Abstract
Study Objectives:
Determine the relationship of non-rapid eye movement (NREM) electroencephalographic (EEG) spectral measures and the response to cognitive behavioral therapy (CBT) in primary insomnia (PI).
Design:
Patients with PI were randomly assigned to CBT or a placebo intervention (PC). Ambulatory polysomnography was performed before and after treatment.
Setting:
University medical center sleep laboratory.
Participants:
Thirty PI patients with sleep maintenance difficulty evident in subjective sleep measures.
Interventions:
CBT and PC.
Results:
CBT led to a more rapid decline in EEG delta power over the night, compared with PC. This change was associated with subjective improvement in response to CBT. Furthermore, lower pretreatment peak EEG delta power in the first NREM cycle and a more gradual decline in delta power predicted a better response to CBT. Increased wake time during the day produced by CBT was correlated with an increase in the steepness of the slope of EEG delta power and subjective improvement. Traditional polysomnography measures were associated with the subjective CBT response to a greater degree among patients whose total sleep time estimates better approximated polysomnography-derived total sleep time. In contrast, changes in all-night averaged NREM EEG spectral indices were more strongly related to subjective improvement in individuals who underestimated total sleep time to a greater extent.
Conclusions:
CBT led to a more rapid decline in EEG delta power over the night. This change is linked to the therapeutic effect of CBT, which appears to occur in conjunction with an increase in homeostatic sleep drive. Traditional polysomnography indices and all-night averaged NREM EEG measures appear to be related to subjective improvements with CBT in subsets of patients with PI.
Citation:
Krystal AD; Edinger JD. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. SLEEP 2010;33(5):669-677.
Keywords: Insomnia, Cognitive Behavioral Therapy, EEG, spectral analysis, homeostatic sleep drive
PRIMARY INSOMNIA (PI) IS A COMMON CONDITION THAT HAS A SIGNIFICANT IMPACT ON PUBLIC HEALTH.1–3 BOTH PSYCHOLOGICAL/BEHAVIORAL AND medication treatments have proven to be effective for the treatment of PI in many instances but are all associated with significant limitations.4–9 As a result, there is a need to develop improved treatments for this condition. One factor limiting this effort is that there is limited understanding of the pathophysiology of PI and of the neurophysiologic changes associated with the treatment response. Improved understanding of the pathophysiology of PI and physiologic correlates of the response to treatments would be helpful to develop new therapies that are specifically targeted to the physiologic aberrations that underlie PI symptoms.4
Toward this end, we sought to study the neurophysiologic effects of cognitive behavioral insomnia therapy (CBT), a psychological and behavioral treatment with well-established efficacy.7–9 Only very limited data exist concerning CBT's effects on neurophysiology. One prior study examined the effects of a similar behavioral treatment on the frequency spectrum of the electroencephalogram (EEG) in the presleep period among 12 individuals with sleep-onset insomnia.10 This study showed that individuals with sleep-onset insomnia had greater 13- to 31-Hz relative spectral power (power in the 13- to 31-Hz band divided by the sum of power in all frequency bands) prior to sleep onset than did healthy subjects without subjective reports of sleep-onset difficulty. Following behavioral therapy, those with insomnia showed a decrease in 13- to 31-Hz relative power in their presleep EEG.
Another open-label study evaluated the effects of CBT on non-rapid eye movement (NREM) EEG spectral indices in 9 psychophysiological insomnia patients.11 That study showed that CBT increased the power of delta (0.5-4.75 Hz) activity, reduced the power of sigma (12-16 Hz) and beta (16-30 Hz) and led to a more rapid decline in delta power over the night. Based on these findings, the authors hypothesized that CBT may increase the homeostatic pressure for sleep.
In the current investigation we sought to further evaluate the neurophysiologic effects of CBT. This study builds on the studies discussed above, as well as our prior research comparing all night averaged NREM EEG spectral indices derived from PI patients with sleep maintenance difficulties and from healthy individuals without evidence of sleep difficulty based on subjective measures.4 In our previous study, we found that patients with PI who underestimated their polysomnography-measured sleep time by at least 10% had decreased NREM EEG delta (0.5-3.5 Hz) and elevated alpha-(8.5-12 Hz), sigma-(12.5-16 Hz), and beta-(16.5-30 Hz) power, compared with both noncomplaining control subjects and PI sufferers who more accurately estimated their sleep times. This finding was consistent with the results of a previous study that found an association between increased high-frequency beta and gamma (> 30-Hz) NREM EEG spectral power and underestimation of polysomnography-measured total sleep time (TST) in a PI sample.12 We also found that traditional polysomnographic indices (e.g., sleep-onset latency [SOL], TST, wake time after sleep onset [WASO], number of awakenings, and duration and percentage of sleep in each of the sleep stages) were correlated with subjective sleep measures only among a subset of PI patients who did not underestimate their sleep time. In contrast, the subjective sleep measures of those who underestimated sleep time by at least 10% were correlated with NREM EEG spectral indices and not with traditional polysomnography measures. These findings suggest that, in some individuals with PI, alterations in NREM EEG frequency content are associated with a perception of wakefulness and/or a sense of impaired sleep that goes undetected by the traditionally-scored polysomnogram. For other patients with PI, traditional polysomnographic indices more strongly reflect subjective measures of sleep.
The study reported herein employed the same NREM EEG spectral indices as our prior study but, in addition, examined measures of the time course of sleep EEG delta (0.5-3.5 Hz) power over the night based on the study of Cervena and coworkers on the EEG effects of CBT described above.11 We did not analyze data from the presleep or perisleep onset periods because of the extreme variability of the EEG in drowsiness, the presence of slow-eye movements during drowsiness, and the absence of artifact-free, waking, eyes-closed, nondrowsy data in a large number of subjects.4,13 Furthermore, in our prior study, we did not find evidence that the EEG frequency content in those with PI differed from normal sleepers in stage 1 sleep.4
In addition to the study of Cervena and colleagues, further rationale for the inclusion of indices of the time course of EEG delta power over the night comes from investigations suggesting that these indices are affected by behaviors such as spending excessive time in bed (TIB) and napping, practices that are common among patients with insomnia and are thought to perpetuate PI.14–18 Moreover, the amount of delta power in the early part of the night and the rate of decline in delta power are putative indices of homeostatic “sleep drive” that have been shown to be responsive to the types of manipulations of sleep-disruptive behaviors implemented with CBT. This therapy, in fact, is designed to increase the time awake throughout the day and, thereby, increase “sleep drive.”14–24 As a result, we hypothesized that changes in these delta power indices would correlate with improvement in CBT and, further, that lower initial NREM EEG delta power and a more gradual decline in delta power over NREM episodes might predict a successful response to CBT. Thus, we sought to test whether (1) some individuals have a relative deficit in initial “sleep drive”, as manifested in lower initial NREM EEG delta power and less rapid decline of delta power over time that is associated with their insomnia and (2) this deficit is addressed with CBT.
