Abstract
Objective
To record the MR imaging features of primary central nervous system lymphoma (PCNSL) and compare these features in monofocal and multifocal disease.
Materials and Methods
Twenty-one cases of monofocal disease were compared to five cases of multifocal disease. All patients were examined by non-enhanced and contrast-enhanced MRI. Tumor location, tumor size, signal intensity, enhancement characteristics, age distribution, peritumoral edema, cystic changes, and the presence of calcifications were assessed. The MRI features were compared between the monofocal and multifocal disease cases.
Results
The 26 cases, including both the monofocal and multifocal cases, exhibited 37 lesions. Contrast-enhanced images showed variable enhancement patterns: homogeneous enhancement (33 lesions), ring-like enhancement (2), and 'open-ring-like' enhancement (2). The 'notch sign' was noted in four of 33 homogeneously enhancing lesions. One case of hemorrhage and three cases of cystic formation were observed. Intra-tumoral calcification was not found. The frontal lobe, the corpus callosum and the basal ganglia were commonly affected in both the monofocal and multifocal groups. Tumor size differed significantly between the two groups (t = 3.129, p < 0.01) and mildly or moderately enhanced lesions were more frequently found in the monofocal group (p < 0.05). There was no statistical difference between perifocal edema (p > 0.05) and the signal characteristics (p > 0.05) between the two groups.
Conclusion
Our data show that PCNSL has a variable enhancement pattern on MR images. We first reported two lesions with an 'open-ring' enhancement as well as four cases with a 'notch sign'. Monofocal PCNSL cases typically have larger sized tumors with mild or moderate enhancement.
Keywords: Brain neoplasm, Lymphoma, Magnetic resonance (MR), Computed tomography (CT)
Primary central nervous system lymphoma (PCNSL) is a rare disease, accounting for 6% of all intracranial malignant tumors and for 1-2% of all lymphomas (1-3). The incidence of PCNSL has recently been on the rise, both in immunocompromised and immunocompetent populations (4, 5). A common characteristic of PCNSL is multiplicity (2, 3, 6-11).
Unlike most other brain tumors, radical surgical excision of PCNSLs is not warranted because the lesions are highly infiltrative (12). Even partial tumor resection appears to be a negative prognostic factor (13). PCNSL is a chemosensitive and radiosensitive tumor and an early diagnosis may shift the treatment from extensive surgery to radiotherapy (14). The accurate diagnosis of a PCNSL is crucial for the treatment and prognosis of the patient.
The typical imaging features of PCNSLs have been described and characterized in previous studies (1-3, 14-18). But, previous reports have not yet systematically compared the different imaging features of monofocal and multifocal PCNSLs. This comparison would be helpful for the differential diagnosis and prediction of clinical prognosis to compare monofocal and multifocal PCNSLs (19). Therefore, this study reviews 26 cases of PCNSL in immunocompetent patients with the goal (a) of identifying the clinical and neuroradiological features and (b) to compare the MRI features of PCNSL in cases of monofocal and multifocal disease.
MATERIALS AND METHODS
A retrospective review of brain tumors in the pathology archives of our institution, from 1996 to 2007, revealed 26 cases of PCNSL. Only patients without a history of acquired immunodeficiency syndrome or other congenital immunodeficiency disorders were included in the study. This retrospective study was performed with the approval of the review board and ethics committee of our institution.
The evaluated patient group consisted of twelve women and fourteen men ranging between 19 and 73 years of age (average 51.5 ± 14.9 years) at the time of observation. Both non-enhanced and contrast-enhanced MR images were acquired for all patients. Twenty-one scans were performed at 1.5T, whereas five scans were performed at 0.5T.
The MR images at 1.5T (Horizontal, GE Medical Systems, Milwaukee, WI) were acquired with a slice thickness of 8 mm. Axial pre-contrast spin echo (SE) T1 weighted image (WI) were obtained in all patients. Additional pre-contrast scans were available for some patients: axial SE T2WI (18 patients) or axial fluid attenuated inversion recovery sequence (FLAIR) (19 patients) so that all 26 patients had at least a T2WI or FLAIR sequence; coronal pre-contrast SE T1WI and T2WI (6 patients). Subsequently, an axial gadolinium-diethylenetriaminepentaacetate (Gd-DTPA)-enhanced SE T1WI was obtained in all patients. An additional coronal Gd-DTPA-enhanced SE T1WI was obtained in 12 patients; whereas, a sagittal enhanced SE T1WI was obtained in 14 patients.
