Abstract
Study Objective:
Sleep is regulated by homeostatic and circadian processes. Slow wave activity (SWA; 1-4 Hz) in the NREM sleep electroencephalogram (EEG) reflects sleep homeostasis. Activity of faster EEG frequencies (10-25 Hz) is thought to be under influence of circadian factors. The relative contribution of both processes to the distribution of sleep and wakefulness and EEG activity in rodents remains uncertain.
Design:
Continuous EEG recording in rats in constant dark conditions (DD) were performed and a sleep deprivation protocol consisting of 2 h sleep deprivation followed by 2 h of rest (2h/2h) was applied for 48 h to obtain a constant sleep pressure.
Settings:
Basic sleep research laboratory.
Patients or Participants:
Adult male Wistar rats.
Intervention:
Sleep deprivation.
Measurements and Results:
Under the 2h/2h protocol, the circadian modulation of waking, NREM and REM sleep was markedly reduced compared to the baseline, affecting the frequency of vigilance state episodes and the duration of REM sleep and waking episodes. In contrast, NREM sleep episode duration still showed a daily modulation. Consecutive 2h values of SWA in NREM sleep were stabile during the 2h\2h protocol, while NREM sleep EEG activity within the higher frequencies (7-25 Hz) still demonstrated strong circadian modulation, which did not differ from baseline.
Conclusions:
In rats, the daily modulation of REM sleep is less pronounced compared to NREM sleep and waking. In contrast to SWA, activity in higher frequencies (7-25 Hz) in the NREM sleep EEG have an endogenous circadian origin and are not influenced by sleep homeostatic mechanisms.
Citation:
Yasenkov R; Deboer T. Circadian regulation of sleep and the sleep EEG under constant sleep pressure in the rat. SLEEP 2010;33(5): 631-641.
Keywords: EEG, slow wave activity, NREM sleep, circadian
SLEEP IS A COMMON BEHAVIOR OBSERVED IN ALL KNOWN MAMMALIAN SPECIES. ACCORDING TO A WELL-ESTABLISHED MODEL OF SLEEP REGULATION, the timing of sleep and wakefulness is regulated by the interaction of a homeostatic (S) and a circadian (C) process.1,2 The sleep homeostat regulates sleep depth and the circadian pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus provides the sleep homeostat with a circadian framework and regulates the timing of sleep.3–5
During normal, undisturbed, sleep, the status of the sleep homeostat is reflected by the activity in the slow wave range (1-4 Hz) of the EEG during NREM sleep, which is an indicator of the discharge of sleep need.6 This notion has become evident from a clear dose-response relation between slow wave activity (SWA) and prior waking duration in many mammalian species, including humans.7–12 Moreover, the time course of SWA could be modeled successfully in three mammalian species on the basis of prior sleep and wakefulness, providing further supporting evidence for a strong interdependence between SWA in the NREM sleep EEG and prior sleep and waking duration.11,13,14
The circadian regulation of sleep is less well understood. In humans, application of sleep deprivation paradigms revealed a complex interaction between the homeostatic and circadian components of sleep regulation. This was investigated with a “nap protocol,” in which 30 min of bed rest was alternated with 60 min of enforced wakefulness for several days,15,16 and with a “forced desynchrony protocol,” in which subjects are exposed to a 28-h day with approximately 19 h of wakefulness and 9 h of bed rest.17 In both protocols, NREM and REM sleep showed modulations over the endogenous circadian cycle with a trough around the late evening shortly before habitual bedtime, increased NREM sleep during the night and subjective morning, and increased REM sleep in the early morning.16,17 Slow wave activity showed a small circadian modulation, whereas sigma activity (12.0-15.0 Hz), the EEG marker of the sleep spindles in humans, demonstrated a clear circadian modulation with high activity during the early subjective night and low activity in the early morning.17
In rodents this type of research has not been performed. In most laboratory rodents (mice, hamsters, rats) sleep-wake distribution shows clear day-night changes under light-dark (LD) conditions with high levels of sleep during the light period (rest) and low levels in the dark when the animals are active. This pattern does not change significantly at the transition from LD cycle to constant dark (DD) conditions18,19 and continues in prolonged DD.20–23 EEG slow wave activity increases in the first day in DD in rats,18 but not in mice19 while EEG activity in higher frequencies (> 9.25 Hz) in NREM sleep is not influenced by this transition18,24,25 and is thought to be under strong endogenous influence of the circadian clock.18,26
Of particular interest are the changes in the theta frequency range (5-7 Hz) of the waking EEG, which seem to be influenced by sleep homeostatic mechanisms,27 and between 7-11 Hz in the NREM sleep EEG, which represents spindle frequency activity (SFA) in the rat.28–32 It was proposed that cortical SWA and SFA have a reciprocal relationship based on the rhythmic interplay within the thalamocortical neuronal networks.28,33 Accordingly, there may be a negative correlation between SWA and the activity of sleep spindles, and this relationship was confirmed in the human sleep EEG,34,35 indicating that both frequencies may be under the influence of the homeostatic mechanisms. This negative relationship has been confirmed under several sleep deprivation protocols in humans.36,37 More recent experiments, applying repeated naps demonstrated both a homeostatic and a circadian component in SFA.38,39 Whether both influences exist in rodents remains to be established.
Developing a rodent model where circadian sleep-wake regulation can be investigated separate from homeostatic mechanisms will enable research towards determining the genetic, molecular, and neuronal basis of the circadian changes in sleep and waking, and EEG frequency activity. To investigate the influence of the circadian clock on sleep regulatory processes in rodents we applied a “short day” sleep deprivation protocol, similar to the nap protocol in humans, to rats adapted to DD conditions for at least a week. Applying the short-day sleep deprivation protocol consisted of 2 h sleep deprivation alternated with 2 h rest and continued for 2 days. It should stabilize sleep propensity over the 24-h circadian day, and lead to an equal distribution of behaviour (vigilance states), as well as a constant level of SWA in the NREM sleep EEG. Simultaneously, prolonged constant DD eliminates the influence of the previous light-dark cycle. From a chronobiological point of view, this protocol is adequate to rule out any environmental influence on the daily modulation of vigilance states or EEG variables. Any daily change observed must be endogenous, i.e. originating from within the animal itself.3,4,40
METHODS
Animals
All experiments were approved by the Animal Experiments Ethics Committee of the Leiden University Medical Center. Adult male Wistar rats (n = 8), weight 312.3 g ± 3.9 (SEM) at time of surgery were used. Before the experimental procedures the animals were kept individually in standard cages (41 cm × 36 cm × 30 cm) in a 12-h light–12-h dark schedule (LD 12:12; lights-on from 09:00 to 21:00) with food and water available ad libitum. Under deep anesthesia (Hypnorm, 0.5 mL/kg, i.m., combined with Dormicum,5 mg/mL, i.p.) the rats were fixed in a stereotaxic frame and implanted with 2 EEG screw electrodes (Plastics One, Roanoke, VA; 1.4 mm diameter) and 2 EMG patch electrodes (Plastics One, Roanoke, VA; 0.35 mm diameter) as described previously.20,22,23 Briefly, one EEG electrode was placed over the right hemisphere (2.0 mm lateral to the midline, 3.5 mm caudal to the bregma) above the somatosensory cortex, while the other was placed above the cerebellum (midline, 2 mm caudal to the lambda) as a reference. Two EMG electrodes were inserted between the neck muscles and skin. The wire branches of all electrodes were set in a plastic pedestal (Plastics One, Roanoke, VA) which was fixed to the skull with dental cement and 3 additional support screws. The rats recovered for at least 7-10 days subsequent to the surgery.