This study also allowed us the opportunity to replicate our previous finding showing that traditional polysomnography indices and all-night averaged NREM EEG relative power indices are not correlates of subjective measures of sleep when studied across all individuals with PI. Instead, the relationship of these indices with subjective measures of sleep is dependent on the degree to which patients with PI underestimate their sleep time compared with polysomnography-derived TST.4 In the present investigation, we sought to test the hypothesis that the subjective improvement with CBT might follow a similar pattern.
To test these hypotheses, we analyzed traditional polysomnography indices, all-night averaged NREM EEG relative power indices, and measures of the time course of sleep EEG delta power in individuals with PI before and after receiving a 6-week course of CBT (n = 16) or a placebo control therapy (PC) (n = 14).
METHODS
Participants
Study patients were 30 individuals with PI selected from a larger study in which 75 patients with PI were randomly assigned to receive CBT, PC, or relaxation therapy.9 The subjects included here were all the CBT- and PC-treated subjects from the parent study for whom artifact-free (see below) NREM EEG data were available at both pretreatment and posttreatment time points. This included 16 patients who received CBT and 14 who received PC. Demographic data for the subjects from these 2 groups appear in Table 1. The subject group included 18 women and 12 men between 40 and 80 years old (mean age 54.9 years), who (1) met diagnostic criteria for PI, (2) were thoroughly screened via structured psychiatric and sleep interviews,25,26 (3) had no significant medical illnesses affecting sleep, (4) had normal thyroid function tests, (5) had fewer than 15 apneas or hypopneas and periodic limb movement-related arousals per hour of sleep, (6) and had a mean WASO of at least 60 minutes in 2 weeks of sleep-log monitoring.
Table 1.
Demographic and baseline data for subjects with primary insomnia who received CBT or PC
| CBT (n = 16) | PC (n = 14) | |
|---|---|---|
| 7 men | 5 men | |
| Sex | 10 women | 8 women |
| Age, y | 54.1 (11.5) | 55.8 (9.9) |
| Baseline mean sleep-diary data | ||
| SOL, min | 24.9 (16.1) | 22.8 (16.2) |
| WASO, min | 97.3 (46.2) | 115.2 (52.3) |
| TST, min | 361.1 (44.8) | 350.8 (66.7) |
| Sleep efficiency, % | 75.9 (9.9) | 71.8 (11.4) |
| Sleep quality, score | 2.9 (0.6) | 2.9 (0.5) |
| TIB, min | 470.4 (49.2) | 489.1 (49.7) |
| Baseline polysomnographic data | ||
| SOL, min | 16.5 (11.1) | 17.0 (15.5) |
| WASO, min | 95.5 (44.9) | 109.3 (48.6) |
| TST, min | 360.9 (81.5) | 348.4 (79.3) |
| Sleep efficiency, % | 78.5 (10.6) | 72.7 (12.7) |
Data are presented as mean (SD), except sex, which is shown as a number.
CBT refers to cognitive behavioral therapy; PC, placebo-controlled condition; SOL, sleep-onset latency; WASO, wake time after sleep onset; TST, total sleep time; TIB, time in bed.
There were no significant differences between groups on any of these variables.
Polysomnography
Subjects underwent a single night of ambulatory polysomnography prior to their treatment (CBT or PC) and following the completion of their treatment.9 The polysomnography montage included 2 EEG channels (C3-M2, Oz-Cz), 1 chin electromyography channel, 2 channels of electrooculography (left eye-M1, right eye-M2), 1 channel of airflow (nasal-oral thermistor), and 2 channels of anterior tibialis electromyography (right and left legs). Studies were all conducted using Oxford 9000 series recorders (Oxford Medical, Inc., Tampa, Fl.).27–29 The EEG data were digitized at 128 Hz with 8-bit accuracy with filter settings of 0.5 and 64 Hz (with −3 dB roll off at both ends and a third-order Butterworth antialiasing filter at 64 Hz) using an Oxford Vision System (Oxford Instruments Inc., Oxford, UK). The digitized data were scored in 30-second epochs using standard scoring criterion4,30 by ADK, who is board certified in sleep medicine and was unaware of the subject group, time (pretreatment vs posttreatment), and response to treatment. The results were used to generate the traditional polysomnography indices used in analyses (TST, WASO, SOL, sleep efficiency percentage [100 × TST /TIB], number of awakenings, number of arousals, and time spent in each sleep stage). Polysomnography data were always collected on a night during the 2-week period in which sleep-log data were collected (see Sleep Log section).
