Abstract
Study Objective:
To study the neurophysiological changes in attention and memory functions in shift work sleep disorder (SWSD), using event-related brain potentials (ERPs).
Participants:
9 healthy night workers (NW) (mean age = 40 y; SD ± 8.9 y); 8 night workers meeting diagnostic criteria for SWSD (mean age = 37 y ± 9.4 y) and 9 healthy day workers (DW) (mean age = 35 y ± 7.3 y).
Methods and Procedure:
Using standard PSG the sleep related measures (TIB, TST, SOL, SE, and sleep stage distribution) were obtained prior to EEG/ERP study. Measures of habitual sleep were obtained from 2 week sleep logs and sleepiness was assessed with standardized measures. Using 32-EEG leads the ERPs to 3 types of sounds (novel, duration deviant, and simple tone) were obtained. The mismatch negativity (MMN) reflecting memory processing and P3a-reflecting the shift of involuntary attention were obtained.
Statistical Analysis:
The statistical comparisons of ERPs and sleep related parameters were performed using repeated measured ANOVAs and t-tests where appropriate.
Results:
Patients with SWSD had reduced TST and increased WASO relative to healthy workers. ERP results demonstrated significant attenuation of MMN amplitude over frontal regions in SWSD patients relative to NW and DW. In the SWSD patients, the P3a was increased to novelty across frontocentral brain regions with respect to the same locations in healthy controls.
Conclusion:
The ERP evidence of sensory memory reduction and attentional hyper-reaction to novel sound in conjunction with disturbed sleep suggests the need for more neurophysiological studies in SWSD workers.
Citation:
Gumenyuk V; Roth T; Korzyukov O; Jefferson C; Kick A; Spear L; Tepley N; Drake CL. Shift work sleep disorder is associated with an attenuated brain response of sensory memory and an increased brain response to novelty: an ERP study. SLEEP 2010;33(5):703-713.
Keywords: Shift Work Sleep Disorder, Event-Related brain potentials (ERP), MMN, P3a
THE OBJECTIVE ASSESSMENT OF THE CONSEQUENCES OF SLEEPINESS ON COGNITIVE FUNCTIONS IS PARTICULARLY RELEVANT IN THE MANAGEMENT OF shift work. Shift work has been shown to impair performance on variety of tasks as well as quality of work across different occupations.1,2 In contrast to the literature regarding cognitive function in shift work, evidence for the impact of shift work as well as shift work sleep disorder (SWSD) on neurophysiological measures of brain function is limited. SWSD is prevalent3 and is diagnosed when clinically significant symptoms of insomnia and/or excessive sleepiness are present in shift workers and cannot be accounted for by another sleep disorder or medical condition (ICSD-2, 2005). Exposures to major circadian and homeostatic challenges affect cognition in patients with SWSD. However, there have been no studies assessing neurophysiological aspects of brain function specifically related to attention and memory that may occur in conjunction with SWSD.
Excessive sleepiness is the main symptom reported by night shift workers, in part, because their daytime sleep is fragmented and can be reduced by 2–4 h.2,4 Since sleep loss leads to deficits in brain functioning,5 it is possible that significant sleep disturbance in night shift workers may alter activity of neuronal circuitry underlying their attention and memory. The major misalignment of circadian rhythms associated with shift work also contributes to cognitive deficits.
The neurophysiology of the attention system can be evaluated by the P300 component of event-related brain potentials (ERP). More specifically, changes in attention can be assessed with the P3a component that reflects the involuntary switching of attention toward an attention-eliciting event, for example, a novel sound such as a dog barking or a car horn.6–8 It has been shown that the prefrontal cortex is a critical element of the neural circuitry that generates the P3a. However, there is strong evidence for a distributed network of cortical regions generating the P3a including the auditory cortex,9 posterior hippocampus,10 temporoparietal junction,11 and anterior cingulate gyrus.12 ERP findings from previous studies looking at the relationship between neural system underlying the P3a and sleepiness or insomnia13–15 suggest that the P3a response may be useful for assessment of neurophysiological changes in involuntary attention occurring with changes in sleep-wake function associated with SWSD. For example, it was shown that disturbed sleep is related to a reduced amplitude of P3a,13 suggesting that an intact involuntary attention switching system reflected by the P3a is critically dependent upon adequate sleep.15
Another auditory ERP component that has been used widely in sleep research is mismatch negativity (MMN). MMN has been used most commonly to assess neurocognitive functions related to auditory sensory memory.16,17 Auditory sensory memory is an automatic process involving transient storage of sensory information arising from incoming acoustic stimuli until they can be integrated with previous stimuli or recalled from memorized auditory events. MMN generation depends on the ability of auditory system to remember attributes of frequently presented sounds and thus provides a noninvasive measure of sensory memory.18 Whereas the P3a is associated with attention switching to a novel event in an unattended auditory stream, the MMN is associated with pre-attentive initiation of an attention switch to deviant sounds.18 Importantly, MMN is an involuntary electrical brain response and therefore can be elicited independent of voluntary attention. Thus, MMN provides an unbiased estimate of sensory memory processing without the influence of an individual's motivation or cooperation.
It has been shown that excessive sleepiness is associated with reduced amplitude of MMN at frontal and central brain regions, but not at temporal locations.19,20 Studies indicate that the fontal lobe subcomponent of MMN associated with initiation of an automatic attention switch might be more sensitive to sleepiness than the temporal lobe MMN component that originates from auditory cortices, which in turn reflects sensory-memory function.18–21 These data are consistent with neuroimaging data that show a reduction of frontal lobe activity following sleep deprivation.5 In addition, these data suggest that both MMN and P3a brain responses are potentially useful tools for the study of neurophysiological changes in attention and memory function in SWSD.
Although the frontal subcomponents of MMN and P3a are related to attention switching, there are critical differences between them. The frontal subcomponent of MMN is associated with pre-attentive initiation of an attention switch to deviant sounds, whereas the P3a frontal-central component reflects attention switching to novel events in an unattended auditory stream.18 The current study uses these auditory ERP measures to evaluate the impact of SWSD on the neurophysiology of memory and attention. In addition to assessing the differences in MMN and P3a associated with night work, we also compared data from the night workers to a control group of day workers assessed using the same tasks.