Experimental Protocol
All the experiments were carried out under constant DD condition to avoid the daily influences by light-dark changes, and to stabilize the circadian environment. Animals were placed into the experimental chambers equipped with an infrared camera for visual monitoring and connected through a flexible cable and a counterbalanced swivel system to the recording setup. Drinking activity was recorded by infrared sensors placed around the nipple of the water bottle to establish the timing of the subjective day and night of the animal.20,22,41,42 The drinking onset was determined by visual interpolation and only those animals were included in the study where the individual onset of increased waking in the baseline corresponded with the onset of drinking activity (circadian time [CT] 12). After at least one week of habituation to the experimental environment, a 24-h baseline day was recorded, starting at the onset of the subjective day (CT 0). Subsequently the short-day deprivation protocol (2h/2h)—2 h of sleep deprivation followed by 2 h of a rest—was performed for 48 h. The last 24 h are used in the present analysis (Figure 1B). Sleep deprivation was carried out by “gentle handling,” which consisted of mild interruption by moderate noise or introducing an interesting materials in the cages as soon as the animals appeared drowsy or slow waves were observed in the EEG.
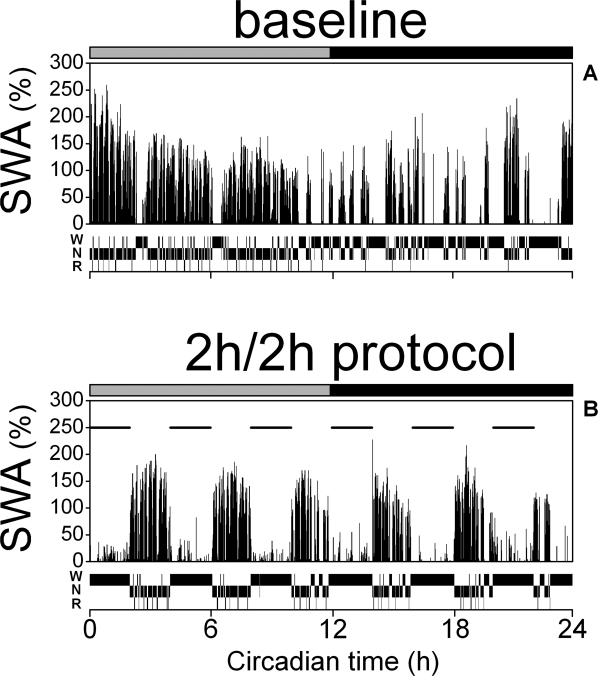
Figure 1.
Slow wave activity and vigilance states in constant dark conditions. Representative individual example of SWA (EEG spectral power density between 1-4 Hz) and vigilance states (waking, W; NREM sleep, N; REM sleep, R) during the 24-h baseline day (A) and the last 24-h of the 2h/2h sleep deprivation protocol (B). Both days start at rest onset (CT 0). SWA is plotted as percentage of the mean EEG SWA in NREM sleep during the baseline 24 h. Each data point represents 1 min as the average of six 10-sec epochs. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Horizontal lines on B match the 2-h sleep deprivation periods. Note the stable level of SWA across 2-h rest phases of the 2h/2h deprivation protocol.
Data Acquisition
The EEG and EMG were simultaneously and continuously recorded for 72 h. The signals were amplified (amplification factor ~ 2000), and band-pass filtered (EEG: 0.5-30.0 Hz, −40 dB/decade; EMG 15.0-40.0 Hz, −40 dB/decade) by a Neurolog System (Digitimer Ltd., Welwyn Garden City, UK), digitized (sampling rate 100 Hz) in 10-sec epochs and automatically stored on hard disk (Spike2, Power1401, CED, Cambridge, UK). A fast Fourier transformation routine with a 10-sec window was performed off-line (MATLAB, The MathWorks Inc., Natick, MA) for the computation of EEG power density spectra within the frequency range 0.5-25.0 Hz. The manual scoring of vigilance states based on the EEG and EMG recordings was performed according to standardized criteria for rats.20,22,43 NREM sleep, REM sleep, and waking were determined, and artifacts were excluded for subsequent analysis.
In this study the second 24-h day (CT 0-24) of the 2h/2h deprivation protocol (when the amount of sleep and SWA in NREM sleep achieved a relatively stable level; see Results) is analyzed and compared to the corresponding 24-h (CT 0-24) of the baseline recording. The average amount of the vigilance states (waking, NREM sleep, REM sleep and REM sleep per total sleep time) and EEG spectral data were analyzed in 4-h intervals, 12-h intervals, and over the whole 24-h circadian period and compared to corresponding baseline intervals. In a separate analysis, the amount of vigilance states were computed for the 2-h rest phases during the 2h\2h day, again analyzed in 4-h, 12-h, and 24-h circadian intervals, and compared with the corresponding intervals of the baseline day (Figure 3, Table 2).
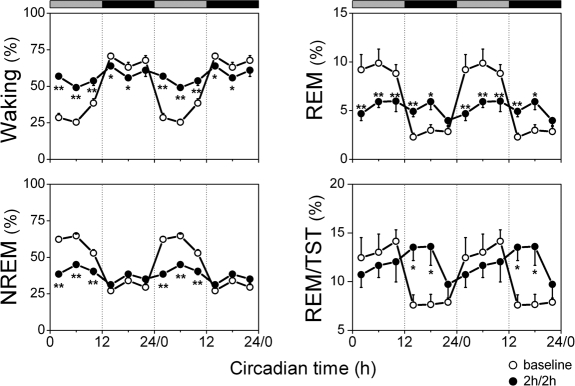
Figure 3.
Amount of vigilance states during the undisturbed 2-h periods of the 2h/2h deprivation protocol and corresponding baseline intervals. Vigilance states (waking, NREM sleep, REM sleep) and REM/TST are plotted as percentage of recording time. The curves connect 2-h mean values (n = 8) for the 24-h of the 2h/2h protocol (dots) and corresponding baseline intervals (circles). Data are double plotted to visualize the daily rhythm. Rest onset (CT 0) was determined per individual animal. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Significant differences between the 2h/2h protocol and corresponding intervals of baseline are indicated by *(P < 0.05) and **(P < 0.01), 2-tailed paired t-test after significant rANOVA, factor “day.”
Table 2.
Amount of vigilance states and REM sleep per total sleep time in baseline and in undisturbed 2h periods of the 2h/2h deprivation protocol
| Circadian time (h) | NREM (%) |
REM (%) |
Waking (%) |
REM/TST (%) |
||||
|---|---|---|---|---|---|---|---|---|
| baseline | 2h/2h | baseline | 2h/2h | baseline | 2h/2h | baseline | 2h/2h | |
| CT 0-12 | 57.6 (2.4) | 69.9 (1.1)** | 8.1 (1.1) | 10.8 (1.3)** | 34.2 (2.5) | 19.1 (2.2)** | 12.3 (1.6) | 13.2 (1.3) |
| CT 12-24 | 30.3 (3.6)§§ | 60.2 (2.9)**§ | 2.1 (0.5)§§ | 9.7 (0.9)** | 69.0 (4.4)§§ | 31.5 (3.4)**§ | 5.9 (0.9)§ | 14.0 (1.3)** |
| CT 0-24 | 43.9 (2.9) | 65.1 (1.4)** | 5.1 (0.5) | 10.2 (1.0)** | 51.6 (3.2) | 25.3 (1.3)** | 10.5 (0.9) | 13.6 (1.3) |
Mean values in % (± SEM); n = 8, for 12-h intervals (CT 0-12, subjective day; CT 12-24, subjective night) and 24-h intervals (CT 0-24, the experimental day). REM/TST, REM per total sleep time. *Significant differences between corresponding intervals of baseline and deprivation protocol (2h/2h). §Comparison between subjective day (CT 0-12) and night (CT 12-24). *§P < 0.05, **§§P < 0.01, 2-tailed paired t-test
Vigilance states episodes frequency and duration were automatically determined for undisturbed 2-h phases of the 2h/2h protocol and compared with corresponding baseline intervals. The distribution of the vigilance state episode duration and frequency was obtained based on an algorithm developed in previous studies in rats on the basis of 8-sec epochs.44 In our study, NREM sleep, REM sleep, and waking episode were considered for analysis if they lasted ≥ 20 sec. Vigilance states episodes were terminated if followed by 2 consecutive 10-sec epochs not scored as the corresponding vigilance state or 7 non-consecutive epochs not scored as the corresponding vigilance state.