EEG Analysis
Much of the EEG analysis was conducted as previously described.4 This analysis was carried out on a single C3-M2 (left central to right mastoid) EEG channel. All epochs of data included in spectral analysis were free of movements, artifacts, arousals, or transitions between sleep stages. We included data only from stage 2 and slow-wave sleep because it was only with NREM EEG data that we found both differences between patients with PI and control subjects and correlations between EEG measures and subjective sleep ratings.4 Also, because our prior study found that the results were the same when stage 2 and slow-wave sleep data were combined or analyzed separately, the data are presented in combined form herein as NREM.4
The EEG data underwent autoregressive high-pass filtering and Hanning windowing and were subsequently decomposed in 2-second epochs using the fast Fourier transform into 6 frequency bands averaging over both time and frequency: delta (0.5-3.5 Hz), theta (4.0-8.0 Hz), alpha (8.5-12 Hz), sigma (12.5-16 Hz), beta (16.5-30 Hz), and gamma (30.5-60 Hz).4 These bands were used in our prior work and chosen to be comparable to those used by others carrying out sleep EEG spectral analysis.4,31–33 This analysis was carried out as in our prior study using custom software written in Visual C++ by ADK, utilizing MATLAB (The MathWorks, Inc., Natick, MA) for computing fast Fourier transforms.4 The results of the spectral analysis were verified as previously described.4 For each of these frequency bands, we computed power in μV2. Based on the findings of our previous study, relative spectral power was used for all-night averaged EEG spectral indices. Relative EEG power was computed by dividing the power in each band by the sum of the power across all bands.12 We logarithmically transformed both absolute and relative power data because we found that this transform best led to distributions that approximated the normal distribution, as has been previously reported.34–36
As described above, we also derived measures of EEG absolute delta power in the first NREM period and the slope of absolute delta power over the night because such measures reportedly are affected by naps and wake time throughout the day.14,15,20 Because several related measures have been studied to reflect these phenomena and there is no consensus about which is optimal, we computed the average, peak (the greatest delta power in any 30-second epoch in a NREM period), and total (summed) power separately for each NREM episode and computed the best-fit exponential functions of these measures across NREM periods. We also studied delta power as a percentage of total delta power over the night, as was done in prior studies of NREM EEG delta dynamics.14,15,20,37–40 An advantage of studying delta power indices as a percentage is that it decreases the effects of interindividual variations in power that are unrelated to the dynamic changes over the early portion of the night.
A challenge facing the computation of such indices is how to best define NREM periods, given that epochs of NREM sleep are frequently interspersed with brief state changes. This is particularly likely for individuals with insomnia. Other investigators have defined NREM periods as at least 15 minutes of NREM sleep that is (1) terminated by a REM episode of at least 5 minutes14,41 or (2) terminated by at least 5 minutes of wakefulness or any amount of REM sleep.37–40,42 Because, in an exploratory analysis we found that many subjects had very few NREM periods using the first criterion, we used the latter of these 2 criteria.
Using this NREM period definition, we first carried out exploratory correlation analyses with the indices of delta dynamics (delta power in the first NREM episode and the slope of delta power) derived using each of the 3 forms of delta power employed in prior research (average, peak, and total delta power) to determine which form of delta power best represented all 3 methods in terms of having the highest correlation with the indices derived using the other 2 types of delta power indices. Although the correlations were high across all the measures, the strongest correlations were found for the measures derived from peak delta power: (1) peak delta power for the first NREM period of the night, expressed as a percentage of the total delta power over the night, and (2) the slope of peak delta power over NREM periods. It is important to note that this slope was the best-fit line of logarithmically transformed data and so represents a best-fit exponential function. Based on these exploratory findings, the percentage peak NREM EEG delta power for NREM period 1 and the best-fit slope (exponential) of peak NREM EEG delta power in NREM periods were the focus of the analyses.
Sleep Logs
Subjective estimates of nocturnal sleep were obtained from sleep logs maintained by subjects for a 2-week baseline period and for 2 weeks following CBT or PC. From these logs, we derived the following measures that were employed based on our previous analysis: WASO, TST, sleep efficiency, sleep quality (5-point rating),and the degree of perceived restedness upon arising each day (5-point scale).4 The a priori primary outcome measure for this study was WASO derived from the average of the 2 weeks of sleep log data. This variable was chosen as the primary outcome measure because all subjects enrolled were required to have sleep maintenance insomnia based on this measure. Sleep-log WASO was employed in all outcome analyses. All sleep-log variables included in the comparison of CBT and PC were also derived from 2-week averages. Sleep-log data for the single day prior to polysomnography were used to determine the time awake over the course of the day prior to lights out for the polysomnogram. Analysis of the difference between self-reported TST and polysomnography-derived TST employed sleep-log TST data from the night of the polysomnogram.
Treatments Administered
The 2 treatments employed in this study, CBT and PC, were both administered at 6 weekly sessions.
Cognitive behavioral therapy
CBT was carried out, as has been previously described in detail.9 Briefly stated, this treatment consisted of sleep education (via standardized audiocassette recording) designed to correct unrealistic sleep expectations and unhelpful sleep beliefs as well as a behavioral regimen designed to correct sleep-disruptive habits. The latter consisted of combined stimulus-control and-sleep restriction instructions that asked patients to (1) establish a standard wake-up time; (2) get out of bed whenever awake for more than 20 minutes; (3) avoid reading, watching TV, eating, worrying, and other sleep-incompatible behaviors in the bed and bedroom; (4) refrain from daytime napping; and (5) limit their total TIB to a specific individually prescribed amount. Initial TIB prescriptions were mean TST (obtained from diaries) + 30 minutes (to allow for normal SOL and brief awakenings). The subsequent 5 treatment sessions were used to reinforce instructions and to make needed adjustments in TIB prescriptions. TIB was increased by 15 minutes each week in which subjects had a mean sleep efficiency of at least 85% according to the sleep logs and reported daytime sleepiness. TIB was decreased by 15 minutes each week subjects had an average sleep efficiency of less than 80%.
PC condition
PC was also administered, as it had been in 1 of our prior studies.9 PC was modeled after the quasi-desensitization protocol described by Steinmark and Borkovec.43 This placebo has been shown to be associated with credibility as an insomnia treatment and a high expectation of positive outcome. PC was presented as a means of desensitizing the “conditioned arousal” that prolongs nocturnal awakenings. The therapist helped each subject develop a hierarchy of 12 activities in which the subject engaged upon awakening in the middle of the night (e.g., opening eyes, looking at the clock, etc.). The therapist also instructed the subject to develop 6 imaginal scenes of himself or herself engaged in neutral activities during the daytime (e.g., watching TV, reading the newspaper, etc.). Subjects were then taught to pair images of each neutral scene with each item on the 12-item hierarchy with 2 items reviewed at each of the 6 treatment sessions.
Statistical Analysis
Analysis was carried out using SAS statistical software (SAS Institute, Inc. Carey, NC) using 2-tailed tests of significance. To assess for differences between CBT and PC, analysis of covariance was carried out controlling for age and sex because of reports indicating that NREM EEG indices may differ as a function of sex and age.37,39,44–46 It should be noted that the parent study was powered to detect differences among treatments in sleep log-derived WASO and not to detect effects with NREM EEG indices.9 Relationships of polysomnography and spectral indices with self-reported improvement with CBT were evaluated with multiple regression analyses that also controlled for age and sex.