At 0.5T (Signa Contour, GE Medical Systems, Milwaukee, WI), MR images were acquired at a slice thickness of 8 mm. Axial SE T1WI and T2WI were obtained before contrast medium administration. Subsequently, axial and sagittal Gd-DTPA-enhanced SE T1WI images were obtained.
CT (HighSpeed, GE Medical Systems, Milwaukee, WI) scans were performed in thirteen patients by 8 mm thick axial plain scan images. Additionally, axial contrast-enhanced images were obtained in three patients.
Two experienced radiologists independently reviewed and analyzed the images retrospectively. All scans were reviewed according to their location, including deep or superficial location in the brain, size, margin, and the characteristics of signal intensity. The presence of calcifications, cystic or necrotic changes, hemorrhage, and characteristics of enhancement were also examined. The presence of calcifications was confirmed on non-enhanced CT scans, if available. Cystic tumor components were defined as areas, which appeared hypointense on T1WI, hyperintense on T2WI and hypointense on FLAIR sequences. Hemorrhage was defined as areas that appeared hyperintense on both T1WI and T2WI. A 'notch sign' was defined as an abnormally deep depression at the tumor margin, whereas 'open-ring' enhancement was defined as when the enhanced ring was not closed.
The degree of edema was judged as follows: a mean diameter of peritumoral edema of less than 10 mm was graded as mild edema, a mean diameter of 11-20 mm as moderate edema, and a mean diameter of more than 20 mm as marked edema.
In the study, the degree of contrast enhancement of the tumor (dCt) was calculated according to literature findings (20), a dCt less than 25 was graded mild enhancement, a dCt between 25-50 was graded as moderate enhancement, and a dCt of more than 50 was graded as marked enhancement.
Histopathology and immunohistochemistry assessments were performed in all cases. For an immunohistochemistry assessment, paraffin sections were treated with monoclonal antibodies against common leukocyte antigen (CLA) and human B-lymphocytes (mAb L26).
The statistical analyses were performed by the statistician of our institution. In brief, a student t-test was used to test the difference in tumor size between monofocal and multifocal disease; whereas, the Chi-square test was used for testing for the difference in peritumoral edema, enhancement degree and signal characteristics. Statistical significance was set at a p-value < 0.05.
RESULTS
Clinical Findings
In the cohort, none of the patients had a history of acquired immunodeficiency syndrome or other congenital immunodeficiency disorders. The clinical signs at the time of presentation were disturbance of intellectual functions and behavioral problems in 17 patients (65%), hemiplegia in 12 (46%), signs of acute intracranial hypertension in eight (31%), cranial nerve deficits in seven (27%), and cerebellar syndromes or epileptic seizures in five (19%). The average duration of clinical symptoms at the time of diagnosis was 4.3 months.
Ten cases (39%) were correctly diagnosed as PCNSL. On the other hand, four cases were preoperatively misdiagnosed as gliomas, five cases were misdiagnosed as metastatic tumors, and three cases were misdiagnosed as meningiomas. The four remaining cases were mistakenly diagnosed as an astrocytoma, hemangioblastoma, glomus jugulare tumor, or craniopharyngioma.
Histopathology and Immunohistochemistry
The histological diagnosis could be ascertained in each case by a positive CLA reaction. Based on the Kiel classification, all lymphomas in the cohort were of B-cell origin. Some were further classified into subtypes: diffuse large cell (13 patients), immunoblastic (5), lymphocytic (3), small cleaved (2), or diffuse mixed (3).
Neuroradiological Findings
In our cohort, five patients (19%) had multifocal lesions, which added up to a total of 37 lesions. The neuroradiological appearances of PCNSL were therefore classified as two groups: monofocal and multifocal disease.