To investigate the behaviour of EEG power density in more detail, the mean time course of SWA, SFA (7-11 Hz), and higher frequencies (11-25 Hz) was analyzed in 10-sec intervals within the first 7 min after NREM sleep onset during the undisturbed 2-h intervals of the 2h/2h protocol and the corresponding 2 h of baseline. The average activity in the first 7 min of NREM sleep was calculated for subsequent statistical analysis.
Statistical Analysis
For data analyses, the SPSS statistical software (SPSS 16.0.2, SPSS Inc., Chicago, IL) was used. One-way analysis of variance for repeated measures (rANOVA) was applied to test the uniformity level of the data across the experimental days; 2-way rANOVA with a mixed-design model including the between-factor day (baseline or 2h/2h protocol) and the within-factor interval (6 time points across the experimental day) was applied to test the effect of repeated sleep deprivation depending on time of the day. All P values were based on Huynh-Feldt (H-F) corrected degrees of freedom, but the original degrees of freedom are reported. Two-tailed paired t-test was applied for the comparisons between the baseline day and the 2h/2h protocol (day or interval as a factor). All P-values derived from paired t-test and rANOVA were set at 0.05 level of significance.
To detect circadian modulation in the non-uniformly distributed data (which demonstrated a significant daily modulation with rANOVA, factor interval) a fitting function (cosinor method) with a 24-h period was applied. The indexes of the goodness of fit (GF) from 0 (best) to 1 (worse) with 95% confidence levels are reported.
RESULTS
A representative example of EEG SWA and vigilance states distribution shows that during the baseline recording period (Figure 1A), sleep predominated during the subjective day (CT 0-12) and occurred only occasionally during the subjective night (CT 12-24). SWA in the NREM sleep EEG gradually decreased during the subjective day and increased in the course of the subjective night. The 2h/2h sleep deprivation paradigm changed both the time course of SWA and the vigilance states distribution (Figure 1B). Each 2-h rest period showed relatively large amount of NREM and REM sleep, and SWA in NREM sleep remained at a constant level across the 2-h rest phases of the 2h/2h protocol.
VIGILANCE STATES
During the baseline day, a clear difference between the subjective day (CT 0-12) and night (CT 12-24) was seen in the amount of the vigilance states, with high levels of NREM and REM sleep in the subjective day and high levels of waking in the subjective night, thus demonstrating a robust circadian pattern both for 4-h mean values (Table 1 and Figure 2, indexes of GF after significant one-way rANOVA, factor interval, Waking: GF = 0.041, P < 0.01; NREM: GF = 0.043, P < 0.02; REM: GF = 0.038, P < 0.01; REM/TST: GF = 0.043, P < 0.02) and for 2-h mean values (Table 2 and Figure 3, GF after significant rANOVA, factor interval, Waking: GF = 0.054, P < 0.02; NREM: GF = 0.047, P < 0.02; REM: GF = 0.035, P < 0.01; REM/TST: GF = 0.056, P < 0.02). This circadian modulation decreased dramatically during the 2h/2h protocol, with relatively constant levels over the 24-h day for all vigilance states, both when 2-h sleep deprivations were included in the analyses (Table 1, Figure 2) and when only the 2-h rest periods and corresponding 2-h baseline periods were considered (Table 2, Figure 3).
Table 1.
Amount of the vigilance states and REM sleep per total sleep time over baseline and 2h/2h deprivation protocol
| Circadian time (h) | NREM (%) |
REM (%) |
Waking (%) |
REM/TST (%) |
||||
|---|---|---|---|---|---|---|---|---|
| baseline | 2h/2h | baseline | 2h/2h | baseline | 2h/2h | baseline | 2h/2h | |
| CT 0-12 | 59.9 (1.6) | 41.3 (0.7) ** | 9.3 (1.2) | 5.5 (0.7)** | 30.9 (2.3) | 53.2 (1.0)** | 13.2 (1.6) | 11.7 (1.3)** |
| CT 12-24 | 30.2 (1.9)§§ | 34.9 (2.1)§* | 2.7 (0.4)§§ | 4.9 (0.4)* | 67.1 (2.3)§§ | 60.1 (2.0)§** | 8.0 (0.8)§ | 12.7 (1.2)* |
| CT 0-24 | 44.9 (1.3) | 38.1 (1.2)** | 6.0 (0.5) | 5.2 (0.5) | 49.2 (1.3) | 56.7 (1.0)** | 11.7 (1.0) | 12.2 (1.2) |
Mean values in % (± SEM); n = 8, for 12-h intervals (CT 0-12, subjective day; CT 12-24, subjective night) and over the day (CT 0-24). REM/TST, REM per total sleep time. *Significant differences between the baseline and deprivation protocol (2h/2h). §§Comparison between subjective day (CT 0-12) and night (CT 12-24). *§P < 0.05, §§**P < 0.01, 2-tailed paired t-test
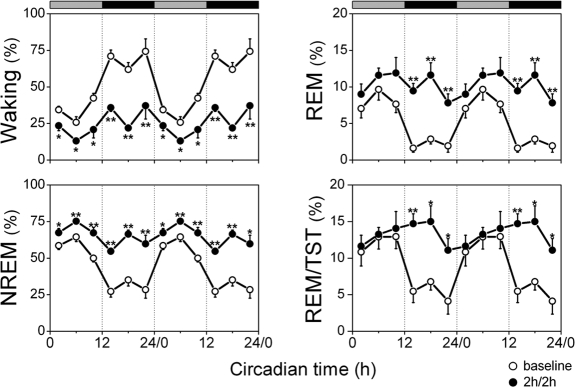
Figure 2.
Amount of vigilance states over baseline and 2h/2h deprivation protocol. Vigilance states (waking, NREM sleep, REM sleep) and REM/TST are plotted as a percentage of recording time. The curves connect 4-h mean values (n = 8) for the 24-h baseline (circles) and the 2h/2h protocol (dots). The data are double plotted to visualize the daily rhythm. Rest onset (CT 0) was determined per individual animal. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Significant differences between the 2h/2h protocol and corresponding intervals of baseline day are indicated by *(P < 0.05) and **(P < 0.01), 2-tailed paired t-test after significant rANOVA, factor “day.”
Across 4-h intervals (including sleep deprivation), the 2h/2h protocol increased the amount of waking and decreased the amount of NREM sleep to levels encountered during the subjective night and, at the same time, stabilized their distribution across the 24-h day (Figure 2, Waking: GF = 0.195, P > 0.05; NREM: GF = 0.174, P > 0.05; after significant rANOVA, factor interval; Waking: F5,35 = 4.2, P < 0.05; NREM: F5,35 = 5.2, P < 0.05). All vigilance states decreased their circadian amplitude as indicated by significant day× intervalinteraction (Waking: F5,70 = 20.2, P < 0.0001; NREM: F5,70 = 21.7, P < 0.0001; REM: F5,70 = 8.8; P < 0.0001; REM/TST: F5,70 = 4.2; P < 0.05). REM sleep and REM/TST remained at an intermediate level and did not show significant changes in their total amount over 24-h (Figure 2, one-way rANOVA, interval: REM: F5,35 = 1.8; P = 0.155; REM/TST: F5,35 = 1.8; P = 0.176; Table 1, 2-tailed t-test, P > 0.05). Total sleep time (TST) over 24-h of the 2h/2h protocol was significantly less (43.3% ± 1.0% SEM) than baseline (50.8% ± 1.3% SEM, 2-tailed paired t-test, P < 0.01).
