Abstract
Study Objectives:
Inescapable shock (IS), an uncontrollable stressor, and presentation of fearful contexts associated with IS produce prominent reductions in REM sleep. We compared sleep in animals trained with IS to that in animals trained with escapable shock (ES), a controllable stressor, in a paradigm in which animals always received shock but could terminate it by their actions.
Design:
Male BALB/cJ mice were implanted with telemetry transmitters for recording EEG and activity. After recovery from surgery, baseline sleep recordings were obtained for 2 days. The mice were then randomly assigned to receive ES (n = 9) or IS (n = 9). ES mice could escape a footshock (20 trials; 0.5 mA; 5.0 sec maximum duration; 1.0 min intervals) by moving to the unoccupied chamber in a shuttlebox. Yoked-control IS mice in a separate shuttlebox received identical footshock. The mice received 2 days of shock training (ST1; ST2) and were re-exposed to the shuttlebox without footshock (context alone).
Setting:
NA.
Patients or Participants:
NA.
Interventions:
NA.
Measurements and Results:
On each training and test day, the mice were returned to their home cages, and EEG and activity were recorded for 20 h. Freezing was scored in the context alone. Compared to baseline, ES mice showed significantly increased REM, and IS mice showed significantly decreased REM after ST1, ST2, and context alone. Total NREM was decreased after shock training only in IS mice. Contextual freezing was enhanced in both ES and IS mice.
Conclusions:
The directionally opposite changes in REM suggest that stressor controllability is an important factor in the effects of stress and stressful memories on sleep.
Citation:
Sanford LD; Yang L; Wellman LL; Liu X; Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. SLEEP 2010;33(5):621-630.
Keywords: Emotion, fear memory, mouse, rapid eye movement sleep, stress
STRESS CAN HAVE A SIGNIFICANT, LONG-LASTING NEGATIVE IMPACT ON HEALTH INCLUDING CHANGES IN BEHAVIOR AND SLEEP. HOWEVER, STRESSORS are often encountered without producing permanent or pathological changes. The difference between successful and unsuccessful coping with stress may involve the resilience of the organism1 as well as characteristics of the stressor. Stressor controllability, along with intensity and predictability, have been found to be important factors in the effects of stress.2 For example, lack of stressor controllability has been suggested to be a factor in the development of posttraumatic stress disorder (PTSD).3,4
Conditioned fear training is conducted with a fear-inducing stressor (usually inescapable shock, [IS])5,6 presented in an experimental paradigm in which the animal receiving training has no control over the stressor. Through association to IS, initially neutral environmental cues and contexts acquire the capacity to elicit behavioral and physiological responses indicative of fear and anxiety, including behavioral freezing,7–9 autonomic responses,10–12 and fear-potentiated startle.5,6
Disturbances in sleep often follow a stressful or traumatic event,13 and we and others have demonstrated that IS and IS-associated fearful cues and contexts produce similar alterations in sleep, including a prominent reduction in rapid eye movement sleep (REM).14,15 By comparison, training with avoidable, signaled shock (a controllable stressor) in a shuttlebox is followed by significant increases in REM,15–18 even though the same stressor (footshock) is initially experienced before the avoidance response is acquired. The results of these studies have typically been focused on demonstrating a correlation between REM and acquisition of the avoidance response.15–18
It is possible to train animals with escapable shock (ES) as a controllable stressor using a shuttlebox paradigm. In this paradigm, the stressor is always experienced but can be terminated by the animal's action. The effects of ES and IS have not been directly compared with respect to post-stress alterations in sleep. Therefore, in this study, we used a yoked design to examine the effects of ES and IS training and of contextual reminders of ES and IS on sleep in BALB/cJ mice, a “behaviorally reactive” strain that shows greater post-stress reductions in REM and alterations in sleep in response to IS compared to C57BL/6J mice.19–21 We also compared mice in the ES and IS conditions for potential differences in gross motor activity and we examined freezing, a common behavioral index of fear,7,22,23 during exposure to the fearful context. Examining freezing enabled us to compare immediate emotional reactivity across conditions and to determine the relationship of initial emotionality to subsequent changes in arousal and sleep.
METHODS
Subjects
The subjects were 18 male BALB/c/J mice weighing 20 to 25 g. All animals were obtained from the Jackson Laboratory, Bar Harbor, Maine. The animals were individually housed after arrival, and food and water were available ad libitum. The recording room was kept on a 12:12 light: dark cycle with lights on from 07:00 to 19:00. Ambient temperature was maintained at 24.5°C ± 0.5°.
Surgery
All mice were implanted intraperitoneally with telemetry transmitters (DataSciences ETA10-F20) for recording EEG and activity as previously described.24 EEG leads from the transmitter body were led subcutaneously to the head, and the free ends were placed into holes drilled in the dorsal skull to allow recording cortical EEG. All surgery was conducted with the mouse under isoflurane (as inhalant: 5% induction; 2% maintenance) anesthesia. Ibuprofen (30 mg/kg, oral) was continuously available in each animal's drinking water for 24 to 48 h preoperatively and for a minimum of 72 h postoperatively. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 05-017).
Training Procedures
The mice were housed and studied in the same room. For recording, individual home cages were placed on a DataSciences telemetry receiver (RPC-1) and the transmitter activated with a magnetic switch. When the animals were not on study, the transmitter was inactivated. Cages and bedding were changed 2 days prior to recording onset for each phase of the experiment and then not disturbed until that phase was complete. After a postsurgery recovery period of 10 to 14 days, uninterrupted sleep recordings were obtained for 2 days.
After habituation to the colony conditions, the mice were randomly assigned to the ES or IS group. Mice in the ES group were able to learn to escape a footshock by moving to the non-occupied chamber in a shuttlebox (Coulbourn Instruments, Model E10-15SC). This terminated shock for the ES group and also terminated shock delivery to yoked-control mice receiving IS in a separate shuttlebox. Thus, both groups received identical amounts of footshock, but the IS mice could not influence the amount of shock they received.