Monofocal Group
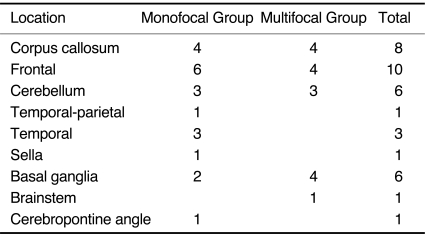
The monofocal group included 21 patients (21 of 26, 11 women and 10 men) that had solitary lesions. The mean age was 53 ± 15.6 years (range 19-71 years). The lesions were primarily located in the frontal lobe (n = 6), the corpus callosum (n = 4), the temporal lobe (n = 3) and the cerebellum (n = 3) (Table 1). Eighteen lesions were located deep within the brain, whereas the other three were located superficially. The mean size of the lesions was 39 ± 1.71 cm (range 2.0-7.0 cm). All lesions in the corpus callosum involved the genu or splenium. On T1WI sequences, all lesions appeared to be hypointense. On T2WI or on FLAIR sequences, 14 lesions appeared to be hyperintense, whereas the remaining seven tumors appeared to be isointense.
Table 1.
Location of Lesions
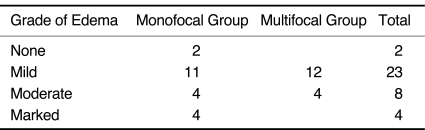
Perilesional edema was present in 19 cases of monofocal tumors, 11 cases of which showed mild, four showed moderate and four showed marked edema. The other two cases of monofocal tumors showed no edema (Table 2). The degree of edema was not consistent with tumor size; some small lesions showed disproportionately marked edema. Lesions in the posterior fossa had no edema or mild edema, while moderate or marked perifocal edema was found in supratentorial lesions.
Table 2.
Edema Grade of Lesions
Cystic changes were found in two cases. The mean size of the two tumors was 2.8 cm (3.0 cm and 2.6 cm). A small round 2 mm diameter cyst was found in one case, whereas a stripy cyst formation was found for the other case. A histologically confirmed subacute hemorrhage, appearing as a patchy hyperintense signal on T1WI, was found in one tumor in the sellar region.
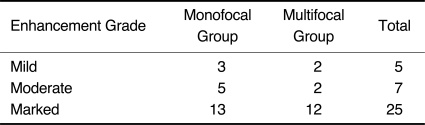
Monofocal lesions showed a variable contrast enhancement ranging from mild (n = 3), to moderate (n = 5), and to marked enhancement (n = 13) (Table 3). The enhancement was homogeneous in 19 cases. In three of these 19 cases, an abnormally deep depression was found at the tumor margin, which we called 'notch sign' (Figs. 1-3). A ring-like enhancement was found in two cases in which the ring was not closed and it was called 'open-ring' enhancement (Figs. 4, 5). The open-ring like enhancement was thick and not uniform.
Table 3.
Enhancement Grade of Lesions
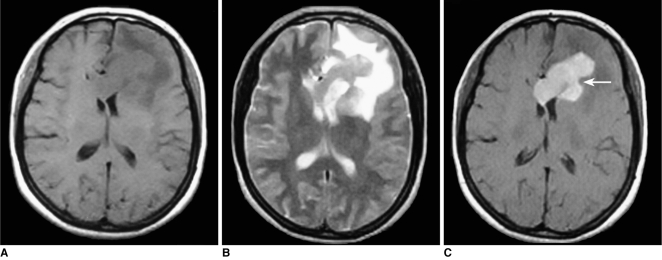
Fig. 1.
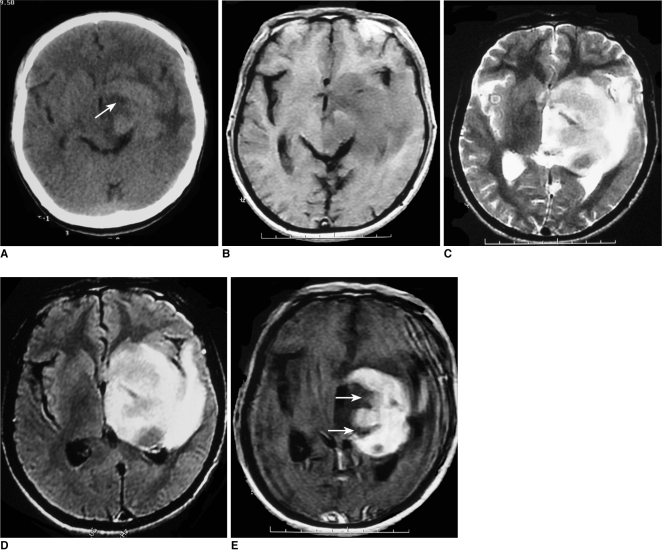
Images of 52-year-old man with lymphoma of left basal ganglia including plain CT image (A), axial T1-weighted image (B), T2-weighted image (C), FLAIR (D) and postcontrast axial T1-weighted image (E). Abnormally deep depression at tumor margin (notch sign) (arrow) was found on post-contrast axial T1-weighted image and plain CT image.
