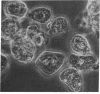
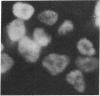
Abstract
Addition of ATP to a highly purified fraction of rat liver nuclei incubated with submicromolar concentrations of Ca2+ and trace amounts of 45Ca2+ resulted in the rapid accumulation of 45Ca2+ in the nuclei. This was associated with an increase in intranuclear free Ca2+ concentration as measured with the fluorescent dye 1-[2-(5-carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2'-a mino-5'- methylphenoxy)ethane-N,N,N',N'-tetraacetic acid (fura-2). Inhibitors of microsomal and mitochondrial Ca2+ translocases had no effect on nuclear Ca2+ sequestration, indicating that it was distinct from previously known intracellular Ca2+-transporting systems. Ca2+ uptake and the associated increase in intranuclear free Ca2+ concentration were prevented by calmidazolium, a potent calmodulin antagonist. Partial characterization of the ATP-stimulated nuclear Ca2+ uptake showed that maximal rates of Ca2+ uptake and increase in intranuclear free Ca2+ level occurred at concentrations of Ca2+ normally present in the cytosol of mammalian cells. Together, these results show that a distinct, ATP- and calmodulin-dependent Ca2+ uptake system exists in liver nuclei. This system may play an important role in the regulation of intranuclear Ca2+-dependent processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachs O., Carafoli E. Calmodulin and calmodulin-binding proteins in liver cell nuclei. J Biol Chem. 1987 Aug 5;262(22):10786–10790. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Means A. R. Potentiation of bleomycin lethality by anticalmodulin drugs: a role for calmodulin in DNA repair. Science. 1984 Jun 22;224(4655):1346–1348. doi: 10.1126/science.6203171. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Delfert D. M., McDonald J. M. Vanadyl and vanadate inhibit Ca2+ transport systems of the adipocyte plasma membrane and endoplasmic reticulum. Arch Biochem Biophys. 1985 Sep;241(2):665–672. doi: 10.1016/0003-9861(85)90593-4. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harper J. F., Cheung W. Y., Wallace R. W., Huang H. L., Levine S. N., Steiner A. L. Localization of calmodulin in rat tissues. Proc Natl Acad Sci U S A. 1980 Jan;77(1):366–370. doi: 10.1073/pnas.77.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova O. G., Savost'ianov G. A., Beliavtseva L. M., Razumovskaia N. I. Issledovanie ATPaznoi aktivnosti i ATP-zavisimoi akkumuliatsii Ca2+ iadrami skeletnykh myshts. Effekty denervatsii i élektricheskoi stimuliatsii. Biokhimiia. 1982 Jul;47(7):1216–1221. [PubMed] [Google Scholar]
- Lotersztajn S., Hanoune J., Pecker F. A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence of an endogenous protein activator distinct from calmodulin. J Biol Chem. 1981 Nov 10;256(21):11209–11215. [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Oakes S. G., Martin W. J., 2nd, Lisek C. A., Powis G. Incomplete hydrolysis of the calcium indicator precursor fura-2 pentaacetoxymethyl ester (fura-2 AM) by cells. Anal Biochem. 1988 Feb 15;169(1):159–166. doi: 10.1016/0003-2697(88)90267-9. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: trapping the covalent enzyme-DNA complex in an active form. Biochemistry. 1987 Jul 14;26(14):4303–4309. doi: 10.1021/bi00388a018. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Twigg J., Patel R., Whitaker M. Translational control of InsP3-induced chromatin condensation during the early cell cycles of sea urchin embryos. Nature. 1988 Mar 24;332(6162):366–369. doi: 10.1038/332366a0. [DOI] [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Williams D. A., Becker P. L., Fay F. S. Regional changes in calcium underlying contraction of single smooth muscle cells. Science. 1987 Mar 27;235(4796):1644–1648. doi: 10.1126/science.3103219. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]