Training took place at the same time on 2 consecutive shock training days (ST1, ST2). The mice were allowed to freely explore the shuttlebox for 5 min after which they were presented with 20 footshocks (0.5 mA, 5.0 sec maximum duration) at 1.0-min intervals. Five min after the last shock, the mice were returned to their home cages. The entire procedure was of approximately 30-min duration and occurred during the fourth hour of the lights-on period. The chamber was thoroughly cleaned with diluted alcohol before each training session. After ST1, the animals were left undisturbed in their home cages until the training session on the following day. After ST2, the animals were left undisturbed other than a weekly cage change until the test day on which they were re-exposed to the shuttlebox without the presentation of footshock to either ES or IS groups. This re-exposure occurred 6 to 7 days after ST2 at the same time and duration (30 min) as the training sessions. On each day, after the mice were returned to their home cages, EEG and activity data were collected for 20 h.
Coulbourn Graphic State software (version 2.1) running on a Pentium-class computer was used to control the administration and timing of footshock to ensure that mice in the ES and IS conditions receive the same duration of footshock. Footshock was produced via Coulbourn Precision Regulated Animal Shockers (Model E13-14) and administered via grid floors in the shuttleboxes.
Determination of Escape Latency and Freezing
Coulbourn Graphic State software (version 2.1) used to control shock presentation recorded the time at shock onset as well as the time of shock termination. Subtracting these 2 values provided the escape latency and an approximation of the duration of shock the mice experienced on each shock trial as these varied with the response and reaction time of the mice in the ES condition. These values were summed for all 20 shock trials on ST1 and ST2 to obtain an estimate of the amount of shock the animals received.
The initial 5-min period prior to shock training and the context re-exposure session was videotaped for subsequent scoring of freezing, defined as the absence of body movement except for respiration.7,22,23 Freezing was scored by trained observers who were blind to condition in 5-sec intervals over the course of the 30 min the mice were in the shock chamber. The percentage time spent in freezing was calculated (FT%: freezing time/observed time × 100) for each animal for each observation period. The analysis of FT% contains data from 8 pair of mice. Recordings from one pair were inadvertently taped over.
Data Recording and Determination of Behavioral State
For recording, individual cages were placed on a DataSciences telemetry receiver (RPC-1) and the transmitter activated with a magnetic switch. When the animals were not on study, the transmitter was inactivated. Telemetry signals (EEG and activity) were processed and collected at 250 Hz by DataSciences Dataquest A.R.T. software (version 3.1). The records were visually scored in 10-s epochs using the SleepSign scoring program.
The EEG and activity data were saved to the hard disk for subsequent offline data analyses and for visual scoring of behavioral state. Epochs were scored by a trained observer as either active wakefulness (AW, activity recorded in epoch), quiet wakefulness (QW, no activity in epoch), NREM, or REM based on EEG and gross whole body activity as previously described.24 Transistor-transistor logic (TTL) pulses generated by the telemetry system when the mice moved around in their cages were counted as a measure of activity. This method provides only a measure of general activity and typically requires gross body movement in order to generate a count.
Data Analyses
The data for sleep and wakefulness parameters and activity were analyzed for the total 20-h recording period and also in 4-h blocks across the recording period. For the analyses of blocks, the data were collapsed to obtain two 4-h analysis blocks in the light period and three 4-h analysis blocks in dark period. The data were analyzed with Group (ES and IS) × Treatment (Baseline, ST1, ST2, and Context) or Group (ES and IS) × Block (Block 1, 2, 3, 4, and 5) mixed-factor analyses of variance (ANOVAs) with repeated measures on Treatment and Block. The escape latency and freezing data were analyzed by t-tests or mixed-factors ANOVA procedures. When indicated by significant ANOVAs, post hoc comparisons among means were conducted with Tukey tests. The following parameters were evaluated: total REM sleep, number of REM episodes, REM sleep duration, REM sleep percentage ([total REM / total sleep] * 100), total NREM sleep, number of NREM episodes, NREM episode duration, and total sleep time (TST). Parameters examined for wakefulness were amounts of AW and QW and activity level.
RESULTS
Escape Latencies and Shock Duration
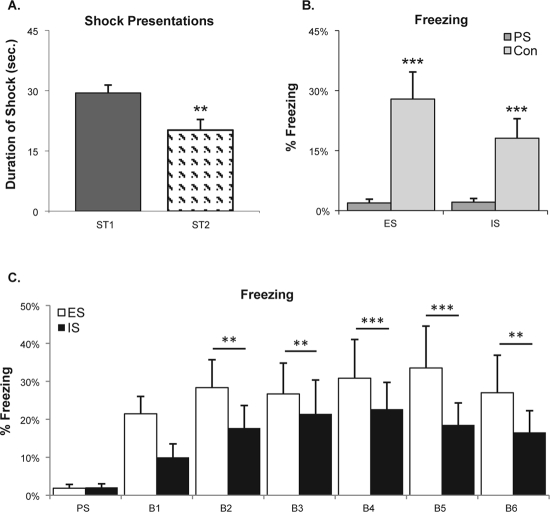
The average escape latency across trials during ST1 (1.47 ± 0.09 s) was significantly greater than during ST2 (1.01 ± 0.13 s), t8 = 3.23, P < 0.01. However, the difference was primarily due to a period of increased escape latencies running from trial 10 to trial 15 on ST1 (not shown). Escape latencies on trials 10, 11, 14, and 15 were significantly greater on ST1 than on ST2, but no other comparisons with trials were significant. The mice showed increased variability in escaping during these trials with 8 of 9 mice showing latencies on one or more trials that ranged from 2.5 s to 5.0 s. Summing the escape latencies showed that total shock received was greater on ST1 than on ST2 (Figure 1A).
Figure 1.
Mean shock duration experienced by the pairs of mice in the escapable shock (ES) and yoked control inescapable shock (IS) conditions and freezing exhibited during re-exposure to the shock context. (A) Total shock duration plotted for shock training days 1 and 2 (ST1, ST2). Significant difference in comparison of ST1 and ST2: **P < 0.01 (t-test). (B) Percent time freezing plotted for the 5-min pre-shock period (PS) when the mice were naïve and for the entire 30-min Context re-exposure (Con) after the mice had experienced ST1 and ST2. Significant difference in comparison of the PS and Con periods: ***P < 0.005 (t-test). (C) Percent time freezing plotted for the 5-min PS period and in 5-min blocks (B1-B6) across the Con period. Significant differences are indicated for comparisons between the PS period and 5-min blocks: **P < 0.01; ***P < 0.001 (Tukey test). Note that compared to the PS period, both ES and IS mice showed significantly increased freezing in the fearful context (B2-B6), but did not significantly differ between groups.
