Abstract
Study Objectives:
The aim was to assess associations between sleep duration, sleep stages, and central obesity in women.
Design:
Cross-sectional study.
Setting:
City of Uppsala, Sweden.
Participants:
Population-based sample of 400 women (range 20-70 years).
Interventions:
Full-night polysomnography and measurement of anthropometric variables.
Measurements and Results:
Sleep duration was inversely related to both waist circumference and sagittal abdominal diameter. Sleep duration remained inversely related to waist circumference (adj. β = −1.22 cm/h; P = 0.016) and sagittal abdominal diameter (adj. β = −0.46 cm/h; P = 0.001) after adjusting for potential confounders. Duration of slow wave sleep (SWS, adj. β = −0.058 cm/min; P = 0.025) and REM sleep (adj. β = −0.062 cm/min; P = 0.002) were both inversely related to waist circumference after adjustments. Moreover, duration of REM sleep was inversely related to sagittal abdominal diameter (adj. β = −0.021 cm/min; P < 0.0001). These associations were stronger in young women (age < 50 years).
Conclusion:
An inverse relationship between short sleep duration and central obesity was found in women after adjusting for confounders. Loss of SWS and REM sleep may be important factors in the association between sleep loss and central obesity.
Citation:
Theorell-Haglöw J; Berne C; Janson C; Sahlin C; Lindberg E. Associations between short sleep duration and central obesity in women. SLEEP 2010;33(5): 593-598.
Keywords: Sleep duration, sleep stages, central obesity, women, population-based
SLEEP DURATION IN THE GENERAL POPULATION HAS DECREASED FROM 8.5 H TO 7.2 H OVER THE LAST 30 YEARS.1,2 IN ADDITION, SLEEP COMPLAINTS ARE common, especially in women.3 Parallel to the decrease in sleep duration, the prevalence of obesity has increased, with several studies finding associations between the two conditions.4–7 An inverse relationship between self-reported sleep duration and obesity has been found in adult men and women, as well as in children.8 Short sleep duration has also been found to predict weight gain and obesity later in life.5,6
Previous studies have primarily focused on self-reported sleep duration in relation to obesity.4–7,9–13 Self-reported sleep is a subjective measure in contrast to polysomnography, which allows for detailed analysis of sleep quantity and quality. Further, earlier studies have examined the association between sleep and body mass index (BMI), whereas the association between sleep and central obesity is less clear. Central obesity is a stronger risk factor than BMI for cardiovascular disease, type 2 diabetes mellitus,14 and mortality.15 Therefore, finding the underlying causes of central obesity and potentially modifiable risk factors is important. Recently, an association between measured sleeping time and waist circumference in elderly men has been reported,16 but no data are available for women or younger populations. Furthermore, the role of sleep architecture is less clear. The aim of this study was therefore to use polysomnography to analyze potential associations between sleep duration, sleep stages, and central obesity in a population-based sample of women aged 20-70 years. By analyzing measured sleep duration and time spent in different sleep stages with polysomnography, this study may enhance our knowledge about the relationships between sleep duration, sleep stages, and central obesity in women.
METHODS
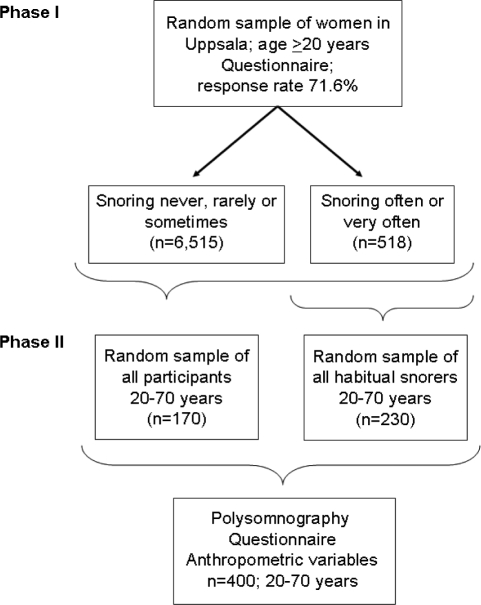
The two-phased, population-based study “Sleep and Health in Women” was conducted between 2000 and 2004. In the first phase, randomly selected women (aged ≥ 20 years) from the population registry of the city of Uppsala, Sweden were sent a questionnaire on sleep disturbances and somatic disorders. A total of 7,051 women responded, giving a response rate of 71.6%. Based on their response to a question on snoring, the participants were categorized into non-snorers (n = 6,515) and snorers (n = 518). In the second phase of the study a sample of 400 women, aged < 70 years (n = 6,112), were selected from the responders in the first phase. Of the 400 women, 230 were selected randomly from the snorers and 170 were selected randomly from the whole group. Such a sampling scheme was employed because one aim of the project “Sleep and Health in women” was to study the association between sleep-disordered breathing and glucose metabolism. Both phases of the study have been described in detail previously.17,18 A flow chart of the study design is depicted in Figure 1.