METHODS
Participants
Subjects for this study were 10 healthy (asymptomatic as to sleep wake function) night workers (mean age = 40 y; SD ± 8.9 y, 4 females, all right handed); 8 night workers meeting diagnostic criteria for SWSD (mean age = 37 y ± 9.4 y, 5 females, all right handed) and 10 healthy day workers (mean age = 35 y ± 7.3 y, 5 females, 2 left handed). Inclusion criteria for the night (NW) and day work (DW) groups were: (1) NW must have worked ≥ 5 night shifts for ≤ 12 h between 21:00 and 08:00 over the past month with a minimum duration of shift work ≥ 3 months; (2) average total 24-h caffeine consumption had to be < 750 mg; (3) no psychiatric or neurological problems; (4) no head injuries or problems with hearing or vision; (5) no sleep disorders confirmed by standard overnight polysomnogram (including respiratory measurement and anterior tibialis electromyograph (apnea-hypopnea index < 10/h and periodic limb movements < 10/h) and clinical evaluation; (6) any CNS active medications were discontinued at least 1 week or 5 half-lives prior to study participation; (7) no history of alcohol/drug abuse. Shift workers meeting criteria for SWSD based on a clinical evaluation and standardized measures of excessive sleepiness (Epworth sleepiness scale [ESS] ≥ 10) or insomnia severity scale [ISI] ≥ 15) were assigned to the SWSD group. Night workers reporting insomnia (ISI ≥ 15 and/or ESS ≥ 10) were excluded from the NW control group.
All participants were in good health based on clinical history, physical examination, and routine laboratory tests, including blood chemistries and a urinalysis drug screen. All participants gave written informed consent which was approved by the Ethics Committee of Henry Ford Hospital and were paid for their participation.
Sleep Screen Methods
As a part of pre-study screening, each subject completed the Horne and Ostberg Morningness-Eveningness Questionnaire (MEQ).22 There were no between-group differences on the MEQ (P > 0.1). The SWSD group consisted of 3 subjects who were morning types and the rest were neithertypes. The NW control group consisted of one morning type, 2 evening types; the remaining subjects were neither types. The DW group consisted of 3 morning types, 2 evening types; the remaining subjects were neither type.
Sleep screening utilized a standardized 8-h polysomnogram (PSG) performed during the day for night workers and at night for day workers. Two weeks prior to the study, each participant was asked to complete a sleep/wake diary to determine habitual sleep patterns.
ERP Methods
The MMN response can be obtained by presenting different auditory stimuli varying in frequency, duration, loudness, spectral pattern, or spatial location. Because the MMN amplitude strongly depends on the probability between the frequently and infrequently presented stimuli, typical MMN paradigms utilize a sequence of stimuli presented with an 80% to 95% probability (standard), while the other, differing slightly, is presented infrequently at a 5% to 20% probability (deviant).18 This paradigm allows an automatic memory trace of frequent auditory event to be stored and compared against infrequent auditory stimuli (e.g., between frequencies, duration, or novelty). Thus the MMN component can be used as a probe of the ability of the brain to form an auditory sensory-memory trace about physical parameters of the auditory event.23,24 The MMN component is elicited at 120-150 ms from deviation onset and can be computed as a difference wave between ERPs to deviant minus ERPs to standard sounds.25
For the current study, the novelty oddball paradigm consisted of 3 types of sounds (simple tone = standard [100 ms], duration deviant sound = deviant [simple tone + 50 ms], and novel sounds) used for MMN and P3a brain response elicitation. This choice was made since it has been shown that processing of novel sounds activates a larger neural network than does a simple tone resulting from the elicitation of a P3a in response to novelty.8 Duration deviance was chosen since it was shown that the processing of sound duration eliciting the MMN is less attenuated by sleep than is frequency deviance in normally subjects.26
ERPs to 3 categories of stimuli were elicited. The frequent standard stimulus was an 800 Hz tone lasting 100 ms and presented with 80% probability. Additionally, 2 types of deviant tones (novel and duration) were employed randomly interspersed among standard tones with a probability of 10% each. The deviant duration stimulus was the same 800 Hz tone, but lasting for 150 ms instead of 100 ms. The novel deviant stimulus was a complex environmental sound (e.g., dog barking, car horn, phone ring). Every novel stimulus was a unique sound, presented only once. All sounds were presented through earplugs binaurally at a 75 dB SPL (sound pressure level) with 5 ms rise/fall time. All stimuli were presented at a constant inter-stimulus interval of 800 ms. A total of 550 trials were presented per session; each session lasted 7.3 min. A total of 4 sessions with a short inter-session break (2 min) was presented to each subject.
During the experiment, each subject lay comfortably on a reclined chair (to reduce muscle artifact and head movement) in an electrically and sound-shielded chamber. Subjects were asked to ignore all presented experimental sounds and keep their attention focused on a subject-chosen silent movie (with subtitles) located about 80 cm from the subject's eyes. The silent movie based on the participant's choice was used for helping our subjects to follow a task instruction to ignore auditory stimuli and to direct their attention away from presented sounds. Online EEG was used to monitor each subject's wake state, and a video camera was used to monitor the subject's behavior during the recording. If subject did fall asleep, the recording session was terminated until the time (not more than 2-4 min) the subject was ready for stay awake to continue the study.
EEG Recording and Procedure
Study start time was 22:30 for night workers and 10:30 for day workers. The electroencephalogram (EEG) and electrooculogram (EOG) were recorded using a 32-EEG channel cap (Easy Cap, Gilching, Germany), with an additional electrode placed on the tip of the nose to serve as a reference. The EOG electrodes were placed below and above the left eye. Impedance was kept < 10 kΩ. The band-pass filter of the analogue amplifier (Neuro Scan, USA) was set from 0.1 to 100 Hz, and the sampling rate was set at 508 Hz.
EEG data were analyzed using Brain Vision Analyzer software (Brain Products GmbH, Gilching, Germany). Data were segmented separately for each stimulus, starting with 100 ms prior to stimulus onset and continuing for 400 ms after the stimulus onset. A band-pass filter ranging from 1 to 20 Hz was applied to segmented data. Trials in which EEG or EOG exceeded ± 70μV were excluded from the average. ERPs in response to the standard tone, novel sounds, and deviant duration sounds were averaged separately. On average, ' 300 trials for the standard tone, and ≥ 100 trials for novel and deviant duration sounds were included for each subject.