During the 2-h rest periods, the amount of waking decreased below baseline levels, whereas NREM sleep increased above baseline in all intervals (Figure 3). REM sleep increased significantly both during the subjective day and night (Table 2, 2-tailed t-test, P < 0.01) which lead to equal amounts of REM sleep over the 2-h rest intervals within the circadian day (Figure 3, one-way rANOVA, interval: F5,35 = 2.0; P = 0.104; Table 2, 2-tailed t-test, P > 0.05). This resulted in a significant increase in REM/TST in the first two-thirds of the subjective night during the 2h/2h sleep deprivation protocol (2-way rANOVA, day × interval, REM/TST: F5,70 = 4.0; P < 0.01), but still with an equal distribution over the 24-h day (Figure 3, one-way rANOVA, factor interval: F5,35 = 1.7; P = 0.20; Table 2, 2-tailed t-test, P > 0.05). Significant differences were found in the amount of NREM sleep during the 2h/2h protocol across the 2-h rest intervals (Figure 3, one-way rANOVA, factor interval: F5,35 = 4.1; P < 0.05) and waking (Figure 3, one-way rANOVA, factor interval: F5,35 = 3.4; P < 0.05) and between the subjective day and night (Table 2, t-test, P < 0.05). However, these daily modulations did not fit significantly to a 24-h cosine function (Figure 3, Waking: GF = 0.214, P > 0.05; NREM: GF = 0.174, P > 0.05).
The above described changes in the vigilance states were the result of temporal changes in the frequency and duration of vigilance state episodes. Therefore, we compared the distribution of vigilance state episode duration and frequency during the 2-h rest phases of the deprivation day with the episodes during the corresponding baseline intervals (Figure 4, Table 3). Under baseline conditions, the episode frequency of all vigilance states demonstrated strong circadian modulation (Figure 4, top panels, NREM: GF = 0.078, P < 0.05; REM: GF = 0.033, P < 0.01; Waking: GF = 0.061, P < 0.05; after significant rANOVA, factor interval). NREM sleep episode duration showed significant circadian modulation as well (Figure 4, bottom left panel, NREM: GF = 0.05, P < 0.02; after one-way rANOVA, factor interval: F5,35 = 6.3, P < 0.05 and Table 3, t-test P < 0.01). The duration of waking episodes showed daily modulation in baseline (one-way rANOVA, factor interval: F5,35 = 7.2, P < 0.01 and Table 3, t-test P < 0.05) but did not reach a significant level in the cosine fit (GF = 0.139, P > 0.05). The daily changes in the frequency of vigilance state episodes in baseline were not always visible in the mean values over the subjective day and night, where NREM sleep and waking episode frequency did not show a significant difference between the 2 phases of the day (Table 3).
Figure 4.
Duration and frequency of vigilance states episodes during baseline and 2h/2h deprivation protocol. Top panels–episode frequencies (events per hour); bottom panels–episode duration (min) for corresponding vigilance states. The curves connect 2-h mean values (n = 8) for the 24-h of the 2h/2h protocol (dots) and corresponding baseline intervals (circles). Data are double plotted to visualize the daily rhythm. Rest onset (CT 0) was determined per individual animal. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Significant differences between the 2h/2h protocol and corresponding intervals of baseline are indicated by *(P < 0.05) and **(P < 0 .01), 2-tailed paired t-test after significant rANOVA, factor “day.”
Table 3.
Duration and frequency of the vigilance states episodes in baseline and 2h/2h deprivation protocol
| Circadian time (h) | NREM |
REM |
Waking |
||||
|---|---|---|---|---|---|---|---|
| baseline | 2h/2h | baseline | 2h/2h | baseline | 2h/2h | ||
| DURATION (min) | CT 0-12 | 5.5 (0.4) | 7.1 (0.5)** | 1.6 (0.1) | 1.5 (0.1) | 1.5 (0.1) | 1.6 (0.3) |
| CT 12-24 | 3.4 (0.4)§§ | 5.6 (0.4)**§§ | 1.5 (0.1) | 1.4 (0.1) | 4.0 (0.8)§ | 2.5 (0.4) | |
| CT 0-24 | 4.5 (0.4) | 6.3 (0.4)** | 1.5 (0.1) | 1.5 (0.1) | 2.5 (0.2) | 2.0 (0.2) | |
| FREQUENCY (events per hour) | CT 0-12 | 6.6 (0.3) | 5.9 (0.4) | 3.1 (0.4) | 4.4 (0.4)** | 12.9 (0.7) | 8.6 (0.9) |
| CT 12-24 | 5.5 (0.5) | 6.2 (0.3) | 1.0 (0.3)§§ | 4.0 (0.3)** | 10.8 (0.9) | 9.2 (1.3) | |
| CT 0-24 | 6.0 (0.3) | 6.0 (0.3) | 2.1 (0.2) | 4.2 (0.4)** | 11.8 (0.5) | 8.9 (1.1)* | |
Mean values (n = 8) of duration (min ± SEM) and frequency (events/h ± SEM) of the vigilance states episodes for 12-h intervals (CT 0-12, subjective day; CT 12-24, subjective night) and for 24-h intervals (CT 0-24, the experimental day). NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep. *Significant differences between corresponding intervals of baseline and deprivation protocol (2h/2h). §Comparison between subjective day (CT 0-12) and night (CT 12-24). *§P < 0.05, **§§P < 0.01, 2-tailed paired t-test
During the 2h/2h protocol, the distribution of all vigilance states changed dramatically as the episode frequency distribution was found to be constant (Figure 4, one-way rANOVA, factor interval, top panels, NREM sleep: F5,35 = 0.4; P = 0.781; REM sleep: F5,35 = 2.4; P = 0.058; waking: F5,35 = 0.7; P = 0.560). This distribution was different from baseline (significant day × interval interaction two-way rANOVA, NREM sleep: F5,70 = 3.5; P < 0.01; REM sleep: F5,70 = 3.2; P < 0.05; waking: F5,70 = 4.7; P < 0.001). NREM sleep episode frequency remained at intermediate levels compared to baseline, whereas the frequency of waking episodes decreased to the lowest baseline values. In contrast, the frequency of REM sleep episodes increased above baseline levels (Figure 4, top middle panel). NREM sleep episode duration still showed significant modulation over the day (Figure 4, bottom left panel, one-way rANOVA, factor interval, F5,35 = 2.8; P < 0.05), but these changes did not reach significance in the cosine fitting procedure (GF = 0.087, P > 0.05). The duration of REM sleep episodes was increased to the highest baseline levels and did not show significant changes over the day (Figure 4, bottom middle panel; one-way rANOVA, factor interval, F5,35 = 0.4; P = 0.821). Waking episode duration reduced its daily circadian amplitude compared to baseline (Figure 4, bottom right panel; 2-way rANOVA, interaction day × interval: F5,70 = 3.5; P < 0.05), due to a significant decrease of episode duration at CT 20-24.
EEG Power Density
The time course of SWA in NREM sleep during the baseline day showed a gradual decrease in the course of the rest phase and a gradual increase in the course of the active phase. This was in contrast with the activity between 7-11 Hz and 11-25 Hz, which both showed high activity at the beginning of the active phase and gradually decreased and reached lowest levels in the first two-thirds of the rest phase, after which a fast increase was observed (Figure 5). All 3 frequency ranges showed highly significant circadian modulation over the 24-h baseline day (Figure 5, SWA: GF = 0.062, P < 0.05; 7-11 Hz: GF = 0.031, P < 0.01; 11-25 Hz: GF = 0.021, P < 0.001; after significant one-way rANOVA, factor interval, SWA: F5.35 = 5.1, P < 0.01; 7-11 Hz: F5.35 = 8.2, P < 0.001; 11-25 Hz: F5.35 = 15.7, P < 0.001).