Figure 1.
Flow chart of the study design
The women filled in questionnaires that included questions on somatic disease, medication, snoring, daytime sleepiness, physical activity, tobacco use, and alcohol consumption. The participants’ physical activity was analyzed by 4 questions adopted from a questionnaire used in a large population-based study on the relationship between physical activity and mortality in women.19 Based on the responses from 6 questions assessing smoking habits,18 the participants were categorized as “current smokers,” “ex-smokers,” or “non-smokers.” Alcohol consumption was assessed by a question requiring details of the weekly consumption of alcoholic beverages. From the response to this question, the total amount of alcohol in grams per week was calculated.
All 400 women underwent a whole night polysomnography using the ambulatory system EMBLA (Flaga Inc., Reykjavik, Iceland). Sleep stages were assessed using electroencephalography, electrooculography and submental electromyography. Continuous measurement of nasal air pressure, oral and nasal airflow, thoracic and abdominal respiratory movement, and oxyhemoglobin saturation was conducted to assess sleep disordered breathing. Electrodes and sensors for the recordings were attached to the participant and connected to the recording system at the sleep laboratory in the evening preceding the polysomnography. All recordings were performed in the women’s home, except in 6 cases, where the women slept at the hospital’s patient hotel. The participants were free to choose when to go to bed and wake up. Data were downloaded to the Somnologica reviewing analysis software (Version 2.0, Flaga Inc., Reykjavik, Iceland) and sleep was scored manually in 30-sec epochs according to standard criteria.20 The polysomnographic recording was considered acceptable when there were ≥ 4 h of sleep recorded and no registration had been lost for ’ 20 min of the night. Six polysomnography recordings were repeated because of poor quality of the first recording. Total sleep time (TST), amounts of different sleep stages (in minutes and percent), and sleep efficiency (i.e., the ratio of TST to the amount of time spent in bed) were collected from the polysomnography information. In addition, the apnea-hypopnea index (AHI; the average number of apneas and hypopneas per hour of sleep) was calculated. Apnea was defined as complete cessation of nasal and oral airflow lasting ≥ 10 sec, while hypopnea was defined as a reduction in airflow of ≥ 50% compared with baseline in combination with ≥ 3% reduction in oxyhaemoglobin saturation or an arousal.
In the morning following the polysomnography the women returned to the laboratory where a research nurse measured height, weight, and central obesity (waist circumference and sagittal abdominal diameter). BMI was calculated as body mass (kg) divided by height (m) squared (kg/m2). Waist circumference was measured at standing, and the sagittal abdominal diameter was measured lying down with the back against the surface beneath.21 Both waist circumference and sagittal abdominal diameter were measured midway between the lower rib margin and the anterior superior iliac crest at the end of normal exhalation. A waist circumference ≥ 88 cm was used to define central obesity according to National Cholesterol Education Program (NCEP) criteria.22
Statistical Analyses
Statistical analyses were performed using Stata 9.0 (Stata Corporation, College Station, TX, USA). Univariate analyses were conducted using the unpaired t-test or the χ2 test to compare baseline data between groups. Associations between sleep duration and measures of obesity were further analyzed using multiple regression analysis. Results from the regression analysis are presented as adjusted estimated β-values with P-values. Statistically significant differences were assumed when P < 0.05. Based on previous analysis of this female population,18 at least 94% of the women who were 46 years of age were classified as being premenopausal, whereas at least 93% of the women 53 years of age were considered postmenopausal. Therefore, when analyzing the influence of age in the present study, the cut-off point was set at 50 years. Interaction analyses were conducted to detect significant differences in the association between sleep duration and age (dichotomized as ≥ 50 years and < 50 years). Interaction analyses were also conducted to detect significant differences in the association between sleep duration and the 2 measures of central obesity in women with and without obstructive sleep apnea (AHI ≥ 15 and < 15, respectively and AHI ≥ 5 and < 5, respectively). A P-value < 0.05 was considered indicating interaction.