Data Analysis
All statistical comparisons of MMN and P3a (to duration and novel stimuli) involved computing difference waves (ERPs in response to duration/novel sounds minus ERPs to the standard tone). The time windows for mean amplitude comparisons were selected based on the peak amplitude of the MMN and P3a responses. Thus, the mean amplitudes of MMN components to novel sounds were measured within 120-150 ms from stimulus onset, while MMN to duration sounds was measured within 220-260 ms time window. Mean amplitude for P3a was measured within a 200-270 ms time window for novel sounds and 300-350 ms for duration deviant stimuli.
To validate the presence of the MMN and P3a for novel and duration deviant sounds, the mean amplitudes measured at the frontal (F3, Fz, F4) and central electrodes (C3, Cz, C4) were compared against zero using a t-test. The between-group differences and scalp distribution of the MMNs and P3a responses were statistically compared with 3-way ANOVAs including the following factors (Group [DW vs. NW vs. SWSD], Frontality [frontal electrodes F3, Fz, and F4 vs. central electrodes C3, Cz, and C4 vs. parietal electrodes P3, Pz and P4], and Laterality [left-hemisphere electrodes F3, C3, and P3 vs. midline electrodes Fz, Cz, and Pz vs. right-hemisphere electrodes F4, C4, and P4]). Subsequent Newman-Keuls post hoc tests were performed to confirm significance for tested differences.
The sleep parameters were compared between groups using one-way ANOVAs including total sleep time (TST), time in bed (TIB), sleep efficiency (SE), latency to sleep, number of awakenings, sleep stage distribution, ESS, and a 100-mm visual analogue scale (VAS) for sleepiness/alertness.
An α level of 0.5 was used for all statistical tests. Greenhouse-Geisser corrections were used in reporting P values where appropriate.
RESULTS
Sleep and Sleepiness Measures
Subjective measures
As the ESS was used for study entry criteria, the VAS for alertness was used to evaluate subjective sleepiness in each group during the study. As expected, the ESS in SWSD group was significantly higher (F2,22 = 9.44, P < 0.001) than the 2 control groups (NW and DW): SWSD = 11.8 ± 4.0, for NW = 5.3 ± 3.9 and for DW = 4.7 ± 2.8. Post hoc testing revealed that NW and DW were not significantly different in ESS. The VAS was not significantly different between any of the groups (F2,22 = 0.37, P < 0.7) SWSD = 41.6 ± 18.5; NW = 32.5 ± 11.0; and DW = 39.3 ± 30.8. Again, as expected the SWSD group had significantly higher ISI = 13 ± 6.7 (F2,22 = 11.00, P < 0.005) than NW (5.7 ± 2.5) and DW (1.7 ± 4.3). The comparison between NW and DW groups was not significant for ISI.
Sleep diary data showed that SWSD patients and night workers without SWSD spent less time in bed per 24 h (including naps)—7.4 h and 6.4 h than DW = 8.5 h (± SD; Table 1). The comparison of TIB between groups showed that NW and SWSD groups had significantly less TIB than DW (F2,22 = 5.34, P < 0.01). TST was lower in SWSD = 6.2 h and NW = 6.4 h compared to DW = 8.2 h (F2,22 = 7.18, P < 0.004); subsequent post hoc test revealed that NW and SWSD slept less (P < 0.01) than DW based on sleep diary reports. SE was lower in SWSD = 85% than in NW = 95% and DW = 96% (F2,22 = 4.83, P < 0.01), and post hoc test revealed that SWSD group was significantly lower in SE than healthy NW and DW. Number of naps as well as their duration in both night workers and SWSD groups were significantly more frequent and longer than day workers (F2,22 = 4.10, P < 0.04) (see Table 1).
Table 1.
Means (± SD) for 2–week sleep diary data obtained from day workers, night workers, and SWSD patients prior to the study. (P-values derive from between-group comparison.)
| Day Workers | Night Workers | SWSD | P-value | |
|---|---|---|---|---|
| TIB (h) | 8.5 ± 1.1 | 6.7 ± 0.9 | 7.4 ± 1.0 | 0.01 |
| TST (h) | 8.2 ± 1.1 | 6.4 ± 0.8 | 6.2 ± 0.7 | 0.004 |
| SOL (min) | 14.2 ± 6.7 | 8.8 ± 3.1 | 23.0 ± 21.1 | ns |
| SE (%) | 96.3 ± 2.0 | 95.0 ± 1.3 | 85.0 ± 0.7 | 0.01 |
| Naps (min) | 7.0 ± 16.8 | 38.4 ± 26.4 | 33.0 ± 46.4 | 0.04 |
Objective measures
A standardized 8-h PSG demonstrated significant differences between the groups on several sleep parameters (Table 2). The SWSD group showed less TST (5.96 h) than the NW (7.0 h) and DW (7.2 h) (F2,22 = 3.91, P < 0.04) groups. Sleep efficiency was also significantly (F2,22 = 3.99, P < 0.04) lower in the SWSD group relative to NW and DW (75.5% vs. 88.6% vs. 89.9%, respectively). WASO was significantly (F2,22 = 4.25, P < 0.03) higher in the SWSD group (107.0) than in DW (30.3). No significant between-group differences in sleep onset latency and sleep stage distribution were found.
Table 2.