Figure 5.
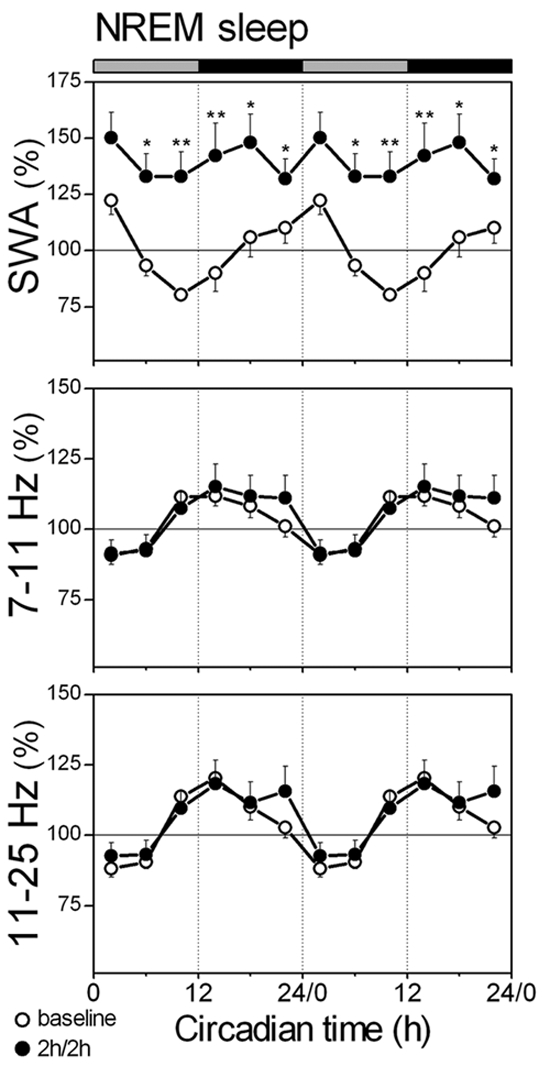
SWA (1-4 Hz), SFA (7-11 Hz), and higher frequency activity (11-25 Hz) in NREM sleep. Data are plotted as percentage of the mean activity of the corresponding frequency during the baseline 24-h. The curves connect 2-h mean values (n = 8) for the 24-h of the 2h/2h protocol (dots) and corresponding baseline intervals (circles). Data are double plotted to visualize the daily rhythm. Rest onset (CT 0) was determined per individual animal. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Significant differences between the 2h/2h protocol and corresponding intervals of baseline are indicated by *(P < 0.05) and **(P < 0.01), 2-tailed paired t-test after significant rANOVA, factor “day.”
During the 2h/2h protocol SWA in NREM sleep increased above baseline levels during all intervals except between CT 0-4, and no significant daily modulation of SWA was observed (Figure 5, one-way rANOVA, factor interval: F5,35 = 2.2; P = 0.096). Remarkably, the power density in the range of 7-11 Hz and 11-25 Hz still showed circadian modulation (Figure 5, 7-11 Hz: GF = 0.039, P < 0.01; 11-25 Hz: GF = 0.068, P < 0.05; after significant one-way rANOVA, factor interval, 7-11 Hz: F5,35 = 11.8; P < 0.001; 11-25 Hz: F5,35 = 11.7; P < 0.001), which did not differ from baseline (2-way rANOVA, day × interval, 7-11 Hz: F5,70 = 1.1; P = 0.347; 11-25 Hz: F5,70 = 1.7; P = 0.148).
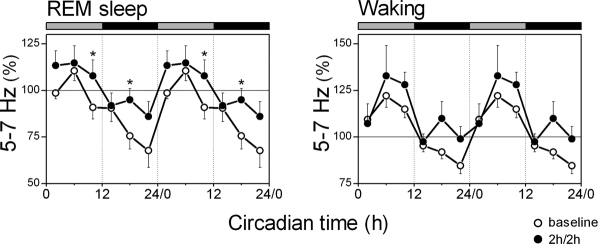
Analysis of the 5-7 Hz band of the EEG in REM sleep and waking during the baseline revealed that the activity gradually decreased during the active phase and rapidly increased after the transition to the rest phase both in REM sleep and waking, thus demonstrating a significant circadian pattern (Figure 6, REM: GF = 0.07, P < 0.05; Waking: GF = 0.022, P < 0.01; after significant one-way rANOVA, factor interval, P < 0.001). During the 2h/2h protocol, EEG 5-7 Hz activity in REM sleep still showed significant modulation over the day (GF = 0.063, P < 0.05 after one-way rANOVA, factor interval: F5,25 = 5.4, P < 0.01). The 5-7 Hz activity in waking did not change significantly compared to baseline (one-way rANOVA, factor day: F1,11 = 0.1, P =0.722; 2-way rANOVA, day × interval: F5,55 = 1.1; P = 0.354) and still showed significant variation over the 2-h intervals (one-way rANOVA, factor interval: F5,25 = 7.9, P < 0.01), although this did not reach significant level in the cosinor fitting procedure (GF = 0.117, P > 0.05).
Figure 6.
Theta activity (5-7 Hz) in REM sleep and waking. Data are plotted as percentage of the mean activity of the corresponding frequency during the baseline 24-h. The curves connect 2-h mean values (n = 8) for the 24 h of the 2h/2h protocol (dots) and corresponding baseline intervals (circles). Data are double plotted to visualize the daily rhythm. Rest onset (CT 0) was determined per individual animal. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Significant differences between the 2h/2h protocol and corresponding intervals of baseline are indicated by *(P < 0.05), 2-tailed paired t-test after significant rANOVA, factor “day.”
To analyze the dynamics of EEG power density in NREM sleep in more detail, the average time course of SWA (1-4 Hz), SFA (7-11 Hz), and higher frequencies (11-25 Hz) were analyzed for the first 7 min within a NREM sleep episodes (Figure 7). In the baseline day, SWA progressively increased, reaching peak activity within 2-5 min after the onset of NREM sleep, and then gradually decreased (Figure 7, SWA, top panel). During the first 4-h interval of baseline (CT 0-4), EEG SWA in the first 7 min of NREM sleep reached a mean activity level of 117.4% ± 5.4%. This decreased to 91.8% ± 3.5% between CT 4-8, and the lowest mean level was found between CT 8-12 (79.0% ± 1.9%). At the end of the subjective night (CT 18-24), SWA reached the average level of 107.4% ± 7.1%. These mean levels in SWA showed highly significant circadian modulation over the baseline day (GF = 0.049, P < 0.02 after one-way rANOVA, factor interval: F5,35 = 7.1; P < 0.001). During the 2h/2h protocol, this circadian variation was abolished (GF = 0.436, P > 0.05 after one-way rANOVA, factor interval: F5,35 = 3.3; P < 0.05).
Figure 7.