The study was approved by the Ethics Committee of the Medical Faculty at Uppsala University and all participants gave their informed consent before participating.
RESULTS
Seven hundred thirty-seven women were asked to participate in the second phase of “Sleep and Health in Women,” and 400 accepted. The reasons for refusing participation were: lack of time (14.9%), ill or pregnant (7.5%), technical reasons or language difficulties (3.6%), not willing (45.4%), and reasons unknown (28.7%). The non-responders were younger (43.8 ± 14.1 y [mean ± SD] versus 47.2 ± 11.3 y; P = 0.0003) than the responders. They also had lower BMI (24.9 ± 4.6 kg/m2 versus 25.8 ± 4.7 kg/m2; P = 0.009) and were more often physically inactive (23% versus 16%; P = 0.038), compared with responders. There was no difference in self-reported sleep duration (7.0 ± 1.2 h versus 6.9 ± 1.2 h; P = 0.39), smoking (26% versus 23%; P = 0.37), or presence of somatic disease (37% versus 39%; P = 0.58) between responders and non-responders.
The centrally obese women (waist circumference ≥ 88 cm) in the study were older, had higher BMI, and had a lower level of physical activity than women without central obesity (Table 1). In addition, the centrally obese women had shorter sleep duration, less sleep efficiency, less slow wave sleep (SWS), less REM sleep, and higher AHI. The two groups did not differ significantly in either smoking status or alcohol consumption (Table 1). No difference in sleep efficiency was observed between the women randomly selected from the snoring group in Phase I and women selected from the whole group (85.9% ± 13.1% versus 84.6% ± 10.7%; P = 0.26).
Table 1.
Characteristics of the study populationa
| Central obesity (NCEP criteriab) |
P-value | ||
|---|---|---|---|
| Yes (n = 182) | No (n = 218) | ||
| Age (years) | 53.0 ± 9.7 | 47.7 ± 11.9 | < 0.0001 |
| Total sleep time (minutes) | 372.3 ± 73.9 | 397.2 ± 65.3 | 0.0004 |
| Slow wave sleep (minutes) | 33.7 ± 22.9 | 40.8 ± 22.8 | 0.002 |
| REM sleep (minutes) | 63.2 ± 29.5 | 77.5 ± 28.9 | < 0.0001 |
| Sleep efficiency (%) | 82.2 ± 13.1 | 87.5 ± 9.9 | < 0.0001 |
| Apnea-hypopnea index | 18.8 ± 19.0 | 9.1 ± 9.7 | < 0.0001 |
| Body mass index (kg/m2) | 30.4 ± 4.7 | 23.5 ± 2.4 | < 0.0001 |
| Sagittal abdominal diameter (centimeters) | 23.5 ± 0.21 | 18.7 ± 0.14 | < 0.0001 |
| Level of physical activity | < 0.0001 | ||
| High | 18 (10.1) | 48 (22.5) | |
| Medium | 127 (71.0) | 151 (70.9) | |
| Low | 34 (19.0) | 14 (6.6) | |
| Smoking | 0.12 | ||
| Non-smoker | 72 (40.5) | 109 (50.7) | |
| Ex-smoker | 63 (35.4) | 65 (30.2) | |
| Current smoker | 43 (24.2) | 41 (19.1) | |
| Alcohol consumption (grams/week) | 55.8 ± 58.0 | 54.9 ± 58.5 | 0.90 |
Results are presented as mean ± SD or n (%).
