Abstract
Study Objectives:
Women experience insomnia more frequently than men. Menstrual cycle changes in reproductive hormones and circadian rhythms may contribute to sleep disruptions. Our aim, therefore, was to clarify the interaction between menstrual and circadian processes as it affects sleep.
Design:
Participants entered the laboratory during the mid-follicular (MF) and mid-luteal (ML) phases of their menstrual cycle for an ultra-rapid sleep-wake cycle (URSW) procedure, consisting of 36 cycles of 60-min wake episodes alternating with 60-min nap opportunities. This procedure concluded with an ad libitum nap episode.
Setting:
Time-isolation suite.
Participants:
Eight unmedicated, physically and mentally healthy females with regular ovulatory menstrual cycles.
Interventions:
N/A
Measurements:
Polysomnographic sleep from nocturnal sleep episodes and 60-min naps; subjective alertness; core body temperature (CBT); salivary melatonin; urinary estradiol; and urinary progesterone.
Results:
Increased CBT values at night and decreased CBT amplitude were observed during ML compared to MF. Circadian phase of CBT and the circadian melatonin profile were unaffected by menstrual phase. All analyzed sleep parameters showed a circadian variation throughout the URSW procedure, with no menstrual phase differences observed for most, including slow wave sleep (SWS). The circadian variation of REM sleep duration, however, was sensitive to menstrual phase, with reduced REM sleep during ML at circadian phase 0° and 30°.
Conclusions:
Moderate but significant changes in REM sleep across the menstrual and circadian cycles were observed. These results support an interaction between circadian and menstrual processes in the regulation of REM sleep.
Citation:
Shechter A; Varin F; Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. SLEEP 2010;33(5):647-656.
Keywords: Circadian rhythms, sleep, menstrual cycle, core body temperature, melatonin, ultra-rapid sleep-wake cycle
SLEEP COMPLAINTS ARE PREVALENT IN WOMEN, WHO, DESPITE FINDINGS OF UNAFFECTED POLYSOMNOGRAPHIC (PSG) SLEEP MACROSTRUCTURE,1 ARE 1.5-2 times as likely to report insomnia symptoms as men.2 The variation of reproductive hormones across the menstrual cycle, including progesterone, which is low throughout the follicular phase (FP) and rises during the luteal phase (LP), can affect sleep.3 Overall, previous studies documenting nocturnal PSG sleep indicate sleep onset latency (SOL), sleep efficiency (SE), and slow wave sleep (SWS) are stable across the menstrual cycle, whereas decreases in REM sleep are observed during LP compared to FP.3 Nevertheless, PSG findings are not unequivocal, and inconsistencies persist regarding the variation of SWS4–6 and REM sleep across the menstrual cycle.6–9
Changes in reproductive hormones are also thought to affect the expression of circadian rhythms, though findings are equivocal.3 During LP, high progesterone levels are associated with significant alterations in core body temperature (CBT) rhythms, including increased daily values of 0.3°-0.4°C, blunted nocturnal decline, and reduced circadian amplitude.10–13 Inconsistencies appear when considering the timing of the temperature rhythm across the menstrual cycle, as studies have reported phase delays7,12 or unchanged phase4,10,11 during LP compared to FP. As for melatonin, a pineal hormone whose high nocturnal secretion pattern makes it a reliable circadian marker, it remains unclear whether its rhythm is affected by menstrual phase. There are prior reports of decreases,13 increases,14 phase-delays,12 or no change15 in melatonin secretion during LP compared to FP. Finally, though limited in number, studies investigating the circadian variation of cortisol across the menstrual cycle have been similarly inconsistent, with rhythms either phase-advanced, phase-delayed, or decreased in amplitude during LP.16
Modifications in circadian rhythms across the menstrual cycle are pertinent since sleep is regulated by a complex interaction between circadian and homeostatic processes.17 Experiments manipulating the timing of the sleep-wake cycle (e.g., by forced desynchrony or ultra-rapid sleep-wake cycle [URSW] procedures17–19) have illustrated the circadian variation of sleep propensity and architecture, and how these are related to CBT and melatonin rhythms. Specifically, increased sleep and REM sleep propensity as well as decreased SOL and REM sleep onset latency (ROL) are observed near the CBT nadir when endogenous melatonin levels are high.17,19
In the present study, we utilized the URSW at the mid-follicular (MF) and mid-luteal (ML) phases of the menstrual cycle to quantify the circadian variation of sleep propensity and organization in healthy women. This design allowed us to observe nocturnal sleep at different menstrual phases, to study variation in circadian rhythms of CBT and melatonin across the menstrual cycle under highly controlled conditions, and to document how the circadian variation of sleep is modified by different phases of the menstrual cycle. This investigation will therefore explore the interaction between circadian and menstrual processes as it affects sleep and alertness in healthy women. As such, this line of investigation has the potential to shed light on factors contributing to the increased susceptibility for sleep disruptions and reports of poor sleep in women.2
METHODS
Participants
Eight physically and mentally healthy women (mean age ± SD: 26 ± 2.67; mean height ± SD: 164.14 ± 1.19 cm; mean weight ± SD: 59.6 ± 3.5 kg) with regular menstrual cycles (26-32 ± 3 days) were studied. Participants confirmed ovulation via plasma progesterone test on day 21 of the menstrual cycle preceding experimental procedures. All were nulliparous, not currently breast feeding, and free of hormonal contraceptives and gynecological pathology. Exclusion criteria included shift work or transmeridian travel within the past 3 months. All were drug free and good sleepers with no evidence of psychopathology or premenstrual dysphoric disorder.
Prior to entry, participants maintained a regular schedule of 8 h sleep/darkness per day for ≥ 3 weeks, confirmed by sleep-wake log, calls to the laboratory at wake/bed-times, and wrist-worn actigraphy (Actiwatch, Mini-Mitter, Bend, OR, USA) the week preceding admission. The Douglas Mental Health University Institute Research Ethics Board approved all procedures and all participants provided informed consent.
Design
Participants entered the laboratory for 5 days during the MF (days 5-9 after menses) and ML (days 19-23 after menses) phases of their menstrual cycle. Each visit was scheduled on a separate menstrual cycle to allow recuperation, and the phase at first visit was balanced such that 4 participants had their initial visit at MF. Procedures during both visits were identical.