Means (± SD) for 8-h PSG obtained from day workers (during night sleep), night workers, and SWSD patients (during day sleep)
| Day Workers | Night Workers | SWSD | P-value | ||
|---|---|---|---|---|---|
| TIB (h) | 8.0 ± 0.02 | 8.0 ± 0.02 | 7.9 ± 0.11 | ns | |
| TST (h) | 7.2 ± 0.40 | 7.0 ± 0.50 | 5.96 ± 1.56 | 0.04 | |
| SE (%) | 89.9 ± 4.87 | 88.6 ± 6.71 | 75.0 ± 19.10 | 0.04 | |
| SOL (min) | 17.0 ± 18.82 | 6.2 ± 3.49 | 15.0 ± 9.7 | ns | |
| WASO (min) | 30.3 ± 21.07 | 48.9 ± 30.79 | 107.0 ± 91.58 | 0.03 | |
| Stage 1 (%) | 5.4 ± 5.62 | 4.3 ± 1.86 | 5.0 ± 3.63 | ns | |
| Stage 2 (%) | 57.1 ± 4.65 | 62.0 ± 7.38 | 61.0 ± 9.85 | ns | |
| Stage 3 (%) | 16.4 ± 5.54 | 10.7 ± 9.39 | 11.0 ± 11.40 | ns | |
| REM (%) | 21.0 ± 4.99 | 22.9 ± 4.06 | 22.0 ± 2.38 | ns |
Obligatory N1 to Novel Sounds
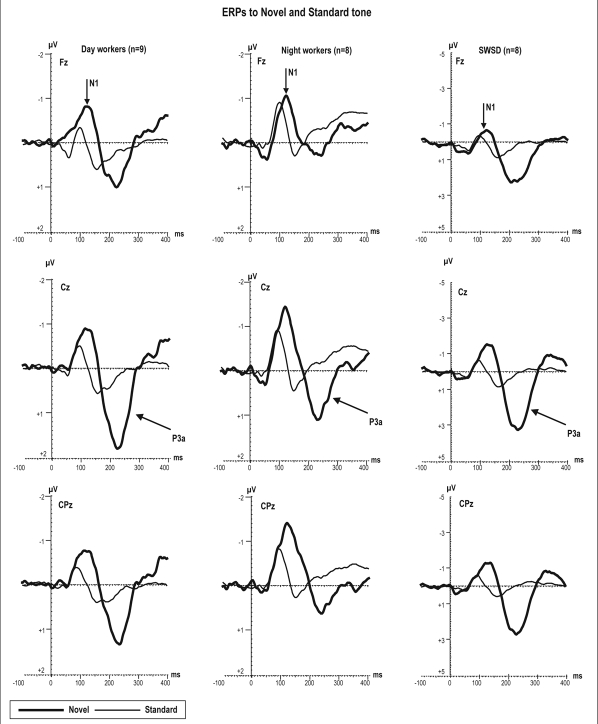
ERP data for 3 participants (2 NW and 1 DW) were excluded from the analyses due to extensive artifact. Thus data from 8 night workers, 8 night workers with SWSD, and 9 day workers are presented. The grand-averaged ERP waveforms representing the standard tone and novel stimuli for each group are superimposed in Figure 1. All groups showed response to auditory stimuli. The one-way ANOVA did not show significant group differences (F2,22 = 0.25, P = 0.7 ns) in amplitude of the obligatory N1 component for either standard or novel and deviant auditory stimuli (Table 3).
Figure 1.
Grand average of ERPs at frontal, central, and centroparietal electrodes elicited by novel sounds and standard tones during oddball task in which all subjects were instructed to ignore all sounds while watching self-chosen silent movie. Note, the scale of the ERP amplitude is enlarged for SWSD group with respect to the 2 control groups.
Table 3.
Mean amplitude (± SD) for sensory (N1) measured at Cz electrode and cognitive (MMN measured at Fz and P3a measured at Cz) ERPs
| ERP Amplitude | SWSD | Night Workers | Day Workers | P-value |
|---|---|---|---|---|
| N1 to STD | −0.49 μV (± 0.3) | −0.84 μV (± 0.6) | −0.56 μV (± 0.2) | ns |
| N1 to NOV | −0.86 μV (± 1.1) | −1.20 μV (± 1.0) | −1.20 μV (± 0.6) | ns |
| N1 to DEV | −0.39 μV (± 1.0) | −0.7 μV (± 0.7) | −0.52 μV (± 0.5) | ns |
| MMN to NOV | −0.96 μV (± 1.5) | −0.76 μV (± 0.5) | −1.2 μV (± 0.6) | ns |
| MMN to DEV | −0.19 μV (± 1.2) | −0.88 μV (± 0.6) | −1.33 μV (± 0.5) | 0.04 |
| P3a to NOV | 3.49 μV (± 2.0) | 1.51 μV (± 1.2) | 1.82 μV (± 0.5) | 0.03 |
| P3a to DEV | 0.58 μV (± 1.6) | 0.39 μV (± 0.6) | 0.50 μV (± 0.4) | ns |
NOV, novel; DEV, deviant duration; STD, standard
MMN to Novel Sounds
Figure 2 shows novel minus standard-tone difference waves to novel sounds for each group. The negative-polarity deflection represents MMN responses to novel stimuli with a peak latency of around 130 ms from stimulus onset and a mean amplitude measured at Fz −1.2 μV vs. −0.8 μV vs. −1.0 μV for day, night workers, and the SWSD group, respectively. In all groups, the MMN mean amplitudes to novel sounds were significantly different from zero (t = 4.7, P < 0.0002 for DW; t= 4.0, P < 0.001 for NW; and t = 2.3, P < 0.03 for the SWSD).
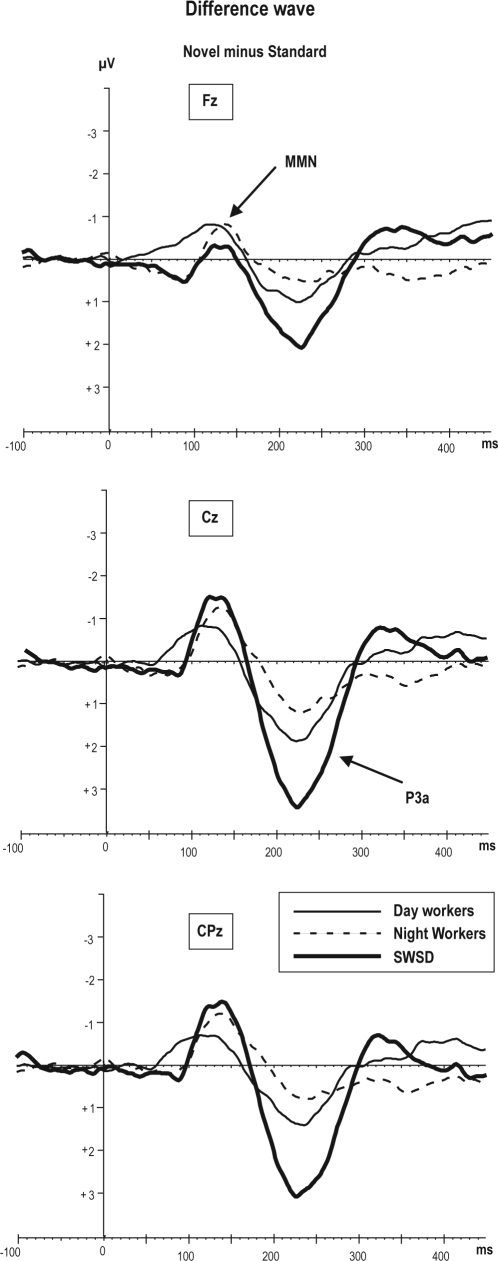
Figure 2.