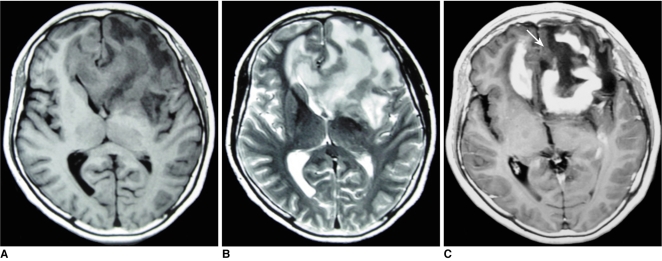
Fig. 3.
Images of 49-year-old woman with primary central nervous system lymphoma including pre-contrast axial T1-weighted image (A), pre-contrast T2-weighted image (B), post-contrast axial T1-weighted image (C). 'Notch sign' (arrow) was found after contrast injection.
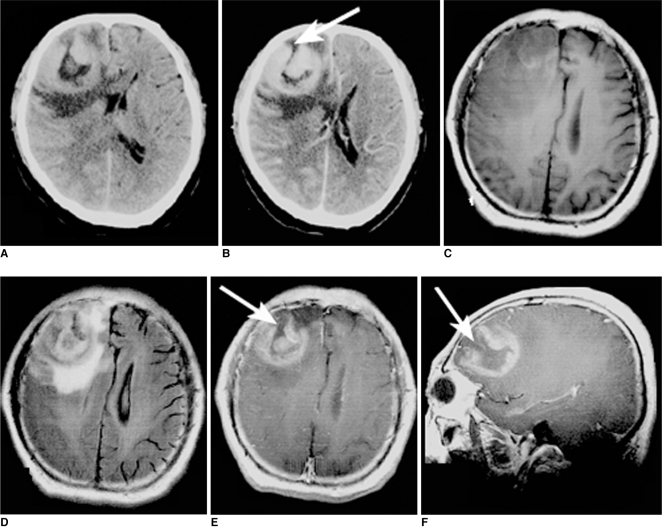
Fig. 4.
Images of 57-year-old man with lymphoma of right frontal lobe including plain CT image (A), contrast CT image (B), axial T1-weighted image (C), FLAIR (D), postcontrast axial T1-weighted image (E) and sagittal T1-weighted image (F). On plain CT image; lesion was hyperdense. 'Open-ring' enhancement (arrows) was found and was characterized as thick and not uniform.
Fig. 5.
Images of 56-year-old woman with lymphoma involving frontal lobe bilaterally including pre-contrast axial T1-weighted image (A), pre-contrast T2-weighted image (B) and post-contrast axial T1-weighted image (C). 'Open-ring' enhancement (arrow) was found and was characterized as thick and not uniform.
Of the 21 patients with monofocal lesions, ten received a plain CT scan. Furthermore, of these ten patients three had an available enhanced CT scan. All 10 lesions appeared slightly hyperdense compared to the normal brain. A marked and homogeneous enhancement was found in all three cases available with the enhanced CT scan.
Multifocal Group
Five patients (19%, 1 woman, 4 men) had multifocal lesions. The mean age of these five patients was 45.8 ± 9.5 years (range 36-61 years). One patient had two lesions, three patients had three lesions, and one patient had five lesions, for a total of 16 lesions. The mean lesion size was 2.2 ± 1.36 cm (range 0.5-6.2 cm). Two cases involved both sides of the tentorium, one case with two lesions involved the cerebellar hemispheres bilaterally, whereas the other two cases had only supratentorial lesions. The lesions involved the frontal lobe (n = 4), corpus callosum (n = 4), basal ganglia (n = 4), cerebellum (n = 3) and the brainstem (n = 1) (Table 1). Moreover, the lesions involved deep white or gray matter in the brain (n = 12) or a superficial location (n = 4). Multifocal lesions showed a hypointense signal on T1WI and a slightly hyperintense signal on T2WI or FLAIR imaging.