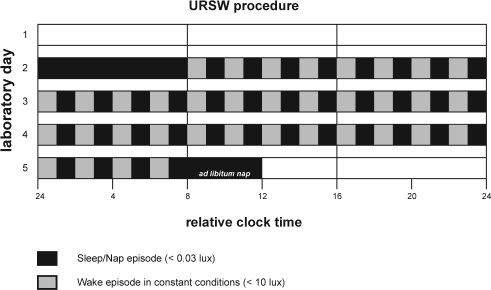
Each visit lasted 5 days in time isolation and began with an 8-h sleep episode based on the habitual sleep times of the preceding 3-week schedule (mean ± SD; bedtime: 00:03 ± 00:39; wake time: 08:03 ± 00:39). This schedule was comparable between visits. Upon awakening, participants began the URSW, consisting of 60-min wake episodes alternating with 60-min nap opportunities, for a total 36 wakes and naps spanning 3 circadian cycles (Figure 1). When not completing experimental procedures (e.g., psychometric testing, saliva and urine sampling) during wake periods, participant activities included reading, listening to music, watching movies and conversing with technical staff. The 72-h procedure concluded with an ad libitum nap episode. Constant conditions, including semi-recumbent posture, a time-cue free environment, iso-caloric snacks (1x/wake), and dim lights during wake episodes (< 10 lux) were maintained throughout the URSW. Naps occurred in darkness (< 0.03 lux).
Figure 1.
Illustration of the laboratory experimental protocol. Participants entered the laboratory on 2 occasions for a duration of 5 days: i.e. during the MF (scheduled on days 5-9 after menses onset) and the ML (scheduled on days 19-23 after menses onset) phases of their menstrual cycle. Each visit was scheduled on a separate menstrual cycle to allow recuperation, and the phase at first visit was balanced such that 4 participants had their initial visit at MF and 4 at ML. After entering the lab in the evening of Day 1 for a period of acclimatization (light levels: 150 lux), participants were put to bed for an 8-h nocturnal sleep episode (light levels: < 0.03 lux) scheduled at their habitual time. Upon awakening, participants began the 72-h URSW procedure which consisted of 36 cycles of 60-min wake episodes in constant conditions (small isocaloric snacks at each wake episode; light levels: < 10 lux) alternating with 60-min nap episodes (light levels: < 0.03 lux), all in semi-recumbent posture. This was concluded with an ad libitum nap episode (light levels: < 0.03 lux). The protocol for a participant with a habitual sleep time from 00:00 to 8:00 is illustrated.
Measures and Data Processing
For all nocturnal sleep and naps, PSG recordings, including central and occipital electroencephalogram, electrooculogram and submental electromyogram (EMG), were made on a computerized system (Harmonie, Stellate Systems, Montreal, QC, Canada) at a sampling rate of 250 Hz, and high- and low-pass filtered at 0.3 Hz and 35 Hz, respectively. Sleep was visually scored in 30-sec epochs according to standard criteria.20 The amount of each sleep stage was determined and expressed as minutes and percent of the sleep period (SP; from sleep onset to final awakening). Total sleep time (TST) was the sum of sleep stages 1 to 4 plus REM sleep. SE was TST divided by the time from lights-off to lights-on (for the 8-h nocturnal sleep episode) or by 30 circadian degrees (for naps throughout the URSW), multiplied by 100. SOL was the time from lights-off to the first appearance of ≥ 2 epochs of stage 1 sleep, or the first appearance of any deeper stage. NREM sleep was the sum of sleep stages 2 to 4. ROL was the time from sleep onset to the first appearance of REM sleep. If a nap contained no incidence of REM sleep, a value of 60 (i.e., the full duration of the nap period) was assigned for ROL. The organization of sleep across the nocturnal SP was analyzed by dividing the SP into 3 segments of equal duration. Periodic leg movements in sleep (PLM) and apnea/hypopnea were ruled out during the first PSG recording. For PLM, EMGs of the left and right anterior tibialis were recorded. Leg movements of 0.5-5.0 sec in duration occurring at intervals of 4.0-90.0 sec and clustered in groups ≥ 4 were considered PLMs, in accordance with Coleman's criteria.21 Respiratory parameters were monitored with bucconasal thermistance and airflow pressure transducer. AASM recommended criteria22 for defining apnea (≥ 90% reduction in airflow for ≥ 10 sec) and hypopnea (≥ 30% reduction in airflow for ≥ 10 sec) were used, and all participants had an apnea/hypopnea index below 5.
A post-sleep questionnaire administered after awakening from the 8-h nocturnal sleep episode assessed subjective sleep quality (SSQ; numeric rating from 0 to 6, with 0 being extremely bad and 6 being extremely good) for the preceding sleep episode. Subjective alertness following the full sleep episode was assessed via numeric rating (from 1 to 9, with 1 being not at all sleepy and 9 being definitely sleepy). A post-nap questionnaire administered ∼2 min after waking from each nap throughout the URSW assessed SSQ for the preceding nap. Subjective alertness (10-cm bipolar visual analog scale [VAS] with 0 cm being extremely sleepy and 10 cm being extremely alert) was also assessed at that time. A second subjective alertness assessment was administered in the middle of all wake episodes (∼30 min after lights-on).
CBT was continuously monitored (4x/min) throughout the URSW via a thermistor (Steri-Probe, Cincinnati Sub-Zero Products Inc., Cincinnati, OH, USA) inserted 10 cm into the rectum, connected to an in-house data acquisition system. Data were inspected for probe malfunctions or “slips,” which were discarded. To minimize confounding effects accompanying the transition from nocturnal sleep to the start of the URSW, CBT data from the first 8 h of the URSW were removed. After collapsing all CBT data into 1-min bins, a dual-harmonic regression model23 without serial correlated noise was applied to individual CBT curves for the last 64 h of the URSW. Circadian phase was defined as time of fitted minimum of this model. Circadian amplitude was defined as the mean-to-trough difference of the first harmonic of the regression.24 The fitted CBT minimum obtained during the first day of the URSW was assigned circadian phase 0° and used to assign a circadian phase from 0° to 359.9° for all outcome parameters throughout the URSW (see below).