Grand average of difference wave obtained by subtracting the standard ERPs from novel ERPs. The difference wave to the novel sounds reflects 2 components: negative deflection is MMN at 130 ms and positive deflection is P3a at 230 ms.
Group differences
There was no significant main effect of group for MMN amplitude to novel sounds.
Frontality
There was a significant main effect of frontality (F2,42 = 6.17, P < 0.004). Across groups, the MMN amplitude to novel sounds was larger at the central recorded site with respect to frontal and parietal (Cz < Fz < Pz).
Laterality
A significant main effect of laterality revealed that the MMN amplitude to novel sounds was larger at midline compared to right or left hemisphere (F2,42 = 7.19, P < 0.003).
Laterality interaction
There was a significant group × laterality interaction (F4,44 = 2.99, P < 0.03) showing a smaller amplitude of MMN to novel stimuli in the right hemisphere in the SWSD group (−0.5 μV) compared to NW (−0.9 μV) and DW (−1.0 μV) groups as determined by post hoc comparisons (all P < 0.05). Post hoc test revealed no significant differences between DW and NW across midline and both hemispheres in MMN amplitude to novel sounds.
There was no significant group × frontality interaction observed.
P3a to Novel Sounds
Figure 1 illustrates the large positive P3a deflection at 200-300 ms to novel sounds with respect to standard tones in all groups. The difference wave (novel sounds minus standard tone) (Figure 2) shows the P3a in all groups was different from 0 μV in the SWSD (t = −6.5, P < 0.001), NW (t = −3.61, P < 0.006), and DW (t = −3.16, P < 0.001).
Group differences
There was a main effect of group in P3a amplitude (F2,22 = 4.22, P < 0.03). Post hoc testing revealed that the SWSD group had overall larger P3a amplitude as compared to NW and DW groups (2.2 μV vs. 0.8 μV vs. 0.9 μV; P < 0.03; Table 3), whereas no significant differences between NW and DW groups was observed in P3a amplitude.
Frontality
There was a significant main effect of frontality (F2,44 = 19.99, P < 0.0001) across all groups in P3a amplitude to novel sounds. The amplitude of this response is maximal at central site (e.g., Cz) and lower at frontal and parietal sites.
Laterality
There was a significant main effect of laterality (F2,44 = 11.07, P < 0.001). Post hoc test revealed that the amplitude of P3a was significantly (P < 0.003) larger at midline sites with respect to right and left hemispheres. The difference in P3a between right and left hemispheres was not significantly different.
Frontality and laterality interaction
There was a significant group × frontality interaction (F4,44 = 3.16, P < 0.02) indicating that the SWSD group showed the largest P3a amplitude over frontal (2.6 μV) and central (2.8 μV) electrodes as compared to the same locations in NW (0.8 μV and 1.2 μV) and DW (0.9 μV and 1.3 μV). Whereas the P3a amplitude measured over parietal locations was not significantly different between groups (SWSD = 1.1 μV, NW = 0.6 μV, and DW = 0.7 μV; P = 0.5, ns)
The group × laterality interaction for the P3a to novel sounds did not reach significance (F4,44 = 2.36, P = 0.06 ns). However, there was a tendency for increased P3a amplitude to occur over midline and left hemisphere in the SWSD group relative to control groups, with minimal group differences over the right hemisphere.
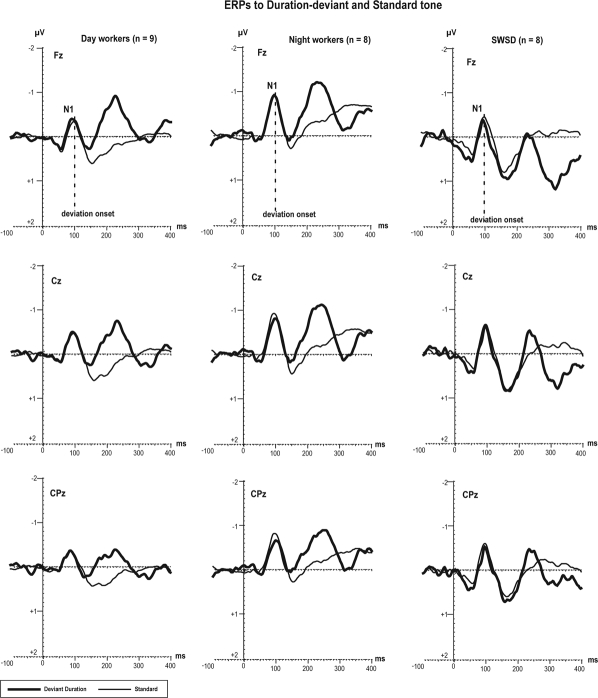
Obligatory N1 to Deviant Duration Sounds
Figure 3 represents the grand averaged ERP waveforms in response to the standard tones and deviant duration sounds for each group. Because we used deviant duration sounds (standard tone = 100 ms; deviant tone = 150 ms), the deviation could only be detected starting at 100 ms after stimulus onset. In the 3 groups, the ERP response to deviant duration sounds showed 2 negative deflections at latencies of 100 (in all groups, see Fz electrode on Figure 3) and 250 ms from stimulus onset (in the NW and DW groups at Fz electrode, with minimal amplitude in the SWSD group; Figure 3).
Figure 3.
Grand average of ERPs elicited by deviant duration (lasting 150 ms) and standard tones (lasting 100 ms). The deviation onset is depicted at 100 ms from stimulus onset.