Freezing in Fearful Context
There was a significant main effect for treatment in comparisons of FT% in the 5-min pre-shock period to that in the entire 30-min Context exposure period (F1,28 = 24.93, P < 0.001), but no significant difference in FT% between the ES and IS mice (Figure 1B). Comparisons of freezing also were made between the pre-shock period and the 30-min Context exposure period divided into six 5-min blocks (Figure 1C). The ANOVA revealed a significant main effect for blocks (F6,84 = 5.20, P < 0.0002). Compared to the pre-shock period, there was significantly increased freezing in blocks 2-6 of the Context period. However, the ES and IS mice did not significantly differ in FT% in any 5-min block during re-exposure to the Context.
Effects of IS and ES on REM
The baseline recordings of the ES and IS mice did not differ for any sleep parameter we examined. In general, compared to baseline recordings, ES resulted in significant post-stress increases and IS resulted in post-stress decreases in all REM parameters that we examined (Figure 2). Significant increases for ES and decreases for IS were found for both shock training and re-exposure to the training Context.
Figure 2.
REM parameters plotted as 20-h totals for baseline (Base), the 2 shock training days (ST1, ST2) and Context (Con). A. Total REM. B. REM percentage (total REM time/ total sleep time). C. Number of REM episodes. D. REM episode duration. Significant differences between ES and IS: *P < 0.05; **P < 0.01; ***P < 0.001. Significant differences in ES compared to Base: +P < 0.05; ++P < 0.01; +++P < 0.001. Significant differences in IS compared to Base: #P < 0.05; ##P < 0.01; ###P < 0.001. All comparisons were conducted with Tukey tests.
The ANOVA for total REM over the 20-h recording period revealed a significant Group effect (F1,16 = 58.66, P < 0.00001) and a significant Group × Treatment Interaction (F3,48 = 37.55, P < 0.00000001). A significant Group effect (F1,16 = 32.96, P < 0.00004) and a significant Group × Treatment Interaction (F3,48 = 30.42, P < 0.00000001) were also found for REM percentage. Post hoc comparisons within subjects found similar patterns for both total REM (Figure 2A) and REM percentage (Figure 2B). Total REM and REM percentage was significantly increased in the ES group in ST1, ST2, and Context compared to baseline; and significantly decreased in the IS group in ST1, ST2, and Context compared to baseline. Total REM and REM percentage in ST1, ST2, and Context was significantly greater in the ES group than the yoked IS group.
The ANOVA for REM episodes (Figure 2C) for the 20-h recording period found a significant Group effect (F1,16 = 32.79, P < 0.00004) and a significant Group × Treatment Interaction (F3,48 = 18.33, P < 0.00000005). The number of REM episodes was significantly increased in the ES group in ST2 and Context compared to baseline and significantly decreased in the IS group in ST1, ST2, and Context compared to baseline. The number of REM episodes did not differ in the ES and IS groups during baseline, but were significantly higher in the ES group compared to the IS group in ST1, ST2, and Context.
The ANOVA for REM episode duration (Figure 2D) for the 20-h recording period found a significant Group effect (F1,16 = 7.51, P < 0.015) and a significant Group × Treatment Interaction (F3,48 = 11.10, P < 0.000012). REM episode duration in the ES group was increased in ST1, ST2, and Context compared to baseline and was significantly decreased in the IS group in Context compared to baseline. REM episode duration in the ES group was greater than in the IS group in ST1, ST2, and Context.
REM parameters were also analyzed in 4-h blocks to determine if there were differences in the time course of changes across groups (Table 1). Total REM and REM percentage were significantly elevated in the ES group compared to the IS group in each analysis block. The ES group also exhibited a greater number of REM episodes, though the difference in block 5 of the dark period during ST1 did not reach significance. ES mice exhibited greater REM episode duration in the dark period on ST1 (blocks 3, 4, and 5) and Context (blocks 3 and 5).
Table 1.
REM parameters in 4-h blocks across the 20-h recording period for baseline, shock training day 1 (S1), shock training day 2 (S2), and the context re-exposure day (Context) in mice trained with escapable shock (ES) and yoked control mice trained with inescapable shock (IS).
| Total REM | Group | Block 1 | Block 2 | Block 3 | Block 4 | Block 5 |
|---|---|---|---|---|---|---|
| Baseline | ES | 9.15 (1.16) | 7.37 (1.09) | 5.63 (1.21) | 9.48 (1.93) | 13.43 (1.16) |
| IS | 9.13 (0.63) | 7.65 (0.80) | 4.31 (1.24) | 12.96 (1.13) | 15.50 (1.19) | |
| S1 | ES | 10.83 (1.57) | 13.30 (1.52) | 6.96 (1.47) | 13.74 (2.30) | 23.00 (1.15) |
| IS | 6.26 (91.00)* | 5.91 (0.67)* | 2.96 (1.43) | 5.80 (1.31)* | 13.94 (1.24)* | |
| S2 | ES | 13.65 (1.81) | 13.63 (1.84) | 7.74 (1.82) | 14.96 (1.62) | 19.91 (1.53) |
| IS | 5.19 (0.89)* | 5.31 (0.81)* | 2.33 (0.63) | 6.50 (1.01)* | 10.56 (1.15)* | |
| Context | ES | 12.83 (1.78) | 13.93 (1.81) | 7.37 (1.46) | 13.09 (1.22) | 19.20 (0.85) |