Saliva samples were collected twice during each wake episode (∼5 and ∼55 min after lights-on), and assayed in duplicate for their content in melatonin using direct saliva melatonin radioimmunoassay 125I-labelled tracer (Participants 01-04: Stockgrand Ltd, Guilford, Surrey, UK, coefficient of variation: 8.5%, lower limit of detection: 2 ng/mL; Participants 05-08: Buhlman, Alpco Diagnostics, Windham, NH, USA, mean intra- and interassay coefficients of variation: 7.9% and 9.8%, respectively, lower limit of detection: 2 ng/mL). Sample concentrations below the lower limit of detection were assigned a value of zero. This was observed in (mean ± SEM) 19.04% ± 6.15% of the samples for MF and 26.01% ± 7.23% of the samples for ML, which was not significantly different between menstrual phases (t7 = −1.05, P = 0.33). Melatonin profile included dim light melatonin onset (DLMOn), dim light melatonin offset (DLMOff), midpoint of secretion (time-point between DLMOn and DLMOff), duration of secretion (time from DLMOn to DLMOff), amplitude, and time of fitted maximum (i.e., circadian phase). DLMOn and DLMOff were defined as the times of upward and downward crossing of the 24-h average, respectively.25 Amplitude and fitted maximum were determined with the first harmonic of a 3-harmonic regression model to individual melatonin curves.26
To document ovulation, in-lab morning urine samples were assayed for estradiol and progesterone concentration. Assays were performed on the Beckman Coulter DxI 800 system, using Beckman reagents for chemiluminescence immunoassays (Beckman Coulter Inc, Brea, CA, USA; estradiol coefficient of variation: 10.7%; progesterone coefficient of variation: 6.8%).
Statistical Analyses
Normality of data was verified with the Shapiro-Wilk test, with obtained values of P > 0.05 indicating a normal distribution. Normality testing for data undergoing ANOVA analyses was conducted on the residuals of the raw data.27 Paired-samples t-tests were used to analyze amplitude and phase of CBT, DLMOn, DLMOff, duration, midpoint, amplitude, and phase of salivary melatonin, and ovarian hormone concentrations between MF and ML. To further account for the order of visits and first night effects, a one-way repeated measures ANCOVA with “menstrual phase at first visit” as a covariate was used to analyze nocturnal sleep measures at MF and ML. Sleep across the nocturnal SP was analyzed with a 2-way repeated measures ANOVA (factors: menstrual phase x third-of-night).
To explore the circadian variation of sleep throughout the URSW, each 30-sec scored sleep epoch was assigned a circadian phase between 0° to 359.9°, relative to the CBT minimum at 0°. Data were then folded every 24 h and binned into 30° circadian bins (blocks of 2 h) yielding a 12-point curve spanning the 24-h day. In that manner, data obtained during similar 30° circadian phases throughout the 3 days of the URSW were combined. SOL and ROL were allocated based on circadian phase at lights out for each nap episode. Subjective alertness (post-nap and mid-wake assessments), CBT (throughout wakes and naps spanning the URSW), and salivary melatonin data were analyzed in a manner similar to PSG sleep results. Analyses utilized 2-way repeated-measures ANOVAs (factors: menstrual phase x circadian phase). Subsequent simple main effects tests and/or Tukey HSD post hoc comparisons were used when appropriate. Before the start of experimental procedures, we hypothesized a specific and directional change (i.e. a reduction) in nocturnal REM sleep during ML. This a priori prediction later formed the basis of a series of 4 Tukey HSD pairwise comparisons applied to the URSW REM sleep data (see Results). All data are expressed as mean ± SEM.
RESULTS
Ovarian Hormones
In all participants, urinary estradiol and progesterone increased from MF to ML. Mean (± SEM) MF and ML estradiol were 596.3 ± 131.0 pmol/L and 1112.5 ± 293.7 pmol/L, respectively (t7 = −2.15, P = 0.07). Mean (± SEM) progesterone increased significantly (t7 = −4.13, P = 0.004) at ML (37.51 ± 8.3 nmol/L) compared to MF (14.1 ± 3.8 nmol/L).
Nocturnal PSG Sleep
Nocturnal sleep recordings for 2 participants at ML were lost due to technical difficulties. To maintain a within-subject design, analyses focused on a total of 12 recordings, reflecting both menstrual phases in 6 participants.
Table 1 contains a summary of PSG measures obtained during nocturnal 8-h sleep episodes at MF and ML. No significant menstrual phase differences were observed for TST, SE, ROL, stage 1 sleep, stage 2 sleep, SWS (stage 3+4), NREM sleep, wake after sleep onset (WASO), or movement time (MT). SOL significantly increased at ML compared to MF (F1,4 = 13.95, P = 0.02). During ML compared to MF, REM sleep % significantly decreased (F1,4 = 8.06, P = 0.04).