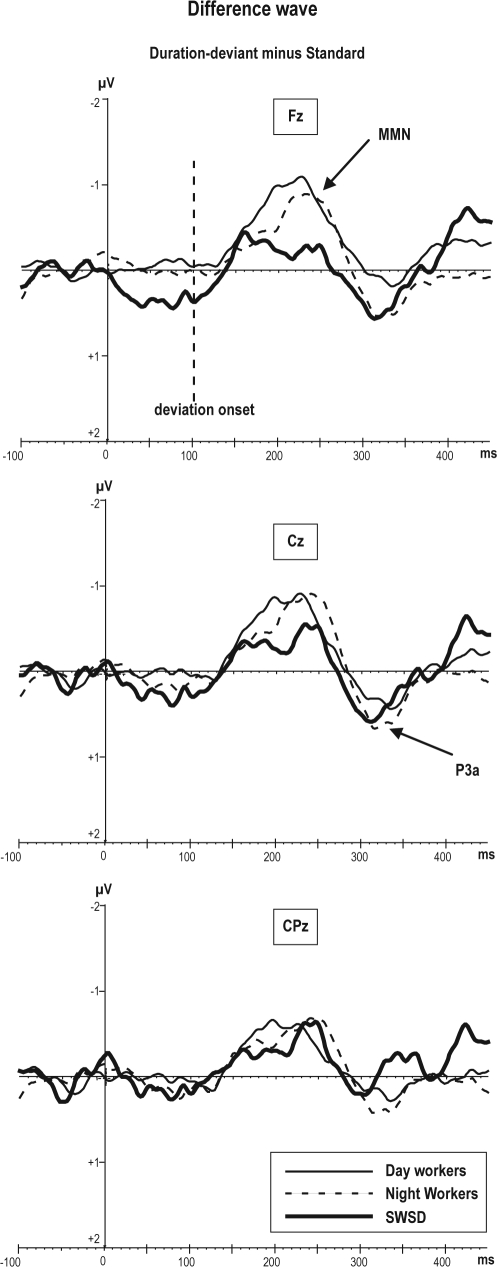
MMN to Deviant Duration Sounds
The difference wave corresponding to deviant duration sounds at 250 ms (Figure 4) reflects an MMN response recorded from frontal, central, and parietal electrodes in the 3 groups. The mean amplitude of MMN measured at Fz was different from 0 μV in NW (−0.8 μV; t = 3.85, P < 0.001), DW (−1.3 μV; t = 4.01, P < 0.001), but was not significantly different from 0μV in the SWSD group (−0.19 μV, P = 0.6 ns).
Figure 4.
Grand average of difference wave obtained by subtracting the standard ERPs from deviant duration ERPs. The difference wave to the deviant duration sounds reflects broad negative deflection between 200-240 ms which is represent MMN to duration
Group differences
Although the MMN amplitude across all locations, was lower in the SWSD group (−0.5 μV) as compared to NW (−0.7 μV) and DW (−0.8 μV), the main effect of group differences in MMN response to duration deviant did not reach significance (F2,22 = 0.33, P = 0.7 ns).
Frontality
The main effect of frontality showed significant differences between frontal (–0.7 μV) central (−0.8 μV) and parietal (−0.3 μV) regions. (F2,44 = 7.68, P < 0.001). Post hoc testing revealed that frontal and central sites showed higher amplitude relative to parietal (P < 0.004), with no significant difference between frontal and central sites.
Laterality
There was a main effect of laterality (F2,44 = 6.84, P < 0.02). Post hoc testing revealed that MMN amplitude in the left hemisphere was significantly lower (−0.5 μV, P < 0.01) than in the right (−0.7 μV) and midline (−0.8 μV).
Frontality interaction
There was a significant group × frontality interaction (F4,42 = 4.52, P < 0.004) for the MMN to duration deviant, indicating that the SWSD group had decreased amplitude of MMN at the left frontal site (F3 = −0.12 μV) with respect to the same location in NW (−0.9 μV) and DW (−1.2 μV) groups. Post hoc testing confirmed that this difference was significant (P < 0.003), whereas differences between NW and DW was not (P = 0.5). Additionally, post hoc testing revealed that other locations (midline and parietal) were not significantly different between groups for MMN to duration of deviant tones.
There was no significant group by laterality interaction observed.
P3a to Duration Deviant
The amplitude of P3a response to duration of deviant sounds was measured at the Cz electrode and compared against 0 μV in DW (0.5 μV; t = −2.07, P < 0.05), NW (0.4 μV; t = −1.61, P < 0.05, ns), and in the SWSD (0.58 μV; t = −2.87, P < 0.05) groups.
The statistical analysis did not revealed group, frontality, or laterality differences between P3a amplitude to duration deviant.
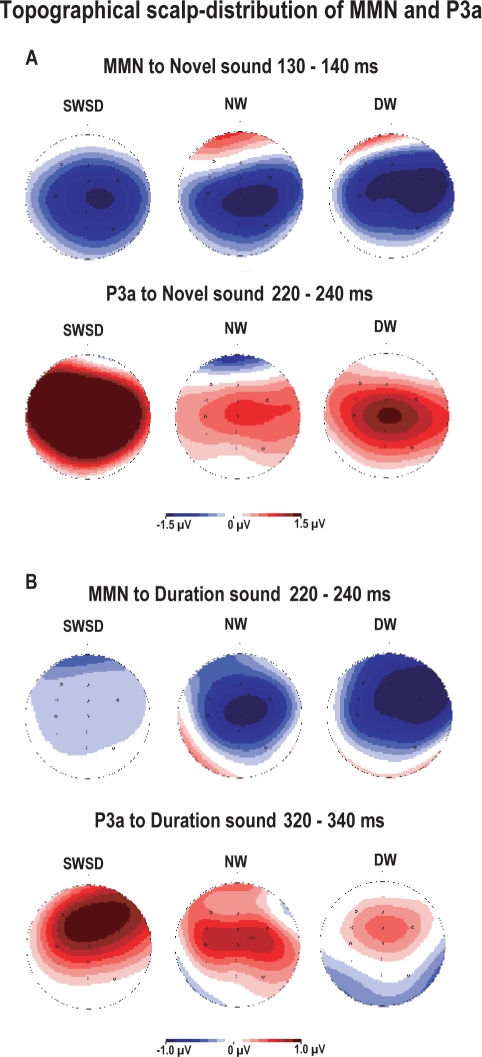
The surface potential maps were used to evaluate the distribution of brain activity associated with MMN and P3a components to novel and duration deviant sounds. Figure 5 shows the topographical maps for each group. Although the MMN is typically distributed over frontal and temporal scalp regions, as illustrated in the control groups (see Figure 5 for MMN scalp distribution which is depicted by blue color), the SWSD group showed more central distribution of MMN to novel sound and less frontocentral voltage distribution for MMN amplitude to duration deviant. In contrast to MMN, the P3a response related to novelty (depicted by red) shows a more spread scalp distribution of activity over central and parietal regions in the SWSD group relative to controls (see Figure 5A). For deviant duration, the topographical maps show a similar localization of the voltage maxima corresponding to P3a; however, the extent of the P3a distribution was greater in night workers relative to day workers (Figure 5B), suggesting the involvement of a larger area of task-related neuronal activation in P3a generation.