Of the 16 lesions, 12 had mild peritumoral edema, whereas the remaining four moderate edema (Table 2). A small round cyst with a 1.5 mm in diameter was found in one case.
Following the contrast agent (Gd-DTPA) injection, 12 lesions in four patients showed marked enhancement, whereas four lesions in two patients showed mild or moderate enhancement (Table 3). Enhancement was homogeneous in 14 lesions and ring-like in two lesions. The lesion with the small cyst formation showed heterogeneous enhancement. One lesion with a 'notch sign' was found. The ring of enhancement was completely closed in the two cases with ring-like enhancement. The co-existence of ring-like and homogeneous enhancement as well as of a 'notch-sign' and homogeneous enhancement was found in one case. No subependymal spread was found in the group.
One patient received both a plain and enhanced CT scan. The lesions showed hyperattenuation and moderate homogeneous enhancement after contrast injection.
Statistical Analysis
Tumor sizes in the monofocal group were significantly larger than the multifocal group (p = 0.00355). Lesions with mild or moderate enhancement were more frequent in the cases of monofocal disease (p = 0.017). There were no significant differences for perifocal edema (p = 0.054) and signal changes (p = 0.164) between the two groups.
DISCUSSION
This study involve the evaluation of the MRI findings of 26 histologically confirmed cases of PCNSL in immunocompetent patients and compares the imaging characteristics of monofocal disease with those of multifocal disease. In principle, our study confirms previous results regarding the appearance of PCNSL on MR images. However, we further detailed two characteristic enhancement features of the tumors that might be helpful in the differential diagnosis.
The mean age at diagnosis in this study was 51.5 ± 14.9 years, which is lower than in previously reported data (6, 21). In other publications, PCNSL usually occurred in the sixth decade (15, 22). There was no significant difference for mean age between monofocal and multifocal lymphomas.
Male predominance is generally recognized at a ratio of up to 1.2-1.7 (23, 24) in PCNSL. Our data confirms this with a ratio of male cases to female cases of 1.3.
Multiplicity of PCNSL is a common characteristic (2, 3). The highest reported number of lesions can be as high as eight (10). We present one case with five lesions. The frequency of the multifocal tumor shows great variation (range 0-50%) in immunocompetent patients (8, 11).
In our patient cohort, most lesions affected the frontal lobe, the corpus callosum, and the basal ganglia, which is consistent with previous studies (8, 16). No statistical difference was found for tumor location between monofocal and multifocal disease (p > 0.05). The classical 'mirror image' or 'butterfly pattern' - caused by lesions involving the genu or splenium in a symmetrical pattern - was found in four cases. We located 24% (9 of 37) of the lesions in the posterior fossa, which is higher than previously reported (12%) (7, 8). PCNSL located both above and below the tentorium is reported to be rare (11), and consistently, only 8% of the patients (2 of 26) in our study involved both sides of the tentorium.
An image analysis showed that the lesion size for monofocal disease is significantly larger than for multifocal disease at the time of diagnosis (3.8 cm versus 2.2 cm), which is consistent with previously reported results (3.9 cm versus 1.64 cm) (7, 9). The reason for the difference in lesion size is unclear. We hypothesize that multifocal tumors present symptoms at an earlier stage (and thus at a smaller size), because multiple lesions are likely to have a larger effect than single lesions.
Mild or moderate peritumoral edema is a common feature of PCNSL (11); it is generally less pronounced than in metastases or high grade gliomas (25). The fact that marked peritumoral edema is uncommon is probably due to the infiltrative nature of the tumor (3, 26). Our data support this assessment, with 84% of the evaluated lesions with mild to moderate edema. However, we found two lesions without edema and this is consistent with previous reports (2, 27). We also report four lesions with marked edema which were not reported previously as well. Contrary to Coulon's study, which reports lesser edema in the multifocal group than in the monofocal group (3), we found no statistical significance between the groups.