| IS | 5.39 (0.57)* | 5.69 (0.87)* | 1.19 (0.40) | 6.13 (1.43)* | 10.87 (1.15)* | |
| F3,48 = 4.53, P < 0.007 | F3,48 = 12.58, P < 0.000003 | F1,16 = 12.17, P < 0.003 (Gp) | F3,48 = 13.24, P < 0.000002 | F3,48 = 12.92, P < 0.000003 | ||
| REM Percent | ||||||
| Baseline | ES | 6.93 (0.61) | 6.76 (0.98) | 9.75 (1.56) | 9.96 (1.39) | 11.51 (0.73) |
| IS | 6.64 (0.53) | 7.86 (0.91) | 10.23 (90.79) | 12.50 (1.00) | 11.53 (0.64) | |
| S1 | ES | 7.38 (1.04) | 11.54 (1.54) | 15.82 (1.12) | 14.66 (0.84) | 15.50 (0.78) |
| IS | 4.18 (0.68)* | 5.33 (0.70)* | 6.18 (1.84)* | 7.05 (1.10)* | 9.23 (0.80)* | |
| S2 | ES | 8.98 (1.10) | 11.65 (1.37) | 15.75 (1.73) | 14.09 (0.65) | 14.28 (0.93) |
| IS | 3.41 (0.62)* | 4.62 (0.65)* | 6.34 (1.28)* | 7.46 (0.71)* | 7.91 (0.93)* | |
| Context | ES | 8.58 (1.06) | 11.56 (1.53) | 15.49 (0.80) | 13.41 (0.54) | 14.97 (0.90) |
| IS | 3,67 (0.44)* | 4.68 (0.70)* | 4.85 (1.34)* | 8.09 (0.99)* | 7.59 (0.67)* | |
| F3,48 = 5.79, P < 0.0018 | F3,48 = 14.92, P < 0.000001 | F3,48 = 6.623, P < 0.0008 | F3,48 = 3.10, P < 0.000002 | F3,48 = 15.96, P < 0.000001 | ||
| REM Count | ||||||
| Baseline | ES | 16.00 (0.88) | 13.67 (2.28) | 8.56 (1.61) | 12.33 (2.16) | 15.78 (1.32) |
| IS | 15.89 (1.41) | 11.56 (1.12) | 6.84 (2.27) | 15.00 (1.15) | 17.67 (1.21) | |
| S1 | ES | 15.78 (2.02) | 18.33 (2.12) | 8.67 (2.14) | 13.00 (2.14) | 22.22 (1.21) |
| IS | 9.78 (1.37)* | 9.78 (1.26)* | 4.33 (1.77) | 8.00 (1.86)* | 19.00 (1.26) | |
| S2 | ES | 20.44 (2.82) | 19.33 (2.33) | 9.78 (2.04) | 16.44 (1.53) | 21.44 (1.33) |
| IS | 10.33 (1.71)* | 9.67 (1.04)* | 3.78 (0.93) | 8.44 (1.01)* | 13.78 (1.46)* | |
| Context | ES | 20.33 (2.77) | 20.00 (2.51) | 9.28 (2.20) | 14.33 (1.15) | 19.44 (1.37) |
| IS | 9.00 (1.05)* | 10.33 (1.16)* | 2.67 (0.94) | 8.00 (1.72)* | 15.00 (1.42)* | |
| F3,48 = 4.19, P < 0.019 | F3,48 = 3.51, P < 0.02 | F1,16 = 7.58, P < 0.014 (Gp) | F3,48 = 7.39, P < 0.0003 | F3,48 = 5.06, P < 0.004 | ||
| REM Duration | ||||||
| Baseline | ES | 0.56 (0.05) | 0.57 (0.04) | 0.61 (0.07) | 0.75 (0.04) | 0.87 (0.05 |
| IS | 0.59 (0.04) | 0.68 (0.08) | 0.68 (0.06) | 0.89 (0.09) | 0.89 (0.06) | |
| S1 | ES | 0.70 (0.06) | 0.75 (0.08) | 0.91 (0.13) | 1.04 (0.05) | 1.06 (0.07) |
| IS | 0.61 (0.07) | 0.67 (0.08) | 0.56 (0.05)* | 0.70 (0.09)* | 0.74 (0.06)* | |
| S2 | ES | 0.67 (0.06) | 0.70 (0.05) | 0.72 (0.06) | 0.91 (0.05) | 0.92 (0.02) |
| IS | 0.53 (0.05) | 0.54 (0.05) | 0.60 (0.08) | 0.76 (0.09) | 0.79 (0.09) | |
| Context | ES | 0.65 (0.04) | 0.70 (0.07) | 0.86 (0.07) | 0.92 (0.05) | 1.02 (0.06) |
| IS | 0.61 (0.05) | 0.54 (0.05) | 0.51 (0.04)* | 0.75 (0.08) | 0.75 (0.08)* | |
| F3,48 = 3.71, P < 0.018 | F3,48 = 4.47, P < 0.008 | F3,48 = 4.73, P < 0.006 | ||||
Indicated F values for ANOVAs conducted on each block are Group main effects (indicated by Gp) or Group × Treatment interactions. Significant differences between ES and IS mice within blocks are indicated for each recording day (*P < 0.05, Tukey test).
NREM and Total Sleep Time
The analysis of NREM and TST revealed significant Treatment effects, but there were few significant differences between ES and IS mice. ANOVAs for the 20-h recording period revealed significant Treatment effects for total NREM (F3,48 = 3.08, P < 0.04) and TST (F3,48 = 3.18, P < 0.032). Post hoc comparisons across Treatment conditions found increased total NREM (Figure 3A) and TST (Figure 3D) on ST2 compared to baseline; however, no significant differences were found when the ES and IS groups were considered separately. No significant differences were found for the number of NREM episodes (Figure 3B) or NREM episode duration (Figure 3C) when the entire 20-h recording period was considered.
Figure 3.
NREM parameters and total sleep time plotted as 20-h totals for baseline (Base), the 2 shock training days (ST1, ST2) and Context (Con). A. Total NREM. B. Number of NREM episodes. C. NREM episode duration. D. Total sleep time.
Analysis of total NREM in 4-h blocks also revealed only significant Treatment effects (block 1 [F3,48 = 3.72, P < 0.018] and block 5 [F3,48 = 5.26, P < 0.003]). In block 1, NREM was significantly increased during ST2 compared to baseline (P < 0.014), and in block 5, NREM was significantly increased during ST1 compared to baseline (P < 0.0014). The ANOVA for numbers of NREM episodes revealed significant Treatment effects for block 4 (F3,48 = 3.46, P < 0.024) and block 5 (F3,48 = 3.87, P < 0.014). This was due to a significant decrease in the number of NREM episodes during ST1 compared to baseline during block 4 and compared to ST2 during block 5. No other comparisons of numbers of NREM episodes were significant.
The ANOVA for NREM episode duration revealed a significant Group effect for block 4 (F1,16 = 14.41, P < 0.002). This was primarily due to a difference between ES (1.76 ± 0.14) and IS (1.88 ± 0.19) during ST1 (P < 0.024). Significant Treatment effects were found for block 1 (F3,48 = 4.43, P < 0.008) with significant increases in NREM episode duration during ST1 (P < 0.03) and Context (P < 0.01) compared to baseline and for block 5 (F3,48 = 4.05, P < 0.012) with a decrease on ST2 compared to ST1 (P < 0.012); however, no significant differences were found between ES and IS mice.