Figure 5.
Brain surface evoke potential maps of voltage amplitude corresponding to MMN and P3a for novelty (A) and deviant duration (B). Blue color represents negative polarity and red color represents positive polarity of ERPs.
DISCUSSION
The main findings of the present study are: (1) SWSD patients had elevated amplitude of the P3a response, reflecting hyperreaction to novelty compared to both healthy day workers and night workers. (2) SWSD patients showed reduced brain response involved with processing of temporal parameters of auditory stimuli (duration deviant). This was observed in SWSD subjects specifically at frontal regions as compared to the control groups. (3) SWSD subjects showed attenuated brain activity when processing complex sounds (novel sounds) relative to both control groups. This effect was most prominent in the right hemisphere. Our ERP findings regarding MMN responses are consistent with previous studies showing that brain function is affected by sleep disruption,27 specifically frontal regions where sleep deprivation reduces brain metabolism.5 Objective PSG data showed that the SWSD group had reduced TST and increased WASO with lower sleep efficiency relative to the two control groups. These results in clinically diagnosed patients suggest that night shift workers with SWSD do have increased sleep disruption relative to their non-affected shift work counterparts. It remains unclear if sleep disturbance in these patients is due to circadian misalignment relative to non-affected shift workers or similar circadian misalignment, or an inherent inability to sleep at adverse circadian phase, possibly due to hyperarousal.3,28
In the present study, both night shift worker groups reported a habitual total sleep time that was about 2 h less (including naps) than the day workers. Interestingly, PSG with a fixed time in bed of 8 h resulted in comparable sleep durations in the night work and day work control groups. With regard to this objective sleep assessment (8-h PSG), healthy night workers slept during the daytime as well as day workers during the nighttime, suggesting that circadian rhythms in our control night shift workers may have adapted successfully to daytime sleep schedules. Alternatively, they may still have shifted circadian rhythms, but simply were able to easily sleep at adverse circadian phases. In contrast, their subjective assessment of TST was different from day workers, suggesting that night shift workers even without SWSD may not obtain sufficient sleep on a regular basis.
Electrophysiological results of the present study demonstrate that the auditory sensory component N1 to all stimuli was intact for all groups with little variation. In previous studies, the N1component was shown to be enhanced after 24 h and 36 h of sleep deprivation with respect to baseline.20 Based on this study, we may infer that our SWSD subjects are not severely sleep deprived, since their N1 is not significantly different from the N1 obtained in controls. In support to this conclusion, a previous study29 indicated that long-term changes in sleep more strongly influence the attention system than do short-term changes in sleep duration. Based on our inclusion criteria for the study, our shift workers have been working on their night shifts for much longer than in simulated shift work study designs. That is why acute sleep deprivation or simulation studies may not be applicable to clarify potential neurophysiological changes that occur due to regular shift work.
With respect to neurophysiological changes associated with cognitive components such as the P3a, our study demonstrated that SWSD patients show increased brain activity to novel sounds reflected by enlarged P3a amplitude over central and parietal regions as compared to controls. In previous studies, it was shown that sleep deprivation reduced the amplitude of P300 to auditory stimuli over frontalcentral brain regions, but increased amplitude over central-parietal regions.30 However, our SWSD subjects showed increased P3a amplitude specifically to novel sounds across all recorded sites with respect to controls. The larger P3a amplitude may be related to the insomnia present in the SWSD group. Devoto et al. showed that insomnia patients have enhanced P300 amplitude after “bad sleep quality” nights with respect to “good sleep quality” nights, suggesting that in primary insomniacs cortical hyperarousal is not a stable characteristic, but is associated with the poor quality on a given night. A study of chronic primary insomniacs,31 showed ERP evidence of greater cortical arousal during sleep (reflected by elevated ERP amplitude to deviant sounds) especially upon awakening in the morning. Because our SWSD patients showed higher ISI and WASO and low SE, in addition to greater amplitude of P3a to novel sounds, we may conclude that SWSD patients, like insomnia patients, may have higher cortical arousal to novelty. This then results in their inability to ignore novel events appearing out of their attentional focus. This clearly can be the mediator of the deficits in concentration and increased distractibility32 reported in ADHD samples.
Unlike the P3a to novel sounds, the MMN amplitude associated with memory was attenuated in the SWSD group, consistent with previous studies showing that MMN is attenuated by sleepiness.19,20 In contrast to our results, the MMN amplitude and latency in OSA patients were not affected by moderate sleepiness (ESS = 15) in the study of Gosselin et al. This apparent inconsistent finding might be explained by differences in methodological parameters (e.g., stimulus interval, and type of deviance) of paradigm and stimuli between their and our study. It is well known that MMN amplitude is affected by the inter-stimulus interval, degree of deviance, and other parameters.18 A more likely explanation of this discrepancy could be that OSA and SWSD differentially impact memory and involuntary attention processes evaluated by ERPs.
A limitation of our study is the lack of data on circadian phase in day and night workers. Time-of-day fluctuations in the cognitive performance of day workers have been reported in many behavioral studies. From these studies, the main conclusion is that core body temperature (CBT) as a measure of circadian variation is related to level of performance: when CBT is high neurobehavioral performance also tends to be high, whereas low CBT or high melatonin secretion is associated with reduced performance and alertness.18,33–35 In our study, we used a task that did not depend on behavioral performance and attentional input, which demonstrated differences in neurophysiological aspects of cognitive processing in patients with SWSD relative to night work controls and day workers. Thus, differences in ERP measures found in the present study may have been related to differences in circadian phase. Standard markers of circadian phase were not obtained in the protocol precluding any circadian interpretation of the effects observed. Due to the paucity of data available regarding circadian fluctuations in ERP components, future studies of SWSD should assess circadian and ERP measures concomitantly.