On pre-contrast MR images PCNSL is known to appear as hypo- or iso-intense on T1WI, and hyper- or iso-intense on T2WI (3, 15-17). Consistent with and the findings above, we did not detect a statistical difference between the monofocal and the multifocal groups. In contrast, the enhancement after injection of Gd-DTPA is known to be variable (9, 15, 17), and in multifocal disease, can even enhance to a different degree within the same patient (11, 28); even tumors without any enhancement were reported (9-11, 28). In our cohort, all the lesions showed enhancement, but the degree and pattern was variable. Most lesions (25 of 37) showed marked enhancement, while the rest (12 of 37) exhibited mild or moderate enhancement. Mild or moderate enhancement was more frequent in monofocal disease.
As for the enhancement pattern, our data and previous studies concur in that enhancement is most commonly homogeneous (14-18). Ring-like enhancement can be found in immunodeficient patients, but is rare in immunocompetent patients (7, 17). Lesions with gyral-like and a radial enhancement pattern has been reported previously (3, 27, 29). To the best of our knowledge, this study is the first to report two specific enhancement patterns: 1) Two tumors showed an open-ring enhancement, which is considered highly specific for atypical brain demyelination (30, 31). However, the ring of enhancement is thin and uniform in brain demyelination, whereas in PCNSL, the ring is thick and not uniform. 2) We report four homogeneously enhanced tumors with a 'notch sign'. The findings suggest that PCNSL should be suspected when lesions show these two specific enhancement patterns. Thus, the two features may be helpful for the differential diagnosis of brain tumors. The specificity will need to be tested in a larger series of brain tumors.
The typical CT finding of PCNSL is that of a primarily iso- or hyperdense space-occupying mass with marked homogeneous enhancement (7, 8). In this study, all lesions (n = 12) with available CT data showed these features.
Furthermore, there are several uncommon findings for PCNSL in immunocompetent patients. First, cystic changes are a very uncommon finding in PCNSL in immunocompetent patients and when present, are usually small single cysts (3, 11, 28). In the present patient population, three small intratumoral cysts were present (average diameter 3 mm), which is in line with previously reported data (11). Secondly, intratumoral calcifications are rare in PCNSL, and to our knowledge, only two cases of PCNSL with calcification before treatment have been reported (3, 11). Therefore, calcifications must be considered as a useful imaging characteristic to differentiate PCNSL from other intracranial tumors that have a tendency to calcify. However, calcifications are more frequently found after the patient has undergone chemotherapy or radiation treatment (3, 14). Lastly, very few cases with intratumoral hemorrhage have been reported in the literature (3, 7, 27). Tumors with hemorrhage have a wide distribution. Our study includes one lesion with chronic hemorrhage in the sellar region.
There are some limitations in the study. In our cohort, only 10 patients (9 with monofocal lesion, 1 with multifocal lesion) had complete follow-up data; therefore, the difference in clinical prognosis between the two groups could not be compared. The slice thickness used for MRI or CT was 8 mm, which was thicker compared with the 5 mm slice thickness usually used in many hospitals. The difference in slice thickness may affect the detection rate of small parenchymal lesions. Although, to the best of our knowledge, this is the first report of the 'notch sign' and 'open ring sign' in PCNSL and the specificity for the differential diagnosis needs to be tested in a large series of brain tumors.
In summary, our study of the MR imaging data of 26 cases of PCNSL in immunocompetent patients concurs with previously described patient populations. Tumors are mostly present in the 5th or 6th decade and they can be monofocal or multifocal. Lesions are typically located in the frontal lobe, corpus callosum, or the basal ganglia, and may present with a 'butterfly sign'. On pre-contrast MR images tumors usually appear hypo- or iso-intense on T1WI and hyperintense on T2WI. Further, the tumors are typically surrounded by edema and post Gd-DTPA enhancement is commonly homogeneous but variable. To the best of our knowledge, we are the first to report an 'open-ring' and a 'notch sign' as enhancement patterns. Calcifications, cyst formations, and hemorrhage are rare findings and are rather indicative of other diseases. Considering the variable appearance of PCNSL in either monofocal or multifocal disease, awareness of the tumor appearance spectrum reviewed in this study would be helpful for a correct diagnosis.