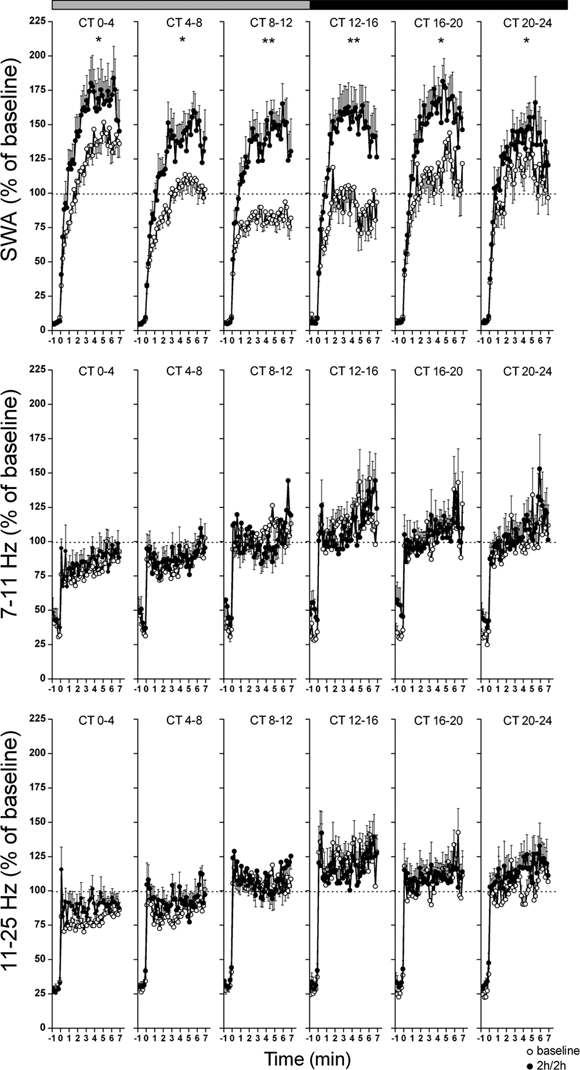
Time course of SWA, SFA (7-11 Hz), and higher frequency activity (11-25 Hz) within the first 7 min of NREM sleep onset. Data are plotted as percentage of the mean activity of the corresponding frequency during the baseline 24 h. The curves connect 10-sec mean values (n = 8) of corresponding activity for the last minute before, and first 7 min after, a NREM episode. Each panel represents mean values (n = 8) for undisturbed 2-h periods of the 2h/2h deprivation protocol and corresponding baseline intervals. The horizontal bars on a top indicate the rest (grey) and active (black) phase. Significant differences between the average levels of activity in the 2h/2h protocol and corresponding baseline intervals are indicated by *(P < 0.05) and **(P < 0.01), 2-tailed paired t-test after significant rANOVA, factor “day.”
Activity between 7-11 Hz and 11-25 Hz in NREM sleep EEG (Figure 7, middle and bottom panels) demonstrated a dissimilar pattern within the 7 min episodes, as well as over 24-h compared to SWA, and showed no significant difference in the mean activity levels between baseline and the 2h/2h protocol (one-way rANOVA, factor day, 7-11 Hz: F1,14 = 0.1; P = 0.766; 11-25 Hz: F1,14 = 0.6; P =0.465). Within the frequency band 7-25 Hz, average activity reached maximum levels almost immediately within the first minute of the NREM sleep episode, but afterwards gradually increased (Figure 7, 7-11 Hz, middle panel) or remained stable (Figure 7, 11-25 Hz, bottom panel) within the first 7 min of the NREM sleep episode. For both frequency ranges, average activity in baseline demonstrated the lowest values in the first 2 intervals of the rest phase (CT 0-8; 7-11 Hz: 82.0% ± 1.7%; 11-25 Hz: 81.5% ± 1.5%) and higher stable levels over the subsequent intervals (CT 8-24; 7-11 Hz: 105.2% ± 2.0%; 11-25 Hz: 110.2% ± 2.2%). This pattern remained in the 2h/2h schedule. Circadian changes were observed for both 7-11 Hz and 11-25 Hz activity in baseline (7-11 Hz: GF = 0.027, P < 0.005; 11-25 Hz: GF = 0.024, P < 0.005 after one-way rANOVA, factor interval, 7-11 Hz: F(5,35) = 18.3; P < 0.001; 11-25 Hz: F5,35 = 26.7; P < 0.001) and during the 2h/2h protocol (7-11 Hz: GF = 0.053, P < 0.02; 11-25 Hz: GF = 0.074, P < 0.05 after rANOVA, factor interval,7-11 Hz: F5,35 = 9.8; P < 0.001; 11-25 Hz: F5,35 = 11.4; P < 0.001).
DISCUSSION
The 2h/2h sleep deprivation paradigm in the rat under DD conditions resulted in a relatively stable level of sleep over the circadian day. The animals spent only 7.5% more of the 24-h in wakefulness compared to baseline; this time was composed of NREM sleep during the baseline day. The amount of REM sleep over 24 h did not change significantly. The amount of REM sleep was slightly reduced during the subjective day, and increased during the 2-h rest periods in the subjective night. Despite the mild influence on TST over 24 h, the application of 2h/2h protocol had dramatic effects on the distribution of sleep and, at the same time, was remarkably successful in stabilizing the amount of SWA in the NREM sleep EEG.
Although sleep and wakefulness were almost evenly distributed and a constant DD condition was applied, a clear circadian fluctuation of EEG power density in the faster frequencies (> 7 Hz; see below), as well as a daily modulation in the vigilance state episode duration could still be observed. In our study, the duration of NREM sleep did not lose daily modulation under influence of the 2h/2h protocol, whereas REM sleep did. The duration of REM sleep episodes was constant and high over the 2h/2h experimental day. This indicates that daily modulation of NREM sleep episode duration is under relatively strong circadian control, compared to REM sleep episode duration, which seems to respond more strongly to sleep homeostatic demands. At the same time, the frequency of the episodes noticeably stabilized for all vigilance states under the 2h/2h protocol. NREM sleep episode frequency remained at an intermediate level, whereas the frequency of waking episodes was decreased.
Our findings are in contrast to previous notions, which propose a functional relationship between NREM and REM sleep episode duration.45 REM sleep propensity would accumulate in the course of a NREM sleep episode, which would predict similar changes in the duration of NREM and REM sleep episodes from baseline to the 2h/2h protocol.
In the present protocol, waking episode duration in particular seemed to be almost independent of sleep homeostatic processes and determined primarily by the phase of the circadian clock. To a lesser extent, the same holds true for NREM sleep, although the diurnal amplitude was reduced. In contrast, REM sleep episode duration appeared to be very susceptible to the moderate increase in sleep pressure, and the influence of the circadian clock was not visible during the 2h/2h protocol. The remaining daily modulation in the amount of waking and NREM sleep in the 2h/2h protocol was caused by daily changes in waking and NREM sleep episode duration.
The 2-h values of SWA in the NREM sleep EEG did not show a significant circadian modulation during the 2h/2h protocol. This phenomenon was observed previously in SCN lesioned rats,46 Guinea pigs,47 and winter adapted Djungarian hamsters.48 In all these cases NREM sleep was equally distributed across the 24-h day, as in the present results. This constant pattern of SWA is in accordance with the 2-process model of sleep regulation,1,2,6 which predicts that SWA in the NREM sleep EEG changes as a function of prior waking duration; presumably, when the amount of waking is evenly distributed over 24 h, SWA will not show a significant modulation. Indeed, the present data indicate that sleep pressure across 4-h intervals over the 24-h day was almost constant and higher than baseline during all intervals except CT 0-4 (when the amount of SWA did not differ from baseline). These facts demonstrate that the 2h/2h protocol resulted in both stabilization and a moderate increase in sleep pressure. A slight daily modulation was seen in SWA in the first 7 min of a NREM sleep episode, indicating that changes in SWA observed on the basis of hourly values may not reflect what happens within a separate NREM sleep episode.