Table 1.
Polysomnographic sleep measures at the MF and ML phases of the menstrual cycle
| Sleep stages during nocturnal sleep episodes | MF (n = 6) | ML (n = 6) | P-value | Sleep stages averaged across all naps | MF (n = 8) | ML (n = 8) | P-value |
|---|---|---|---|---|---|---|---|
| TST (min) | 437.6 ± 8.3 | 423 ± 6.5 | 0.40 | TST (min) | 29.7 ± 1.3 | 29.2 ± 1.3 | 0.50 |
| SE (%) | 93.0 ± 1.2 | 89.5 ± 1.6 | 0.11 | SE (%) | 49.7 ± 2.1 | 48.9 ± 2.3 | 0.65 |
| SOL (min)a | 7.2 ± 1.9 | 16.0 ± 2.7 | 0.02 | SOL (min) | 28.6 ± 1.5 | 28.8 ± 1.6 | 0.84 |
| ROL (min) | 87.2 ± 16.9 | 99.8 ± 19.1 | 0.71 | ROL (min)a | 48.1 ± 1.4 | 50.0 ± 1.5 | 0.05 |
| Stage 1 (%) | 2.8 ± 0.6 | 2.8 ± 0.4 | 0.99 | Stage 1 (%) | 8.3 ± 1.7 | 8.2 ± 1.4 | 0.92 |
| Stage 1 (min) | 12.2 ± 2.7 | 11.9 ±1.6 | 0.87 | Stage 1 (min) | 2.5 ± 0.4 | 2.1 ± 0.2 | 0.14 |
| Stage 2 (%) | 61.4 ± 3.6 | 63.9 ± 2.6 | 0.19 | Stage 2 (%) | 51.1 ± 4.3 | 52.9 ± 4.5 | 0.40 |
| Stage 2 (min) | 269.4 ± 18.3 | 269.8 ± 10.5 | 0.91 | Stage 2 (min) | 18.3 ± 1.3 | 19.5 ± 1.4 | 0.18 |
| SWS (%) | 10.6 ± 2.6 | 10.6 ± 1.2 | 0.91 | SWS (%) | 7.7 ± 1.5 | 7.1 ± 1.2 | 0.36 |
| SWS (min) | 45.4 ± 10.5 | 44.75 ± 5.6 | 0.82 | SWS (min) | 3.4 ± 0.7 | 3.1 ± 0.6 | 0.27 |
| NREM (%) | 71.9 ± 1.7 | 74.4 ± 1.6 | 0.08 | NREM (%) | 58.8 ± 4.4 | 60.0 ± 4.4 | 0.53 |
| NREM (min) | 314.8 ± 8.8 | 314.6 ± 6.8 | 0.96 | NREM (min) | 21.7 ± 1.2 | 22.5 ± 1.4 | 0.23 |
| REM (%)a | 25.2 ± 2.0 | 22.7 ± 1.9 | 0.04 | REM (%) | 11.6 ± 0.9 | 10.3 ± 1.4 | 0.24 |
| REM (min) | 110.6 ± 9.9 | 96.2 ± 8.5 | 0.12 | REM (min) | 5.4 ± 0.4 | 4.5 ± 0.5 | 0.08 |
| WASO (%) | 3.6 ± 1.6 | 5.5 ±1.7 | 0.39 | WASO (%) | 23.4 ± 4.1 | 23.8 ± 3.9 | 0.76 |
| WASO (min) | 17.3 ± 7.8 | 26.2 ± 8.3 | 0.39 | WASO (min) | 14.1 ± 2.8 | 14.2 ± 2.8 | 0.83 |
| MT (%) | 1.2 ± 0.3 | 1.3 ± 0.2 | 0.44 | MT (%) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.71 |
| MT (min) | 5.6 ± 1.2 | 6.0 ± 1.1 | 0.38 | MT (min) | 0.16 ± 0.0 | 0.16 ± 0.0 | 0.83 |
| MT + WASO (%) | 4.8 ± 1.6 | 6.8 ± 1.5 | 0.32 | MT + WASO (%) | 23.6 ± 4.1 | 24.0 ± 3.9 | 0.75 |
| MT + WASO (min) | 22.9 ± 7.7 | 32.2 ± 7.4 | 0.32 | MT + WASO (min) | 14.3 ± 2.3 | 14.4 ± 2.3 | 0.93 |
| # REM episodes | 4.17 ± 0.3 | 4.5 ± 0.2 | 0.49 | ||||
| mean REM efficiency (%) | 93.3 ± 2.2 | 97.1 ± 0.8 | 0.37 | ||||
| mean REM length (min) | 26.5 ± 1.3 | 21.3 ± 1.5 | 0.07 | ||||
| last REM episode: # fragments | 2.0 ± 0.5 | 1.3 ± 0.2 | 0.40 | ||||
| last REM episode: efficiency (%) | 94.3 ± 4.0 | 97.4 ± 1.9 | 0.42 | ||||
| last REM episode: length (min) | 27.8 ± 3.0 | 17.8 ± 2.2 | 0.07 |
MF, mid-follicular; ML, mid-luteal; TST, total sleep time; SE, sleep efficiency; SOL, sleep onset latency; ROL, REM sleep onset latency; SWS, slow wave sleep; WASO, wake after sleep onset; MT, movement time
All values are mean ± SEM; P-values in left panel provided for one-way repeated measures ANCOVA (with menstrual phase at first visit as covariate); P-values in right panel provided for 2-tailed paired-samples t-tests
indicates significant menstrual phase difference (P ≤ 0.05)
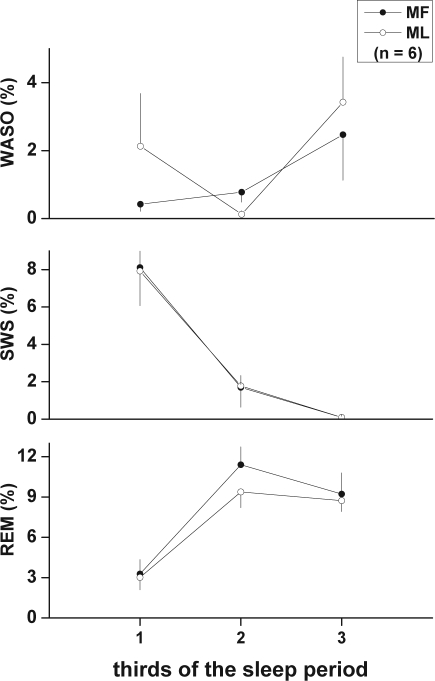
The variation of WASO, SWS, and REM sleep throughout the nocturnal SP was also analyzed by expressing each in minutes and percent per third of the SP at MF and ML. A significant main effect of time was observed for SWS (min: F2,10 = 12.71, P = 0.002; %: F2,10 = 12.41, P = 0.002; Figure 2 middle panel) and REM sleep (min: F2,10 = 44.18, P < 0.0001; %: F2,10 = 44.77, P < 0.0001; Figure 2, lower panel), with typical decreases and increases observed, respectively, across the SP. A significant main effect of menstrual phase was observed for REM sleep % (F1,5 = 8.23, P = 0.035), with an apparent decrease observed during the second third of the SP in ML compared to MF (Figure 2, lower panel).
Figure 2.
Variation of wake after sleep onset (WASO), slow wave sleep (SWS), and REM sleep across thirds of the nocturnal sleep period (SP; from sleep onset to final awakening) in the MF and ML phases of the menstrual cycle. Amounts per third were expressed as % of the full SP. A significant main effect of time within the SP was observed for SWS % and REM sleep % (P < 0.01), and a significant main effect of menstrual phase was observed for REM sleep % (P = 0.035), by 2-way ANOVA for repeated measures. Values are mean ± SEM.
Subjective Sleep Quality and Alertness Following the Nocturnal Sleep Episode
SSQ reflecting the 8-h nocturnal sleep episode was decreased (t7 = 2.27, P = 0.057) in ML (mean ± SEM: 2.6 ± 0.3) compared to MF (mean ± SEM: 3.7 ± 0.4). Subjective alertness upon awakening from the nocturnal sleep episode was significantly decreased during ML compared to MF (MF: 2.9 ± 0.6, ML: 5.1 ± 0.5; t7 = −3.0, P = 0.02).