The ANOVA for TST across 4-h blocks found significant Treatment effects in block 1 (F3,48 = 3.07, P < 0.04) and block 5 (F3,48 = 6.08, P < 0.002). In both blocks, TST was significantly increased in ST1 compared to baseline (block 1, P < 0.03; block 5, P < 0.0007). The analysis of block 4 revealed a significant Group × Treatment interaction (F3,48 = 3.97, P < 0.02) due to a significant decreases in TST in ST1 (P < 0.03) and Context (P < 0.03) compared to baseline in the IS mice. No other comparisons were significant.
Wakefulness and Activity
ES and IS mice did not significantly differ in amount of AW (Figure 4A), QW (Figure 4B), or total activity (Figure 4C) when the entire 20-h recording period was considered. However, the amount of QW differed significantly across Treatment conditions (F3,48 = 6.27, P < 0.002) and was reduced in ST1 (P < 0.002), ST2 (P < 0.006), and Context (P < 0.04) compared to baseline. There was also a Treatment effect for total activity (F3,48 = 3.83, P < 0.015) with significantly increased activity in Context (P < 0.02) compared to baseline.
Figure 4.
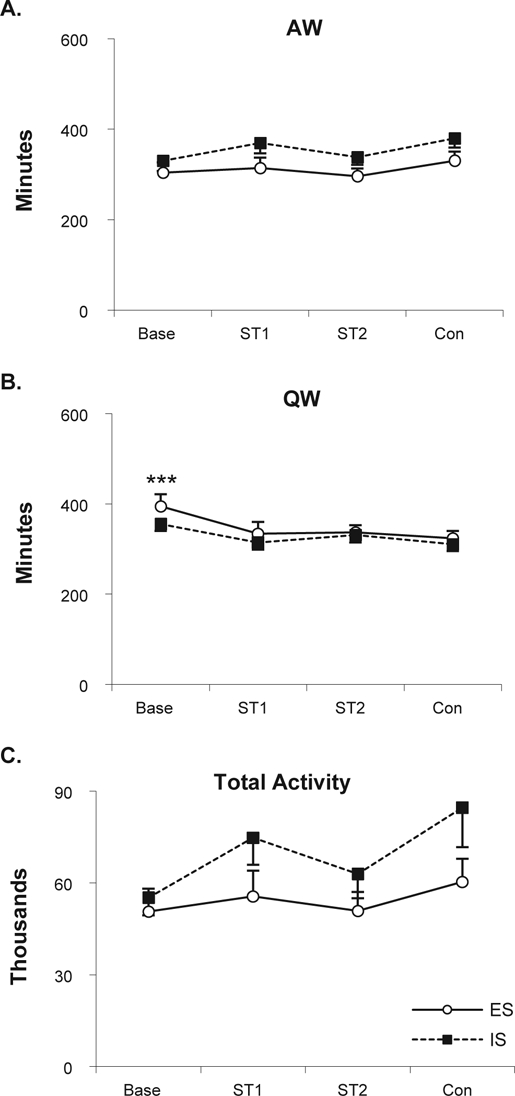
Waking parameters and total activity plotted as 20-h totals for baseline (Base), the 2 shock training days (ST1, ST2) and Context (Con). A. Active wakefulness. B. Quiet wakefulness. C. Total activity. Significant difference between ES and IS: ***P < 0.001 (Tukey test).
Analyses of these data in 4-h blocks also did not reveal any significant differences between the ES and IS mice, though ES and IS produced similar differences across days in some measures as indicated by significant Treatment effects (AW: block 1 [F3,48 = 2.80, P < 0.05], block 4 [F3,48 = 3.27, P < 0.029], and block 5 [F3,48 = 3.09, P < 0.04]; QW: block 1 [F3,48 = 24.37, P < 0.000001] block 4 ([F3,48 = 3.60, P < 0.019], and block 5 [F3,48 = 3.96, P < 0.013]; activity: block 1 [F3,48 = 7.17, P < 0.0005] and block 4 [F3,48 = 3.61, P < 0.02]). Post hoc tests for AW did not find significant differences among conditions in block 1. Amounts of AW in block 4 were decreased in Context (P < 0.05) compared to baseline and amounts of AW in block 5 were decreased in ST1 (P < 0.28) compared to baseline. QW in block 1was decreased in ST1 (P < 0.00017), ST2 (P < 0.00017), and Context (P < 0.00017) compared to baseline. QW in block 5 was decreased in Context (P < 0.01) and in ST1 (P < 0.007) compared to baseline. Activity was significantly increased in Context compared to baseline (P < 0.0004) and ST2 (P < 0.02) in block 1 and significantly increased in Context compared to baseline (P < 0.02) in block 4.
DISCUSSION
The first major finding of this study is that controllable stress, as modeled by ES, was followed by significant and dramatic increases in REM, whereas uncontrollable stress, as modeled by IS, was followed by significant decreases in REM. The increase in REM after ES was pronounced and persisted over the course of the 20-h post-stress recording. By comparison, decreases in REM after IS can occur without recovery REM in mice19,20 and rats.25,26 For example, while we did not specifically examine longer recording periods in this study, BALB/cJ mice have shown a return to baseline levels of REM on the day after shock training and on the day after context re-exposure,20 and no rebound in REM in recordings made for ten consecutive days after shock training.27 Changes in other sleep and arousal states and activity showed fewer differences between the ES and IS groups, though both ES and IS produced changes across the different Treatment days.
The second major finding of this study was that re-exposure to the training context without presentation of footshock produced changes in REM that were directionally similar to those seen during training. Re-exposure to the ES context was followed by increases in REM, whereas re-exposure to the IS context was followed by decreases in REM. The fact that contextual reminders of both controllable and uncontrollable stress produce enhancements in freezing compared to the pre-shock period and alterations in behavior and sleep similar to those seen when the footshock stressor was presented indicates that learning occurred and that memories of the stressful events were formed.
The third major finding was that fear memory, as indicated by freezing, dissociated from post-stress REM. That is, directionally different changes in REM occurred even though FT% in the context was statistically equivalent in the ES and IS mice.
