Abstract
An insulin analogue that embodies two distinct structural modifications, each of which independently increases insulin activity, has been synthesized and evaluated for biological activity. The analogue, des-(B26-B30)-[AspB10,TyrB25-NH2]insulin is the most potent insulin analogue yet described; it displays an 11- to 13-fold higher activity than natural insulin. The findings are discussed with regard to the receptor-binding domains of insulin.
Full text
PDF
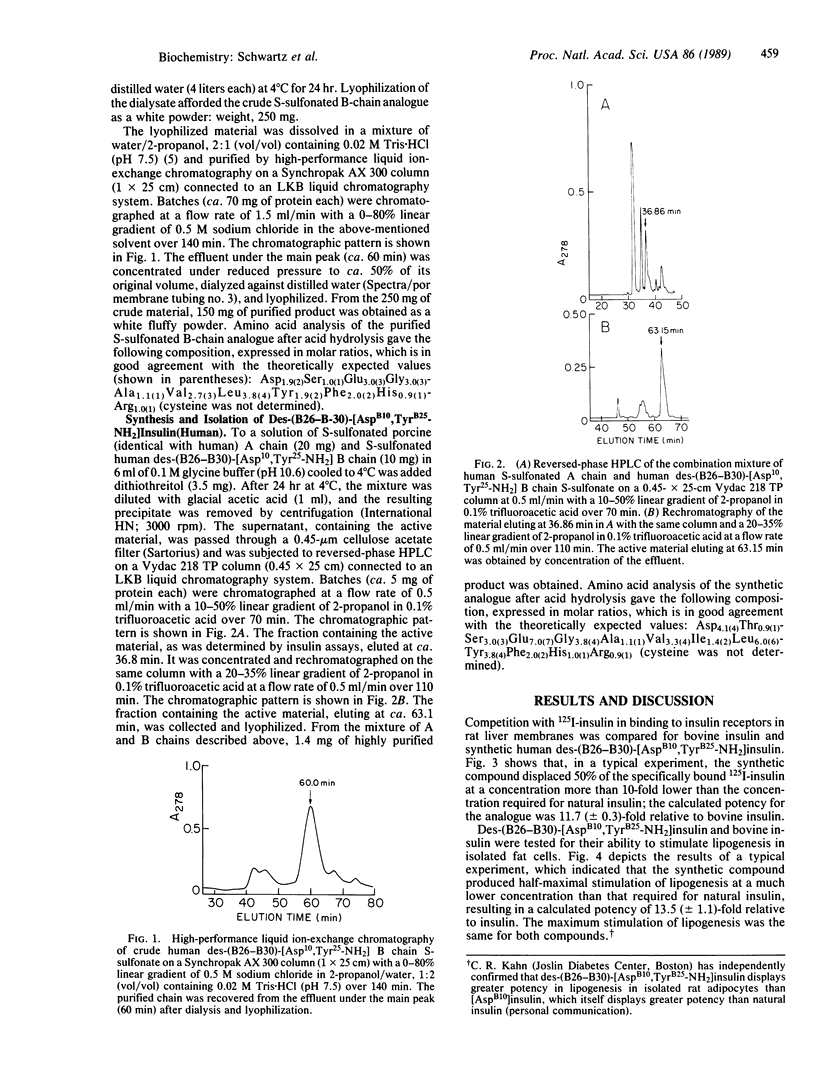
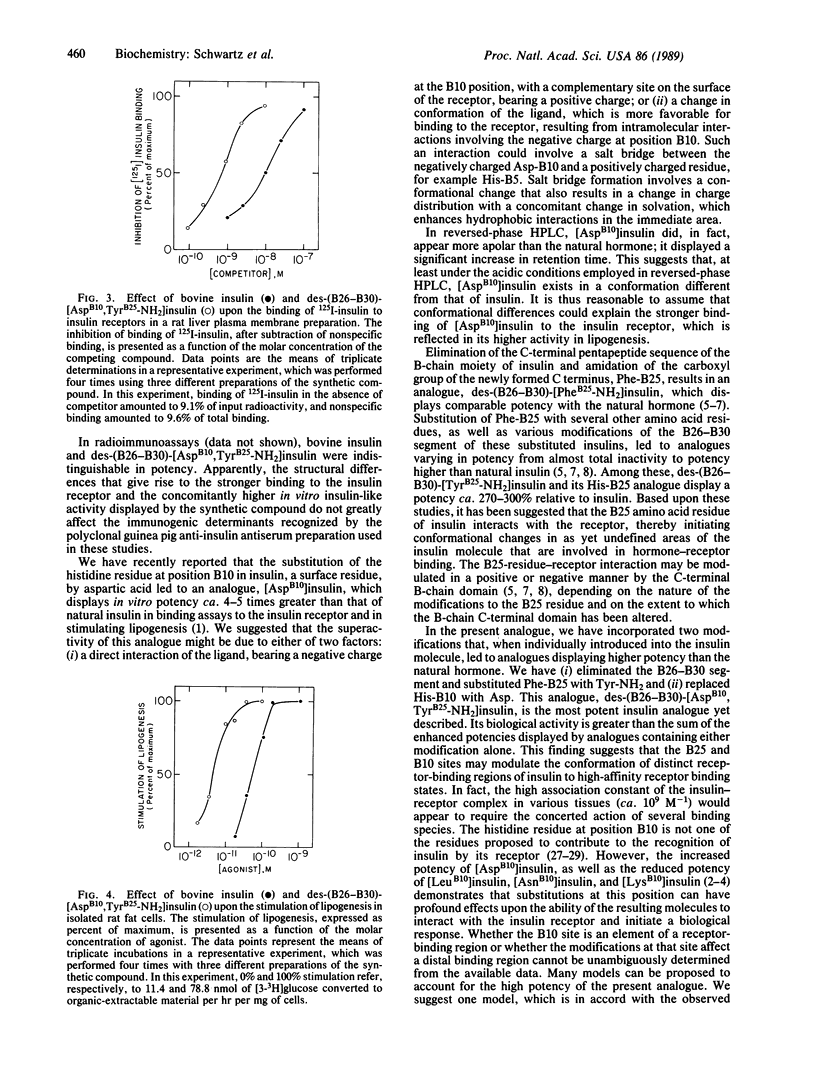

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blundell T. L., Wood S. P. Is the evolution of insulin Darwinian or due to selectively neutral mutation? Nature. 1975 Sep 18;257(5523):197–203. doi: 10.1038/257197a0. [DOI] [PubMed] [Google Scholar]
- Burke G. T., Schwartz G., Katsoyannis P. G. Nature of the B10 amino acid residue. Requirements for high biological activity of insulin. Int J Pept Protein Res. 1984 Apr;23(4):394–401. doi: 10.1111/j.1399-3011.1984.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Casaretto M., Spoden M., Diaconescu C., Gattner H. G., Zahn H., Brandenburg D., Wollmer A. Shortened insulin with enhanced in vitro potency. Biol Chem Hoppe Seyler. 1987 Jun;368(6):709–716. doi: 10.1515/bchm3.1987.368.1.709. [DOI] [PubMed] [Google Scholar]
- Cosmatos A., Ferderigos N., Katsoyannis P. G. Chemical synthesis of [des(tetrapeptide B27--30), Tyr(NH2)26-B] and [des(pentapeptide B26--30), Phe(NH2)25-B] bovine insulins. Int J Pept Protein Res. 1979;14(5):457–471. doi: 10.1111/j.1399-3011.1979.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Fischer W. H., Saunders D., Brandenburg D., Wollmer A., Zahn H. A shortened insulin with full in vitro potency. Biol Chem Hoppe Seyler. 1985 May;366(5):521–525. doi: 10.1515/bchm3.1985.366.1.521. [DOI] [PubMed] [Google Scholar]
- Gattner H. G. B-Kettenverkürzung von polymergebundenem Insulin mit Pepsin, I. Darstellung und Eigenschaften von Des-Pentapeptid (B26-30)-Rinderinsulin. Hoppe Seylers Z Physiol Chem. 1975 Sep;356(9):1397–1404. [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Ginos J., Cosmatos A., Schwart G. Synthesis of destetrapeptide B27-30 human (porcine) insulin. A biologically active insulin analog. J Am Chem Soc. 1973 Sep 19;95(19):6427–6434. doi: 10.1021/ja00800a045. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Ginos J., Schwartz G. P., Cosmatos A. Synthesis of a biologically active truncated insulin. Des(pentapeptide B23-30) human (porcine) insulin. J Chem Soc Perkin 1. 