PSG Sleep Throughout the URSW
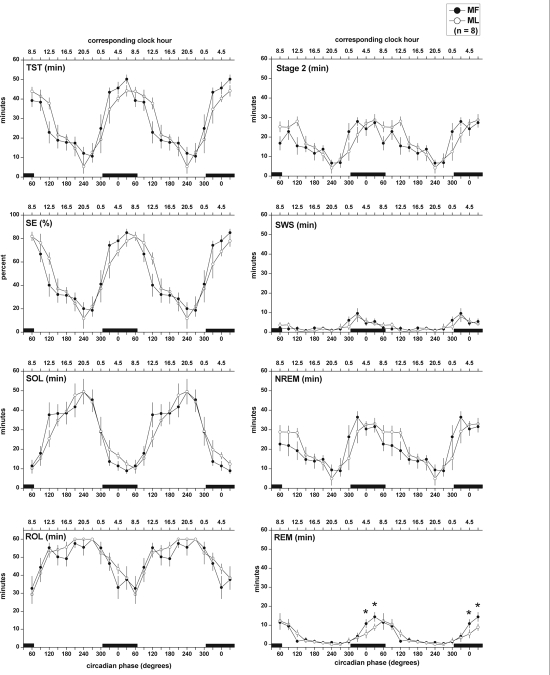
Figure 3 illustrates the variation of PSG sleep measures throughout the URSW at both menstrual phases. Two-way repeated measures ANOVA revealed a significant main effect of circadian phase for TST, SE, SOL, ROL (Figure 3, left panels), and minutes of stage 2, SWS, NREM, and REM sleep (Figure 3, right panels; for all: F11,77> 8.00, P < 0.0001). Sleep propensity, as indicated by the SOL minimum, reached a maximum at the end of the projected habitual nocturnal sleep episode. The peaks of SWS and REM sleep, which were narrower than the peak of stage 2 sleep, were observed at the start and end of the projected nocturnal sleep period, respectively. No menstrual phase differences were observed for TST (F1,7 = 0.73, P = 0.42), SE (F1,7 = 0.40, P = 0.55), SOL (F1,7 = 0.02, P = 0.89), stage 2 sleep (F1,7 = 3.51, P = 0.10), SWS (F1,7 = 1.53, P = 0.26), and NREM sleep (F1,7 = 1.01, P = 0.35). Menstrual phase differences for REM sleep (F1,7 = 3.01, P = 0.12), and ROL (F1,7 = 4.06, P = 0.08) did not reach statistical significance, either. However, a series of Tukey pairwise comparisons on REM sleep data spanning times throughout the habitual nocturnal sleep episode (i.e., circadian phases from 300° to 60°), which were conducted in order to test the validity of an a priori prediction of decreased nocturnal REM sleep during ML, confirmed significantly decreased REM sleep at ML compared to MF at circadian phase 0° and 30° (P < 0.05). When sleep stages were averaged across all naps, a menstrual phase difference in ROL (t7 = −2.36, P = 0.05) was observed, with values increased during ML compared to MF (Table 1).
Figure 3.
Variation of polysomnographic sleep measures across the circadian cycle at the MF and ML phases of the menstrual cycle obtained throughout the URSW procedure. TST: total sleep time; SE: sleep efficiency; SOL: sleep onset latency; ROL: rapid eye movement sleep onset latency; SWS: slow wave sleep. Two-way ANOVA for repeated measures revealed a significant main effect of circadian phase for all reported sleep parameters (P < 0.0001). *indicates significant menstrual phase differences (P < 0.05) in REM sleep occurring at circadian phase 0° and 30° during ML compared to MF, by Tukey pairwise comparisons. Each 30-sec scored sleep epoch was assigned a circadian phase between 0° to 359.9°, relative to the CBT minimum at 0°. Data were then folded every 24 h, and binned into 30° circadian bins (blocks of 2 h) yielding a 12-point curve spanning the 24-h day. In that manner, data obtained during similar 30° circadian phases throughout the three days of the URSW were combined. SOL and ROL were allocated based on circadian phase at lights out for each nap episode. Data are plotted at the middle of each bin, such that 0° reflects data in the 345° to 15° bin, 30° reflects data from 15° to 45° bin, etc. Data are repeated over 2 consecutive days for illustration purposes. Black bars represent time of projected habitual nocturnal sleep episode (mean ± S.D. circadian phase at bedtime and wake time are 305.9 ± 0.7 and 65.9 ± 0.7 circadian degrees, respectively). All values are mean ± SEM.
Subjective Sleep Quality and Alertness Throughout the URSW
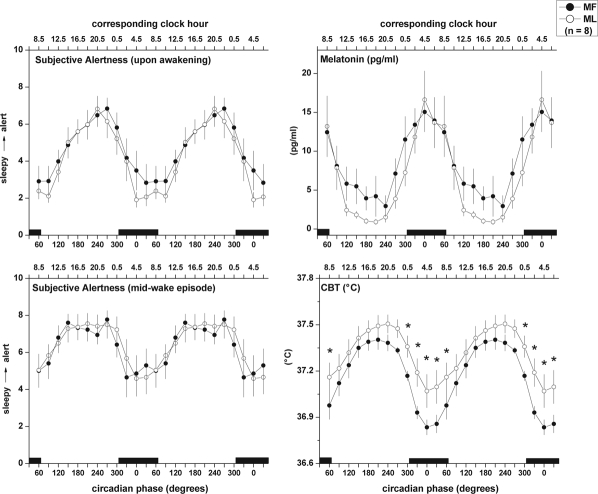
Two-way repeated measures ANOVA applied to SSQ of naps revealed a significant circadian variation (F11,77 = 18.89, P < 0.0001), with a pattern similar to the variation of SE throughout the URSW. Menstrual phase had no effect on SSQ (F1,7 = 0.99, P = 0.35). Subjective alertness as assessed by the VAS either at the start or middle of wake episodes showed a significant circadian rhythm (start: F11,77 = 19.43, P < 0.0001; middle: F11,77 = 7.96, P < 0.0001; Figure 4, left panels), with no menstrual phase differences (start: F1,7 = 0.14, P = 0.28; middle: F1,7 = 0.11, P = 0.75).
Figure 4.
Circadian variation of subjective alertness, salivary melatonin, and core body temperature (CBT) at the MF and ML phases of the menstrual cycle obtained throughout the URSW. Two-way ANOVA for repeated measures revealed a significant main effect of circadian phase for measures of subjective alertness, melatonin, and CBT (P < 0.001), and a significant menstrual x circadian phase interaction for CBT only (P = 0.05). *indicates significant simple main effects tests reflecting menstrual phase differences (P < 0.05). CBT was continuously recorded throughout the URSW. Salivary melatonin samples were obtained twice during each wake episode. Subjective alertness (10-cm VAS; 0: extremely sleepy, 10: extremely alert) was assessed ∼2 min and ∼30 min after waking from each nap episode (upper and lower left panels, respectively). Data were first assigned a circadian degree (0° to 359.9°), then averaged into 30° bins, and finally folded every 24 h to combine data occurring at similar circadian phase throughout the 72-h URSW. Data are plotted at the middle of each bin, such that 0° reflects data in the 345° to 15° bin, 30° reflects data from 15° to 45° bin, etc. Data are repeated over 2 consecutive days for illustration purposes. Black bars represent time of projected habitual nocturnal sleep episode (mean ± S.D. circadian phase at bedtime and wake time are 305.9 ± 0.7 and 65.9 ± 0.7 circadian degrees, respectively). All values are mean ± SEM.