RESULTS
Demographic data, baseline polysomnography indices, and baseline 2-week averaged sleep-log data for the CBT and PC subjects included in analyses appear in Table 1. There were no significant differences in any of these variables between the treatment groups.
Effects of CBT vs PC
The change in diary- and polysomnography-derived indices of sleep with CBT versus PC appear in Table 2. Inter-treatment differences were the same as previously reported in the publication of the parent study, which included 9 more subjects receiving CBT and 11 more receiving PC.9
Table 2.
Effects of CBT and PC on the change in sleep-diary and traditional polysomnography indices of sleep
| Mean sleep-diary data | CBT (n = 16) | PC (n = 14) |
|---|---|---|
| SOL, min | −7.3 (9.9) | −1.7 (11.6) |
| WASO, mina | −48.6 (29.2) | −25.8 (20.6) |
| TST, min | 22.4 (37.6) | 14.5 (36.5) |
| Sleep efficiency, %a | 10.6 (6.1) | 5.2 (4.9) |
| Sleep quality, score | 0.56 (0.6) | 0.23 (0.5) |
| TIB, mina | −48.4 (29.4) | −13.1 (31.4) |
| Polysomnographic data | ||
| SOL, min | −3.2 (10.4) | 1.2 (7.5) |
| WASO, mina | −28.1 (20.9) | −6.5 (28.7) |
| TST, min | 10.1 (51.9) | −5.1 (49.3) |
| Sleep efficiency, %a | 5.8 (8.2) | 1.2 (6.3) |
Data are presented as mean (SD) CBT refers to cognitive behavioral therapy
PC, placebo-controlled condition; SOL, sleep-onset latency; WASO, wake time after sleep onset; TST, total sleep time; TIB, time in bed
P < 0.05.
Sleep Diary–Derived Sleep Indices
The differences between CBT and PC in 2-week averaged sleep-diary indices were the same as previously reported in the publication of the parent study, which included 9 more subjects receiving CBT and 11 more receiving PC (See Table 2).9 CBT led to a significantly greater decrease than PC in self-reported WASO and a significantly greater increase in sleep efficiency. There were no significant treatment-group differences for diary-derived SOL, TST, or sleep quality. An a priori-defined measure of CBT adherence for this study was the pretreatment-to-posttreatment change in the average TIB derived from the sleep diaries. A greater decrease in TIB from before to after treatment indicated better CBT adherence. As reported with the parent study subject population, we found that CBT led to a significantly greater decrease in sleep diary–derived TIB (48.4 min), as compared with PC (13.1 min) (P < 0.05), indicating reasonable CBT treatment adherence.9
Traditional Polysomnography-Derived Sleep Indices
CBT was associated with significantly greater improvement in WASO and sleep efficiency, as compared with PC (See Table 2). No differences between CBT and PC were found for SOL and TST. These findings are the same as reported for the parent study except that a significant effect on TST was found in the parent-study analysis.9
Indices of the time course of NREM EEG delta power
CBT led to a significantly greater increase in the rate of exponential decline (the slope became more negative) in delta power over NREM periods across the night compared with PC (F = 4.5, P < 0.05) (See Table 3). Although CBT led to a numerically greater increase in peak delta power in the first NREM period expressed as percentage of total delta power over the night, this effect was not significant (F = 3.6, P < 0.07). No sex or age effects were found for the delta power indices.
Table 3.
Effects of CBT and PC on the change in spectral power-derived indices
| Peak 1 delta % powera | Delta slopeb | Delta power | Theta power | Alpha power | Sigma power | Beta power | |
|---|---|---|---|---|---|---|---|
| CBT (n = 16) | 0.066 (.26) | −0.00018 (.001) | 1.4 (2.9) | −0.029 (0.06) | −0.41 (.9) | −0.52 (1.1) | −0.13 (.5) |
| PC (n = 14) | −0.22 (.37) | 0.0012 (.001) | 2.2 (2.3) | −0.037 (0.09) | −0.46 (.8) | −0.50 (.7) | −0.23 (.3) |
Data are shown as mean posttreatment minus pretreatment differences (SD). Differences in power indices were computed with logarithmically transformed data. Delta, alpha, sigma, and beta power is relative power (power in the indicated frequency band divided by the sum of power in all bands and therefore dimensionless) averaged over the entire night. Peak 1 delta % power is the peak delta power in the first non-rapid eye movement period expressed as a percentage of total power over the night. Delta slope is the slope of the best-fit line of peak delta power over the night. Because it is fit to logarithmically transformed power, it is the exponent of the best-fit exponential function.
P < 0.10 for cognitive behavioral therapy (CBT) vs placebo-controlled condition (PC) difference.
P < 0.05 for CBT vs PC difference.
NREM EEG relative spectral power indices averaged over the night
No significant CBT-versus-PC differences were found for NREM EEG relative spectral power for any frequency band (See Table 3). We repeated this analysis with all-night averaged NREM EEG absolute spectral power indices and confirmed that the lack of significant treatment effects was not due to the use of relative power measures.
Relationship of the Change in polysomnography and Spectral Indices with Self-Reported Improvement with CBT
Traditional polysomnography derived sleep indices
No significant relationships between the change in sleep-diary WASO and the change in any traditional polysomnography-derived indices (WASO, TST, SOL, sleep efficiency, or percentage of stage 1 or stage 2 sleep, slow-wave sleep, or REM sleep) with CBT were found.