Waist circumference ≥ 88 cm
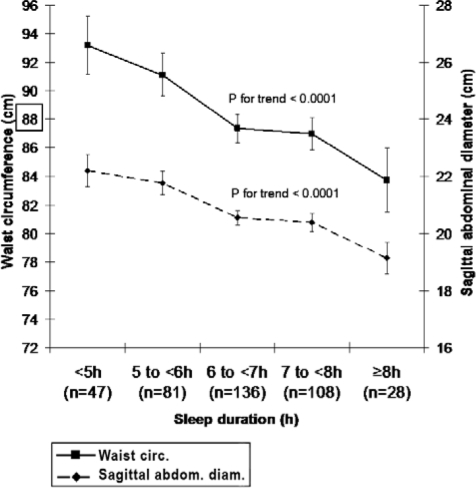
A significant negative association was observed between TST and waist circumference, and TST and sagittal abdominal diameter in unadjusted data. There was a mean difference of 9 cm in waist circumference and 3 cm in sagittal abdominal diameter between women sleeping < 5 h and women sleeping ≥ 8 h (Figure 2).
Figure 2.
Waist circumference and sagittal abdominal diameter in relation to sleep duration. Results are presented as mean ± SE. The box indicates the limit for central obesity according to National Cholesterol Education Program (NCEP) criteria.22
No interactions were detected between the selection group from Phase I (snorer or from the whole group) and any of the variables of sleep when using waist circumference (P for interaction = 0.18-0.97) or abdominal sagittal diameter (P for interaction = 0.35-0.80) as the dependent variable. Furthermore, no interactions were observed between AHI (≥ 15 and < 15) and TST when using waist circumference (P = 0.25) or sagittal abdominal diameter (P = 0.30) as the dependent variable. This was also true when using the cut off point AHI 5 (P = 0.37 and 0.16, respectively). Therefore, the multivariate analysis model was performed in the whole group adjusting for AHI as a continuous variable (Table 2). There was an inverse relationship between TST (hours) and both measures of central obesity after adjusting for confounders. This adjusted relationship showed that an increase by 1 hour in TST was associated with a decrease of 1.24 cm in waist circumference and 0.46 cm in abdominal sagittal diameter. SWS (in minutes) was significantly associated with waist circumference, and there was a trend for an association between SWS (in minutes) and sagittal abdominal diameter. Duration of REM sleep was associated with both measures of central obesity. Moreover, the association between SWS (minutes and percent) and waist circumference remained significant also after adjustment for BMI. Both TST (hours) and REM sleep (minutes and percent) was further significantly associated with sagittal abdominal diameter also when adjusting for BMI (data not shown).
Table 2.
Associations between total sleep time and measures of central obesity after adjustment for potential confoundersa,b
| Waist circumference (cm) |
Sagittal abdominal diameter (cm) |
|||
|---|---|---|---|---|
| Adj. β-value | P-value | Adj. β-value | P-value | |
| Total sleep time (hours) | −1.24 | 0.016 | −0.46 | 0.001 |
| Slow wave sleep (minutes) | −0.058 | 0.025 | −0.013 | 0.068 |
| Slow wave sleep (%) | −0.20 | 0.050 | −0.034 | 0.21 |
| REM sleep (minutes) | −0.062 | 0.002 | −0.021 | < 0.0001 |
| REM sleep (%) | −0.15 | 0.074 | −0.057 | 0.013 |
| Sleep efficiency (%) | −0.13 | 0.014 | −0.043 | 0.003 |
| Age (years) | 0.11 | 0.069 | 0.037 | 0.020 |
| Exercise level | ||||
| High | 0 | 0 | ||
| Medium | 3.21 | 0.045 | 1.34 | 0.002 |
| Low | 10.95 | < 0.0001 | 3.39 | < 0.0001 |
| Smoking | ||||
| Non-smoker | 0 | 0 | ||
| Ex-smoker | 1.06 | 0.43 | −0.12 | 0.73 |
| Currentsmoker | 1.29 | 0.41 | −0.10 | 0.81 |
| Alcohol consumption (grams/week) | 0.0061 | 0.55 | 0.0008 | 0.77 |
| Apnea-hypopnea index | 2.53 | < 0.0001 | 0.59 | < 0.0001 |
Results are presented as adjusted estimated β-values with P-values.