Initial post-stress decreases and subsequent increases in sleep occur in response to relatively mild stressors that animals may experience repeatedly. A simple example of a repeated mild stressor is routine cage changes that experimental animals experience as part of normal husbandry; this stressor produces significant alterations in their sleeping and living environments. The stress induced by cage change may be related to fear and novelty,15, 28,29 a view supported by behavioral and physiological observations, including increased rearing and grooming, increased exploratory behavior, and increased heart rate and blood pressure in rats after a cage change.29 The lost sleep is recovered, and the responses are consistent with recurring weekly cage change schedules, suggesting that the animals do not habituate over time.29 There appears to be no evidence that this experience induces pathology. Another example of a mild stressor is open field exposure, which may produce anxiety and an opportunity for exploration. Sleep after exposure to an open field also is characterized by an initial decrease followed by an increase in REM.21 The increases in REM are positively correlated to the amount of exploration in the open field in mice,21 which may reflect an adaptive process of small rodents as they cope with potential challenges posed by a new environment.15,21,28 Initial decreases in REM and subsequent increases in REM also are found with naturalistic stressors for which animals may have evolved coping mechanisms, including social stress30 and simulated predation in wild caught rats.31 Thus, post-stress increases in REM and sleep in general may play a positive functional role in responses to stressors that are experienced during wakefulness.
Increased REM has been reported to be positively correlated with performance in learning paradigms including avoidance learning in a shuttlebox,16,32,33 spatial learning the Morris water maze,34 and operant learning.35 Learning may also take place in other paradigms (e.g., open field, exposure to novel objects as noted above) in which REM is increased. The ES and IS paradigms we used are also learning paradigms, as illustrated by improved performance across days in the ES group and the fact that contextual reminders of ES and IS produced directional changes in sleep similar to those seen on the shock training days. Thus, the role of REM is unlikely simply to be to promote memory consolidation in these two paradigms. This also is suggested by the fact that similar increases or decreases in REM can occur across multiple days of shock training. Another consideration is that the changes in REM and differences between the ES and IS training groups appear disproportional for learning a relatively simple set of associations and stimulus-response behaviors. At present, it is not possible to determine the precise factors that result in the directionally different alterations in REM; however, there likely are interactions between stressor controllability, stress-related learning and memories, and stress-related emotions.
Significant directional differences in REM with controllable and uncontrollable stress may indicate stress-induced divergence in processes linking memory consolidation, synaptic plasticity, and sleep.36 Best et al.37 reviewed work in the hippocampus and proposed that pyramidal neurons change from a firing pattern that supports long-term potentiation (LTP) in wakefulness to one that supports depotentiation during REM; thus, putatively “resetting” the hippocampus after memories have been transferred to the frontal cortex and clearing the way for the formation of future memories. It also has been suggested that REM functions to weaken unwanted memory traces in the cortex.38 If true, reductions in REM induced by uncontrollable stress could impair this process.
Impaired resetting of the hippocampus is not likely to be the only factor in the differences observed in post-stress sleep, as both contextual and cued fear associated with uncontrollable stress have similar effects on sleep; yet only contextual fear is thought to require the hippocampus8,39 (note, though, that the hippocampus appears to be required for contextual information modulating the expression of cued fear40). However, both ES and IS paradigms have a significant emotional component, and given that the amygdala mediates behavior associated with emotional stress41 and has a role in emotional memory,42 the directionally different amounts of REM may be involved in differential processing emotional aspects of the memories. The involvement of REM in fear memory is also suggested by our finding that extinguished fear is followed by normalized amounts of REM and NREM, whereas continued fear is followed by reduced REM.43 Recent work in humans has led to suggestions that REM may play an important role in consolidating memories for aversive events and in “decoupling” memory of such an event from its emotional charge.44,45 However, both ES and IS have a significant emotional component, and while the directionally different amounts of REM observed with ES and IS suggest potentially differential processing of emotional aspects of the memories, the strong fear responses in both ES and IS mice suggest other neurobiological processing in addition to emotional valence or strength.
A role for the amygdala in modulating fear- and stress-induced changes in sleep has also been demonstrated. Recent work in our laboratory found that IS preceded by microinjections of vehicle or the GABAA agonist, muscimol, selectively reduced REM and increased Fos expression, a marker of neural activation,46–49 in LC; whereas microinjection of the GABAA antagonist, bicuculline, into the central nucleus of the amygdala (CNA) prior to IS attenuated the reduction in REM and attenuated Fos expression in LC to levels seen in non-shocked controls.50 Additional work in our laboratory has found that local administration of the corticotropin releasing factor antagonist, antalarmin, into CNA attenuated the reduction in REM induced by contextual fear and also reduced Fos expression in the hypothalamic paraventricular nucleus (PVN), locus coeruleus (LC), and dorsal raphe nucleus (DRN). However, behavioral freezing in the fearful context was not reduced. These data suggest that the amygdala plays a significant role in modulating REM in stressful situations.
A recent study demonstrated that chemical lesions of CNA and the bed nucleus of the stria terminalis (BNST) prevented reductions in REM in rats exposed to a dirty cage previously inhabited by a conspecific.51 This finding is consistent with an interpretation that the lesions removed important relay sites by which the amygdala regulates REM. Interestingly, this study found Fos activation in the vicinity of CNA and the example figure in the paper shows significant Fos activation in the lateral CNA, but not medial CNA.51 However, consistent with functional studies demonstrating that blocking activation in CNA decreases REM,28,52 both IS and fearful auditory cues that produce significant reductions in REM do not produce Fos activation in either lateral or medial CNA.53 Recent work suggests that the medial CNA is involved in mediating the short-term effects of fear, whereas the lateral CNA and BNST mediate longer term fear or anxiety,54–57 suggesting the possibility of alternate pathways by which the amygdala can influence REM regulatory regions. CNA also is differentially responsive to stressful odors. For instance, Fos is activated in CNA by trimethylthiazoline (a component of fox feces), but not cat odor.58 Whereas both trimethylthiazoline and cat odor were stressful, as indicated by increased defecation, only cat odor decreased grooming and increased escape attempts.58 Thus, one cannot discount the possibility that Fos activation in the CNA (and BNST) could have been produced by olfactory cues and did not reflect neural activity related to reductions in REM seen in the dirty cage. Regardless, current evidence suggests that the amygdala may regulate fear and anxiety and mediate their effects on sleep and arousal via multiple pathways. Further work will be required to determine the potential differential roles of the lateral and medial CNA in regulating stress-induced alterations in REM.