In sum, the present findings demonstrate that in night workers with SWSD the frontal brain areas showed insufficiency in sensory memory processes reflected by decreasing MMN amplitude relative to healthy controls. During passive oddball task the novel sounds captured the attention of SWSD patients to a greater extent than controls which makes processing of novelty for SWSD patients more demanding with greater neuronal requirements (over frontocentral areas).
DISCLOSURE STATEMENT
This was a principal investigator (Dr. Drake) initiated study supported by Cephalon, Inc. Dr. Gumenyuk has received research support from Cephalon and Takeda. Dr. Roth has received grants from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport. He has served as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, élan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Ceullular, Jazz, Johnson and Johnson, King, Lundbeck McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Additionally, Dr. Roth has served as a speaker for Cephalon, Sanofi, and Takeda. Dr. Drake has received research support from Takeda, Cephalon, Sanofi-Aventis, Sepracor, and AmericInn Hotels. The other authors have indicated no other financial conflicts of interest
ACKNOWLEDGMENTS
We thank the all our participants for their contribution to this study, especially our night shift workers who did dedicate their time off for this study. Also we thank the two anonymous reviewers for their constructive and thoughtful comments on this paper.
Footnotes
A commentary on this paper appears in this issue on page 579.
REFERENCES
- 1.Folkard S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. 2008;25:215–24. doi: 10.1080/07420520802106835. [DOI] [PubMed] [Google Scholar]
- 2.Akerstedt T. Altered sleep/wake patterns and mental performance. Physiol Behav. 2007;90:209–18. doi: 10.1016/j.physbeh.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 4.Walsh JK, Tepas DI, Moses PD, editors. The EEG sleep of night and rotating shift workers. New York: Spectrum Publications; 1981. [Google Scholar]
- 5.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–43. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- 7.Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- 8.Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neurosci Biobehav Rev. 2001;25:355–73. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 9.Opitz B, Mecklinger A, Friederici AD, von Cramon DY. The functional neuroanatomy of novelty processing: integrating ERP and fMRI results. Cereb Cortex. 1999;9:379–91. doi: 10.1093/cercor/9.4.379. [DOI] [PubMed] [Google Scholar]
- 10.Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–9. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 11.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–83. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 12.Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. Neuroreport. 1997;8:3029–37. doi: 10.1097/00001756-199709290-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gosselin N, Mathieu A, Mazza S, Decary A, Malo J, Montplaisir J. Deficits in involuntary attention switching in obstructive sleep apnea syndrome. Neurosci Lett. 2006;408:73–8. doi: 10.1016/j.neulet.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 14.Devoto A, Violani C, Lucidi F, Lombardo C. P300 amplitude in subjects with primary insomnia is modulated by their sleep quality. J Psychosom Res. 2003;54:3–10. doi: 10.1016/s0022-3999(02)00579-2. [DOI] [PubMed] [Google Scholar]
- 15.Salmi J, Huotilainen M, Pakarinen S, Siren T, Alho K, Aronen ET. Does sleep quality affect involuntary attention switching system? Neurosci Lett. 2005;390:150–5. doi: 10.1016/j.neulet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Atienza M, Cantero JL, Dominguez-Marin E. Mismatch negativity (MMN): an objective measure of sensory memory and long-lasting memories during sleep. Int J Psychophysiol. 2002;46:215–25. doi: 10.1016/s0167-8760(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 17.Colrain IM, Campbell KB. The use of evoked potentials in sleep research. Sleep Med Rev. 2007;11:277–93. doi: 10.1016/j.smrv.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naatanen R. Attention and brain function. New Jersey: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 19.Sallinen M, Lyytinen H. Mismatch negativity during objective and subjective sleepiness. Psychophysiology. 1997;34:694–702. doi: 10.1111/j.1469-8986.1997.tb02144.x. [DOI] [PubMed] [Google Scholar]
- 20.Raz A, Deouell LY, Bentin S. Is pre-attentive processing compromised by prolonged wakefulness? Effects of total sleep deprivation on the mismatch negativity. Psychophysiology. 2001;38:787–95. [PubMed] [Google Scholar]
- 21.Naumann A, Bierbrauer J, Przuntek H, Daum I. Attentive and preattentive processing in narcolepsy as revealed by event-related potentials (ERPs) Neuroreport. 2001;12:2807–11. doi: 10.1097/00001756-200109170-00011. [DOI] [PubMed] [Google Scholar]
- 22.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 23.Korzyukov O, Alho K, Kujala A, et al. Electromagnetic responses of the human auditory cortex generated by sensory-memory based processing of tone-frequency changes. Neurosci Lett. 1999;276:169–72. doi: 10.1016/s0304-3940(99)00807-1. [DOI] [PubMed] [Google Scholar]
- 24.Kujala T, Tervaniemi M, Schroger E. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol. 2007;74:1–19. doi: 10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Naatanen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118:2544–90. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Ruby P, Caclin A, Boulet S, Delpuech C, Morlet D. Odd sound processing in the sleeping brain. J Cogn Neurosci. 2008;20:296–311. doi: 10.1162/jocn.2008.20023. [DOI] [PubMed] [Google Scholar]
- 27.Koslowsky M, Babkoff H. Meta-analysis of the relationship between total sleep deprivation and performance. Chronobiol Int. 1992;9:132–6. doi: 10.3109/07420529209064524. [DOI] [PubMed] [Google Scholar]
- 28.Blatter K, Cajochen C. Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol Behav. 2007;90:196–208. doi: 10.1016/j.physbeh.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 30.Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clin Neurophysiol. 2005;116:211–22. doi: 10.1016/j.clinph.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Bastien CH, St-Jean G, Morin CM, Turcotte I, Carrier J. Chronic psychophysiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31:887–98. doi: 10.1093/sleep/31.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumenyuk V, Korzyukov O, Alho K, Escera C, Naatanen R. Effects of auditory distraction on electrophysiological brain activity and performance in children aged 8-13 years. Psychophysiology. 2004;41:30–6. doi: 10.1111/1469-8986.00123. [DOI] [PubMed] [Google Scholar]
- 33.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277(3 Pt 2):R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277(4 Pt 2):R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 35.Monk TH, Folkard S, Wedderburn AI. Maintaining safety and high performance on shiftwork. Appl Ergon. 1996;27:17–23. doi: 10.1016/0003-6870(95)00048-8. [DOI] [PubMed] [Google Scholar]