β-values are adjusted for age, level of physical activity, smoking status, alcohol consumption, and apnea-hypopnea index.
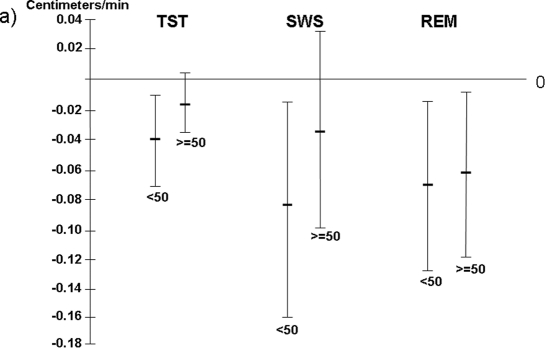
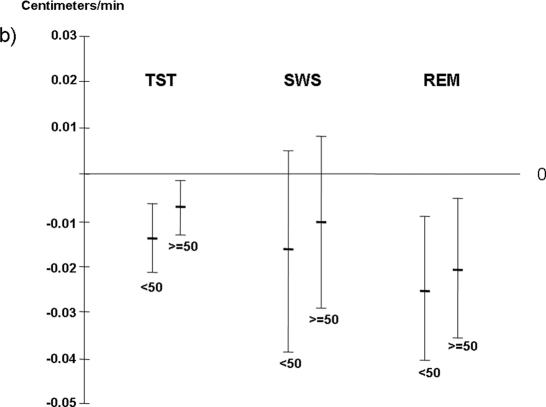
There was a significant interaction between age (< 50 years and ≥ 50 years) and TST when sagittal abdominal diameter was used as the dependent variable (P = 0.048), and a trend between these 2 variables when waist circumference served as the dependent variable (P = 0.07). A significant negative association between TST and both waist circumference and sagittal abdominal diameter was seen in women < 50 years after adjusting for confounders (Figure 3a and 3b). In addition, TST was associated with sagittal abdominal diameter in women ≥ 50 years (Figure 3a). When analyzing independent associations of different sleep stages with central obesity in the 2 age groups, both SWS duration and REM sleep duration were significantly inversely related with waist circumference in the younger women; REM sleep duration also showed a relationship with waist circumference in women ≥ 50 years (Figure 3a). REM sleep duration was further significantly associated with sagittal abdominal diameter in both age groups (Figure 3a and 3b).
Figure 3a.
Total sleep time and different sleep stages in relation to waist circumference in women < 50 years and ≥ 50 years.
Figure 3b.
Total sleep time and different sleep stages in relation to sagittal abdominal diameter in women < 50 years and ≥ 50
Results are presented as a decrease in cm of waist circumference per one-minute increase of different sleep variables. Results are adjusted for age, physical activity, AHI, smoking and alcohol consumption. TST = Total sleep time; SWS = Slow wave sleep.
DISCUSSION
The main result of this population-based study is the independent inverse relationship between objectively measured sleep duration, sleep stages, and central obesity in women. The negative association between sleep duration and measures of central obesity remained significant also after adjusting for BMI. In addition, there were independent negative associations between SWS and central obesity and between REM sleep and central obesity. These associations were stronger in the younger (< 50 years) than in the older (≥ 50 years) women.
Several studies have reported a significant inverse relationship between self-reported sleep duration and weight.5–7,10,23,24 Two studies using self-reported sleep duration have failed to demonstrate any significant independent association with central obesity.25,26 The wider age range in these two studies as compared with our study may have contributed to the discrepant findings. By using PSG to measure sleep duration (TST), we found a negative linear association with central obesity after controlling for confounding variables. Our observations also indicate that less than 6-7 hours of sleep is associated with a substantial and gradual increase in waist circumference and abdominal sagittal diameter. In contrast to our results, two previous studies using objectively measured sleep duration (measured by wrist activity monitoring or PSG) found no relationship to obesity.12,13 However, the use of BMI as the factor of obesity may have contributed to these discrepant findings.