In BALB/c mice, the basolateral amygdala, LC and DRN are strongly activated by IS53,59 as indicated by Fos. Fos in these and other regions is differentially activated by ES and IS. In a previous study, we trained C57BL/6J (B6) mice for three days with ES and IS using a shuttlebox paradigm similar to the one used in the current study.59 On the third day, we measured Fos expression in the amygdala, PVN, laterodorsal tegmental nucleus, LC, and DRN. Fos expression was greater in all regions after IS compared to ES and a handling control group that explored the shuttlebox but never received footshock. However, Fos expression after ES was greater only in PVN compared to the handling control group. These results demonstrate that controllability reduces stressor-induced neural activity (as indicated by Fos) in brain regions implicated in the stress response and in the regulation of arousal. This reduction may, in part, account for differences in arousal and sleep in the aftermath of uncontrollable and controllable stress.
Our measure of shock duration also indicates escape latency, as it was measured from shock onset until entrance into the “safe” side of the chamber. While the ES mice showed a decrease in mean escape latency across the two training days, there was considerable variability in escape latency within and across days. Escape latencies on individual trials ranged from 0.3 sec to 5.0 sec (6 mice received the maximum shock on one or more trials on ST1), and 62 of the 360 total shock trials (inclusive of all animals and all trials across days) had escape latencies greater than 2.0 sec. This variability in escape latencies in the ES mice provided a wide range of shock durations administered to the ES and yoked IS mice. In our previous work on the effects of IS on sleep and arousal in mice, we have found that 20 training trials with 0.2 mA footshocks applied for 0.5 sec produces significant alterations in arousal, including reductions in REM.19,20,53 It is possible that the unpredictable and longer duration of the shock experienced in this study could have been more stressful than the set duration footshocks used in other studies. However, even with the mice receiving greater footshock, there was a clear difference in the amount of post-stress REM shown by the ES and IS groups.
Behavioral freezing has been used to evaluate fear, with greater FT% being interpreted as indicating stronger fear reactions.7,22,23 Thus, within-subject comparisons of FT% during the initial pre-shock period to that during the context after shock had been experienced should reflect whether the shock chamber had acquired fear-inducing characteristics. FT% did not significantly differ across groups in the fearful context suggesting that the intervening shock presentations had induced fearful behavior in both groups. Interestingly, in a previous study using B6 mice, we found enhanced FT% during shock training with ES compared to shock training with IS in yoked mice.59 In addition, compared to B6 mice trained with IS, B6 mice trained with ES also showed reduced Fos activation in brain regions involved in the stress response. Exposure to the shock chamber would have been a fearful experience for both ES and IS groups; thus, it is doubtful that greater freezing simply reflected greater fear in this situation.
The directionally different changes in REM produced by ES and IS in this study were dramatic. However, it should be noted that, compared to other strains, BALB/c mice exhibit greater anxiety-like behavior on a variety of behavioral tests60 and have been suggested to exhibit trait or pathological anxiety.61,62 In addition, relative to B6 mice, BALB/c mice show greater reductions in REM after IS, and after presentation of IS-associated fearful conditioned cues and contexts.19,20 BALB/c mice exhibit more persistent reductions in the time spent in sleep, particularly in REM after exposure to mild stressors including an open field,21 presentation of a novel object in the home cage and cage change,63 and show less REM rebound after restraint stress.64 Thus, it is possible that the extent of the difference between the ES and IS conditions are greater in this strain. Additional studies are needed to determine the size of effect of these paradigms in other mouse strains and other species.
In summary, controllable and uncontrollable stress, as modeled by ES and IS, produced directionally different alterations in post-stress REM. Contextual reminders of ES and IS also produced significant changes in REM similar to those seen with the initial stressor. The alterations in REM occurred even though mice trained with either ES and IS showed behavioral freezing on re-exposure to the context. This training paradigm should be valuable for understanding the role sleep may play in determining the persisting effects of stressful experiences.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was by supported by NIH research grants MH61716 and MH64827.
REFERENCES
- 1.Yehuda R, Flory J D, Southwick S, Charney D S. Developing an Agenda for Translational Studies of Resilience and Vulnerability Following Trauma Exposure. Annals of the New York Academy of Sciences. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 2.Natelson B H. Stress hormones and disease. Physiol Behav. 2004;82:139–43. doi: 10.1016/j.physbeh.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 3.Bolstad B R, Zinbarg R E. Sexual victimization, generalized perception of control, and posttraumatic stress disorder symptom severity. J Anxiety Disord. 1997;11:523–40. doi: 10.1016/s0887-6185(97)00028-5. [DOI] [PubMed] [Google Scholar]
- 4.Foa E B, Zinbarg R, Rothbaum B O. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 5.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. The role of the amygdala in conditioned fear., in The Amygdala: Neurobiological aspects of emotion, memory and mental dsyfunction. In: Aggleton J., editor. New York: Wiley-Liss Inc.; 1992. pp. 255–305. [Google Scholar]
- 7.Blanchard R J, Blanchard D C. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 8.Phillips R G, LeDoux J E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 9.Paylor R, Tracy R, Wehner J, Rudy J. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- 10.Nijsen M, Croiset G, Diamant M, Stam R, Delsing D, de Wied D, Wiegant V. Conditioned fear-induced tachycardia in the rat: vagal involvement. Eur J Pharmacol. 1998;350:211–222. doi: 10.1016/s0014-2999(98)00261-1. [DOI] [PubMed] [Google Scholar]
- 11.Misslin R. The defense system of fear: behavior and neurocircuitry. Neurophysiol Clin. 2003;33:55–66. doi: 10.1016/s0987-7053(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 12.Stiedl O, Tovote P, Ogren S O, Meyer M. Behavioral and autonomic dynamics during contextual fear conditioning in mice. Auton Neurosci. 2004;115:15–27. doi: 10.1016/j.autneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 14.Pawlyk A C, Jha S k, Brennan F X, Morrison A R, Ross R J. A rodent model of sleep disturbances in posttraumatic stress disorder: The role of context after fear conditioning. Biol Psychiatry. 2005;57:268–77. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Sanford L D, Xiao J, Liu X, Yang L, Tang X. Influence of avoidance training (AT) and AT cues on sleep in C57BL/6J (B6) and BALB/cJ (C) mice. Sleep. 2005;28:A6–A7. [Google Scholar]
- 16.Datta S, Saha S, Prutzman S L, Mullins O J, Mavanji V. Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat. J Neurosci Res. 2005;80:727–37. doi: 10.1002/jnr.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C, Kitahama K, Valatx J L, Jouvet M. Increased paradoxical sleep in mice during acquisition of a shock avoidance task. Brain Res. 1974;77:221–30. doi: 10.1016/0006-8993(74)90786-0. [DOI] [PubMed] [Google Scholar]