Indices of the time course of NREM EEG delta power
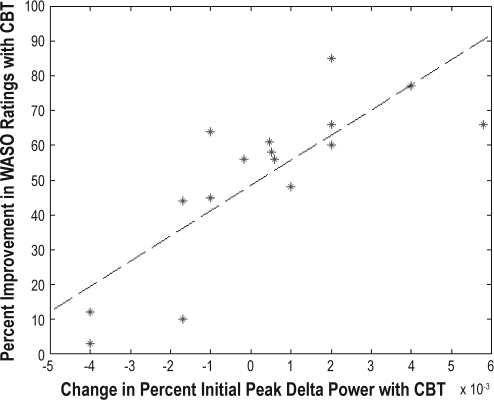
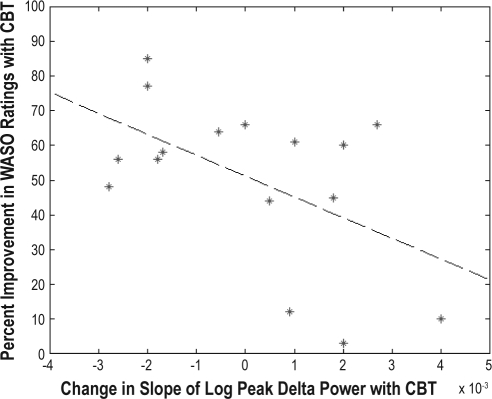
An increase in the peak delta power for the first NREM period (R2 = 0.63, F = 24, P < 0.0003) and an increase in the steepness of the decline in delta power over the night (R2= 0.25, F = 4.7, P < 0.05) were associated with greater self-reported improvement in WASO with treatment (See Figures 1–2). No significant age or sex effects were found. This analysis was repeated for the PC subjects, and no association was found between subjective improvement and EEG delta power indices. We also found that a greater increase in wake time over the course of the day with CBT was significantly associated with an increase in the steepness of the slope of delta power over the night (R2 = 0.32, F = 5.8, P < 0.04), though the relationship with the change in initial delta power was not significant (R2 = 0.21, F = 3.4, P < 0.09). Furthermore, greater improvement in self-reported WASO was significantly associated with a greater increase in the period of wake time before going to bed achieved with CBT (R2 = 0.50, F = 14, P < 0.003).
Figure 1.
Scatter plot of the change in the percentage of peak electroencephalographic delta power in the first non-rapid eye movement sleep period with cognitive behavioral therapy (CBT) vs the percentage of improvement in subjective wake time after sleep onset (WASO) ratings with CBT. The dotted line indicates the best-fit line.
Figure 2.
Scatter plot of the change in the slope of the logarithm of the percentage of peak electroencephalographic delta power in the first non-rapid eye movement sleep period with cognitive behavioral therapy (CBT) vs the percentage of improvement in subjective wake time after sleep onset (WASO) ratings with CBT.
NREM EEG relative spectral power indices averaged over the night
No significant relationships were found between the change in sleep diary–derived WASO with CBT and the change in any all-night averaged relative spectral power indices.
Accuracy of estimation polysomnography-derived TST and the relationship of self-reported improvement and polysomnography-derived and all-night averaged spectral power
Based on our prior study, we carried out additional regression analyses to assess whether the relationship of self-reported improvement with CBT and both traditional polysomnography indices and all-night averaged spectral indices might be dependent on accuracy with which subjects estimated their polysomnography-derived TST.4 The pretreatment difference between polysomnography-derived TST and sleep-diary–derived TST estimates on the night of the sleep study served as the measure of estimation accuracy. The regression analyses included the following predictor variables: TST estimation accuracy, changes with CBT in the traditional polysomnographic and averaged spectral power indices, and the interaction of TST estimation accuracy and the polysomnography and spectral power measures.
For traditional polysomnography indices, we found that the degree of improvement in sleep diary WASO with CBT was significantly predicted by the interactions of improvements in polysomnographic WASO (R2 = 0.29, F = 6.9, P < 0.02) and sleep efficiency (R2 = 0.28, F = 6.5, P < 0.03) with the degree of underestimation of TST.4 Subjects with less underestimation of TST at baseline who had greater improvement in polysomnographic WASO and polysomnographic sleep efficiency with treatment also experienced greater subjective improvement in WASO with CBT. For all-night averaged NREM spectral power measures, we found that greater improvement in self-reported WASO was significantly associated with the interaction of less underestimation of TST at baseline and (1) an increase in delta relative power (R2 = 0.35, F = 6.6, P < 0.03) with CBT and (2) a decrease in alpha (R2 = 0.51, F = 12.5, P < 0.005), sigma (R2 = 0.32, F = 5.6, P < 0.04, and beta (R2 = 0.35, F = 6.1, P < 0.04) relative power with CBT.
Pretreatment prediction of subsequent response to CBT
We also carried out additional regression analyses to determine if pretreatment traditional polysomnography and spectral power indices might predict the CBT treatment response. These analyses indicated that lower peak delta power in the first NREM period (R2 = 0.33, F = 8.0, P < 0.02) and a slower decline in peak delta power over the night (R2 = 0.23, F = 4.9, P < 0.05) were both associated with greater subjective improvement with CBT. No significant effects were found for traditional polysomnography indices, all-night averaged NREM spectral power measures, age, or sex.
DISCUSSION
Whereas this study must be considered preliminary, there were a number of notable findings. We found evidence for a relationship among the subjective response to CBT, indices of the time course of sleep EEG delta power, and an increase in the amount of wake time throughout the day with CBT. Specifically we found that (1) CBT led to a more rapid decline in peak EEG delta power over NREM periods compared with the placebo treatment; (2) CBT was associated with an increase in the period of waking throughout the day that, in turn, was correlated with the change in the slope of delta power over the night with treatment; (3) changes in the period of wakefulness throughout the day and changes in the slope of EEG delta power were both associated with the subjective improvement in WASO with CBT; and (4) lower initial peak EEG delta power in the first NREM period and more gradual decline in peak EEG delta power over NREM periods prior to treatment predicted a greater subjective response to CBT.
We also found support for the hypothesis that traditional polysomnography indices and all-night averaged NREM EEG relative spectral power indices are not related to subjective measures of sleep overall but relate to subjective sleep indices in subgroups of those with PI. Our analyses indicate that changes in traditional polysomnography indices did not correlate with the subjective improvement in WASO for the CBT group as a whole, but changes in these indices correlated with subjective improvement to a greater degree among those individuals with more accurate estimation of polysomnography TST. In contrast, for those whose TST estimates were shorter than polysomnography-derived TST to a greater extent, subjective improvement was unrelated to traditional polysomnography indices but was associated with a decrease in all-night averaged NREM delta relative power and increased all-night averaged higher frequency activity.