In comparison with other measures of central obesity, sagittal abdominal diameter has a stronger correlation with measured metabolic variables, including insulin sensitivity.21 The association between objectively measured sleep duration and sagittal abdominal diameter remained significant even after adjusting for BMI, which indicates that short sleep duration is more strongly related to central obesity than to general obesity, irrespective of which of the two methods for assessment of abdominal obesity is used.
Short sleep duration could have an impact on obesity by influencing lifestyle and habits. Lack of sleep may cause daytime sleepiness and fatigue, which can lead to restriction of physical activity and, in turn, start a vicious circle of short sleep duration, physical inactivity, and weight gain.27 Moreover, less time for sleep gives more time for eating.28 Stress and emotional disturbance has also been associated with both sleep duration and obesity and could be a potential link between the two.12 Unfortunately, the present study does not have data on emotional stress.
Loss of both SWS and REM sleep were independently associated with central obesity in the present study, and the associations remained significant after adjusting for BMI, indicating a stronger relationship with central obesity than general obesity. Our study also shows that duration of SWS and REM sleep, rather than the percentage of different sleep stages, is of importance in central obesity. One recent study in elderly men assessed associations between sleep architecture and body composition, showing a relationship between SWS and waist circumference.16 However, no relationship was seen between REM sleep and body composition. The older age group and different gender may explain the discrepant findings.
Growth hormone, mostly secreted during SWS, is suppressed by reduced sleep.29 Growth hormone deficiency because of hypopituitarism is associated with visceral obesity, which is reversed by growth hormone replacement therapy.30 In the present study SWS was independently associated with waist circumference, indicating that the relationship between short sleep duration and central obesity could be mediated through an abnormal growth hormone secretion pattern. Furthermore, the amount of REM sleep may have an impact on cortisol levels in that a trend toward elevated levels of cortisol has been shown with reduced amounts of REM sleep.31 Because REM sleep was independently associated with both measures of central obesity (waist circumference and sagittal abdominal diameter), the relationship could be mediated through increased cortisol levels.
Menopausal state has been suggested to have an influence on sleep and sleep quality.32,33 In addition, menopause is also often related to weight gain.34 However, in the present female population, the influence of short sleep duration and reduced duration of SWS or REM sleep on accumulation of abdominal fat was most pronounced in women < 50 years (when the women were assumed to be premenopausal). This finding indicates that the mechanisms underlying the development of abdominal obesity may start to operate early in life. This observation finds support from the NHANES cohort, in which both men and women younger but not older than 50 years demonstrated an inverse univariate relationship between reported short sleep duration and increased prevalence of overweight or obesity.5 Multivariate analysis of data from a cohort of clinic-based patients showed no age-related difference in risk of obesity, but there was a difference between genders, with women sleeping < 8 h having an increased risk of obesity in contrast to men.35
The present study was conducted in a large population of women. Nonetheless, there are some limitations to consider when interpreting the results. Because the response rate in this study was relatively modest (54%), it could have affected the results. The non-responders were younger, had lower BMI, and were more often physically inactive. Still, there was no difference in self-reported sleep duration, smoking, or presence of somatic disease between responders and non-responders. There was only one night of polysomnography performed. Polysomnography studies have an inherent risk of a “first-night effect,” due to disturbance from the polysomnography equipment leading to shorter sleep duration. This may have restricted sleep duration in our study, because only 27 of the women slept ≥ 8 h. On the other hand, this might also be a true observation of sleep, in the sense that sleep duration has decreased over the past decades,1,2 and PSGs were not performed solely on weekends when people tend to sleep longer. Furthermore, PSG is the only method available for recording different sleep stages. In our population-based sample, there was a deliberate oversampling of snorers. Consequently, the prevalence of OSA and central obesity can be assumed to be higher in this sample than in the general population.18 However, adjusting for AHI or using sleep efficiency instead of TST as an independent variable did not significantly change any of the reported results. Furthermore, the study only included women, which may limit the generalizability of our results.