Activity in the NREM sleep EEG frequencies above 7 Hz still showed clear circadian modulation that did not differ from baseline. This is the first demonstration that faster EEG frequencies in rodents are not influenced by sleep homeostatic mechanisms and show circadian modulation with an endogenous origin. Circadian rhythms originate from the circadian clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus3–5; however, the mechanism by which this clock determines the activity in the higher frequencies of the NREM sleep EEG remains unclear. It has been suggested that melatonin, which is released during the (subjective) dark period is influencing the EEG.49 However, melatonin should then exert opposite effects in day active animals compared to night active animals, because activity in faster frequencies of the EEG also shows an opposite pattern.25 Other hormones with a circadian release pattern, such as hypocretin/orexin and cortisol,50,51 may cause circadian changes in sleep or the sleep EEG. As an alternative hypothesis, it was proposed that daily fluctuations in brain temperature may cause circadian changes in EEG activity in frequencies above 20 Hz.52 Higher brain temperatures during the active phase may increase the firing rate of individual neurons, which in turn, would increase EEG activity in the faster frequencies. Whether the 2h/2h protocol influences body temperature is unclear. A similar nap protocol in humans, who were carefully controlled for body posture, did not change the circadian time course in body temperature.53 Therefore, the 2h/2h protocol may also not have changed body temperature levels within NREM and REM sleep when the animals are in the same sleeping posture. The present data show that frequencies above 7 Hz in the NREM sleep EEG of the rat display endogenous circadian modulation, which is not influenced by sleep homeostatic mechanisms.
The present results can be compared with an experiment in humans, in which subjects were forced to sleep for 30 min every 90 min. In this nap protocol, the amount of sleep dropped from the normal 7-8 h over the 24-h baseline day to less than 5 h15,54; therefore, the sleep deprivation in these studies was much more severe than in our experiments in the rat. Nevertheless, clear circadian modulation of NREM and REM sleep was observed under the nap protocol in humans, with low amounts of NREM and REM sleep shortly before normal bedtime (22:00-23:00) and high levels of NREM and REM sleep shortly after the core body temperature minimum.16,54 Although in our experiments the sleep deprivation was much milder than in the nap protocol in humans, the distribution of sleep seemed to be influenced more in the rat. In particular, REM sleep distribution was strongly influenced by the 2h/2h protocol. Application of the forced desynchrony protocol is another well-accepted way to investigate circadian modulation of sleep and the sleep EEG in humans.17,55 Similar to the nap protocol, it was shown that the lowest level of sleep was found around 22:00-23:00, and the highest levels of NREM sleep when the body temperature was low. REM sleep peaked shortly after the trough in core body temperature.17 In our protocol, NREM sleep was slightly reduced during the subjective night, and waking was increased by a similar amount. However, a short distinct period of markedly increased waking (like that in humans at 22:00-23:00) and a clear peak in REM sleep propensity were not found. This is probably attributable to the ultradian sleep-wake pattern of the rat, which renders this species more flexible in choosing its sleep time, but it could also be due to the larger sampling intervals (4 h vs. 1.5 h in humans) applied in the present experiment.
In the forced desynchrony protocol, it was shown that when sleep-wake dependent changes were evened out, a small but significant circadian modulation in SWA in the NREM sleep EEG could still be observed.17 This might be related to circadian variations in REM sleep duration56,57 or circadian variation in sleep time in the preceding episodes.17 In the human EEG, frequencies between 11-16 Hz showed large circadian amplitude changes. The 12.25-13.0 Hz range and the 13.75-15.5 Hz range showed circadian modulation that were 180 degrees out of phase.55 The activity in frequencies below 11 Hz was primarily dependent on the duration of prior wakefulness, whereas frequencies above 16 Hz (16-25 Hz) were not strongly influenced by either of the two.
In general, the comparison of EEG power density spectra between species is rather difficult. The outcome of the results in EEG activity obtained in humans depends on electrode placement and may be typical for humans and not for rodents. This may also explain why the negative correlation between SWA and spindle frequency activity in baseline sleep in humans34 was not found in our analysis of the time course of the 7-11 Hz frequency range within the first 7 min of NREM sleep. In our protocol, we applied an electrode configuration similar to those applied in several previous studies in the rat and other rodent studies, and our baseline results do not differ from previous findings showing a gradual decrease in SWA in the course of the rest phase and a subsequent increase during the active phase.11,18,24,43,59–61 In addition, changes in the faster EEG frequencies are consistent with previous findings.18,24,25 Thus, we conclude that the robust circadian modulation of the faster frequencies in the 2h/2h protocol is a genuine endogenous circadian rhythm.
The reported data were achieved with sleep deprivation comprising 50% of the total recording time. A different relationship between rest and sleep deprivation duration would have resulted in a different overall sleep pressure level; this may have influenced the results. It also was not possible to control the protocol for an equal distribution of food and water intake or to control for posture of the animals, as is done in a constant routine protocol. Nevertheless, the present results give new insights into sleep regulatory mechanisms in the rat. The duration of waking episodes shows daily modulation, which, compared to LD values in the literature,44 remains virtually intact in DD and under the 2h/2h protocol when sleep pressure is constant. In contrast, the amount of REM sleep and REM sleep episode frequency and duration lose their circadian modulation under these conditions. NREM sleep shows intermediate behavior, with a slight reduction in the circadian amplitude of episode duration. NREM sleep EEG frequencies in a range 7-25 Hz behaved in a circadian-dependent way similar to that during baseline, and thus appear to be under strong control of the circadian clock.
We demonstrate here that the 2h/2h protocol is a valuable new tool to investigate the circadian regulation of sleep. All together, the data show that the duration of waking and NREM sleep episodes and activity in the higher frequencies are clearly under control of the circadian clock; the sleep homeostat seems to exert its influence on the amount of waking and NREM sleep, mainly through changes in episode frequency. In contrast to humans, the circadian clock in the rat seems to have only weak control over the occurrence of REM sleep, which responds very strongly to mild changes in homeostatic sleep demand by changes in episode frequency and duration.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Dr. J. H. Meijer for comments and advice on the manuscript. This research was supported by the European Union (Grant LSHM-CT-2005-518189) and the Netherlands Organization for Scientific Research (NWO, grant 818.02.016).