- 18.Smith C, Lapp L. Prolonged increases in both PS and number of REMS following a shuttle avoidance task. Physiol Behav. 1986;36:1053–7. doi: 10.1016/0031-9384(86)90479-8. [DOI] [PubMed] [Google Scholar]
- 19.Sanford L D, Tang X, Ross R J, Morrison A R. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- 20.Sanford L D, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003;26:527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 21.Tang X, Xiao J, Liu X, Sanford L D. Strain differences in the influence of open field exposure on sleep in mice. Behav Brain Res. 2004;154:137–47. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard R J, Blanchard D C. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 23.Doyáere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–65. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 24.Tang X, Sanford L D. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002;25:691–9. [PubMed] [Google Scholar]
- 25.Tang X, Yang L, Sanford L D. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep. 2005;28:1235–44. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 26.Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–62. doi: 10.1016/0031-9384(91)90041-l. [DOI] [PubMed] [Google Scholar]
- 27.Sanford L D, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 28.Sanford L D, Parris B, Tang X. GABAergic regulation of the central nucleus of the amygdala: implications for sleep control. Brain Research. 2002;956(2):276–84. doi: 10.1016/s0006-8993(02)03552-7. [DOI] [PubMed] [Google Scholar]
- 29.Duke J L, Zammit T G, Lawson D M. The effects of routine cage-changing on cardiovascular and behavioral parameters in male Sprague-Dawley rats. Contemp Top Lab Anim Sci. 2001;40:17–20. [PubMed] [Google Scholar]
- 30.Meerlo P, Turek F W. Effects of social stimuli on sleep in mice: non-rapid-eye-movement (NREM) sleep is promoted by aggressive interaction but not by sexual interaction. Brain Res. 2001;907:84–92. doi: 10.1016/s0006-8993(01)02603-8. [DOI] [PubMed] [Google Scholar]
- 31.Lesku J A, Bark R J, Martinez-Gonzalez D, Rattenborg N C, Amlaner C J, Lima S L. Predator-induced plasticity in sleep architecture in wild-caught Norway rats (Rattus norvegicus) Behav Brain Res. 2008;189:298–305. doi: 10.1016/j.bbr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Smith C, Young J, Young W. Prolonged increases in paradoxical sleep during and after avoidance-task acquisition. Sleep. 1980;3:67–81. [PubMed] [Google Scholar]
- 33.Portell-Cortes I, Marti-Nicolovius M, Segura-Torres P, Morgado-Bernal I. Correlations between paradoxical sleep and shuttle-box conditioning in rats. Behav Neurosci. 1989;103:984–90. doi: 10.1037//0735-7044.103.5.984. [DOI] [PubMed] [Google Scholar]
- 34.Smith C, Rose G M. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- 35.Smith C, Wong P T. Paradoxical sleep increases predict successful learning in a complex operant task. Behav Neurosci. 1991;105:282–8. doi: 10.1037//0735-7044.105.2.282. [DOI] [PubMed] [Google Scholar]
- 36.Shaffery J P, Lopez J, Bissette G, Roffwarg H P. Rapid eye movement sleep deprivation revives a form of developmentally regulated synaptic plasticity in the visual cortex of post-critical period rats. Neurosci Lett. 2006;391:96–101. doi: 10.1016/j.neulet.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Best J, Diniz Behn C, Poe G R, Booth V. Neuronal models for sleep-wake regulation and synaptic reorganization in the sleeping hippocampus. J Biol Rhythms. 2007;22:220–32. doi: 10.1177/0748730407301239. [DOI] [PubMed] [Google Scholar]
- 38.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–4. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 39.Desmedt A, Garcia R, Jaffard R. Differential modulation of changes in hippocampal-septal synaptic excitability by the amygdala as a function of either elemental or contextual fear conditioning in mice. J Neurosci. 1998;18:480–487. doi: 10.1523/JNEUROSCI.18-01-00480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–6. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis M, Whalen P J. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 42.LeDoux J E. Emotional memory systems in the brain. Behav Brain Res. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 43.Wellman L L, Yang L, Tang X, Sanford L D. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep. 2008;31:1035–42. [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida M, Pearsall J, Buckner R L, Walker M P. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker M P. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 46.Melia K R, Ryabinin A E, Schroeder R, Bloom F E, Wilson M C. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–38. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cullinan W E, Herman J P, Battaglia D F, Akil H, Watson S J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 48.Chowdhury G M, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–82. doi: 10.1016/s0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 49.Mongeau R, Miller G A, Chiang E, Anderson D J. Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J Neurosci. 2003;23:3855–68. doi: 10.1523/JNEUROSCI.23-09-03855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Yang L, Wellman L L, Tang X, Sanford L D. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep. 2009;32:888–96. doi: 10.1093/sleep/32.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cano G, Mochizuki T, Saper C B. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–84. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanford L D, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–8. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Tang X, Sanford L D. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991:1–17. doi: 10.1016/j.brainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Davis M, Walker D L, Miles L, Grillon C. Phasic vs Sustained Fear in Rats and Humans. Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker D, Yang Y, Ratti E, Corsi M, Trist D, Davis M. Differential effects of the CRF-R1 antagonist GSK876008 on fear-potentiated, light- and CRF-enhanced startle suggest preferential involvement in sustained vs phasic threat responses. Neuropsychopharmacology. 2009;34:1533–42. doi: 10.1038/npp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker D L, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 57.Walker D L, Miles L A, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staples L G, McGregor I S, Apfelbach R, Hunt G E. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–47. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Tang X, Sanford L D. Stressor controllability and Fos expression in stress regulatory regions in mice. Physiol Behav. 2009;97:321–6. doi: 10.1016/j.physbeh.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang X, Orchard S M, Sanford L D. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–69. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 61.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–9. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 62.Tang X, Orchard S M, Sanford L D. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–69. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 63.Tang X, Xiao J, Parris B S, Fang J, Sanford L D. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/J mice. Physiol Behav. 2005;85:419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Meerlo P, Easton A, Bergmann B M, Turek F W. Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R846–54. doi: 10.1152/ajpregu.2001.281.3.R846. [DOI] [PubMed] [Google Scholar]