CBT and Salivary Melatonin Throughout the URSW
CBT amplitude was significantly reduced at ML compared to MF (Table 2; t7 = 3.61, P = 0.01). A significant negative correlation was found between urinary progesterone concentration and CBT amplitude (r = −0.51, P = 0.04). Time of fitted minimum of CBT was unchanged between menstrual phases (Table 2; t7 = 0.93, P = 0.40). A significant main effect of menstrual phase was observed for CBT, indicating elevated overall CBT values throughout ML compared to MF (F1,7 = 7.70, P = 0.03; Figure 4, lower right panel). A significant menstrual phase x circadian phase interaction was also observed for CBT throughout the URSW, with significantly elevated values during ML versus MF occurring between circadian phase 300° to 60° (F11,77 = 1.88, P = 0.05; Figure 4, lower right panel).
Table 2.
Circadian profile of CBT and salivary melatonin at the MF and ML phases of the menstrual cycle
| MF (n = 8) | ML (n = 8) | P-value | |
|---|---|---|---|
| CBT amplitude (°C)a | 0.31 ± 0.03 | 0.23 ± 0.03 | 0.01 |
| CBT fitted minimum (clock time) | 04:34 ± 00:25 | 04:20 ± 00:22 | 0.40 |
| DLMOn (clock time) | 22:13 ± 00:32 | 22:49 ± 00:24 | 0.28 |
| DLMOff (clock time) | 08:10 ± 00:25 | 08:16 ± 00:31 | 0.91 |
| Melatonin duration of secretion (hours) | 09:57 ± 00:46 | 09:26 ± 00:27 | 0.62 |
| Melatonin midpoint (clock time) | 03:11 ± 00:18 | 03:32 ± 00:25 | 0.43 |
| Melatonin AUC (24-h) | 228.97 ± 30.00 | 164.11 ± 42.00 | 0.30 |
| Melatonin amplitude (pg/mL) | 6.34 ± 1.27 | 7.75 ± 1.51 | 0.22 |
| Melatonin fitted maximum (clock time) | 03:14 ± 00:59 | 04:16 ± 00:45 | 0.32 |
CBT, core body temperature; DLMOn, dim light melatonin onset; DLMOff, dim light melatonin offset; AUC, area under the curve
All values are mean ± SEM;P-values provided for 2-tailed paired-samples t-tests
indicates significant menstrual phase difference by paired-samples t-test (P < 0.05)
Two-way repeated measures ANOVA revealed a circadian variation of salivary melatonin with a peak during the habitual dark/sleep period at MF and ML (Figure 4, upper right panel; F11,77 = 10.07, P < 0.0001), though no menstrual phase difference was observed (F1,7 = 0.67, P = 0.44). DLMOn (t7 = −1.18, P = 0.28), DLMOff (t7 = −0.12, P = 0.91), duration (t7 = 0.51, P = 0.62), midpoint of secretion (t7 = −0.83, P = 0.43), AUC (t7 = 1.12, P = 0.30), and circadian amplitude (t7 = −1.36, P = 0.22) and phase (t7 = −1.06, P = 0.32) were comparable between MF and ML (Table 2).
DISCUSSION
We investigated how the menstrual cycle influences ovarian hormones, nocturnal sleep, and the circadian variation of CBT, melatonin and sleep in healthy women.
Increased estradiol and progesterone during ML compared to MF confirmed ovulation in all participants. Reinforcing others,10–13 we observed these ovarian hormone changes to be coincident with significantly altered CBT rhythms during ML, including reduced nocturnal decline and decreased circadian amplitude, which was correlated with increased progesterone concentration. Circadian phase of CBT, however, was unchanged between menstrual phases in agreement with those who utilized constant conditions to minimize masking effects.13,15 Likewise, we found all aspects of the circadian salivary melatonin profile to be unaffected by menstrual phase. Most studies examining melatonin at different menstrual phases3 did not use constant conditions to control for masking effects, and were inconsistent. Using a modified constant routine, Wright and Badia15 found no difference in salivary melatonin across the menstrual cycle, whereas Shibui and colleagues, utilizing an URSW similar to ours found a decreased AUC for plasma melatonin during LP, though DLMOn, DLMOff, phase, and duration were similar.13
We observed moderate but significant menstrual cycle changes in nocturnal sleep. Confirming others, we observed decreased REM sleep during ML compared to MF.4,28 Our finding of lengthened SOL during ML contradicts prior reports,4,5,7,28 but is consistent with the expected relationship between increased CBT and reduced sleep propensity reported across the circadian cycle.17 Our results support the conclusion that sleep homeostasis is unaffected by menstrual phase.3 This was illustrated by the similarity in total nocturnal SWS and the time course of SWS across the SP at both menstrual phases. A contrast was also noted between TST and SOL, with the finding of increased nocturnal SOL during ML compared to MF indicating weakened sleep initiation mechanisms but maintained sleep quality at different menstrual phases. Like others,29 our participants reported decreased nocturnal subjective sleep quality during the luteal phase. This apparent discrepancy between objective PSG-based estimates and subjective questionnaire-based estimates of sleep quality across the menstrual cycle has also been observed by others.30
Our findings of a circadian variation for TST, SE, SOL, ROL, stage 2, SWS, NREM, and REM sleep are consistent with others.17,19 Interestingly, the circadian variation of SWS peaked early in the night whereas that of REM sleep occurred at the end of the night.
We found menstrual-related changes in REM sleep, which decreased during ML compared to MF. Though the study by Shibui and colleagues did not report the full PSG sleep profile, and only described the variation of sleep propensity across menstrual and circadian phases, they reported that within the 09:00-16:30 (daytime) time range, the number of naps containing SWS was increased during LP compared to FP.13 We observed SWS throughout the URSW to be unaffected by menstrual phase. Though similar, our experimental design differed in that our participants had an 8-h nocturnal sleep opportunity, whereas their participants were sleep deprived for 24 h preceding the start of the URSW. This situation could have increased the homeostatic pressure for SWS and may have thus confounded the expression of REM sleep in their study. Moreover, by scheduling 60-min naps, as opposed to the 10-min naps used by Shibui et al., we increased the opportunity for observing REM sleep. Increased homeostatic sleep drive, combined with short nap opportunities could thus have resulted in a displacement of REM sleep expression in favor of SWS in the Shibui et al. study. A full description of REM sleep characteristics across the circadian cycle was not reported in their study, however.
