, Mok YJ, et al. A phase II study of biweekly dose-intensified oral capecitabine plus irinotecan (bXELIRI) for patients with advanced or metastatic gastric cancer. Br J Cancer. 2007;96:1514–1519. doi: 10.1038/sj.bjc.6603752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takayama T, Sato Y, Sagawa T, Okamoto T, Nagashima H, Takahashi Y, Ohnuma H, Kuroiwa G, Miyanishi K, Takimoto R, et al. Phase I study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Br J Cancer. 2007;97:851–856. doi: 10.1038/sj.bjc.6603957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yashiro M, Shinto O, Nakamura K, Tendo M, Matsuoka T, Matsuzaki T, Kaizaki R, Ohira M, Miwa A, Hirakawa K. Effects of VEGFR-3 phosphorylation inhibitor on lymph node metastasis in an orthotopic diffuse-type gastric carcinoma model. Br J Cancer. 2009;101:1100–1106. doi: 10.1038/sj.bjc.6605296. [DOI] [PMC free article] [PubMed] [Google Scholar]