In conclusion, a relationship was observed between short sleep duration and central obesity in women, even after controlling for potential confounders. This relationship was most pronounced in women aged < 50 years. Both loss of SWS and REM sleep was associated with central obesity and may affect the relationship between sleep duration and central obesity.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Berne has participated in speaking engagements for Astellas, MSD, Novo Nordisk, Sanofi-Aventis, and Amgen. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Lars Berglund, UCR-Uppsala Clinical Research Center, Uppsala, for his statistical advice.
This study was financially supported by the Swedish Heart Lung Foundation and by the Uppsala County Association against Heart and Lung Diseases.
Footnotes
A commentary on this article appears in this issue on page 573.
REFERENCES
- 1.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 2.Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27:293–8. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg E, Janson C, Gislason T, Björnsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: Can gender differences be explained by differences in psychological status? Sleep. 1997;20:381–7. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 6.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 7.Kohatsu ND, Tsai R, Young T, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–5. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 8.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes (Lond) 2006;30:1080–5. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 9.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- 12.Vgontzas AN, Lin HM, Papaliaga M, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008;32:801–9. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 13.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 15.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 16.Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–90. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theorell-Haglöw J, Lindberg E, Janson C. What are the important risk factors for daytime sleepiness and fatigue in women? Sleep. 2006;29:751–7. doi: 10.1093/sleep/29.6.751. [DOI] [PubMed] [Google Scholar]
- 18.Svensson M, Lindberg E, Naessen T, Janson C. Risk factors associated with snoring in women with special emphasis on body mass index: a population-based study. Chest. 2006;129:933–41. doi: 10.1378/chest.129.4.933. [DOI] [PubMed] [Google Scholar]
- 19.Lissner L, Bengtsson C, Björkelund C, Wedel H. Physical activity levels and changes in relation to longevity. A prospective study of Swedish women. Am J Epidemiol. 1996;143:54–62. doi: 10.1093/oxfordjournals.aje.a008657. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages in human subjects. Washington DC: U.S National Public Health Service, U.S. Government Printing Office; 1968. [Google Scholar]
- 21.Riserus U, Arnlov J, Brismar K, Zethelius B, Berglund L, Vessby B. Sagittal abdominal diameter is a strong anthropometric marker of insulin resistance and hyperproinsulinemia in obese men. Diabetes Care. 2004;27:2041–6. doi: 10.2337/diacare.27.8.2041. [DOI] [PubMed] [Google Scholar]
- 22.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Adamkova V, Hubacek JA, Lanska V, et al. Association between duration of the sleep and body weight. Physiol Res. 2009;58(Suppl 1):S27–31. doi: 10.33549/physiolres.931853. [DOI] [PubMed] [Google Scholar]
- 24.Magee CA, Iverson DC, Caputi P. Sleep duration and obesity in middle-aged Australian adults. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.373. [DOI] [PubMed] [Google Scholar]
- 25.Ko GT, Chan JC, Chan AW, et al. Association between sleeping hours, working hours and obesity in Hong Kong Chinese: the ‘better health for better Hong Kong’ health promotion campaign. Int J Obes (Lond) 2007;31:254–60. doi: 10.1038/sj.ijo.0803389. [DOI] [PubMed] [Google Scholar]
- 26.Fogelholm M, Kronholm E, Kukkonen-Harjula K, Partonen T, Partinen M, Harma M. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes (Lond) 2007;31:1713–21. doi: 10.1038/sj.ijo.0803663. [DOI] [PubMed] [Google Scholar]
- 27.Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355–65. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73:2467–75. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel K, Leproult R, Colecchia EF, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol. 2000;279:R874–83. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- 30.Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl A):S10–17. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 32.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–49. [PMC free article] [PubMed] [Google Scholar]
- 33.Kalleinen N, Polo-Kantola P, Himanen SL, et al. Sleep and the menopause - do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 2008;14:97–104. doi: 10.1258/mi.2008.008013. [DOI] [PubMed] [Google Scholar]
- 34.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buscemi D, Kumar A, Nugent R, Nugent K. Short sleep times predict obesity in internal medicine clinic patients. J Clin Sleep Med. 2007;3:681–8. [PMC free article] [PubMed] [Google Scholar]