The Time Course of EEG Delta Power and the Subjective Response to CBT
The results of this study suggest a relationship between the therapeutic effect of CBT as a treatment for PI and the dynamics of EEG delta power. The data indicate that a relatively lower peak EEG delta power in the first NREM cycle and slower decline of peak EEG delta power over NREM cycles prior to treatment predict a greater likelihood of response to CBT, which may achieve its therapeutic effect in conjunction with an increase in the steepness of the slope of delta power. An increase in the speed of decline of EEG delta power over the night was reported to occur with CBT in a prior open-label study of 9 patients with psychophysiological insomnia.11 An increase in the steepness of the decline of peak EEG delta power over cycles has also been reported to occur in sleep-deprivation studies that increase the period of waking prior to bedtime and presumably increase sleep drive, need, or pressure.13–24
The implied link of the therapeutic CBT effect and an increase in sleep drive is further supported by evidence that the observed changes in EEG delta-power dynamics and improvement with CBT were both associated with an increase in the period of waking throughout the day among those receiving this therapy. Hence, as has been the case in experimental studies, increasing the period of wakefulness prior to bedtime with CBT enhances sleep drive and appears to be associated with its therapeutic effect in PI.13–24 It is important to note, however, that the apparent increase in sleep drive with CBT does not reflect that individuals are sleepier after treatment because of sleep deprivation. Although there may be a sleep-deprivation effect in the early phases of treatment, there is no evidence for a sustained decrease in sleep with CBT (see Table 2). Whereas this suggests that CBT is not simply a sleep-deprivation intervention, our findings could be interpreted to suggest that an important component of CBT is increasing time awake prior to going to bed at night and that adherence with this aspect of CBT accounts for some of its therapeutic effects. Two components of the CBT were aimed at increasing the time awake over the course of the day: sleep restriction and nap avoidance. However, it cannot be ruled out that other aspects of the treatment—such as establishing a standard wake-up time, getting out of bed whenever awake for more than 20 minutes (stimulus control), and avoiding sleep-incompatible behaviors in the bed and bedroom—might have had an impact on delta power by diminishing arousal and/or modulating circadian aspects of sleep. In this regard, our study must be viewed as focusing on only 1 component of CBT related to sleep homeostasis and the associated neurophysiology. Future studies will be needed to assess the neurophysiologic effects of arousal and circadian aspects of CBT.47,48
Thus, the findings of this study suggest the hypothesis that CBT exerts its therapeutic effect in association with increasing wake time throughout the day, which, in turn, is associated with an increase in sleep drive as reflected in an increase in the steepness of the decline in delta power over time. Individuals for whom delta power declines relatively rapidly prior to treatment presumably stand to gain relatively little from this aspect of the CBT intervention. As a result, they fail to respond to treatment. In contrast, individuals with low pretreatment sleep drive are more likely to improve with CBT, which achieves its effect by addressing the relative lack of sleep drive, as reflected in of the steepness of the decline in delta power over time. For such individuals without a pretreatment sleep-drive deficit, other treatments might be preferred, such as pharmacotherapy or more extensive cognitive therapies. Further studies are needed to replicate these findings and to determine whether indices of the time course of sleep EEG delta power could be of clinical utility in optimizing treatment selection.
The Relationships of Traditional Polysomnography Indices and All-night Averaged NREM EEG Measures with Subjective Improvement with CBT
The results of this study suggest that neither traditional polysomnography indices nor all-night averaged NREM EEG indices reflect the subjective response to CBT in all patients with PI. Changes in these measures do not appear to be intrinsic to the process by which CBT improves people's perception of their sleep. Instead, the findings are consistent with our prior report.4 As in that study, we found that averaged relative power indices in the delta and 3 higher-frequency bands are related to subjective WASO estimates in those who tend to underestimate their polysomnography-measured TST to a greater degree prior to CBT. Also as previously found, patients with more accurate estimates of TST have a greater association between their subjective WASO and traditional polysomnography sleep measures.4
That traditional polysomnography measures correlate with subjective measures of sleep more in individuals whose estimates of sleep correspond with polysomnographic sleep measures prior to treatment could reflect a selection effect. Those subjects for whom subjective sleep estimates match polysomnography estimates well prior to treatment have been selected for good polysomnography/subjective agreement. Those individuals for whom there is a large mismatch prior to treatment have already demonstrated that traditional polysomnographic indices do not reflect their subjective assessments, and it is not surprising, therefore, that these indices do not reflect their degree of subjective improvement with treatment.
These findings are consistent with the hypothesis that there are 2 types of neurophysiologic alterations in patients with PI. One is characterized by alterations in traditional polysomnography indices indicative of a problem with the quantity and/or timing of sleep. The other is marked by an alteration in physiology that occurs during sleep in the form of decreased delta and increased higher-frequency all-night averaged NREM EEG relative power.4 The reason that a tendency to underestimate sleep with respect to polysomnography is specifically associated with alterations in all-night averaged NREM EEG frequency content is unclear. It is also not clear whether the identification of these 2 types of neurophysiologic alterations in patients with PI has implications for understanding the pathophysiology of PI. For example, the findings could reflect differences in disorder severity among subgroups of patients with PI. However, to the extent that traditional polysomnography measures and spectral power indices reflect the pathophysiology of PI, our data suggest that PI may not have 1 unified pathophysiology but that the physiologic mechanisms may differ among subgroups.
Some preliminary evidence links less delta and greater high-frequency NREM EEG relative power to a relative hyperarousal and greater awareness during periods when there is traditional polysomnographic evidence of NREM sleep.4,12 This has been the basis for speculation that those with altered frequency content may be individuals with PI who are relatively hyperaroused. Whereas they appear to be asleep by traditional scoring criteria, they may have relatively intact awareness and therefore tend to underestimate their sleep time.4,12,49–52 Further studies are needed to assess whether these different pathophysiologic mechanisms are represented in individuals with PI, to determine their relative frequency of occurrence, and to begin to tease apart their underlying causes.