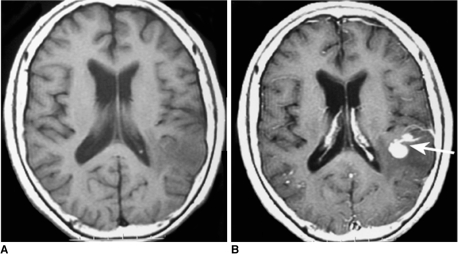
Fig. 2.
Images of 71-year-old woman with lymphoma of left temporal lobe including pre-contrast axial T1-weighted image (A), post-contrast axial T1-weighted image (B). 'Notch sign' (arrow) was found on post-contrast T1-weighted image.
References
- 1.Gutmann J, Kendall B. Unusual appearances of primary central nervous system non-Hodgkin's lymphoma. Clin Radiol. 1994;49:696–702. doi: 10.1016/s0009-9260(05)82663-7. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe M, Tanaka R, Takeda N, Wakabayashi K, Takahashi H. Correlation of computed tomography with the histopathology of primary malignant lymphoma of the brain. Neuroradiology. 1992;34:36–42. doi: 10.1007/BF00588431. [DOI] [PubMed] [Google Scholar]
- 3.Coulon A, Lafitte F, Hoang-Xuan K, Martin-Duverneuil N, Mokhtari K, Blustajn J, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002;12:329–340. doi: 10.1007/s003300101037. [DOI] [PubMed] [Google Scholar]
- 4.Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H. Pathology with clinical correlations of primary central nervous system non-Hodgkin's lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer. 1994;74:1383–1397. doi: 10.1002/1097-0142(19940815)74:4<1383::aid-cncr2820740432>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Nitta T, Uda K, Ebato M, Ikezaki K, Fukui M, Sato K. Primary peripheral-postthymic T-cell lymphoma in the central nervous system: immunological and molecular approaches to diagnosis. J Neurosurg. 1995;82:77–82. doi: 10.3171/jns.1995.82.1.0077. [DOI] [PubMed] [Google Scholar]
- 6.Slone HW, Blake JJ, Shah R, Guttikonda S, Bourekas EC. CT and MRI findings of intracranial lymphoma. AJR Am J Roentgenol. 2005;184:1679–1685. doi: 10.2214/ajr.184.5.01841679. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Reese DF, Scheithauer BW. Radiographic findings in 32 cases of primary CNS lymphoma. AJR Am J Roentgenol. 1986;146:271–276. doi: 10.2214/ajr.146.2.271. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, O'Neill BP, Banks PM, Reese DF. Central nervous system lymphoma: histologic types and CT appearance. Radiology. 1988;167:211–215. doi: 10.1148/radiology.167.1.3279454. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BA, Fram EK, Johnson PC, Jacobowitz R. The variable MR appearance of primary lymphoma of the central nervous system: comparison with histopathologic features. AJNR Am J Neuroradiol. 1997;18:563–572. [PMC free article] [PubMed] [Google Scholar]
- 10.Küker W, Nägele T, Korfel A, Heckl S, Thiel E, Bamberg M, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72:169–177. doi: 10.1007/s11060-004-3390-7. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins CN, Colquhoun IR. Characterization of primary intracranial lymphoma by computed tomography: an analysis of 36 cases and a review of the literature with particular reference to calcification haemorrhage and cyst formation. Clin Radiol. 1998;53:428–434. doi: 10.1016/s0009-9260(98)80271-7. [DOI] [PubMed] [Google Scholar]
- 12.Onda K, Wakabayashi K, Tanaka R, Takahashi H. Intracranial malignant lymphomas: clinicopathological study of 26 autopsy cases. Brain Tumor Pathol. 1999;16:29–35. doi: 10.1007/BF02478899. [DOI] [PubMed] [Google Scholar]
- 13.Bataille B, Page P. Prognostic factors and primary cerebral lymphoma. Neurochirurgie. 1997;43:385–387. [French] [PubMed] [Google Scholar]