1974;11(0):1311–1317. doi: 10.1039/p19740001311. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Tometsko A., Zalut C., Johnson S., Trakatellis A. C. Studies on the synthesis of insulin from natural and synthetic A and B chains. I. Splitting of insulin and isolation of the S-sulfonated derivatives of the A and B chains. Biochemistry. 1967 Sep;6(9):2635–2642. doi: 10.1021/bi00861a001. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Zalut C., Harris A., Meyer R. J. Analogs of insulin. 1. Synthesis of destripeptide B 28-30 bovine insulin and destripeptide B 28-30 porcine (human) insulin. Biochemistry. 1971 Oct 12;10(21):3884–3889. doi: 10.1021/bi00797a014. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Ogawa H., Burke G. T., Chanley J. D., Katsoyannis P. G. Critical role of the A2 amino acid residue in the biological activity of insulin: [2-glycine-A]- and [2-alanine-A]insulins. Biochemistry. 1984 Mar 27;23(7):1405–1413. doi: 10.1021/bi00302a011. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- NICOL D. S., SMITH L. F. Amino-acid sequence of human insulin. Nature. 1960 Aug 6;187:483–485. doi: 10.1038/187483a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa S. H., Tager H. S. Role of the COOH-terminal B-chain domain in insulin-receptor interactions. Identification of perturbations involving the insulin mainchain. J Biol Chem. 1987 Sep 5;262(25):12054–12058. [PubMed] [Google Scholar]
- Nakagawa S. H., Tager H. S. Role of the phenylalanine B25 side chain in directing insulin interaction with its receptor. Steric and conformational effects. J Biol Chem. 1986 Jun 5;261(16):7332–7341. [PubMed] [Google Scholar]
- Pullen R. A., Lindsay D. G., Wood S. P., Tickle I. J., Blundell T. L., Wollmer A., Krail G., Brandenburg D., Zahn H., Gliemann J. Receptor-binding region of insulin. Nature. 1976 Feb 5;259(5542):369–373. doi: 10.1038/259369a0. [DOI] [PubMed] [Google Scholar]
- Schwartz G. P., Burke G. T., Katsoyannis P. G. A superactive insulin: [B10-aspartic acid]insulin(human). Proc Natl Acad Sci U S A. 1987 Sep;84(18):6408–6411. doi: 10.1073/pnas.84.18.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. P., Burke G. T., Katsoyannis P. G. [12-asparagine-B] human insulin. An analogue with modification in the hydrophobic core of insulin. Int J Pept Protein Res. 1981 Feb;17(2):243–255. doi: 10.1111/j.1399-3011.1981.tb01989.x. [DOI] [PubMed] [Google Scholar]