When assessed either immediately after awakening from naps or at the middle of wake episodes, we observed a significant circadian variation of subjective alertness, with no menstrual cycle effects. This is inconsistent with the findings of Shibui and colleagues,13 who reported increased LP sleepiness during the daytime (09:00-16:30), though here too, the effects of the 24-h sleep deprivation could have affected vigilance levels during wake periods in their study. Interestingly, our participants did demonstrate significantly increased subjective feelings of sleepiness during ML compared to MF when assessed after awakening from the nocturnal sleep episode, which may suggest a menstrual cycle modulation of sleep inertia in the morning after the completion of a full sleep episode.
Circadian rhythms of melatonin secretion, body temperature and sleep propensity are related, as prior research has linked increases in circulating melatonin, increased distal heat loss, decreased CBT and reduced sleep latencies.31 Sleep is usually initiated on the declining limb of the CBT curve, and the CBT nadir is associated with peak sleep propensity.17 Conversely, times of high CBT are associated with increased SOL, as well as decreased TST, SE, and REM sleep.17 Results obtained in our nocturnal comparisons between MF and ML revealed similar associations between sleep and CBT.
Circadian rhythms of CBT are generated by the suprachiasmatic nucleus (SCN) via a projection to the dorsal subparaventricular zone and then the medial preoptic region32 and preoptic anterior hypothalamus (POAH), which is involved in thermoregulation.33 In ovulatory menstrual cycles, there is a modulation of the endogenous CBT cycle illustrated by elevated nocturnal minima, blunted circadian amplitude and increased mesor of ∼0.3°C during LP as shown by Cagnacci et al.12 and our present data. This is likely due to progesterone, which is thermogenic and acts by altering the firing rate of thermosensitive neurons in the POAH.34 Cagnacci and colleagues suggested that altered CBT across the menstrual cycle results from a resistance to the hypothermic effects of melatonin during LP. This was illustrated by administering exogenous melatonin during daytime hours, which elicited a CBT decline during FP but was ineffective during LP.12 Recording throughout 24 h, the authors also documented a blunted nocturnal CBT decline despite normal melatonin levels,12 a finding consistent with our results.
The POAH, as an integrator between melatonin, body temperature, and sleep, contains melatonin receptors, as well as warm- and cold-sensitive neurons that are more or less active during sleep, respectively.33 A study demonstrated that cutaneous warming of the peripheral skin in humans promotes sleep, possibly via indirect stimulation of warm-sensitive neurons.35 More recently, the same authors reported that in elderly insomniacs, manipulations that cooled CBT and warmed proximal skin resulted in a 28% decrease in SOL, when compared to the opposite combination.36 These observations are consistent with our current results, demonstrating that reduced CBT is associated with a rapid SOL. Therefore, a more detailed investigation of thermoregulatory changes across the menstrual cycle would be interesting to pursue, especially considering the role of CBT, distal- and proximal skin temperature changes in sleep regulation. The sleep-temperature coupling occurring at the POAH may explain the susceptibility of REM sleep propensity to menstrual cycle modulation. The circadian variation of REM sleep is well established, with a peak occurring shortly after the CBT nadir.17 REM sleep can also affect temperature regulation, as a result of suppressed POAH neuronal thermosensitivity during this sleep stage.37 A finding that is particularly important for individuals experiencing disturbed sleep and/or altered REM sleep was that REM sleep % can be increased by distal skin warming in young and elderly healthy individuals, whereas it can be reduced by the same manipulation in elderly insomniacs38 and narcoleptic patients.39 However, the interpretation of our data in light of these prior findings is difficult since skin temperature manipulations in the aforementioned study38 did not result in CBT changes, whereas the effects of CBT on sleep parameters were of primary interest in our study. Interestingly, when the circadian variation of sleep was studied in a group of narcoleptics using a 90-min URSW (30-min sleep/60-min wake for 72 h), REM sleep was observed to occur at all times of day, unlike the pattern in healthy controls, where it was limited to early morning hours.40 An effect of REM sleep on body temperature is also supported by evidence from rats undergoing REM sleep deprivation41 and the effects of tricyclic antidepressants, which are known to reduce REM sleep and disrupt body temperature.42
A direct action of progesterone and/or its neuroactive metabolites (which are agonistic modulators of central nervous system GABAA-receptors) on the sleep system should not be disregarded. In agreement with our findings, exogenous progesterone reduced REM sleep in rats,43 and human male subjects showed either a trend or a significant REM reduction (with no SWS changes) after administration of progesterone44 or a progesterone-receptor agonist,45 respectively.
The results presented here, together with the localization of estrogen and progesterone receptors at the SCN,46 and the report of a reproductive cycle effect on oscillation patterns of PER2 in the limbic forebrain in rats,47 suggests an interaction between the circadian system and the menstrual cycle. Our findings in particular, based on a carefully controlled URSW procedure, illustrate the relationship between the menstrual cycle and associated sex steroid hormones, circadian CBT and REM sleep, and provide a better understanding of the physiological changes associated with the menstrual and circadian cycles and how they affect sleep in women. In summary, healthy women during the ML phase compared to the MF phase experience increased progesterone, elevated nocturnal CBT, reduced circadian amplitude of CBT, and reduced REM sleep throughout the circadian cycle, without altered sleep homeostasis as determined by SWS. This line of research may be helpful in the development of new therapeutic options for sleep complaints in the female population.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Boivin has participated in research sponsored by Transport Canada and Litebook; is CEO and Scientific Director of Alpha Logik, Inc.; has consulted for Servier; and has received the use of equipment from Litebook. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank the research participants and the staff and students of the Centre for Study and Treatment of Circadian Rhythms for their contributions to this investigation. We also thank Dr. Sylvie Rheaume for medical supervision and Francine Duquette for dietary information. We are especially grateful to Dr. Julie Carrier, Manon Robert and Sonia Frenette for their assistance on the sleep analysis.
This study was supported by the Canadian Institute of Health Research (CIHR).