- 14.Dubuisson A, Kaschten B, Lénelle J, Martin D, Robe P, Fassotte MF, et al. Primary central nervous system lymphoma report of 32 cases and review of the literature. Clin Neurol Neurosurg. 2004;107:55–63. doi: 10.1016/j.clineuro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Herrlinger U, Schabet M, Bitzer M, Petersen D, Krauseneck P. Primary central nervous system lymphoma: from clinical presentation to diagnosis. J Neurooncol. 1999;43:219–226. doi: 10.1023/a:1006298201101. [DOI] [PubMed] [Google Scholar]
- 16.Erdag N, Bhorade RM, Alberico RA, Yousuf N, Patel MR. Primary lymphoma of the central nervous system: typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol. 2001;176:1319–1326. doi: 10.2214/ajr.176.5.1761319. [DOI] [PubMed] [Google Scholar]
- 17.Gliemroth J, Kehler U, Gaebel C, Arnold H, Missler U. Neuroradiological findings in primary cerebral lymphomas of non-AIDS patients. Clin Neurol Neurosurg. 2003;105:78–86. doi: 10.1016/s0303-8467(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 18.Cellerier P, Chiras J, Gray F, Metzger J, Bories J. Computed tomography in primary lymphoma of the brain. Neuroradiology. 1984;26:485–492. doi: 10.1007/BF00342686. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg. 1988;68:835–853. doi: 10.3171/jns.1988.68.6.0835. [DOI] [PubMed] [Google Scholar]
- 20.Holodny AI, Nusbaum AO, Festa S, Pronin IN, Lee HJ, Kalnin AJ. Correlation between the degree of contrast enhancement and the volume of peritumoral edema in meningiomas and malignant gliomas. Neuroradiology. 1999;41:820–825. doi: 10.1007/s002340050848. [DOI] [PubMed] [Google Scholar]
- 21.Koeller KK, Smirniotopoulos JG, Jones RV. Primary central nervous system lymphoma: radiologic-pathologic correlation. Radiographics. 1997;17:1497–1526. doi: 10.1148/radiographics.17.6.9397461. [DOI] [PubMed] [Google Scholar]
- 22.Brown JH, Stallmeyer MJ, Lustrin ES, Chew FS. Primary cerebral lymphoma. AJR Am J Roentgenol. 1995;165:626. doi: 10.2214/ajr.165.3.7645482. [DOI] [PubMed] [Google Scholar]
- 23.Reni M, Ferreri AJ, Garancini MP, Villa E. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol. 1997;8:227–234. doi: 10.1023/a:1008201717089. [DOI] [PubMed] [Google Scholar]
- 24.Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43:199–201. doi: 10.1023/a:1006290032052. [DOI] [PubMed] [Google Scholar]
- 25.Jiddane M, Nicoli F, Diaz P, Bergvall U, Vincentelli F, Hassoun J, et al. Intracranial malignant lymphoma. Report of 30 cases and review of the literature. J Neurosurg. 1986;65:592–599. doi: 10.3171/jns.1986.65.5.0592. [DOI] [PubMed] [Google Scholar]
- 26.Thomas M, MacPherson P. Computed tomography of intracranial lymphoma. Clin Radiol. 1982;33:331–336. doi: 10.1016/s0009-9260(82)80283-3. [DOI] [PubMed] [Google Scholar]
- 27.DeAngelis LM. Cerebral lymphoma presenting as a nonenhancing lesion on computed tomographic/magnetic resonance scan. Ann Neurol. 1993;33:308–311. doi: 10.1002/ana.410330313. [DOI] [PubMed] [Google Scholar]
- 28.Terae S, Ogata A. Nonenhancing primary central nervous system lymphoma. Neuroradiology. 1996;38:34–37. doi: 10.1007/BF00593213. [DOI] [PubMed] [Google Scholar]
- 29.Braks E, Urbach H, Pels H, Träber F, Block W, Schild HH. Primary central nervous system immunocytoma: MRI and spectroscopy. Neuroradiology. 2000;42:738–741. doi: 10.1007/s002340000392. [DOI] [PubMed] [Google Scholar]
- 30.Masdeu JC, Quinto C, Olivera C, Tenner M, Leslie D, Visintainer P. Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology. 2000;54:1427–1433. doi: 10.1212/wnl.54.7.1427. [DOI] [PubMed] [Google Scholar]
- 31.Bizzi A, Movsas B, Tedeschi G, Phillips CL, Okunieff P, Alger JR, et al. Response of non-Hodgkin lymphoma to radiation therapy: early and long-term assessment with H-1 MR spectroscopic imaging. Radiology. 1995;194:271–276. doi: 10.1148/radiology.194.1.7997566. [DOI] [PubMed] [Google Scholar]