REFERENCES
- 1.Borbély AA. A two process model of sleep regulation. I. Physiological basis and outline. Human Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–83. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 3.Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Rev. 2005;49:429–54. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 2007;8(Suppl 3):27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Vansteensel MJ, Michel S, Meijer JH. Organization of cell and tissue circadian pacemakers: a comparison among species. Brain Res Rev. 2008;58:18–47. doi: 10.1016/j.brainresrev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier; 2005. pp. 405–17. [Google Scholar]
- 7.Tobler I, Borbély AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 8.Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–19. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 9.Lancel M, Riezen van H, Glatt A. Effects of circadian phase and duration of sleep deprivation on sleep and EEG power spectra in the cat. Brain Res. 1991;548:206–14. doi: 10.1016/0006-8993(91)91123-i. [DOI] [PubMed] [Google Scholar]
- 10.Strijkstra AM, Daan S. Dissimilarity of slow-wave activity enhancement by torpor and sleep deprivation in a hibernator. Am J Physiol. 1998;275:R1110–7. doi: 10.1152/ajpregu.1998.275.4.R1110. [DOI] [PubMed] [Google Scholar]
- 11.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 12.Deboer T, Tobler I. Sleep Regulation in the Djungarian hamster: comparison of the dynamics leading to the slow-wave activity increase after sleep deprivation and daily torpor. Sleep. 2003;26:567–72. doi: 10.1093/sleep/26.5.567. [DOI] [PubMed] [Google Scholar]
- 13.Achermann P, Dijk DJ, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 14.Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci Lett. 1991;130:141–4. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon MA, Dement WC. Sleep studies on a 90-minute day. Electroencephalogr Clin Neurophysiol. 1975;39:145–55. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- 16.Carskadon MA, Dement WC. Distribution of REM sleep on a 90 min sleep-wake schedule. Sleep. 1980;2:309–17. [PubMed] [Google Scholar]
- 17.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobler I, Franken P, Alföldi P, Borbély AA. Room light impairs sleep in the albino rat. Behavioral Brain Res. 1994;63:205–211. doi: 10.1016/0166-4328(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 19.Deboer T, Ruigrok G, Meijer JH. Short light-dark cycles affect sleep in mice. Eur J Neurosci. 2007;26:3518–23. doi: 10.1111/j.1460-9568.2007.05964.x. [DOI] [PubMed] [Google Scholar]
- 20.Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep. 2007;30:257–62. doi: 10.1093/sleep/30.3.257. [DOI] [PubMed] [Google Scholar]
- 21.Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav. 1991;50:373–78. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- 22.Deboer T, Vansteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–90. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 23.Deboer T. Sleep and sleep homeostasis in constant darkness in the rat. J Sleep Res. 2009;18:357–64. doi: 10.1111/j.1365-2869.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 24.Tobler I, Jaggi K. Sleep and EEG spectra in the Syrian hamster (Mesocricetus auratus) under baseline conditions and following sleep deprivation. J Comp Physiol. 1987;161:449–59. doi: 10.1007/BF00603970. [DOI] [PubMed] [Google Scholar]
- 25.Tobler I, Dijk DJ, Borbély AA. Comparative aspects of sleep regulation in three species. In: Horne JA, editor. Sleep'90. Bochum: Pontenagel Press; 1990. pp. 349–51. [Google Scholar]
- 26.Trachsel L, Tobler I, Borbély AA. Electroencephalogram analysis of non-rapid eye movement sleep in rats. Am J Physiol. 1988;255:R27–37. doi: 10.1152/ajpregu.1988.255.1.R27. [DOI] [PubMed] [Google Scholar]
- 27.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillation in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 29.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Puig M, Ushimaru M, Kawaguchi Y. Two distinct activity pattern of fast-spiking interneurons during neocortical UP states. Proc Natl Acad Sci U S A. 2008;105:8428–33. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khazipov R, Sirota A, Leinekuge X, Holmes G, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–61. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 32.Sakata S, Yamamori T, Sakurai Y. 7-12 Hz cortical oscillations: behavioral context and dynamics of prefrontal neuronal ensembles. Neuroscience. 2005;134:1099–111. doi: 10.1016/j.neuroscience.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Steriade M, Curró Dossi R, Nuñez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–17. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aeschbach D, Borbély AA. All-night dynamics of the human sleep EEG. J Sleep Res. 1993;2:70–81. doi: 10.1111/j.1365-2869.1993.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 35.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 36.De Gennaro L, Ferrara M, Bertini M. Effect of slow-wave sleep deprivation on topographical distribution of spindles. Behav Brain Res. 2000;116:55–9. doi: 10.1016/s0166-4328(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 37.Finelli L, Borbély AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–90. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 38.Knoblauch V, Kräuchi K, Renz C, Wirz-Justice A, Cajochen C. Homeostatic control of slow-wave and spindle frequency activity during human sleep: effect of differential sleep pressure and brain topography. Cereb Cortex. 2002;12:1092–10. doi: 10.1093/cercor/12.10.1092. [DOI] [PubMed] [Google Scholar]
- 39.Knoblauch V, Martens W, Wirz-Justice A, Kräuchi K, Cajochen C. Regional differences in the circadian modulation of human sleep spindle characteristics. Eur J Neurosci. 2003;18:155–63. doi: 10.1046/j.1460-9568.2003.02729.x. [DOI] [PubMed] [Google Scholar]
- 40.Mistlberger RE, Rusak B. Circadian rhythms in mammals: formal properties and environmental influences. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier; 2005. pp. 277–85. [Google Scholar]
- 41.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meijer JH, Daan S, Overkamp GJ, Hermann PM. The two-oscillator circadian system of tree shrews (Tupaia belangeri) and its response to light and dark pulses. J Biol Rhythms. 1990;5:1–16. doi: 10.1177/074873049000500101. [DOI] [PubMed] [Google Scholar]
- 43.Franken P, Dijk DJ, Tobler I, Borbély AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 44.Franken P, Tobler I, Borbély AA. Cortical temperature and EEG slow-wave activity in the rat: analysis of vigilance state related changes. Eur J Physiol. 1992;420:500–7. doi: 10.1007/BF00374625. [DOI] [PubMed] [Google Scholar]
- 45.Benington JH, Heller HC. REM-sleep timing is controlled homeostatically by accumulation of REM sleep propensity in non-REM sleep. Am J Physiol. 1994;266:R1992–2000. doi: 10.1152/ajpregu.1994.266.6.R1992. [DOI] [PubMed] [Google Scholar]
- 46.Trachsel L, Edgar DM, Seidel WF, Heller HC, Dement WC. Sleep homeostasis in suprachiasmatic nuclei-lesioned rats: effects of sleep deprivation and triazolam administration. Brain Res. 1992;589:253–61. doi: 10.1016/0006-8993(92)91284-l. [DOI] [PubMed] [Google Scholar]
- 47.Tobler I, Franken P, Jaggi K. Vigilance states, EEG spectra, and cortical temperature in the guinea pig. Am J Physiol. 1993;264:R1125–32. doi: 10.1152/ajpregu.1993.264.6.R1125. [DOI] [PubMed] [Google Scholar]
- 48.Deboer T, Vyazovskiy VV, Tobler I. Long photoperiod restores the 24-h rhythm of sleep and EEG slow-wave activity in the Djungarian hamster (Phodopus sungorus) J Biol Rhythms. 2000;15:429–36. doi: 10.1177/074873040001500508. [DOI] [PubMed] [Google Scholar]
- 49.Dijk DJ, Roth C, Landolt HP, et al. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci Lett. 1995;201:13–6. doi: 10.1016/0304-3940(95)12118-n. [DOI] [PubMed] [Google Scholar]
- 50.Deboer T, Overeem S, Visser NA, et al. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience. 2004;129:727–32. doi: 10.1016/j.neuroscience.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 51.Maywood ES, O'Neill JS, Chesham JE, Hastings MH. Minireview: The circadian clockwork of the suprachiasmatic nuclei-analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–34. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- 52.Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J Sleep Res. 1998;7:254–6. doi: 10.1046/j.1365-2869.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 53.Cajochen C, Knoblauch V, Kräuchi K, Renz C, Wirz-Justice A. Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. NeuroReport. 2001;12:2277–81. doi: 10.1097/00001756-200107200-00046. [DOI] [PubMed] [Google Scholar]
- 54.Dantz B, Edgar DM, Dement WC. Circadian rhythms in narcolepsy: studies on a 90 minute day. Electroencephalogr Clin Neurophysiol. 1994;90:24–35. doi: 10.1016/0013-4694(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 55.Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505:851–8. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beersma DG, Dijk DJ, Blok CG, Everhardus I. REM sleep deprivation during 5 hours leads to an immediate REM sleep rebound and to suppression of non-REM sleep intensity. Electroencephalogr Clin Neurophysiol. 1990;76:114–22. doi: 10.1016/0013-4694(90)90209-3. [DOI] [PubMed] [Google Scholar]
- 57.Brunner DP, Dijk DJ, Tobler I, Borbély AA. Effect of partial sleep deprivation on sleep stages and EEG power spectra: evidence for non-REM and REM sleep homeostasis. Electroencephalogr Clin Neurophysiol. 1990;75:492–9. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- 58.Dijk DJ, Brunner DP, Borbély AA. EEG power density during recovery sleep in the morning. Electroencephalogr Clin Neurophysiol. 1991;78:203–14. doi: 10.1016/0013-4694(91)90034-2. [DOI] [PubMed] [Google Scholar]
- 59.Dijk DJ, Daan S. Sleep EEG spectral analysis in a diurnal rodent: Eutamias sibiricus. J Comp Physiol. 1989;165:205–15. doi: 10.1007/BF00619195. [DOI] [PubMed] [Google Scholar]
- 60.Deboer T, Franken P, Tobler I. Sleep and cortical temperature in the Djungarian hamster under baseline conditions and after sleep deprivation. J Comp Physiol. 1994;174:145–55. doi: 10.1007/BF00193782. [DOI] [PubMed] [Google Scholar]
- 61.Tobler I, Gaus SE, Deboer T, et al. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639–42. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]