REFERENCES
- 1.Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530:565–74. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares CN. Insomnia in women: an overlooked epidemic? Arch Womens Ment Health. 2005;8:205–13. doi: 10.1007/s00737-005-0100-1. [DOI] [PubMed] [Google Scholar]
- 3.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–22. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Baker FC, Driver HS, Paiker J, Rogers GG, Mitchell D. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J Appl Physiol. 2002;92:1684–91. doi: 10.1152/japplphysiol.00919.2001. [DOI] [PubMed] [Google Scholar]
- 5.Parry BL, Mostofi N, LeVeau B, et al. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Res. 1999;85:127–43. doi: 10.1016/s0165-1781(98)00128-0. [DOI] [PubMed] [Google Scholar]
- 6.Ito M, Kohsaka M, Fukuda N, et al. Effects of menstrual cycle on plasma melatonin level and sleep characteristics. Jpn J Psychiatry Neurol. 1993;47:478–9. doi: 10.1111/j.1440-1819.1993.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 7.Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442:729–37. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- 8.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–91. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driver HS, McLean H, Kumar DV, Farr N, Day AG, Fitzpatrick MF. The influence of the menstrual cycle on upper airway resistance and breathing during sleep. Sleep. 2005;28:449–56. doi: 10.1093/sleep/28.4.449. [DOI] [PubMed] [Google Scholar]
- 10.Lee K. Circadian temperature rhythms in relation to menstrual cycle phase. J Biol Rhythms. 1988;3:255–63. [Google Scholar]
- 11.Parry BL, LeVeau B, Mostofi N, et al. Temperature circadian rhythms during the menstrual cycle and sleep deprivation in premenstrual dysphoric disorder and normal comparison subjects. J Biol Rhythms. 1997;12:34–46. doi: 10.1177/074873049701200106. [DOI] [PubMed] [Google Scholar]
- 12.Cagnacci A, Soldani R, Laughlin GA, Yen SS. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–9. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Shibui K, Uchiyama M, Okawa M, et al. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol Psychiatry. 2000;48:1062–8. doi: 10.1016/s0006-3223(00)00912-4. [DOI] [PubMed] [Google Scholar]
- 14.Webley GE, Leidenberger F. The circadian pattern of melatonin and its positive relationship with progesterone in women. J Clin Endocrinol Metab. 1986;63:323–8. doi: 10.1210/jcem-63-2-323. [DOI] [PubMed] [Google Scholar]
- 15.Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103:185–94. doi: 10.1016/s0166-4328(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 16.Parry BL, Martinez LF, Maurer EL, Lopez AM, Sorenson D, Meliska CJ. Sleep, rhythms and women's mood. Part I. Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10:129–44. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Franken P. Interaction of sleep homeostasis and circadian rhythmicity: Dependent or independent systems? In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier; 2005. pp. 418–34. [Google Scholar]
- 18.Carskadon MA, Dement WC. Sleep studies on a 90-minute day. Electroencephalogr Clin Neurophysiol. 1975;39:145–55. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- 19.Munch M, Knoblauch V, Blatter K, et al. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26:1307–19. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems for sleep stages of human subjects. Los Angeles: Brain Information Service, Brain Research Institute, UCLA; 1968. [Google Scholar]
- 21.Coleman RM, Guilleminault C. Sleeping and waking disorders: indications and techniques. Menlo-Park: Addison-Wesley; 1982. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome; p. 265. [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Acandemy of Sleep Medicine; 2007. [Google Scholar]
- 23.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 24.Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: a further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- 25.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 26.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–52. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iles TC. Crossed and hierarchical analysis of variance. In: Fry JC, editor. Biological data analysis: a practical approach. New York: Oxford University Press; 1993. pp. 41–80. [Google Scholar]
- 28.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–35. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 29.Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209–14. doi: 10.1037//0278-6133.16.3.209. [DOI] [PubMed] [Google Scholar]
- 30.Armitage R, Baker FC, Parry BL. The menstrual cycle and circadian rhythms. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier; 2005. pp. 1266–77. [Google Scholar]
- 31.Cajochen C, Krauchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–7. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 32.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–7. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol. 1995;269:R1240–9. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- 34.Tsai CL, Matsumura K, Nakayama T. Effects of progesterone on thermosensitive neurons in preoptic slice preparations. Neurosci Lett. 1988;86:56–60. doi: 10.1016/0304-3940(88)90182-6. [DOI] [PubMed] [Google Scholar]
- 35.Raymann RJ, Swaab DF, Van Someren EJ. Cutaneous warming promotes sleep onset. Am J Physiol. 2005;288:R1589–97. doi: 10.1152/ajpregu.00492.2004. [DOI] [PubMed] [Google Scholar]
- 36.Raymann RJ, Van Someren EJ. Diminished capability to recognize the optimal temperature for sleep initiation may contribute to poor sleep in elderly people. Sleep. 2008;31:1301–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Heller H. Temperature, thermoregulation, and sleep. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: WB Saunders; 2005. pp. 292–304. [Google Scholar]
- 38.Raymann RJ, Swaab DF, Van Someren EJ. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–13. doi: 10.1093/brain/awm315. [DOI] [PubMed] [Google Scholar]
- 39.Fronczek R, Raymann RJ, Overeem S, et al. Manipulation of skin temperature improves nocturnal sleep in narcolepsy. J Neurol Neurosurg Psychiatry. 2008;79:1354–7. doi: 10.1136/jnnp.2008.143610. [DOI] [PubMed] [Google Scholar]
- 40.Dantz B, Edgar DM, Dement WC. Circadian rhythms in narcolepsy: studies on a 90 minute day. Electroencephalogr Clin Neurophysiol. 1994;90:24–35. doi: 10.1016/0013-4694(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12:68–87. [PubMed] [Google Scholar]
- 42.Clark WG, Lipton JM. Changes in body temperature after administration of adrenergic and serotonergic agents and related drugs including antidepressants: II. Neurosci Biobehav Rev. 1986;10:153–220. doi: 10.1016/0149-7634(86)90025-4. [DOI] [PubMed] [Google Scholar]
- 43.Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271(4 Pt 1):E763–72. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- 44.Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep in male subjects. Am J Physiol. 1997;272:E885–91. doi: 10.1152/ajpendo.1997.272.5.E885. [DOI] [PubMed] [Google Scholar]
- 45.Wiedemann K, Lauer CJ, Hirschmann M, Knaudt K, Holsboer F. Sleep-endocrine effects of mifepristone and megestrol acetate in healthy men. Am J Physiol. 1998;274:E139–45. doi: 10.1152/ajpendo.1998.274.1.E139. [DOI] [PubMed] [Google Scholar]
- 46.Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75:296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- 47.Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S. The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci U S A. 2006;103:5591–6. doi: 10.1073/pnas.0601310103. [DOI] [PMC free article] [PubMed] [Google Scholar]