These findings may be of relevance for the development of treatments for PI. Studies of potential new treatments generally seek to employ physiologic sleep measures to provide objective validation for the subjective reports of the subjects. Studies that employ a polysomnographic screening procedure to select individuals with PI who have altered traditional polysomnographic indices are more likely to find concordance of changes in traditional polysomnography and subjective sleep assessments in response to treatment. Although it is understood that the use of such a screening procedure excludes many individuals with PI, our results raise the possibility that such procedures may exclude a subset of subjects who could have a different underlying pathophysiology. This subset seeks and receives clinical treatment for PI but could respond differently to treatment than the selected group. It will be important to study whether traditional research screening procedures could lead to a discrepancy between outcome in research studies and clinical practice. We did not find any evidence in the current study that those who tend to underestimate their objective sleep times respond differently to CBT than do others with PI. However, the capacity to assess this was limited by the number of subjects evaluated. Further studies will be needed to determine if these subgroups of patients with PI respond differentially to insomnia therapies.
Limitations
The major limitation of the present study is the relatively small number of subjects included. This constrained the statistical analysis and limited our ability to study interactions and relationships among the variables. A related limitation is that this study was powered to detect differences in self-reported WASO and not NREM EEG indices.9 As a result, our analyses may have been underpowered to demonstrate significant effects with a number of the NREM EEG indices studied. Additional limitations include (1) a restricted age range (there were no individuals less than 40 years of age in this study), (2) the objective baseline and posttreatment measures were derived from a single night of polysomnography, (3) no objective measures of the period of wakefulness prior to bedtime were employed, and (4) there were no corroborative measures of sleep drive employed, such as the Multiple Sleep Latency Test.
Future Directions
It will be important to carry out studies with a larger number of subjects so as to allow for analyses of the relationships among indices of the time course of delta power, traditional polysomnography measures, and all-night averaged NREM EEG power measures and to make it possible to study interactions among these variables. Studies employing additional polysomnograms and other measures of sleep drive will also be needed. In addition, it remains unknown whether the changes in EEG delta power that were associated with the therapeutic response to CBT might be seen with other effective treatments for PI.
Although we are not aware of any studies addressing this issue, a few studies have reported the effects of zolpidem, 10 mg, on the NREM EEG power spectrum in individuals with PI. One study reported a significant increase in 0.25- to 1-Hz power with treatment,53 whereas another study reported a decrease in 12-Hz power.54 Clearly, additional studies of this nature are needed.
Lastly, the physiologic mechanisms whereby CBT could alter sleep EEG delta power dynamics remain unknown. Several lines of research, primarily involving animal studies, suggest that such effects could involve the basal forebrain adenosine system, though future studies will be required to determine the mechanisms of the observed findings.39,55–62
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Krystal has received grant/research support from Sanofi-Aventis, Cephalon, GlaxoSmithKline, Merck, Neurocrine, Pfizer, Sepracor Inc., Samoxon, Takeda, Transcept, Respironics, Neurogen, Evotec, Astellas, and Neuronetics and has served as a consultant for Abbott, Actelion, Arena, Astellas, Axiom, AstraZeneca, BMS, Cephalon, Eli Lilly, GlaxoSmithKline, Jazz, Johnson and Johnson, King, Merck, Neurocrine, Neurogen, Neuronetics, Novartis, Organon, Ortho-McNeil-Janssen, Pfizer, Respironics, Roche, Sanofi-Aventis, Sepracor Inc., Somaxon, Takeda, Transcept, Astellas, Research Triangle Institute, Kingsdown Inc., and CHDI. Dr. Edinger has received research support form Philips Respironics and Helicor, Inc. and has consulted for Philips Respironics and Kingsdown, Inc.
ACKNOWLEDGMENTS
This research was supported by NIMH grant R01-MH48187 and NHLBI grant R01-HL096492. The results presented in this manuscript were presented in part at the 15th Annual Meeting of the Associated Professional Sleep Societies, Chicago, IL, May 2001.
REFERENCES
- 1.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997 May–Jun;31(3):333–46. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo JL, Gras CB, Garcíia YD, Lapeira JT, del Campo del Campo JM, Verdejo MA. Functional status in the elderly with insomnia. Qual Life Res. 2007 Mar;16(2):279–86. doi: 10.1007/s11136-006-9125-9. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: 1994. [Google Scholar]
- 4.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. Non-REM sleep EEG spectral amplitude correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–640. [PubMed] [Google Scholar]
- 5.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, 3rd, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–7. [PubMed] [Google Scholar]
- 6.National Institute of Mental Health. Consensus conference report: drugs and insomnia-the use of medication to promote sleep. JAMA. 1984;251:2410–2414. [PubMed] [Google Scholar]
- 7.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 8.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Edinger JD, Wohlgemuth WK, Radtke R, Marsh GR, Quillian RE. Cognitive Behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs GD, Benson H, Friedman R. Home-based central nervous system assessment of a multifactor behavioral intervention for chronic sleep-onset insomnia. Behav Therapy. 1993;24:159–74. [Google Scholar]
- 11.Cervena K, Dauvilliers Y, Espa F, Touchon J, Matousek M, Billiard M, Besset A. Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. J Sleep Res. 2004;13:385–93. doi: 10.1111/j.1365-2869.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 12.Perlis ML, Smith MT, Andrew PJ, Orff H, Giles DE. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Santamaria J, Chiappa KH. The EEG of drowsiness. New York, NY: Demos Publications; 1987. [Google Scholar]
- 14.Dijk D-J, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207219. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- 15.Werth E, Dijk D-J, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 16.Dijk D-J, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 17.Tobler I, Borbély AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 18.Brunner DP, Dijk D-J, Borbély AA. Repeated partial sleep deprivation progressively changes the EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 19.Dijk D-J, Cajochen C, Tobler I, Borbély AA. Sleep extension in humans: sleep stages, EEG power spectra and body temperature. Sleep. 1991;14:294–306. doi: 10.1093/sleep/14.4.294. [DOI] [PubMed] [Google Scholar]
- 20.Dijk D-J, Brunner DP, Beersma DGM, Borbély AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 21.Brunner DP, Dijk D-J, Tobler I, Borbély AA. Effect of partial sleep deprivation on sleep stages and EEG power spectra: evidence for non-REM and REM sleep homeostasis. Electroencephalogr Clin Neurophysiol. 1990;75:492–9. doi: 10.1016/0013-4694(90)90136-8. [DOI] [PubMed] [Google Scholar]
- 22.Endo T, Schwierin B, Borbély AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 23.Landolt H-P, Finelli LA, Roth C, Buck A, Achermann P, Borbély AA. Zolpidem and sleep deprivation: Different effect on EEG power spectra. J Sleep Res. 2000;9:175–83. doi: 10.1046/j.1365-2869.2000.00192.x. [DOI] [PubMed] [Google Scholar]
- 24.Dijk D-J, Brunner DP, Borbély AA. EEG power density during recovery sleep in the morning. Electroencephalogr Clin Neurophysiol. 1991;78:203–14. doi: 10.1016/0013-4694(91)90034-2. [DOI] [PubMed] [Google Scholar]
- 25.Schramm E, Hohagen F, Grasshoff MA, et al. Test-retest reliability and validity of a Structured Interview for Sleep Disorders according to DSM-III-R Am J Psychiatry. 1993;150:867–72. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- 26.Structured Clinical Interview for DSM-III-R (SCID) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 27.Edinger JD, Hoelscher TJ, Webb MD, Marsh GR, Radtke RA, Erwin CW. Polysomnographic assessment of DIMS: Empirical evaluation of its diagnostic value. Sleep. 1989;12:315–22. [PubMed] [Google Scholar]
- 28.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14:13–7. [PubMed] [Google Scholar]
- 29.Edinger JD, Fins AI, Sullivan RJ, et al. Laboratory versus home-based polysomnography in the comparison of insomniacs and normal sleepers. Sleep Res. 1996;25:236–44. [Google Scholar]
- 30.Rechtshaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems of sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 31.Perlis M.L., Smith M T, Andrew P J, et al. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Merica H, Blois R, Gaillard J M. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;105:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–82. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasser T, Bacher P, Mocks J. Transformations towards the normal distribution of broad band spectral paramters of the EEG. Electroencephalogr Clin Neurophysiol. 1982;53:119–24. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- 35.Krystal AD, Weiner RD, Coffey CE. The ictal EEG as a marker of adequate stimulus intensity with unilateral ECT. J Neuropsychiatry Clin Neurosci. 1995;7:295–303. doi: 10.1176/jnp.7.3.295. [DOI] [PubMed] [Google Scholar]
- 36.John ER, Ahn H, Prichep L, Treptin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–8. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- 37.Armitage R, Hoffmann R, Fitch T, Trivedit M, Rush J. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000;5:607–17. [PubMed] [Google Scholar]
- 38.Hoffmann R, Hendrickse W, Rush AJ, Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 2000;95:215–25. doi: 10.1016/s0165-1781(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 39.Armitage R, Emslie GJ, Hoffmann RF, Rintelmann J, Rush AJ. Delta sleep EEG in depressed adolescent females and healthy controls. J Affect Disord. 2001;63:139–48. doi: 10.1016/s0165-0327(00)00194-4. [DOI] [PubMed] [Google Scholar]
- 40.Armitage R, Hoffmann R, Madhukar T, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–13. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- 41.Landolt H, Dijk D, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 42.Aeschbach D, Dijk D-J, Borbély AA. Dynamics of EEG spindle frequency activity during extended sleep in humans: relationship to slow-wave activity and time of day. Brain Res. 1997;748:131–6. doi: 10.1016/s0006-8993(96)01275-9. [DOI] [PubMed] [Google Scholar]
- 43.Steinmark SW, Borkovec TD. Active and placebo treatment effects on moderate insomnia under counterdemand and positive demand instructions. J Abnorm Psychol. 1974;83:157–63. doi: 10.1037/h0036489. [DOI] [PubMed] [Google Scholar]
- 44.Rediehs MH, Reis JS, Creason NS. Sleep in old age: focus on gender differences. Sleep. 1990;13:410–24. [PubMed] [Google Scholar]
- 45.Reynolds CF, III, Kupfer DJ, Taska LS, Hoch CC, Sewitch DE, Spiker DG. Sleep of healthy seniors: a revisit. Sleep. 1985;8:20–9. doi: 10.1093/sleep/8.1.20. [DOI] [PubMed] [Google Scholar]
- 46.Smith J, Karacan I, Yang M. Ontogeny of delta activity during human sleep Electroencephalogr Clin Neurophysiol. 1977;43:229–37. doi: 10.1016/0013-4694(77)90130-4. [DOI] [PubMed] [Google Scholar]
- 47.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Qullian RE. Does cognitive-behavioral insomnia therapy alter dysfunctional beliefs about sleep? Sleep. 2001;24:591–9. doi: 10.1093/sleep/24.5.591. [DOI] [PubMed] [Google Scholar]
- 48.Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8:463–7. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- 49.Edinger JD, Krystal AD. Subtyping primary insomnia: Is sleep state misperception a distinct clinical entity? Sleep Med Rev. 2003;7:203–14. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- 50.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 51.Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, III, Kupfer DJ. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Perlis JL, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Subjective-objective discrepancies in psychophysiologic insomnia: a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 53.Monti JM, Vlvarino F, Monti D. Conventional and power spectrum analysis of the effects of zolpidem on sleep EEG in patients with chronic primary insomnia. Sleep. 2000;23:1075–84. [PubMed] [Google Scholar]
- 54.Benoit O, Bouard G, Payan C, Borderies P, Prado J. Effect of a single dose (10 mg) of zolpidem on visual and spectral analysis of sleep in young poor sleepers. Psychopharmacology. 1994;116:297–303. doi: 10.1007/BF02245332. [DOI] [PubMed] [Google Scholar]
- 55.Benington JH, Heller C. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–60. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 56.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strecker RE, Morairty S, Thakkar MM, et al. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 58.Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521:679–90. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benington JH, Kodali SK, Heller C. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 60.Landolt H-P, Werth E, Borbély AA, Dijk D-J. Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Res. 1995;675:67–74. doi: 10.1016/0006-8993(95)00040-w. [DOI] [PubMed] [Google Scholar]
- 61.Landolt HP, Dijk D, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;12:229–38. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- 62.Basheer R, Porkka-Heiskanen T, Strecker RE, Thakkar MM, McCarley RW. Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biol Signals Recept. 2000;9:319–27. doi: 10.1159/000014655. [DOI] [PubMed] [Google Scholar]